Introduction
Liver transplantation remains at present an
effective treatment for patients with end-stage liver disease. Due
to the increasing disparity between the number of patients on the
waiting list and the number of donor organs available (1), the use of livers from donors after
circulatory death (DCD) has been deemed to be a useful strategy to
overcome organ shortage (2,3).
However, compared with the recipients of livers from donors after
brain death, patients who receive DCD liver transplantation have
worse prognoses (3). Additionally,
the incidence of biliary complications and retransplantation is
increasing in the recipients of DCD livers (4). The notable factor resulting in the
poor outcome of patients with DCD liver grafts is
ischemia-reperfusion injury (IRI) (5).
Hepatic IRI results from the combined effect of
multiple pathological responses occurring during ischemia and
reperfusion processes (6). As
livers from DCD donors endure an unpredictable duration of warm
ischemia, they are more susceptible to IRI (6,7).
Presently, IRI has been established to be one of the primary
reasons for post-transplantation complications including chronic
graft rejection and primary graft nonfunction (8). The pathological characteristics of
IRI include an reactive oxygen species (ROS) overabundance,
mitochondrial dysfunction and sodium-potassium pump failure
(9,10). A combination of mitochondrial
dysfunctions in the ischemia phase and the activation of innate
immunity in the reperfusion phase result in a deterioration in
organ function (11,12). Among the notable molecular events
implicated in IRI, an excessive innate inflammatory response has
attracted particular attention. Damage associated molecular
patterns released from the stressed cells bind to pattern
recognition receptors (PRRs), resulting in the activation of innate
immune cells (12,13). Toll-like receptor 4 (TLR4) have
been hypothesized to have a pivotal function in harmful, aseptic
inflammation (13). Therefore, the
present study hypothesized that inhibiting the TLR4 signaling
pathway in DCD livers may reduce tissue reperfusion-induced
inflammation and accordingly improve the quality of DCD grafts.
A number of clinical approaches are presently being
applied to minimize IRI, including ischemia pretreatment (IPC),
normothermic mechane perfusion and hypothermic mechane perfusion
(14,15). Radojkovic et al (16) revealed that IPC exerts a protective
effect on IRI by reducing oxidative stress and inflammation in
vivo. Mergental et al (17) reported that normothermic machine
perfusion increases organ availability for liver transplantation.
Nevertheless, it is difficult to apply these approaches on a wider
scale due to the complicated techniques involved. Comparatively,
pharmacological manipulations of those necessary signaling pathways
are a promising strategy to reduce liver IRI (15). TAK242 is a specific inhibitor of
the TLR4 signaling pathway (18).
It has been reported that TAK242 ameliorates tissue injury by
dampening the innate immune response (19). However, so far it is unclear
whether TAK242 may alleviate IRI in the DCD grafts by inhibiting
the TLR4 signaling pathway.
In the present study, the effects of TAK242 on the
TLR4 signaling pathway, inflammatory cytokine release and tissue
damage were evaluated using a rat DCD model. It was hypothesized
that TAK242 ameliorates hepatic IRI and resultantly improves the
quality of DCD grafts via reducing the release of pro-inflammatory
cytokines and inhibiting the excessive innate immune response. The
present study aimed to determine the effectiveness of the TLR4
inhibitor in reducing liver IRI and improving the quality of DCD
grafts.
Materials and methods
Animals
The present experiment was conducted with the
ethical approval of the Animal Experimental Ethics Committee of
Wuhan University (Wuhan, China) and all experimental procedures
were performed in accordance with the Guidance for the Care and Use
of Laboratory Animals (20). A
total of 24 male Sprague-Dawley (SD) rats (weighing 250–300 g) were
purchased from Beijing Vital River Laboratory Animal Technology
Co., Ltd. (Beijing, China) and maintained at 22–24°C, with a 12-h
light/dark cycle and ad libitum access to food and
water.
Experimental design
Rats were randomly divided into 4 groups (n=6 each),
and all rats were fasted for 12 h prior to the initiation of the
experimental procedures (but without drinking restrictions). For
the control group, the rats were pretreated with intraperitoneal
injections of 0.1% dimethyl sulfoxide (DMSO) as the vehicle for 30
min prior to the surgical operations, and then anesthetized with
pentobarbital sodium (50 mg/kg intraperitoneally) and the livers
without warm ischemia exposure were obtained by midline laparotomy,
followed by 24 h storage at 4°C in University of Wisconsin (UW)
solution (DowDuPont, Inc.). Subsequently, the livers were connected
to an isolated perfused rat liver system (IPRL) and perfused at
37±0.5°C for 1 h. For the TAK242 group, TAK242 was dissolved in
DMSO and diluted with physiological saline. Doses of TAK-242 were
determined based on preliminary tests (using 0.1, 0.5 and 1.0
mg/kg) and on previous studies (18,21).
The preliminary tests revealed that 0.5 and 1.0 mg/kg TAK-242
induced protective effects in DCD livers, and that 1.0 mg/kg
TAK-242 was more effective. The rats were pretreated with an
intraperitoneal injection of TAK242 (1.0 mg/kg) at 37°C for 30 min
prior to all surgical operations and all other operations were
performed consistent with the control group. For the DCD group, the
livers were subjected to in situ warm ischemia at 37°C for
30 min, but all other operations were performed in the same manner
as in the control group. For the DCD+TAK242 group, the rats were
pretreated with TAK242 (1.0 mg/kg) at 37°C for 30 min, as
previously described (22), but
all other procedures were similar between the DCD+TAK242 group and
the DCD group.
DCD model and liver procurement
Following midline laparotomy, cardiac arrest was
induced by an incision of the diaphragm (23), and simultaneously, curved vascular
clamping was used to prevent blood flow in the thoracic aorta.
Following 30 min of warm ischemia at 37°C, the livers were perfused
with 40 ml cold heparinized Ringer's solution (Shanghai Guandao
Biological Engineering Co., Ltd.) via the aorta abdominalis.
Subsequently, the portal vein and infrahepatic vena cava were
cannulated with polyethylene pipes, followed by the ligation of the
superior hepatic caval and the right adrenal veins. The rats in the
control group underwent the same procedure but with an absence of
warm ischemia.
IPRL system
Following cold storage, the livers were connected to
the IPRL system and subjected to normothermic perfusion for 60 min
at 37±0.5°C, as previously described (24). The IPRL system was based on
previously described methods (24). Freshly prepared Krebs-Henseleit
bicarbonate buffer (Sigma-Aldrich; Merck KGaA) with 95%
O2 and 5% CO2 was pumped into the liver via
the portal vein with a peristaltic pump (Shanghai Jingke Industrial
Co., Ltd.). The flow velocity of the perfusate was set at a flow
rate of 15 ml/min. To ensure that portal pressure did not exceed 8
mmHg, pressure sensors (Chengdu Techman Software Co., Ltd.) were
used to monitor portal pressure. The perfusate was serially
collected from the intrahepatic vena at 5, 30 and 60 min. Following
perfusion, the perfusate and tissue samples were stored at −80°C
for later analyses.
Measurement of perfusate
aminotransferase
The perfusates were stored in a refrigerator at
−80°C for 24 h and tested after all liver perfusions have been
completed. The levels of alanine aminotransferase (ALT) and
aspartate aminotransferase (AST) in the perfusate were determined
using an automatic biochemical analyzer (Hitachi, Ltd., Tokyo,
Japan) in the clinical laboratory of Zhongnan Hospital of Wuhan
University.
