Introduction
The effective functioning of the intestinal barrier
is important for the maintenance of homeostasis in this organ, as
the intestinal epithelium acts as the interface between the outer
and inner microenvironments (1).
The intestinal epithelium is a polarized monolayer that functions
as a selective filter, permitting the transport of luminal
nutrients and preventing the passage of harmful agents towards the
inner milieu (1).
Monolayer components, such as absorptive
enterocytes, are connected adjacently at the apical side of the
paracellular membrane by tight junctions (TJs), which have a key
role in the regulation of intestinal permeability (2). TJs are composed of integral
transmembrane proteins, including claudins and occludin (a member
of the TJ-associated MARVEL protein family), and junctional
adhesion molecules (JAM)-A, -B and -C (2). In the intracellular milieu, TJs
interact with the perijunctional actomyosin ring of cytoskeleton
filaments via the periplasmic scaffolding protein zonula
occludens-1 (ZO-1) (2). At the
extracellular level, claudins serve as barrier-enhancers by forming
‘kisses’ that seal the paracellular space, or enable a paracellular
flux of solutes via pore-forming or leaky pathways (3). By contrast, occludin is involved in
the regulation of the flux pathways (3). In the mouse colon, claudins of the
cation-pore (claudin-7, −12 and −15) or cation-barrier (claudin-8)
type increase electrolyte absorption or decrease permeability,
respectively (4–6). In mice, claudin-8 is more strongly
expressed than claudin-12 and −15 in the colon, whereas claudin-7
and occludin are evenly distributed in the intestinal tract
(4–6).
The mucus layer produced by goblet cells is an
extracellular component that serves an important role in intestinal
barrier function. Mucin 2, which blocks the direct contact of
microbiota with the epithelial surface to prevent an inflammatory
reaction, is located within the mucus layer (7). The colon comprises an outer loose
mucus layer rich in microbiota, and an inner layer devoid of
bacteria and tightly attached to the epithelium (7).
Chronic stress can alter the function of the colonic
barrier, leading to increased permeability, bacterial overgrowth,
infiltration of neutrophils and mucus depletion (8–11).
The deleterious effects of chronic stress on barrier function
result in part from the activation of the
hypothalamus-pituitary-adrenal (HPA) axis and the concomitant
release of corticotropin releasing factor, which induces the
activation and degranulation of colonic mast cells (11–15).
Compounds released by mast cells induce a wide array of effects on
neuroendocrine and immune pathways that regulate colonic
permeability and replenish the mucus (11–15).
Stress hormones released via HPA activation, such as adrenal
corticosteroids, are also involved in the control of colonic
permeability (16).
Assays in which mice or rats underwent
water-avoidance stress or crowding stress have been used to
investigate the effects of chronic stress on the protein and mRNA
expression of certain TJ components in the colon (such as ZO-1,
JAM-A, occludin and claudin-1, −2, −5 and −8), mucus properties and
the inflammatory response (11,16–19).
At present, the impact of chronic immobilization
stress on TJ proteins and other biomarkers involved in gut barrier
function are yet to be reported. Therefore, the present study aimed
to conduct an overall analysis of the effects of chronic
immobilization stress on epithelial components (goblet cells, TJ
proteins) and nonepithelial biomarkers (neutrophils, fecal
lactoferrin, proinflammatory cytokines, aerobic bacteria) involved
in colonic homeostasis.
Materials and methods
Animals
A total of 12 6-week-old female BALB/c mice (Unidad
de Production y Experimentacion de Animales de Laboratorio,
Universidad Autonoma Metropolitana Unidad Xochimilco) were housed
in two groups (n=6 in each group) under a 12-h light/dark cycle
(the light phase begins/ends at 7:00 a.m./7:00 p.m.), in a room at
20°C, with a relative humidity of 55%. The mice were allowed free
access to food and water (Laboratory Rodent Diet 5001; LabDiet)
ad libitum. Mice were housed for 2 weeks prior to the
initiation of the stress protocol to adapt to housing conditions.
Animal manipulations were always performed by the same trained
handler between 8:00-11:00 a.m. to reduce the influence of the
circadian cycle on fluctuations in the levels of corticosterone and
adrenocorticotropic hormones. The animal experiments (approval no.
176) were approved by The Comite Interno para el Uso y Cuidado de
Animales de Laboratorio, Universidad Autonoma Metropolitana, Unidad
Xochimilco. Animals were maintained and handled according to the
Mexican federal regulations for animal experimentation and care
(NOM-062-ZOO-1999; Ministry of Agriculture, Mexico City, Mexico)
(20).
Stress protocol
At 8 weeks of age, mice were subjected to restraint
stress or control conditions. During the short-term stress
protocol, a group of mice (n=6) was immobilized on a board, as
previously described (21). In
addition, a control (untreated) group (n=6) was included. For
immobilization, mice were placed in a prone position, and the four
limbs were gently stretched and attached with adhesive tape to an
expanded polystyrene board that was covered with plastic film for
easy cleaning. Immobilization was first applied to the fore feet,
then the hind feet and finally the tail. Very low-adhesion tape was
fastened directly to the skin of the animals on top of which
high-adhesion tape was placed, thus minimizing pain during tape
removal. Adhesive tape was placed on the dorsum of the fore feet,
the foot pads of hind feet, and the middle part of the tail. Curve
strips produced from paperboard-adhesive tape reels were placed
upon the adhesive tape as chewers for mice to prevent
self-inflicted injuries on the fore leg skin. During the assay, the
head of each mouse was allowed to move freely, whereas the twisting
of limbs and tearing of whiskers were reduced. Following
immobilization for 2 h, the adhesive tape was carefully removed in
the following order: Tail, hind feet and fore feet. This protocol
was repeated daily for 4 days, beginning at the same time every
day.
Collection of biological samples
Upon completion of the stress protocol, fecal
pellets were collected in microcentrifuge (2 ml) tubes. All animals
were subsequently sacrificed via exposure to isoflurane and
exsanguination by cardiac puncture. The colon was dissected and
flushed with sterile PBS (pH 7.2) to remove all luminal fecal
content. Then, 1-cm samples of the colon were cut for histological
examination by optical microscopy. Additional 1-cm colonic segments
were collected in sterile pre-weighed microcentrifuge tubes (2 ml)
containing 500 µl thioglycolate broth for bacterial counts. The
colonic mucosa was extracted using a glass microscope slide and
stored at −70°C for western blot and or reverse
transcription-quantitative PCR (RT-qPCR) analyses.