Histopathology and terminal
deoxynucleotidyl-transferase-mediated dUTP nick end labelling
(TUNEL) assay
Liver samples were sliced into small pieces
following 1 h of perfusion and were fixed in 4% buffered
paraformaldehyde at room temperature for 24 h, embedded in paraffin
at 60°C for 1 h and stained with haemotoxylin and eosin (H&E)
at room temperature for 10 min. The severity of liver IRI was
blindly graded according to Suzuki's criteria (25). Briefly, the degree of sinusoidal
congestion, vacuolization/ballooning and hepatocyte necrosis are
graded on a scale from 0 to 4 (25). Histopathological changes were
evaluated by two pathologists who were blinded to this experiment.
Paraffin-embedded tissue sections were examined for apoptosis using
a TUNEL staining assay (Roche Diagnostics, Indianapolis, IN, USA)
according to the manufacturer's protocol and tissue sections were
incubated with fluorescein-dUTP at 37°C for 1 h. Total hepatocytes
and TUNEL-positive cells were counted in 6 randomly selected fields
of view, and the apoptotic index (the percentage of apoptotic cells
in all hepatocytes in each view) was calculated with the use of
Image-Pro Plus 6.0 (Media Cybernetics, Inc., Rockville, MD,
USA).
Immunohistochemistry and
immunofluorescence
The protein expression levels of high-mobility group
box protein b1 (HMGB1) and TLR4 in liver tissues were analyzed by
immunohistochemistry using polyclonal rabbit primary antibodies and
secondary polymeric horseradish peroxidase (HRP)-conjugated
anti-rabbit immunoglobulin G (IgG) antibodies. Livers were fixed in
4% buffered paraformaldehyde at room temperature for 24 h and
embedded in paraffin at 60°C for 1 h. The paraffin-embedded liver
tissues were cut into 3–4 µm-thick sections. Sections were
deparaffinized and rehydrated with a graded xylene and alcohol
series. Antigen retrieval was performed by high temperature and
pressure; 1 mM EDTA (Beijing Solarbio Science & Technology Co.,
Ltd.) was heated to ~100°C, the sections were soaked in EDTA and
heated for ~3–4 min at a pressure of 110 kPa. Sections were
incubated in 3% hydrogen peroxide at room temperature for 10 min.
Subsequently, sections were blocked in 10% BSA (Beijing Solarbio
Science & Technology Co., Ltd.) at room temperature for 30 min.
Section were incubated with rabbit anti-HMGB1 (cat. no. 10829-1-AP;
1:100; ProteinTech Group, Inc.) or rabbit anti-TLR4 (cat. no.
19811-1-AP; 1:200; ProteinTech Group, Inc.) at 4°C overnight, and
incubated with HRP-conjugated anti-rabbit IgG antibodies at room
temperature for 30 min (cat. no. SA00001-2; 1:200; ProteinTech
Group, Inc.). Sections were incubated with diaminobenzidine
(Shanghai Yeasen Biotechnology Co., Ltd.) at room temperature for 5
min. Subsequently, sections were stained using 1.5 mg/ml
hematoxylin at room temperature for 2 min. Sections were visualized
using light microscopy (magnification, ×200). HRP activity was
measured using 3,3′-diaminobenzidine substrate. The expression of
interleukin-6 (IL-6) in liver tissues was analyzed by
immunofluorescence and eight fields of view were randomly selected
to quantify IL-6 expression. Livers were fixed, embedded and
sectioned using the same method mentioned above. Sections were
blocked in 5% BSA (Beijing Solarbio Science & Technology Co.,
Ltd.) at room temperature for 2 h and were incubated with rabbit
anti-IL-6 (cat. no. 21865-1-AP; 1:100; ProteinTech Group, Inc.) at
4°C overnight. The sections were then incubated with Alexa Fluor
555-conjugated goat anti-rabbit (cat. no. P0179; 1:100; Shanghai
Biyuntian Biological Co., Ltd.) and DAPI at room temperature for 1
h (cat. no. C1002; Shanghai Biyuntian Biological Co., Ltd.).
Results were visualized using a fluorescence microscope
(magnification, ×400) and images were analyzed by Image J software
(version 1.51; National Institutes of Health).
Transmission electron microscopy
(TEM)
Tissues were cut into 1–2 mm sections and were fixed
in 2.5% glutaraldehyde at 4°C for 24 h. Subsequently, tissues were
immersed in 1% osmium tetroxide at 4°C for 2 h and were dehydrated
using a graded ethanol series. Later, tissue were embedded in Epon
resin (Thermo Fisher Scientific, Inc.) at 60°C for 36 h. Tissues
were cut into sections (thickness, ~70 nm) using a microtome (Leica
Microsystems Inc.) and stained with uranylacetate and lead citrate.
The extent of liver IRI was evaluated by observing damaged
subcellular organelles under a TEM (Tecnai G2 20 TWIN; FEI; Thermo
Fisher Scientific, Inc., Waltham, MA, USA), performed by the Wuhan
Institute of Virology, Chinese Academy of Sciences (Wuhan, China).
The severity of mitochondrial damage was blindly graded according
to Flameng criteria which evaluates mitochondrial function based on
mitochondrial morphology and grades this on a scale from 0 to 4
(26).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA from liver tissues was extracted using
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.) and RNA
was reverse transcribed into cDNA using the RevertAid First Strand
cDNA Synthesis Kit (Thermo Fisher Scientific, Inc.). RT was
performed at 42°C for 1 h followed by an incubation at 75°C for 5
min. cDNA was separated by 1% agarose gel electrophoresis. The
thermocycling conditions were as follows: 50°C for 2 min, 95°C for
10 min, followed by 40 cycles of 95°C for 10 sec and 60°C for 30
sec. The relative quantity of the assessed genes to the
housekeeping gene were analyzed using the SYBR green PCR Master Mix
(Thermo Fisher Scientific Inc.) according to the manufacturer's
protocol, and performed on a SYBR green quantitative RealTime-PCR
system (Yeasen Biotechnology Co., Ltd., Shanghai, China). The
relative expression of genes was calculated using the
2−∆∆Cq method and β-actin was used as internal standard
(27). Primers used are listed in
Table I.
 | Table I.Nucleotide sequences of primers used
for reverse transcription-quantitative polymerase chain
reaction. |
Table I.
Nucleotide sequences of primers used
for reverse transcription-quantitative polymerase chain
reaction.
| Gene |
| Primer sequence
(5′-3′) |
|---|
| HMGB1 | Forward |
GGCGGCTGTTTTGTTGACAT |
|
| Reverse |
ACCCAAAATGGGCAAAAGCA |
| TLR4 | Forward |
TGTATCGGTGGTCAGTGTGC |
|
| Reverse |
CAGCTCGTTTCTCACCCAGT |
| IL-1β | Forward |
GAGGCTGACAGACCCCAAAAGA |
|
| Reverse |
TCCACAGCCACAATGAGTGACA |
| IL-6 | Forward |
AGCGATGATGCACTGTCAGA |
|
| Reverse |
GGAACTCCAGAAGACCAGAGC |
| TNF-α | Forward |
GTGATCGGTCCCAACAAGGA |
|
| Reverse |
TTTGCTACGACGTGGGCTAC |
| COX2 | Forward |
TCCATTTGTGAAGATTCCTGTGTTG |
|
| Reverse |
TCTCACTGGCTTATGCCGAAA |
| β-actin | Forward |
TGCTATGTTGCCCTAGACTTCC |
|
| Reverse |
GTTGGCATAGGTCTTTACGG |
Western blot analysis
Frozen liver tissues were disrupted in
phenylmethylsulfonyl fluoride (Roche Applied Science, Penzberg,
Germany)-containing RIPA lysis buffer (Beyotime Institute of
Biotechnology, Haimen, China) using a tissue lyser, followed by
centrifugation (24,000 × g for 15 min at 4°C). Following heat
denaturation, Protein concentration was determined using a
bicinchoninic acid assay kit (Beyotime Institute of Biotechnology).
An equal amount of protein (50 µg) was separated on 10% SDS-PAGE
gels, followed by an electrotransfer onto PVDF membranes.
Subsequent to blocking in 5% bovine serum albumin at room
temperature for 2 h, the immunoblots were incubated with primary
antibodies at 4°C overnight, followed by incubation with the
secondary HRP-conjugated anti-rabbit IgG antibody (cat. no.
SA00001-2; 1:8,000; ProteinTech Group, Inc.) for 2 h at room
temperature. All primary and secondary antibodies were from
ProteinTech Group, Inc. (Chicago, IL, USA) and include rabbit
anti-HMGB1 (cat. no. 10829-1-AP; 1:1,000; ProteinTech Group, Inc.),
rabbit anti-TLR4 (cat. no. 19811-1-AP; 1:1,000; ProteinTech Group,
Inc.), rabbit anti-IL-1β (cat. no. 16806-1-AP; 1:500; ProteinTech
Group, Inc.), rabbit anti-IL-6 (cat. no. 21865-1-AP; 1:500;
ProteinTech Group, Inc.), rabbit anti-mitochondrially encoded
cytochrome c oxidase II (COX2; cat. no. 12375-1-AP; 1:500;
ProteinTech Group, Inc.) and rabbit anti-β-actin (cat. no.
20536-1-AP; 1:1,000; ProteinTech Group, Inc.). The bands were
visualized using enhanced chemiluminescent reagent (Wuhan Boster
Biological Technology, Ltd.) and quantified by using the Image-J
software (version 1.51; National Institutes of Health).
Malondialdehyde (MDA) and ROS
assays
To evaluate oxidative stress and lipid peroxidation,
the levels of MDA and ROS were measured using commercial
colorimetric assay kits (Nanjing Jiancheng Bioengineering
Institute, Nanjing, China) according to the manufacturer's
protocol.
Statistical analysis
Data were analyzed using SPSS 15.0 statistical
software (SPSS, Inc., Chicago, IL, USA) and the results are
expressed as the mean ± standard deviation. Differences between
groups were analyzed using a one-way analysis of variance followed
by a Kruskal-Wallis test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Effect of TAK242 on the function and
pathology of DCD livers
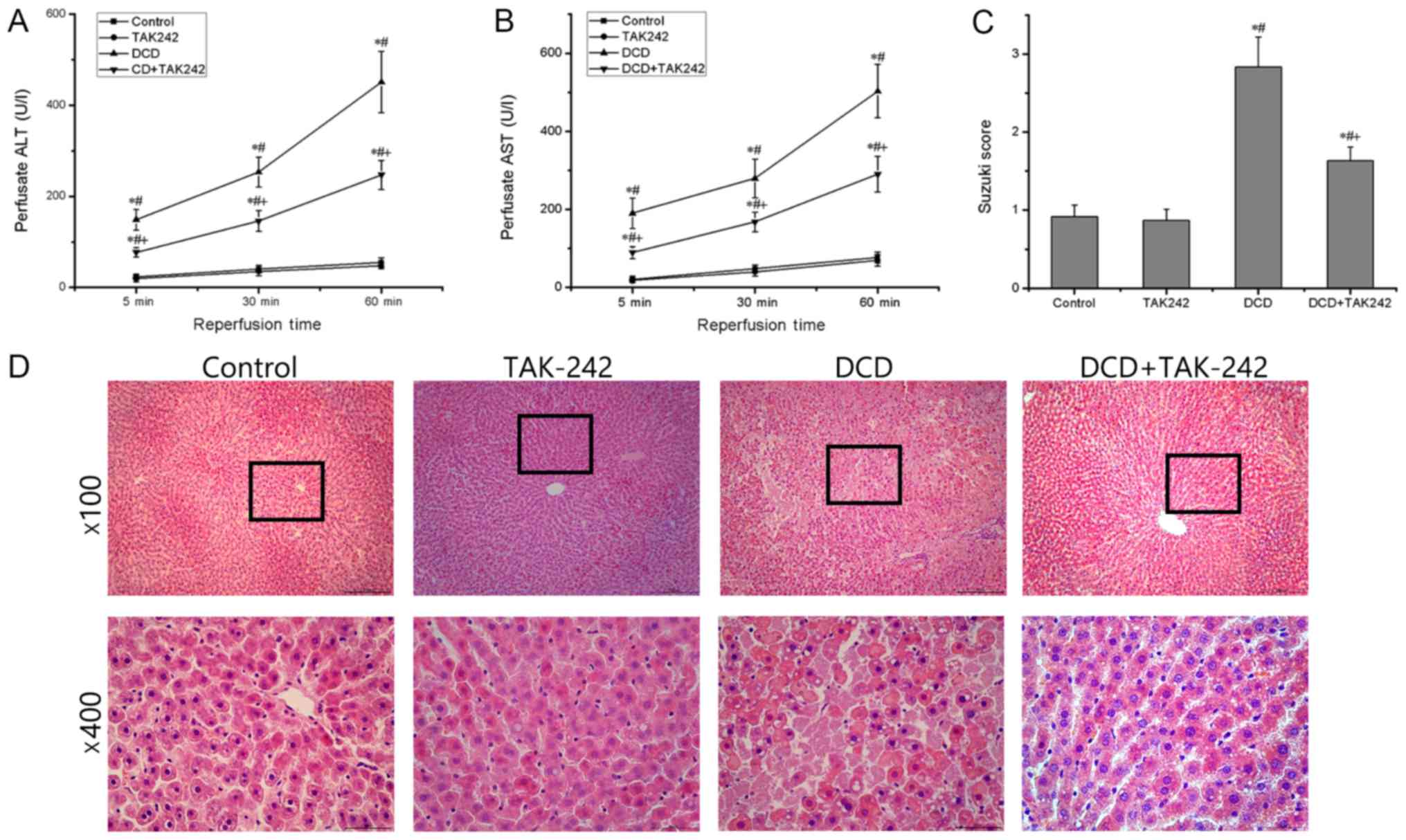
First, the present study determined the leakage of
ALT and AST into the liver perfusate, which are indicative of DCD
liver injury and altered metabolic functions. As presented in
Fig. 1A and B, the levels of two
transaminase in the perfusate were significantly increased at each
time point assessed in the DCD group when compared with those in
the control group (P<0.05). There was no statistically
significant difference in the level of transaminases between the
control group and the TAK242 group (P>0.05). However, TAK242
pretreatment for 30 min significantly decreased the levels of ALT
and AST in the perfusate compared with those in the DCD group
(P<0.05), suggesting that TAK242 pretreatment improves the
metabolic functions of the DCD livers. H&E staining revealed
that there were significantly more infiltrated inflammatory cells
and necrotic hepatocytes in the DCD group compared with in the
control group, whereas they were significantly attenuated in the
DCD+TAK242 group compared with the DCD group (Fig. 1D). Furthermore, the Suzuki scores,
used for the assessment of liver damage and hepatocyte death
(25), identified no statistically
significant differences between the control group and the TAK242
group (P>0.05) but were significantly lower in the DCD+TAK242
group when compared with the DCD group (P<0.05; Fig. 1C). These results indicate that
pretreatment with TAK242 alleviates the IRI of the DCD livers.