Neutral and acid mucin staining
procedure
Colon tissues were fixed with 4% paraformaldehyde
for 30 min at 37°C and embedded in paraffin. Tissues were cut into
7-µm-thick sections. Neutral or acid mucins in colonic samples were
detected by staining with periodic acid-Schiff (PAS) or alcian blue
(AB), respectively. Following deparaffinization with xylene for 30
min at 60°C and rehydration in a graded alcohol series, samples
were incubated in 0.5% periodic acid for 15 min at room
temperature, washed with water and incubated with Schiff's reagent
for 30 min at room temperature. The samples were then washed in
warm water. For AB staining, samples were incubated in 3% acetic
acid for 3 min at room temperature and then incubated in AB
solution (1% in 3% acetic acid) for 15 min at room temperature. The
slides were then washed in water, as previously described (22). The morphology of PAS- and
AB-positive cells was visualized by light microscopy. Stained cells
were counted in five randomly-selected fields of view (area of each
field of view, 0.05 mm2) from each mouse (magnification,
×40; ECLIPSE E 600; Nikon Corporation). The number and size of
cells were analyzed using Image-Pro Plus 5.1 software (Media
Cybernetics, Inc.).
Determination of fecal
lactoferrin
The determination of fecal lactoferrin was conducted
using a protocol based on an indirect ELISA with certain
modifications (23). The weight of
two fecal samples was measured from the difference between the
weight of the tubes before and after collection. Then, feces were
fully homogenized in 500 µl of collection buffer [PBS with protease
inhibitor cocktail (cOmplete Mini; cat. no. 11836153001;
Roche Diagnostics) and 0.1% sodium deoxycholate (cat. no. D5670;
Sigma-Aldrich; Merck KGaA)]. Fecal suspensions were centrifuged at
4°C, for 10 min at 10,000 × g, and supernatants were collected and
stored at −70°C. Total protein content was quantified using the
Bradford method (cat. no. 500-0006; Bio-Rad Protein Assay, Bio-Rad
Laboratories, Inc.). Fecal extracts were analyzed in ≥3 replicates.
The total volume of all reactions was 100-µl. Microtiter plates
(96-well; cat. no. 3590, Costar; Corning, Inc.) were coated with
fecal extracts (total protein content, 50 µg/ml) in
carbonate-bicarbonate buffer (pH 9.6) and incubated overnight at
4°C. The plates were washed three times with 200 µl PBS with 0.05 %
Tween 20. The samples were blocked with 0.5% BSA (cat. no. A2934;
Sigma-Aldrich) (24) in
carbonate-bicarbonate buffer (pH 9.6) for 2 h at 37°C. After
washing, the samples were incubated with rabbit anti-human
lactoferrin (cat. no. L3262; Sigma-Aldrich) in 0.5% BSA/PBST and
incubated overnight at 4°C. Plates were washed and then incubated
for 1 h at 37°C with horseradish peroxidase (HRP)-conjugated goat
anti-rabbit IgG (cat. no. ab97080; Abcam; 1,5,000) in 0.5%
BSA/PBST. The substrate mixture [30% hydrogen peroxide (10 µl) and
10 mg o-phenylenediamine dissolved in 0.05 M
citric-phosphate buffer (50 ml) at pH 5.0] was subsequently added
prior to incubation at room temperature for 20 min. The enzymatic
reaction was stopped with 2.5 M sulfuric acid, and the absorbance
was detected at λ=492 nm using a microplate reader. A standard
curve of human lactoferrin was generated to quantify the
lactoferrin concentration and was divided by the fecal sample
weight to calculate the lactoferrin concentration in ng/g (23).
mRNA expression assays
RNA was isolated from colonic mucosa using TRI
Reagent® (cat. no. TR 118, Molecular Research Center,
Inc.). Total RNA (0.2 µg) was used as template. The RNA was mixed
with 1 µl Oligo dT 15 primer (cat. no. C1101; Promega Corporation)
and sterile injectable water in a total volume of 25 µl. The
reaction mixture was heated at 70°C for 5 min and immediately
cooled on ice. Subsequently, the mixture was incubated with 5 µl
M-MLV 5X reaction buffer, 1.0 µl M-MLV reverse transcripatse (both
from Promega Corporation), 1.5 µl dNTPs mix (Promega Corporation)
and sterile water in a total volume of 25 µl. The reaction mixture
was heated at 42°C for 1 h using a thermocycler. cDNA was
quantified using a spectrophotometer (Nanodrop 2000, Thermo Fisher
Scientific, Inc.), and purity and integrity were evaluated by
electrophoresis on a 1.5% agarose gel. The qPCR reaction was
performed as follows: 20 µl Taq DNA polymerase master mix 1.1X
(cat. no. A120301; Ampliqon, Inc.), 1 µl forward and 1 µl reverse
primers (10 µM), 1 µl cDNA (100 ng), 1 µl EvaGreen Dye (cat. no.
31000; Biotium, Inc.) and sterile water in a total volume of 25 µl.
qPCR was performed using a Gene 6000 Rotor (Qiagen GmbH) as
follows: Initial denaturation at 95°C for 10 min, followed by 40
cycles of 95°C for 10 sec, 60°C for 15 sec, and 72°C for 20 sec.
The relative gene expression values of TJ proteins (occludin,
claudin-2, −4, −7, −12 and −15) and proinflammatory cytokines
(TNF-α, IL-1β, −6 and −8) were determined using the
2−ΔΔCq method (25) and
normalized to the constitutive gene β-actin. Specific
oligonucleotide primers for TJ proteins, cytokines and β-actin
(Table I) were designed using the
sequences provided in the database of genes at the National Center
for Biotechnology Information and Primer3 version 0.4.0 software
(26). The data were analyzed
using RegLinPCR version 2015.3 software (http://www.hartfaalcentrum.nl).
 | Table I.Primer set used for reverse
transcription-quantitative PCR analysis of tight junction proteins
and cytokines. |
Table I.