Effect of TAK242 on oxidative stress
and hepatocytic apoptosis in DCD livers
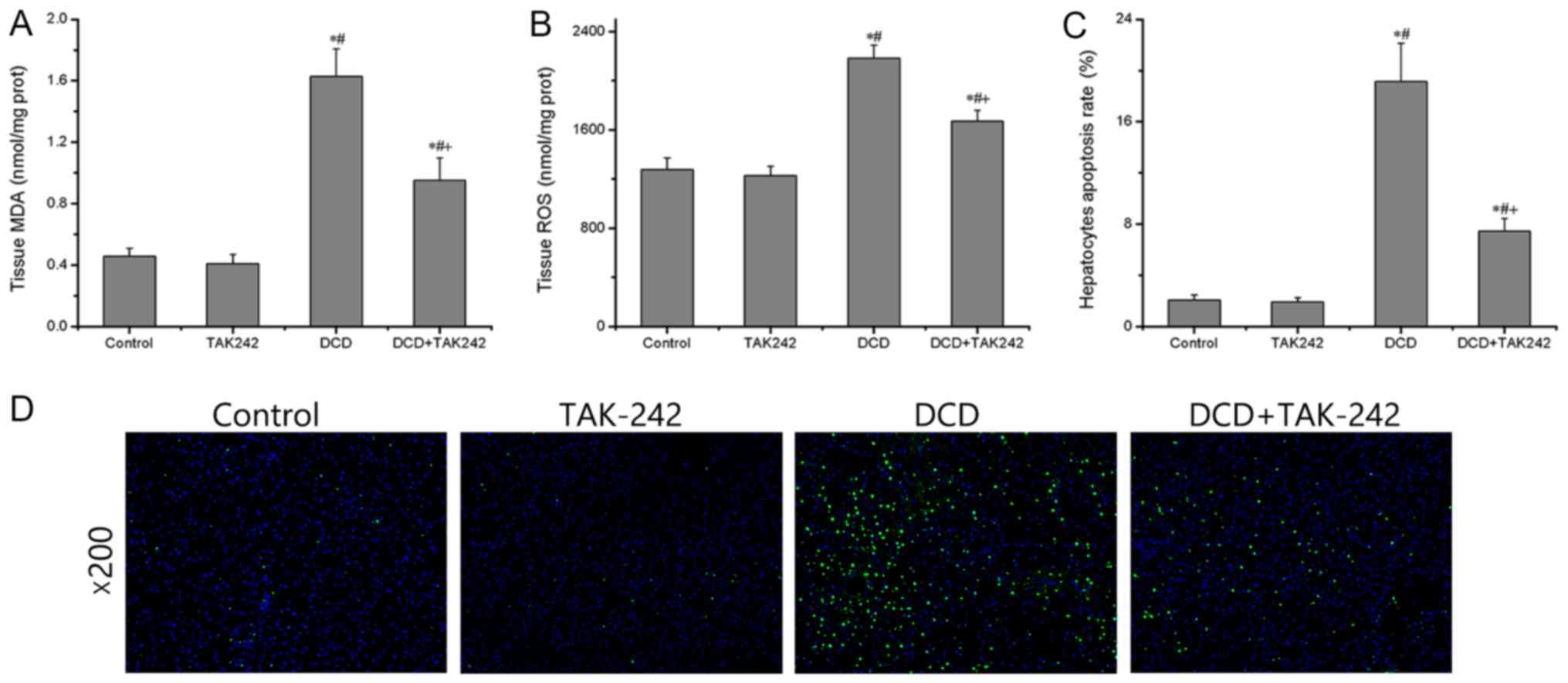
Next, the present study determined the redox status
and hepatic apoptosis in the DCD livers pretreated with or without
TAK242. When compared with the control group, the levels of ROS and
MDA were not significantly different in the TAK242 group
(P>0.05) but significantly increased in the DCD group
(P<0.05), whereas these changes were significantly reversed
following TAK242 pretreatment (P<0.05; Fig. 2A and B), indicating an antioxidant
effect of TAK242. Furthermore, as presented in Fig. 2C and D, the numbers of
TUNEL-positive apoptotic cells in the DCD group were significantly
increased compared with the control group, whereas a significant
decrease in the numbers of apoptotic cells were observed in the
DCD+TAK242 group compared with the DCD group (P<0.05). These
results indicate that pretreatment with TAK242 attenuates oxidative
stress and hepatocytic apoptosis in DCD livers.
Effect of TAK242 on the subcellular
structure of the DCD livers
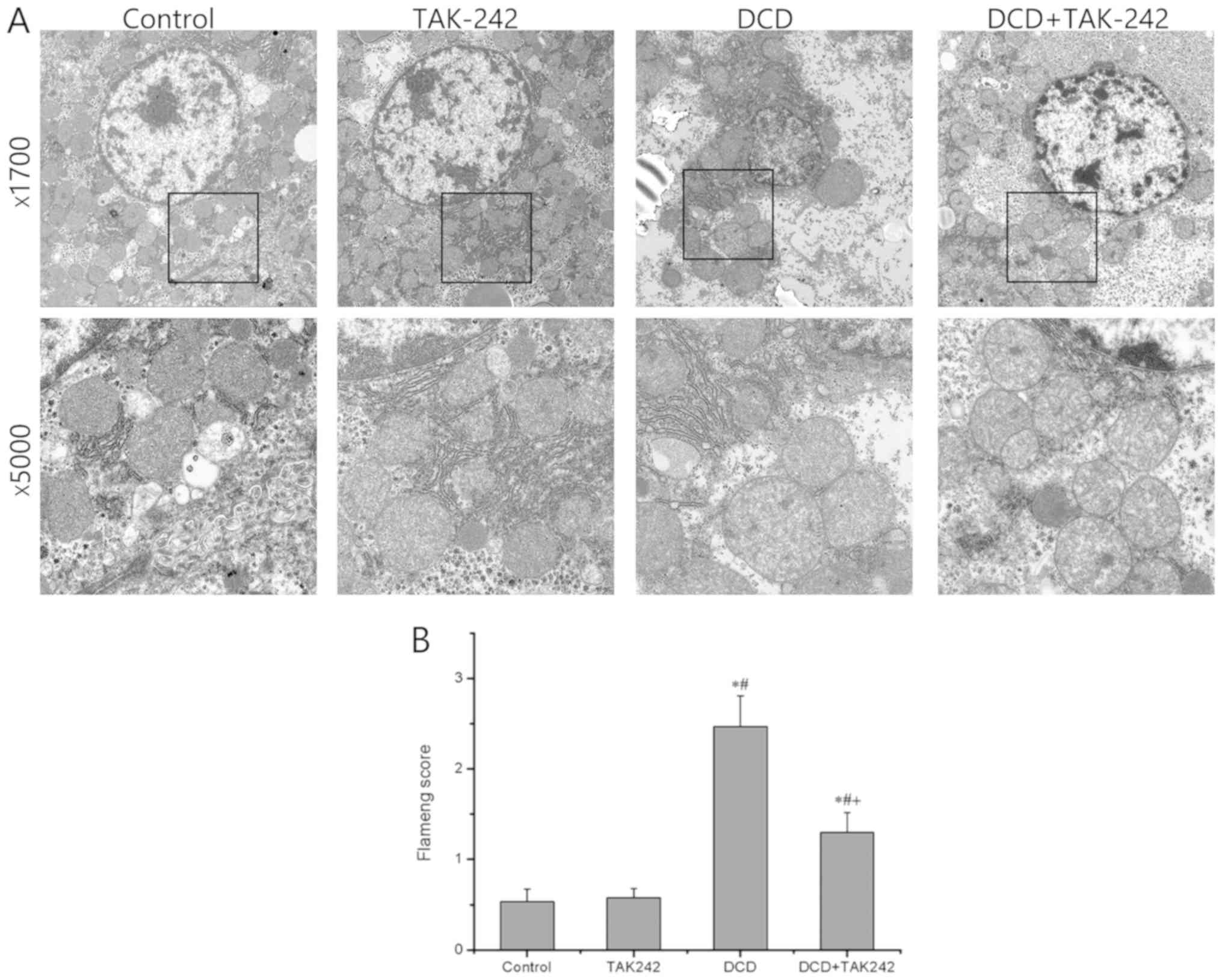
Next, the present study determined the subcellular
structures of the DCD livers pretreated with or without TAK242 by
TEM. As presented in Fig. 3A, when
compared with the control group, the subcellular structure in group
A was essentially normal without obvious changes, whereas the DCD
group exhibited more serious damage to the subcellular structures,
including the appearance of mitochondria swelling, nuclei
irregularity, cellular edema and transparent electronic density
area, whereas these structural changes, including mitochondrial
damage and cellular edema, in the DCD livers were substantially
relieved with the pretreatment with TAK242. As presented in
Fig. 3B, the Flameng scores
exhibited no statistically significant difference between the
control group and the TAK242 group (P>0.05) but were
significantly lower in the DCD+TAK242 group when compared with the
DCD group (P<0.05). These results indicate that the TLR4
inhibitor protects DCD-exposed hepatocytes, partially via
minimizing subcellular organelle damage.