Primer set used for reverse
transcription-quantitative PCR analysis of tight junction proteins
and cytokines.
| Gene | Forward primer
(5′-3′) | Reverse primer
(5′-3′) |
|---|
| β-actin |
ATTGGCAATGAGCGGTTCA |
GGATGCCACAGGACTCCAT |
| Occludin |
ATGTCCGGCCGATGCTCTC |
CTTTGGCTGCTGTTGGGTCTG |
| Claudin-2 |
GGCTGTTAGGCACATCCAT |
TGGCACCAACATAGGAACTC |
| Claudin-4 |
ACAGGTCCTGGGAATCTCCT |
CACTGCATCTGACCTGTCCT |
| Claudin-7 |
CACACGCCTTTAATCCCAGT |
TGATGTCTCCCAAGTCCACA |
| Claudin-12 |
AAGTGGCCGAGGAGGTATTT |
GAGCAGGTCCGCGTTACACA |
| Claudin-15 |
TGAGGCTTGGCTGTTTCTTT |
AAGCCTGGCAGCTTAAAACA |
| IL-6 |
CCCCAATTTCCAATGCTCTCC |
CGCACTAGGTTTGCCGAGTA |
| IL-8 |
TGCATGGACAGTCATCCCC |
ATGACAGACCACAGAACGGC |
| IL-1β |
TGCCACCTTTTGACAGTGATG |
TGATGTGCTGCTGCGAGATT |
| TNF-α |
GATCGGTCCCCAAAGGGATG |
TTTGCTACGACGTGGGCTAC |
Western blot assay
Extracts from colonic mucosa were homogenized in 500
µl lysis buffer (0.1 M Tris-HCl pH 7.5 containing 2% SDS, 10%
glycerol, 5% 2-mercaptoethanol with a protease inhibitor cocktail
(cOmplete Mini; cat. no. 11836153001; Roche Diagnostics) as
previously described (27). Total
protein lysates were quantified using Bradford assay (Bio-Rad
Laboratories, Inc.). In total, 50 µg protein extracts were mixed
with NuPAGE LDS sample buffer (4X; cat. no. NP0007; Invitrogen;
Thermo Fisher Scientific, Inc.), NuPAGE™ Sample Reducing Agent
(10X; cat. no. NP0009; Invitrogen; Thermo Fisher Scientific, Inc.)
and double distilled water. Subsequently, the samples were boiled
at 95°C for 5 min and separated by SDS-PAGE using 10 and 12% gels
for occludin and claudins, respectively. Proteins were transferred
to PVDF membranes (cat. no. IPVH00010, EMD Millipore) and blocked
with 5% milk in TBS-Tween 20 (TBS-T) for 2 h at room temperature.
Membranes were incubated with constant agitation for 1 h at room
temperature with the following primary antibodies (all from Santa
Cruz Biotechnology, Inc.) diluted with 1% milk in TBS-T: Rabbit
anti-occludin (1:500; cat. no. sc-5562); rabbit anti-claudin-2
(1:500; cat. no. sc-133464); goat anti-claudin-4 (1:1,000; cat. no.
sc-17664); goat anti-claudin-7 (1:1,000; cat. no. sc-17670); rabbit
anti-claudin-12 (1:500; cat. no. sc-98608); rabbit anti-claudin-15
(1:500; cat. no. sc-25712) and actin (1:1,000; cat. no. sc-1615).
The membranes were subsequently incubated with continuous agitation
for 1 h at room temperature with HRP-conjugated goat anti-rabbit
IgG (cat. no. 31460, Invitrogen; Thermo Fisher Scientific, Inc.) or
HRP-conjugated rabbit anti-goat IgG (1:5,000; cat. no. 81-1620,
Invitrogen; Thermo Fisher Scientific, Inc.) diluted with 1% milk in
TBS-T. Finally, the blots were developed using a SuperSignal West
Femto enhanced chemiluminescence kit (cat. no. 34096; Thermo Fisher
Scientific, Inc.), and images were captured using a Fusion SL
system (Vilber Lourmat). Protein expression was quantified using
VisionCape Advance version 16.11a software (Vilber Lourmat).
Counting of colonic aerobic
bacteria
After the samples were weighed, 1-cm colonic
segments collected in sterile microcentrifuge tubes (2 ml)
containing thioglycolate broth (500 µl) were fully
homogenized. Colonic suspensions were serially diluted (×10) in
thioglycolate broth, and 10 µl of the serial dilutions were
plated on trypticase soy agar. Following incubation for 48 h at
37°C, the number of colonies was counted to determine the
colony-forming units (CFU)/g.
Statistical analysis
Experimental assays were repeated three times
(n=36), and representative data from one assay (n=12) are
presented. Data are expressed as the mean ± standard deviation
(n=6/group) and were compared using parametric (Student's t-test)
or nonparametric (Mann-Whitney test) tests. All data were analyzed
using SigmaPlot for Windows version 11.1 (Systat Software Inc.).
P<0.05 was considered to indicate a statistically significant
difference.
Results
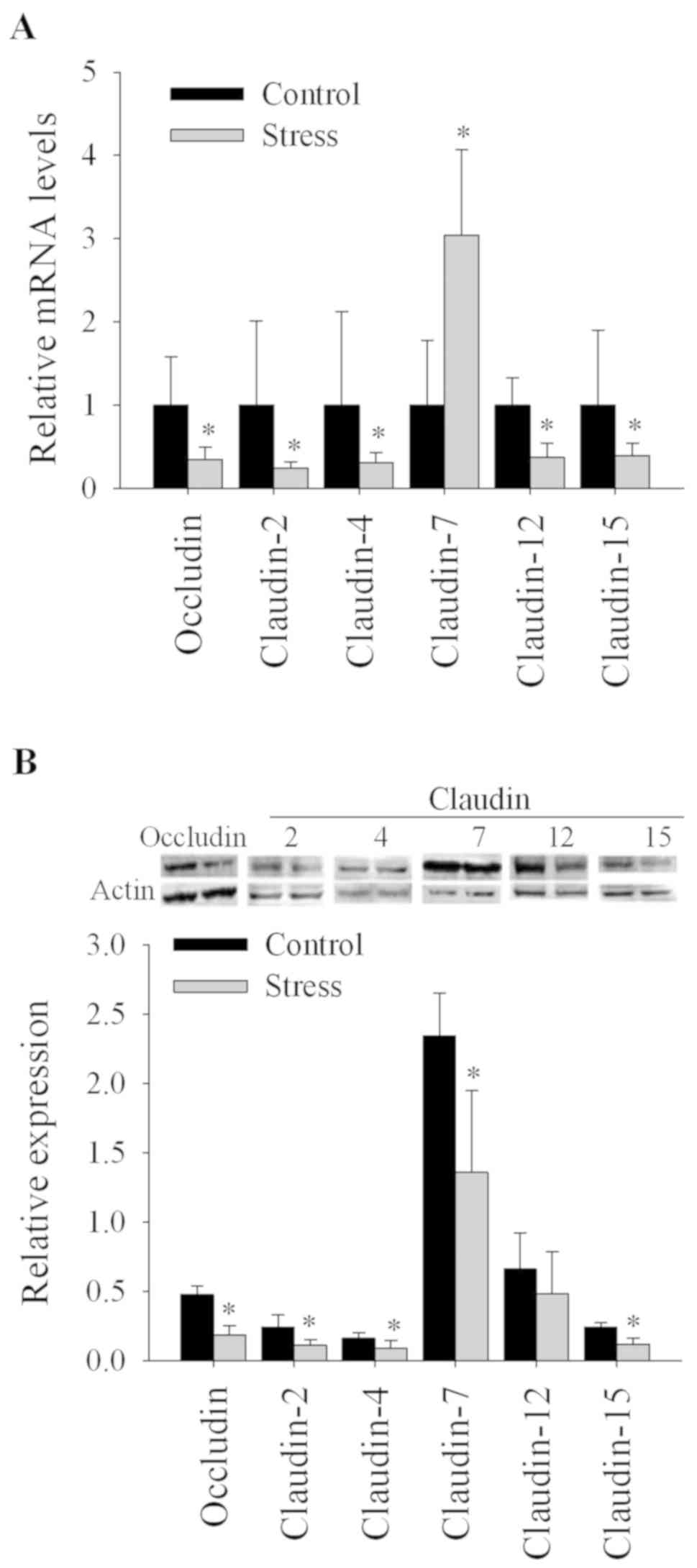
Stress decreases TJ mRNA and protein
expression in the colon
Stress affects the barrier function of the
epithelial cell layer by modulating TJ protein expression (16–19);
therefore, transcriptional and translational expression of TJ
proteins was determined. At the transcriptional level, mice
subjected to short-term repeated immobilization stress exhibited
significantly reduced mRNA expression of occludin, and claudin −2,
−4, −12 and −15 (P<0.05) and increased mRNA levels of claudin-7
(P<0.05) compared with the control group (Fig. 1). At the protein level, stressed
mice exhibited significantly reduced expression of occludin, and
claudin −2, −4, −7 and −15 (P<0.05) compared with control
animals. Additionally, the reduced claudin-12 expression in the
stressed group was not statistically significant (Fig. 1).
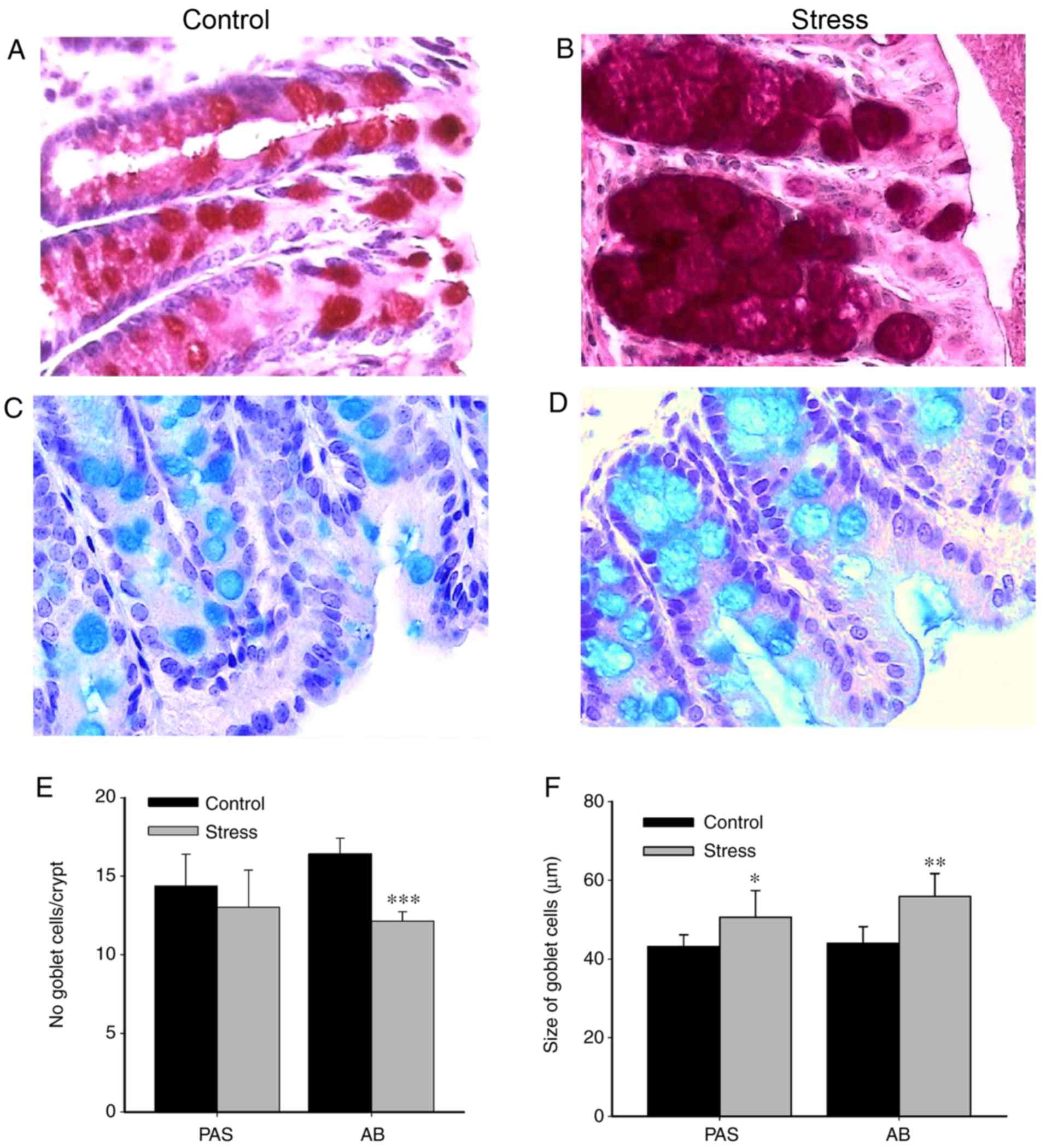
Chronic immobilization stress has no
effect on colonic inflammatory cell responses, but alters goblet
cell cellularity
Chronic stress impairs gut barrier function by
increasing the luminal infiltration of proinflammatory cells
(9,11) and altering intestinal mucosal cells
(28). Therefore, the effect of
immobilization stress on the colonic proinflammatory response was
investigated (Fig. 2). Compared
with the control unstressed group, the colonic infiltration of
polymorphonuclear leukocytes was not significantly altered in the
stressed group (data not shown). Histological analysis was
performed to identify and evaluate the presence of goblet cells,
which are the major producers of mucins. AB and PAS staining were
conducted to detect acid and neutral mucopolysaccharides,
respectively. Compared with the control, goblet cells in the colons
of stressed animals exhibited markedly increased granule density of
neutral (Fig. 2A and B) and acid
mucins (Fig. 2C and D).