Effect of TAK242 on TLR4 signaling
pathway-associated mRNA expression of inflammatory mediators in the
DCD livers
To understand the mechanism behind the protective
effects of TAK242 on the DCD livers, the present study further
determined the expression of HMGB1, TLR4 and their downstream
target genes at the mRNA levels using RT-qPCR analysis. As
presented in Fig. 4, it was
revealed that TAK242 pretreatment, as expected, significantly
inhibited the gene expression of TLR4, IL-1β, IL-6, COX2 and tumor
necrosis factor-α (TNF-α) in comparison with the DCD-alone group
(P<0.05). However, TAK242 did not significantly influence the
gene expression of the cytokine HMGB1. These results support the
concept that TAK242 inhibits the TLR4 signaling pathway and
associated inflammation in DCD livers.
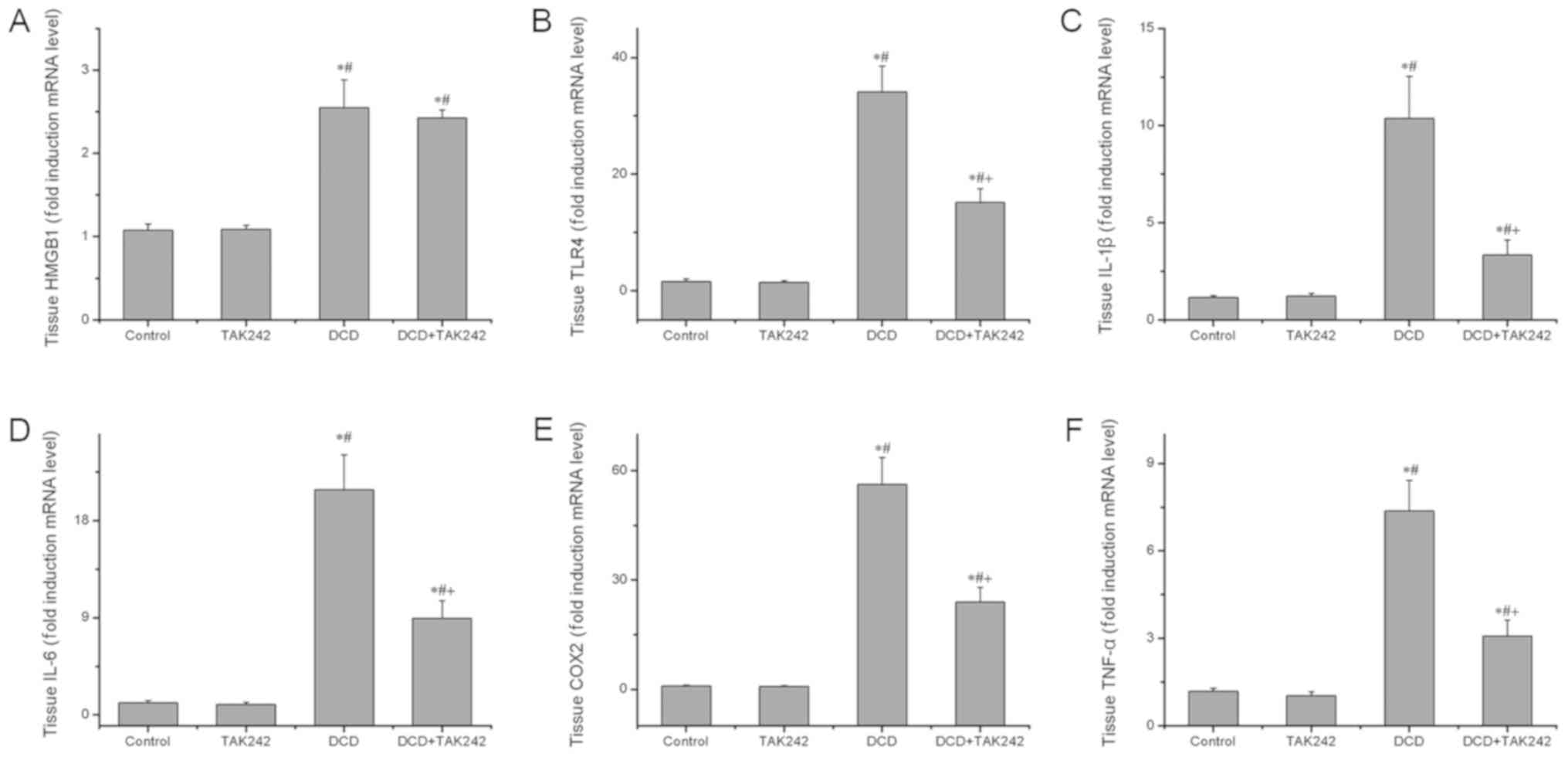 | Figure 4.TAK242 pretreatment reduces the gene
expression levels of HMGB1, TLR4 and TLR4 downstream target genes.
The mRNA expression levels of (A) HMGB1, (B) TLR4, (C) IL-1β, (D)
IL-6, (E) COX2 and (F) TNF-α were assessed using reverse
transcription-quantitative polymerase chain reaction. Three
independent experiments were performed. Data are expressed as the
mean ± standard deviation. *P<0.05 vs. the control group.
#P<0.05 vs. the TAK242 group. +P<0.05
vs. the DCD group. DCD, donors after circulatory death; HMGB1,
high-mobility group box protein b1; TLR4, toll-like
receptor 4; IL, interleukin; COX2, mitochondrially encoded
cytochrome c oxidase II; TNF-α, tumor necrosis factor-α. |
Effect of TAK242 on TLR4 signaling
pathway-associated protein expression of inflammatory mediators in
the DCD livers
Next, the present study determined the effect of
TAK242 on the expression of HMGB1, TLR4 and their downstream
inflammatory mediators in DCD livers at the protein level using
western blot analysis. As presented in Fig. 5, compared with the control group,
TLR4 and their downstream inflammatory mediators did not change
significantly in TAK242 group. But TAK242 pretreatment
significantly downregulated the protein expression levels of TLR4
and its downstream inflammatory mediators IL-1β, IL-6 and COX2,
compared with the DCD group (P<0.05). Again, there was no
statistically significant difference in HMGB1 expression levels
between the DCD group and the DCD+TAK242 group (P>0.05). These
results further confirmed that the TLR4 inhibitor, TAK242, reduces
the production and expression of proinflammatory cytokines and
enzymes in the DCD livers subjected to 24-h cold storage and 1 h
warm perfusion.