Furthermore, the number of PAS-reactive goblet cells was not
significantly different between the control and stressed groups;
however, the number of AB-reactive goblet cells was significantly
decreased in colonic samples from stressed mice stained compared
with the control (P<0.001; Fig.
2E). Conversely, the size of goblet cells was significantly
increased in the stressed group compared with the control (PAS,
P<0.05; AB, P<0.01; Fig.
2F).
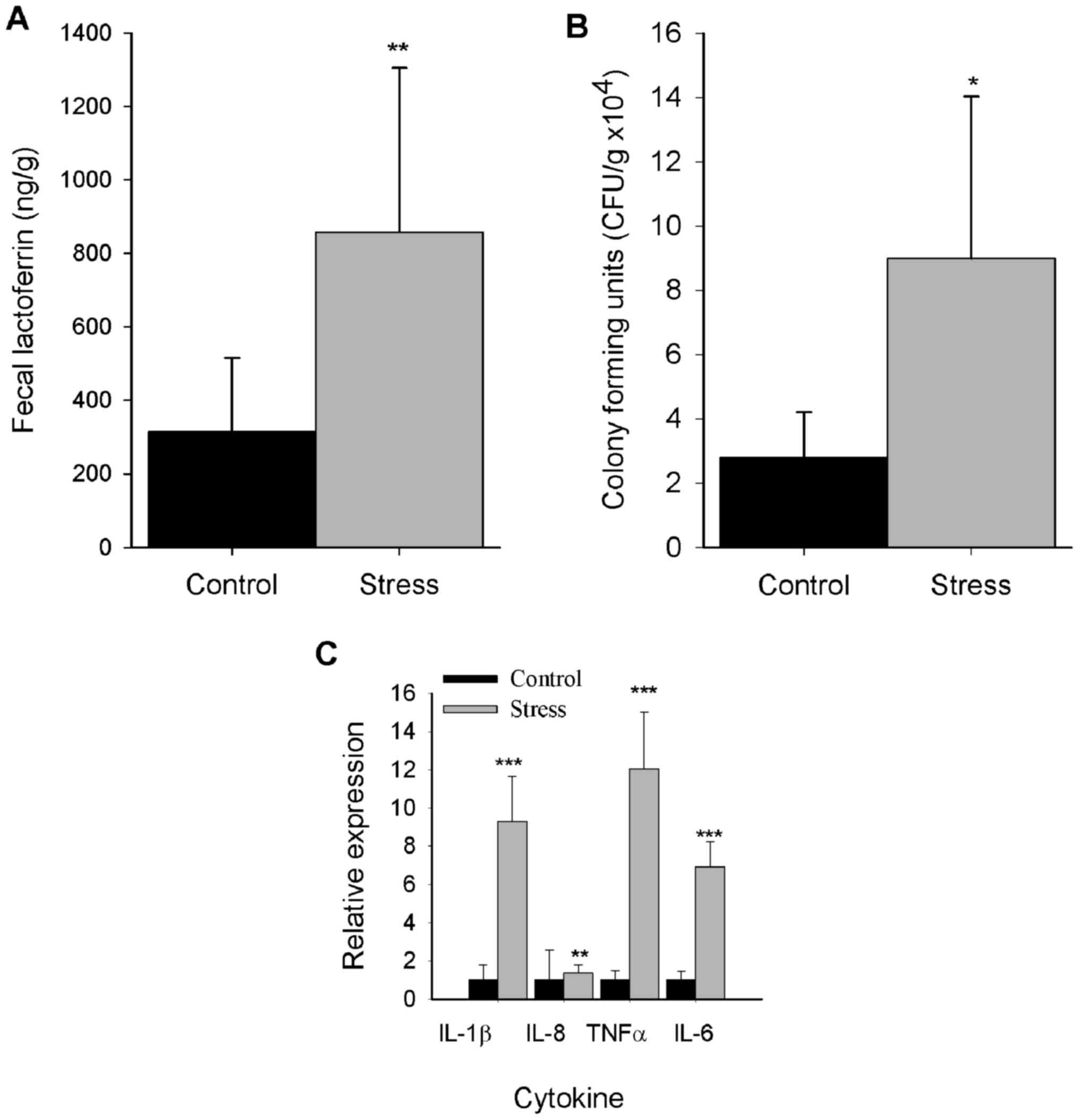
Stress elicits an increase in fecal
lactoferrin levels
Fecal lactoferrin is a noncellular biomarker of
inflammation that serves an important role in gut barrier function
(23,29). Thus, fecal lactoferrin was
quantified to investigate the effects of repeated immobilization
stress on inflammation. As presented in Fig. 3A, fecal extracts from the stressed
group exhibited significantly increased levels of fecal lactoferrin
compared with the control group (P<0.01).