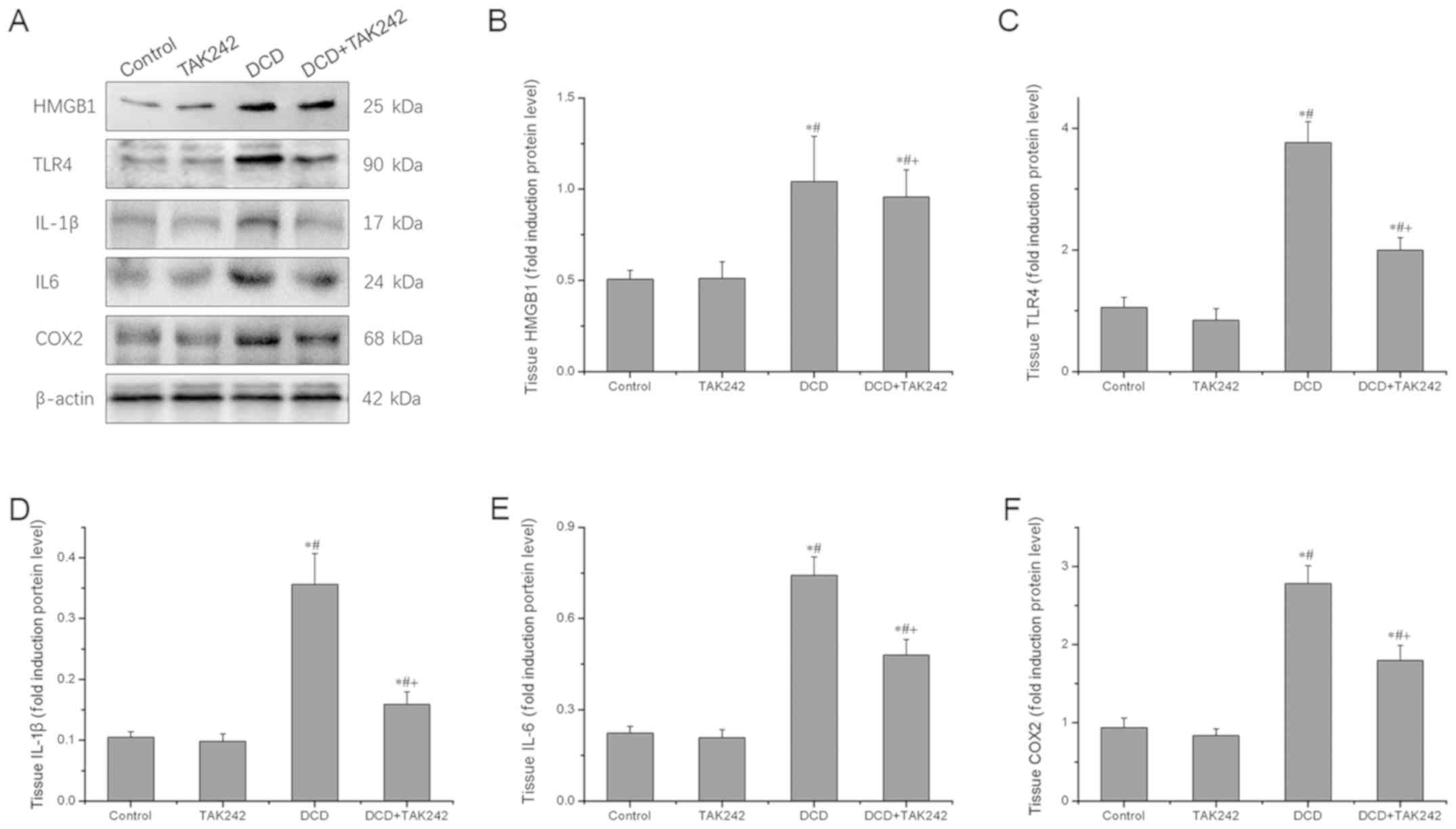 | Figure 5.TAK242 pretreatment reduces the
protein expression levels of HMGB1, TLR4 and TLR4 downstream target
genes. (A) Western blot analysis of HMGB1, TLR4, IL-1β, IL-6, COX2
and β-actin. Representative bands of three independent experiments
are presented. The density of the bands was quantified and
normalized to that of β-actin. Quantification of (B) HMGB1, (C)
TLR4, (D) IL-1β, (E) IL-6 and (F) COX2 expression levels. Data are
expressed as the mean ± standard deviation (n=3). *P<0.05 vs.
the control group. #P<0.05 vs. the TAK242 group.
+P<0.05 vs. the DCD group. DCD, donors after
circulatory death; HMGB1, high-mobility group box protein
b1; TLR4, toll-like receptor 4; IL, interleukin; COX2,
mitochondrially encoded cytochrome c oxidase II; TNF-α,
tumor necrosis factor-α. |
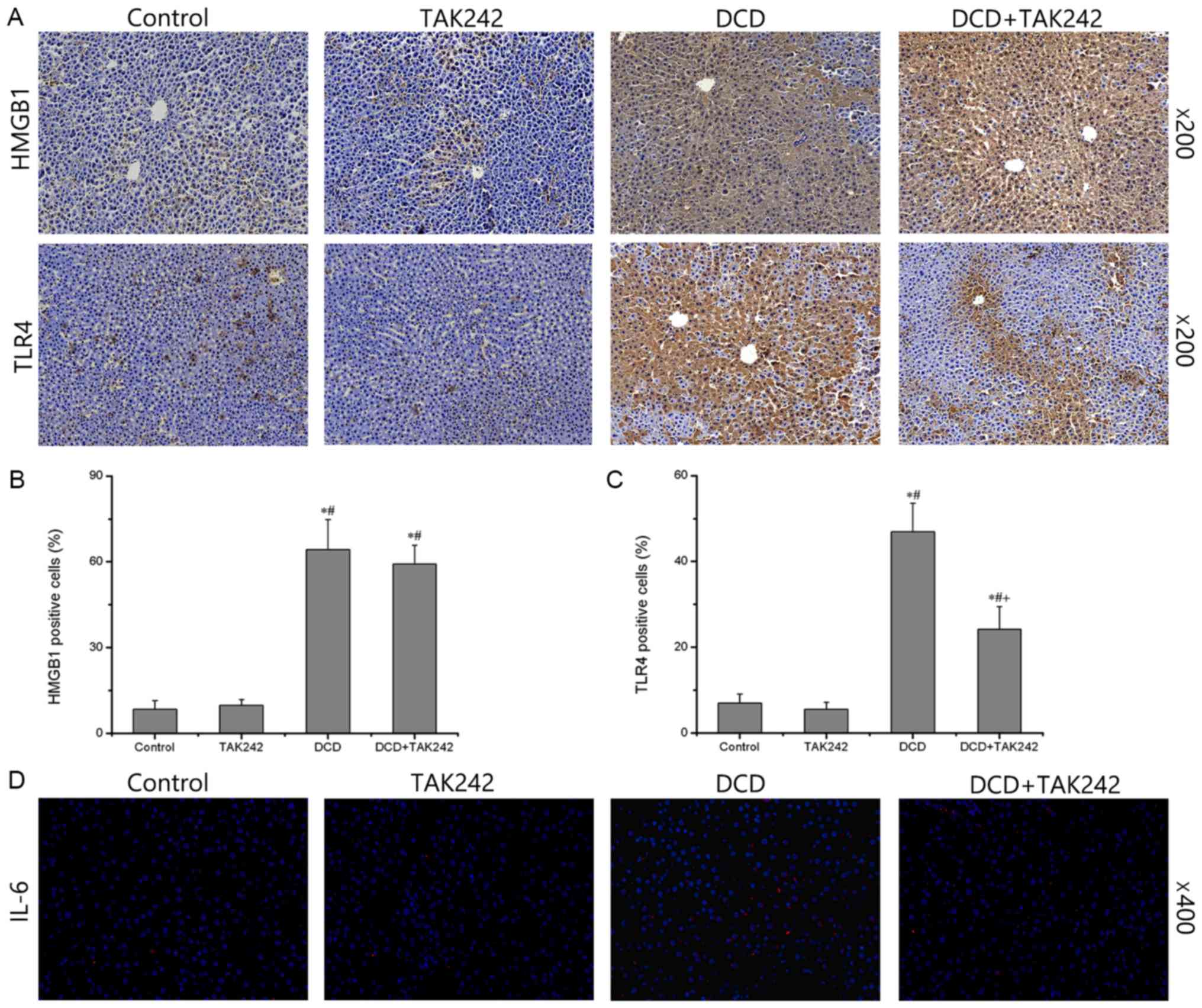
Finally, the present study demonstrated the
downregulation of TLR4 in the DCD livers by immunohistochemical
analysis. When compared with the control group, there were no
significant changes in HMGB1 or TLR4 in the TAK242 group (Fig. 6A). There was no statistically
significant difference in the percentage of HMGB1 and TLR4-positive
cells between the TAK242 and the control group (P>0.05; Fig. 6B and C). The DCD groups exhibited a
significantly increased expression of HMGB1 and TLR4 compared with
the control group (P<0.05; Fig.