Stress induces colonic growth of
aerobic bacteria
Stress induces deleterious effects on intestinal
homeostasis by promoting the overgrowth of microbiota members,
leading to dysbiosis (30);
therefore, the abundance of aerobic bacteria was determined in the
stress model of the present study. Compared with the control, a
significant increase in aerobic bacterial colony formation was
detected in the colons of stressed mice (P<0.05; Fig. 3B).
Stress induces mRNA expression of
proinflammatory cytokines
As previously reported, chronic stress alters TJ
protein expression by eliciting the generation of components
involved in the inflammatory response, such as tumor necrosis
factor (TNF)-α and interleukin (IL)-1β (17). Therefore, the expression of
inflammatory cytokines in colonic mucosa was determined. It was
revealed that chronic stress upregulated the expression TNF-α,
IL-1β, IL-6 (P<0.001) and IL-8 (P<0.01) mRNA levels compared
with the control group (Fig.
3C).
Discussion
The effects of chronic stress on transcriptional
and/or protein expression of TJ proteins has been analyzed in rats
and mice subjected to water-avoidance stress and crowding stress
(16–19); however, to the best of our
knowledge, the present study is the first report of the effects of
chronic immobilization stress on the colonic expression of
occludin, and claudin-2, −4, −7, −12 and −15. It was observed that
immobilization stress downregulated the expression of TJ proteins
at the transcriptional and protein levels.
As previously reported following water-avoidance
stress in mice and rats, stress reduced the transcription and
protein expression of occludin (16–19).
Occludin collaborates with claudin-2 in the regulation of the
paracellular flux of solutes and ultimately barrier function
(3). Occludin serves a role in
promoting a leaky pathway in the epithelium suggesting unrestricted
macromolecule flux; however, claudin-2 is a pore-forming claudin
involved in the restricted diffusion of ions via the pore pathway
(31). It has been hypothesized
that interactions between dephosphorylated or phosphorylated forms
of occludin and claudin-2 via ZO-1 control the regulation of leaky
or pore-forming TJ pathways, respectively (3).
Previous studies using water-avoidance stress or
crowding stress on rats revealed that claudin-2 protein expression
was unchanged or increased, and positively associated with TNF-α
mRNA levels following stress (17,18).
In the present study, repeated immobilization stress downregulated
claudin-2 expression at the transcriptional and protein level,
whereas stress induced an increase in the mRNA expression of TNF-α
and other proinflammatory cytokines, including IL-1β and IL-6. The
discrepancy may reflect independent TNF-α mechanisms underlying
claudin-2 expression during stress responses that may involve
mediators of oxidative burst, such as reactive oxygen species (ROS)
(11,32). It was previously reported that ROS,
such as hydrogen peroxide, decreased the expression of claudin-2
and occludin in polarized Caco-2 cell monolayer cultures (33).
In the present study, immobilization stress
downregulated claudin-4, a pore-sealing claudin, and claudin-7, −12
and −15, which are regarded as pore-forming claudins that permit
cation flux (4,34). Claudin-7 exhibited either increased
mRNA expression or reduced protein expression in stressed animals;
this discrepancy potentially resulted from decreased translation of
claudin-7 mRNA or rapid protein turnover (6). Divergence between the significant
decrease in claudin-12 mRNA and the non-significant effect on
claudin-12 protein (potentially due to data dispersion) was
observed. Despite the mismatch, the findings suggested an
inhibitory effect of stress on claudin-12 expression. In the
present study, claudin-7 was overexpressed; however, the underlying
mechanism remains unclear. Dissimilar to other claudins, claudin-7
is distributed and highly expressed evenly throughout the
intestinal tract, and located more prominently on the basolateral
membrane compared with the apical tips of epithelial cells
(4).
Experimental data from male rats or male BALB/c mice
indicated that chronic water-avoidance stress evoked a mast
cell-dependent response associated with mucus depletion,
concomitant inflammation as determined by increased infiltration of
mononuclear cells and/or neutrophils, and a reduced ratio of
colonic goblet cells to epithelial cells (9,11).
In other studies, histological differences (morphology and
inflammation) in the colons of male mice under restraint stress and
control mice were not observed (35). In the present study, chronic stress
reduced the goblet cell number and induced the enlargement of their
size and granule density, as determined via AB staining. The
stress-induced reduction in goblet cell number may induce
proinflammatory effects by decreasing the mucus layer thickness,
increasing contact between luminal bacteria and the epithelial
surface (36). Furthermore, in the
current study, chronic stress elicited the transcription of IL-8, a
potent chemokine; however, a significant increase in
polymorphonuclear leukocyte infiltration was not observed (data not
shown). As the experiments presented in this study were conducted
in female mice, inconsistencies between the present and previous
findings may arise, in part, due to the effects of sexual
dimorphism of colonic homeostasis (37). Compared with male and
postmenopausal counterparts, young fertile females exhibit more
robust intestinal homeostasis and are less prone to the effects of
chronic stress on the inflammatory response, due in part to the
protective role of estrogens (38).
According to the present findings, immobilization
stress elicited the production of fecal lactoferrin, which is
regarded as an inflammatory biomarker. To the best of our
knowledge, the effects of immobilization stress on colonic
inflammation as determined by fecal lactoferrin have not been
previously reported. As previously demonstrated, the induction of
fecal lactoferrin production is caused by increased luminal
infiltration of circulant neutrophils, as observed in inflammatory
conditions, such as those induced by gut infections and intestinal
bowel disease (23,29). In this assay, increased production
of fecal lactoferrin and IL-8 mRNA (a neutrophil-chemotactic
chemokine) (39) was observed;
however, no significant differences in luminal infiltration of
neutrophils were detected (data not shown). The latter finding may
suggest an inhibitory effect of the stress glucocorticoid response
on colonic neutrophil recruitment and/or proliferation, as
described in models of colitis, including water-avoidance stress
and dinitrofluorobenzene (40,41).
Thus, the stimulation of fecal lactoferrin production suggests an
increased transudation of circulant lactoferrin released by
degranulated neutrophils (29),
demonstrating the complex interplay of neuroendocrine and immune
pathways that regulate the gut barrier function, as observed in
intestinal diseases associated with an emotional component, such as
irritable bowel syndrome (42). In
this study, the overgrowth of aerobic bacteria was observed. It was
previously reported that, under stress conditions, catecholamine
stress hormones separate iron from iron-binding proteins, such as
lactoferrin, promoting iron uptake and eliciting intestinal
bacteria growth (30).