6). TAK242 pretreatment in the DCD livers significantly
decreased the percentage of TLR4 positive cells compared with the
DCD group (P<0.05; Fig. 6C),
However, TAK242 pretreatment in the DCD livers did not
significantly decrease the percentage of HMGB1 positive cells
compared with the DCD group (P>0.05; Fig. 6B). Immunofluorescence revealed that
TAK242 pretreatment in DCD livers reduced the release of IL-6 when
compared with the DCD group (Fig.
6D). These results further indicate that TAK242 reduces TLR4
expression in DCD livers.
Discussion
Hepatic IRI can cause tissue damage in liver surgery
and transplantation, and affects graft regeneration, often
resulting in a poor prognosis (4,5,28).
DCD livers which have experienced serious warm ischemia at the time
of transplantation become particularly vulnerable to IRI (9,10).
Hepatic IRI may trigger an acute inflammatory response which
further exacerbates graft injury (29). One previous study identified that
TLR4 is a major PRR that mediates innate immune responses (30), so targeting TLR4 is considered to
be effective in the reduction of graft injury in liver
transplantation. As TAK242, a specific small molecular inhibitor of
TLR4, has been under clinical development for the treatment of
patients with severe inflammatory pathology (31), in the present study the use of the
TLR4 inhibitor was extended to the improvement of hepatic IRI and
graft quality. It was demonstrated that the TLR4 signaling pathway
is activated by the state of circulatory death, whereas TAK242
pretreatment improves the quality of the liver following
reperfusion in rats, by inhibiting the TLR4 signaling pathway
activation, inflammatory cytokine release, and redox stress.
In the present study, SD rats were used to establish
a model of DCD livers (23). The
livers were preserved in cold UW solution for 24 h and then
connected to a IPRL system for perfusion, so that tissue and
perfusate were easily collected for assessing hepatic IRI. Although
an in vitro IPRL system rather than an orthotopic liver
transplantation model was applied to assess liver quality, the rat
liver transplantation model closely resembles the physiological
state and pathological condition of DCD livers. By reducing
aminotransferase levels in the perfusate and alleviating
mitochondrial damage and hepatic apoptosis, the present study
demonstrated that TAK242 has restorative effects against
TLR4-associated inflammatory responses, and resultantly reduces
mitochondrial component damage and hepatocyte necrosis in addition
to improving hepatic function.
Hepatic IRI is a complex pathological process in
which tissue injury is initiated by ischemia and further aggravated
by the recovery of blood perfusion, with mitochondrial dysfunctions
that characterize hepatic IRI (32). During the ischemic process,
mitochondria produce large amounts of ROS as a result of the
damaged electron transport chain. With the recovery of the oxygen
supply, more ROS are generated from oxidative damage to
mitochondrial components (29,32,33).
The levels of MDA, a product of lipid peroxidation, usually reflect
the degree of oxidative damage and indirectly mirror the degree of
IRI. It was revealed that the increased levels of ROS and MDA in
the DCD livers were significantly reduced by TAK242 (P<0.05).
When mitochondria are severely damaged, they will fail to maintain
their normal morphology, structure and function, resulting in an
influx of extracellular macromolecules that contribute to
mitochondrial swelling and crista disintegration (9). In the model used in the present
study, the mitochondrial damage caused by hepatic IRI was clearly
observed; however, it was significantly improved by pretreatment
with TAK242 (P<0.05). Damaged mitochondria release caspases and
apoptosis-inducing factors and induce apoptosis (9,34,35),
a key mechanism used to maintain the balance of the intracellular
environment. Previous studies have revealed that the excessive
activation of apoptosis is highly associated with the poor
prognosis of DCD livers (36,37).
Hamada et al (38) revealed
that matrix metalloproteinase (MMP)-9 activity may result in the
detachment of hepatocytes from the extracellular matrix and
apoptosis in hepatic IRI. One recent study has revealed that
apoptosis is also implicated in postoperative complications in
liver transplantation (39). The
present study therefore determined DNA fragmentation as a hallmark
of apoptosis in the DCD livers and identified that TAK242
pretreatment substantially reduces oxidative damage and the
apoptosis rate of hepatocytes. Liver fibrosis may be associated
with the repair process following liver ischemia reperfusion injury
(40). The formation of liver
fibrosis takes a number of weeks (41,42),
however, the livers were perfused for 1 h in the present study, and
the quality of the livers were determined according to the necrosis
and apoptosis of the tissue. In future experiments, the association
between MMP-2/-9, cathepsins S/K, liver fibrosis and liver ischemia
reperfusion injury will be assessed via a rat liver transplantation
model.
TLRs are present on innate immune cells in which
they trigger an innate immune response against pathogens (43,44).
In livers, TLRs are mainly expressed on the sinusoidal endothelial
cells and hepatocytes (30,43).
TLR4 has been identified to have a crucial function in the
activation of the innate immune response and contribute to the
pathogenesis of sepsis (44). TLR4
also regulates inflammation through cathepsins (45). Endogenous ligands, including HMGB1
and heat shock proteins, activate the aseptic inflammatory response
by binding to TLR4 in IRI (46).
HMGB1 translocates from the nucleus to the cytoplasm and is then
released to the extracellular space when hepatocytes are exposed to
ischemic and hypoxic conditions (47). Upon binding to TLR4, extracellular
HMGB1 serves as a danger signal that promotes the production of
inflammatory mediators (43,45),
which amplify inflammatory signaling in a positive feedback loop
(48,49). In the present study, the increased
production of cytokines, including IL-1β, IL-6 and TNF-α, are
involved in the inflammatory response and tissue injury. However,
the beneficial effects of TAK242 were achieved, at least partially
through the downregulation of IL-1β, IL-6 and TNF-α. With regard to
the irresponsive nature of HMGB1 to TAK242 pretreatment, one
potential explanation is that TAK242 exerts an inhibitory effect on
TLR4 activation and its downstream signaling pathway, but not on
TLR4 activators, including HMGB1 (50,51).
In summary, the present study demonstrated that the
TLR4 signaling pathway serves a pivotal function in the hepatic IRI
of DCD, promoting the inflammatory cascade and deteriorating
hepatic injury. Furthermore, pretreatment with TAK242 of DCD livers
effectively reduces circulatory death-induced hepatic injury and
decreases inflammatory responses via inhibiting TLR4-mediated
signaling. Therefore, targeting TLR4 signaling may help to
ameliorate hepatic IRI and improve organ function following liver
transplantation. Thus, the results of the present study provide
novel insight into the treatment of hepatic IRI from DCD organ
donors.