In the present study, the permeability of the
epithelial barrier was not determined; however, the function of the
epithelial barrier can be unaffected, despite an increase in
neutrophil transmigration induced by occludin disruption (43). Additionally, the expression of
proinflammatory cytokines was determined exclusively via RT-qPCR
analysis without conducting functional assays (ELISA and/or flow
cytometry). Even with these limitations, the animal study provides
an experimental background to inform research intended for the
development of therapies to treat dysfunctions in which stress has
a prominent proinflammatory role, such as inflammatory bowel
syndrome, and chronic inflammatory diseases in which stress is a
contributing factor, such as ulcerative colitis (44,45).
In conclusion, the findings from the present study
revealed that short-term immobilization stress downregulated the
expression of TJ proteins, and induced a proinflammatory response
involving the production of fecal lactoferrin, the expression of
proinflammatory cytokines at the mRNA level and an overgrowth of
gut aerobic bacteria. The study provides novel insight regarding
the expression of TJ proteins in the intestinal epithelium and
inflammation in response to short-term immobilization stress. These
findings may reflect underlying mechanisms involving crosstalk
between components of the systemic and local neuroendocrine
responses aimed at reestablishing colonic homeostasis in the gut
barrier during and following chronic stress.
Acknowledgements
Not applicable.
Funding
The present study was supported by Secretaría de
Investigación y Posgrado of Instituto Politécnico Nacional
(Marycarmen Godinez-Victoria; project no. 20161692).
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
NMR, TSE and JICS performed the RT-qPCR assays,
analysis and interpretation of data. NMR and MGV performed the
western blotting, analysis and interpretation of data. JPY
performed the histological examination of the colon, analysis and
interpretation of data. RCR and MEDS made substantial contributions
to the conception and design of the study, and drafting the
manuscript.
Ethics approval and consent to
participate
The present study (approval no. 176) was approved by
The Comite Interno para el Uso y Cuidado de Animales de
Laboratorio, Universidad Autonoma Metropolitana Unidad Xochimilco.
Animals were maintained and handled according to The Mexican
federal regulations for animal experimentation and care
(NOM-062-ZOO-1999; Ministry of Agriculture, Mexico City, Mexico)
(20).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zhang K, Hornef MW and Dupont A: The
intestinal epithelium as guardian of gut barrier integrity. Cell
Microbiol. 17:1561–1569. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
France MM and Turner JR: The mucosal
barrier at a glance. J Cell Sci. 130:307–314. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Turner JR, Buschmann MM, Romero-Calvo I,
Sailer A and Shen L: The role of molecular remodeling in
differential regulation of tight junction permeability. Semin Cell
Dev Biol. 36:204–212. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fujita H, Chiba H, Yokozaki H, Sakai N,
Sugimoto K, Wada T, Kojima T, Yamashita T and Sawada N:
Differential expression and subcellular localization of claudin-7,
−8, −12, −13, and −15 along the mouse intestine. J Histochem
Cytochem. 54:933–944. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Holmes JL, Van Itallie CM, Rasmussen JE
and Anderson JM: Claudin profiling in the mouse during postnatal
intestinal development and along the gastrointestinal tract reveals
complex expression patterns. Gene Expr Patterns. 6:581–588. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Inai T, Sengoku A, Guan X, Hirose E, Iida
H and Shibata Y: Heterogeneity in expression and subcellular
localization of tight junction proteins, claudin-10 and −15,
examined by RT-PCR and immunofluorescence microscopy. Arch Histol
Cytol. 68:349–360. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Faderl M, Noti M, Corazza N and Mueller C:
Keeping bugs in check: The mucus layer as a critical component in
maintaining intestinal homeostasis. IUBMB Life. 67:275–285. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bailey MT, Dowd SE, Parry NM, Galley JD,
Schauer DB and Lyte M: Stressor exposure disrupts commensal
microbial populations in the intestines and leads to increased
colonization by Citrobacter rodentium. Infect Immun. 78:1509–1519.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cameron HL and Perdue MH: Stress impairs
murine intestinal barrier function: Improvement by glucagon-like
peptide-2. J Pharmacol Exp Ther. 314:214–220. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Reber SO, Peters S, Slattery DA, Hofmann
C, Schölmerich J, Neumann ID and Obermeier F: Mucosal
immunosuppression and epithelial barrier defects are key events in
murine psychosocial stress-induced colitis. Brain Behav Immun.
25:1153–1161. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Söderholm JD, Yang PC, Ceponis P, Vohra A,
Riddell R, Sherman PM and Perdue MH: Chronic stress induces mast
cell-dependent bacterial adherence and initiates mucosal
inflammation in rat intestine. Gastroenterology. 123:1099–1108.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Santos J, Yang PC, Söderholm JD, Benjamin
M and Perdue MH: Role of mast cells in chronic stress induced
colonic epithelial barrier dysfunction in the rat. Gut. 48:630–636.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Santos J, Yates D, Guilarte M, Vicario M,
Alonso C and Perdue MH: Stress neuropeptides evoke epithelial
responses via mast cell activation in the rat colon.
Psychoneuroendocrinology. 33:1248–1256. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Vicario M, Guilarte M, Alonso C, Yang P,
Martínez C, Ramos L, Lobo B, González A, Guilà M, Pigrau M, et al:
Chronological assessment of mast cell-mediated gut dysfunction and
mucosal inflammation in a rat model of chronic psychosocial stress.
Brain Behav Immun. 24:1166–1175. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Vicario M, Alonso C, Guilarte M, Serra J,
Martínez C, González-Castro AM, Lobo B, Antolín M, Andreu AL,
García-Arumí E, et al: Chronic psychosocial stress induces
reversible mitochondrial damage and corticotropin-releasing factor
receptor type-1 upregulation in the rat intestine and IBS-like gut
dysfunction. Psychoneuroendocrinology. 37:65–77. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zheng G, Wu SP, Hu Y, Smith DE, Wiley JW
and Hong S: Corticosterone mediates stress-related increased
intestinal permeability in a region-specific manner.
Neurogastroenterol Motil. 25:e127–e139. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hattay P, Prusator DK, Tran L and
Greenwood-Van Meerveld B: Psychological stress-induced colonic
barrier dysfunction: Role of immune-mediated mechanisms.
Neurogastroenterol Motil. 29:2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lauffer A, Vanuytsel T, Vanormelingen C,
Vanheel H, Salim Rasoel S, Tóth J, Tack J, Fornari F and Farré R:
Subacute stress and chronic stress interact to decrease intestinal
barrier function in rats. Stress. 19:225–234. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nébot-Vivinus M, Harkat C, Bzioueche H,
Cartier C, Plichon-Dainese R, Moussa L, Eutamene H, Pishvaie D,
Holowacz S, Seyrig C, et al: Multispecies probiotic protects gut
barrier function in experimental models. World J Gastroenterol.
20:6832–6843. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Official Mexican Standard
NOM-062-ZOO-1999, . Technical specifications for the production,
care and use of laboratory animals. Secretary of agriculture,
livestock, rural development, fisheries and Food (SAGARPA).
Official Gazette, Mexican Federal Government. June 18–2001.
|
|
21
|
Bhatia N, Maiti PP, Choudhary A, Tuli A,
Masih D, Khan U, Ara T and Jaggi AS: Animal models in the study of
stress: A review. NSHM J Pharm Healthcare Manage. 2:42–50.
2011.
|
|
22
|
Uni Z, Smirnov A and Sklan D: Pre- and
posthatch development of goblet cells in the broiler small
intestine: Effect of delayed access to feed. Poult Sci. 82:320–327.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Logsdon LK and Mecsas J: A non-invasive
quantitative assay to measure murine intestinal inflammation using
the neutrophil marker lactoferrin. J Immunol Methods. 313:183–190.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xiao Y and Isaacs SN: Enzyme-linked
immunosorbent assay (ELISA) and blocking with bovine serum albumin
(BSA)-not all BSAs are alike. J Immunol Methods. 384:148–151. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rozen S and Skaletsky H: Primer3 on the
WWW for general users and for biologist programmers. Methods Mol
Biol. 132:365–386. 2000.PubMed/NCBI
|
|
27
|
Kyoko OO, Kono H, Ishimaru K, Miyake K,
Kubota T, Ogawa H, Okumura K, Shibata S and Nakao A: Expressions of
tight junction proteins Occludin and Claudin-1 are under the
circadian control in the mouse large intestine: Implications in
intestinal permeability and susceptibility to colitis. PLoS One.
9:e980162014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liévin-L Moal V and Servin AL: The front
line of enteric host defense against unwelcome intrusion of harmful
microorganisms: Mucins, antimicrobial peptides, and microbiota.
Clin Microbiol Rev. 19:315–337. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Siddiqui I, Majid H and Abid S: Update on
clinical and research application of fecal biomarkers for
gastrointestinal diseases. World J Gastrointest Pharmacol Ther.
8:39–46. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sandrini SM, Shergill R, Woodward J,
Muralikuttan R, Haigh RD, Lyte M and Freestone PP: Elucidation of
the mechanism by which catecholamine stress hormones liberate iron
from the innate immune defense proteins transferrin and
lactoferrin. J Bacteriol. 192:587–594. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Al-Sadi R, Khatib K, Guo S, Ye D, Youssef
M and Ma T: Occludin regulates macromolecule flux across the
intestinal epithelial tight junction barrier. Am J Physiol
Gastrointest Liver Physiol. 300:G1054–G1064. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ponferrada A, Caso JR, Alou L, Colón A,
Sevillano D, Moro MA, Lizasoain I, Menchén P, Gómez-Lus ML, Lorenzo
P, et al: The role of PPARgamma on restoration of colonic
homeostasis after experimental stress-induced inflammation and
dysfunction. Gastroenterology. 132:1791–1803. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Iraha A, Chinen H, Hokama A, Yonashiro T,
Kinjo T, Kishimoto K, Nakamoto M, Hirata T, Kinjo N, Higa F, et al:
Fucoidan enhances intestinal barrier function by upregulating the
expression of claudin-1. World J Gastroenterol. 19:5500–5507. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Nevado R, Forcén R, Layunta E, Murillo MD
and Grasa L: Neomycin and bacitracin reduce the intestinal
permeability in mice and increase the expression of some
tight-junction proteins. Rev Esp Enferm Dig. 107:672–676. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Koh SJ, Kim JW, Kim BG, Lee KL and Kim JS:
Restraint stress induces and exacerbates intestinal inflammation in
interleukin-10 deficient mice. World J Gastroenterol. 21:8580–8587.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kim JJ and Khan WI: Goblet cells and
mucins: Role in innate defense in enteric infections. Pathogens.
2:55–70. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bourke CH, Harrell CS and Neigh GN:
Stress-induced sex differences: Adaptations mediated by the
glucocorticoid receptor. Horm Behav. 62:210–218. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Grishina I, Fenton A and Sankaran-Walters
S: Gender differences, aging and hormonal status in mucosal injury
and repair. Aging Dis. 5:160–169. 2014.PubMed/NCBI
|
|
39
|
Andrews C, McLean MH and Durum SK:
Cytokine tuning of intestinal epithelial function. Front Immunol.
9:12702018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Cakir B, Bozkurt A, Ercan F and Yeğen BC:
The anti-inflammatory effect of leptin on experimental colitis:
Involvement of endogenous glucocorticoids. Peptides. 25:95–104.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Rijnierse A, Koster AS, Nijkamp FP and
Kraneveld AD: TNF-alpha is crucial for the development of mast
cell-dependent colitis in mice. Am J Physiol Gastrointest Liver
Physiol. 291:G969–G976. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Camilleri M, Sellin JH and Barrett KE:
Pathophysiology, evaluation, and management of chronic watery
diarrhea. Gastroenterology. 152:515–532.e2. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Lapointe TK and Buret AG: Interleukin-18
facilitates neutrophil transmigration via myosin light chain
kinase-dependent disruption of occludin, without altering
epithelial permeability. Am J Physiol Gastrointest Liver Physiol.
302:G343–G351. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ananthakrishnan AN: Environmental triggers
for inflammatory bowel disease. Curr Gastroenterol Rep. 15:3022013.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Konturek PC, Brzozowski T and Konturek SJ:
Stress and the gut: Pathophysiology, clinical consequences,
diagnostic approach and treatment options. J Physiol Pharmacol.
62:591–599. 2011.PubMed/NCBI
|