Acknowledgements
The authors would like to thank Miss Pei Zhang and
Miss Anna Du from The Department of Core Facility and Technical
Support (Wuhan Institute of Virology), for their assistance with
TEM micrographs.
Funding
The present study was supported by The Natural
Science Foundation of Jiangxi Province (grant no. 20171BAB205010),
The Education Department of Jiangxi Province (grant no. GJJ180014)
and The National Natural Science Foundation of China (grant no.
U1403222).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
XZ and QX designed the experiment, established the
rat models and performed liver perfusion, analyzed and interpreted
data and drafted the manuscript. ZL and WW performed histological
and molecular biological experiments, and interpreted data. CL, PY
and WY performed molecular biological experiments and analyzed
data. JX and QY designed and performed the experiment, and
interpreted the data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present experiment was conducted with the
ethical approval of The Animal Experimental Ethics Committee of
Wuhan University and all experimental procedures were performed in
accordance with The Guidance for The Care and Use of Laboratory
Animals (19).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ortega-Deballon I, Hornby L and Shemie SD:
Protocols for uncontrolled donation after circulatory death: A
systematic review of international guidelines, practices and
transplant outcomes. Crit Care. 19:2682015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bendorf A, Kelly PJ, Kerridge IH,
McCaughan GW, Myerson B, Stewart C and Pussell BA: An international
comparison of the effect of policy shifts to organ donation
following cardiocirculatory death (DCD) on donation rates after
brain death (DBD) and transplantation rates. PLoS One.
8:e620102013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jay C, Ladner D, Wang E, Lyuksemburg V,
Kang R, Chang Y, Feinglass J, Holl JL, Abecassis M and Skaro AI: A
comprehensive risk assessment of mortality following donation after
cardiac death liver transplant-an analysis of the national
registry. J Hepatol. 55:808–813. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dageforde LA, Feurer ID, Pinson CW and
Moore DE: Is liver transplantation using organs donated after
cardiac death cost-effective or does it decrease waitlist death by
increasing recipient death? HPB (Oxford). 15:182–189. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nastos C, Kalimeris K, Papoutsidakis N,
Tasoulis MK, Lykoudis PM, Theodoraki K, Nastou D, Smyrniotis V and
Arkadopoulos N: Global consequences of liver ischemia/reperfusion
injury. Oxid Med Cell Longev. 2014:9069652014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhai Y, Petrowsky H, Hong JC, Busuttil RW
and Kupiec-Weglinski JW: Ischaemia-reperfusion injury in liver
transplantation-from bench to bedside. Nat Rev Gastroenterol
Hepatol. 10:79–89. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhai Y, Busuttil RW and Kupiec-Weglinski
JW: Liver ischemia and reperfusion injury: New insights into
mechanisms of innate-adaptive immune-mediated tissue inflammation.
Am J Transplant. 11:1563–1569. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Deoliveira ML, Jassem W, Valente R,
Khorsandi SE, Santori G, Prachalias A, Srinivasan P, Rela M and
Heaton N: Biliary complications after liver transplantation using
grafts from donors after cardiac death: Results from a matched
control study in a single large volume center. Ann Surg.
254:716–723. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zorov DB, Juhaszova M and Sollott SJ:
Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS
release. Physiol Rev. 94:909–950. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chouchani ET, Pell VR, Gaude E,
Aksentijević D, Sundier SY, Robb EL, Logan A, Nadtochiy SM, Ord
ENJ, Smith AC, et al: Ischaemic accumulation of succinate controls
reperfusion injury through mitochondrial ROS. Nature. 515:431–435.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Szabo G and Petrasek J: Inflammasome
activation and function in liver disease. Nat Rev Gastroenterol
Hepatol. 12:387–400. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Quesnelle KM, Bystrom PV and
Toledo-Pereyra LH: Molecular responses to ischemia and reperfusion
in the liver. Arch Toxicol. 89:651–657. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lu L, Zhou H, Ni M, Wang X, Busuttil R,
Kupiec-Weglinski J and Zhai Y: Innate immune regulations and liver
ischemia reperfusion injury. Transplantation. 100:2601–2610. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Theodoraki K, Karmaniolou I, Tympa A,
Tasoulis MK, Nastos C, Vassiliou I, Arkadopoulos N and Smyrniotis
V: Beyond preconditioning: Postconditioning as an alternative
technique in the prevention of liver ischemia-reperfusion injury.
Oxid Med Cell Longev. 2016:82359212016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bejaoui M, Pantazi E, Folch-Puy E,
Baptista PM, García-Gil A, Adam R and Roselló-Catafau J: Emerging
concepts in liver graft preservation. World J Gastroenterol.
21:396–407. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Radojkovic M, Stojanovic M, Stanojevic G,
Radojkovic D, Gligorijevic J, Ilic I and Stojanovic N: Ischemic
preconditioning vs adenosine vs prostaglandin E1 for protection
against liver ischemia/reperfusion injury. Brazilian J Med Biol
Res. 50:e61852017. View Article : Google Scholar
|
|
17
|
Mergental H, Perera MT, Laing RW, Muiesan
P, Isaac JR, Smith A, Stephenson BT, Cilliers H, Neil DA, Hübscher
SG, et al: Transplantation of declined liver allografts following
normothermic ex-situ evaluation. Am J Transplant. 16:3235–3245.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sha T, Sunamoto M, Kitazaki T, Sato J, Ii
M and Iizawa Y: Therapeutic effects of TAK-242, a novel selective
Toll-like receptor 4 signal transduction inhibitor, in mouse
endotoxin shock model. Eur J Pharmacol. 571:231–239. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li M, Wang ZN, Yang LF, Yan Y, Cai LM, Li
YT, Qiao YK and Chen ZG: TLR4 antagonist suppresses airway
remodeling in asthma by inhibiting the T-helper 2 response. Exp
Ther Med. 14:2911–2916. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Committee for the Update of the Guide for
the care and use of Laboratory Animals, Institute for Laboratory
Animal Research, Division on Earth and Life Studies et al, . Guide
For the care and use of laboratory animals eighth edition
[EB/OL].
|
|
21
|
Zhao Y, Zhao Y, Zhang M, Zhao J, Ma X,
Huang T, Pang H, Li J and Song J: Inhibition of TLR4
signalling-induced inflammation attenuates secondary injury after
diffuse axonal injury in rats. Mediators Inflamm. 2016:47069152016.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yokoi T, Yokoyama Y, Kokuryo T, Yamaguchi
J and Nagino M: Inhibition of Toll-like receptor 4 ameliorates
experimental postischemic injury in the cholestatic liver through
inhibition of high-mobility group box protein b1 (HMGB1) signaling.
Surgery. 163:270–276. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Schlegel A, Graf R, Clavien PA and
Dutkowski P: Hypothermic oxygenated perfusion (HOPE) protects from
biliary injury in a rodent model of DCD liver transplantation. J
Hepatol. 59:984–991. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu Z, Zhang X, Xiao Q, Ye S, Lai CH, Luo
J, Huang X, Wang W, Zeng C, Zhong Z, et al: Pretreatment donors
after circulatory death with simvastatin alleviates liver ischemia
reperfusion injury through a KLF2-dependent mechanism in rat. Oxid
Med Cell Longev. 2017:38619142017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Suzuki S, Toledo-Pereyra LH, Rodriguez FJ
and Cejalvo D: Neutrophil infiltration as an important factor in
liver ischemia and reperfusion injury. Modulating effects of FK506
and cyclosporine. Transplantation. 55:1265–1272. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Flameng W, Borgers M, Daenen W and
Stalpaert G: Ultrastructural and cytochemical correlates of
myocardial protection by cardiac hypothermia in man. J Thorac
Cardiovasc Surg. 79:413–424. 1980.PubMed/NCBI
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mateo R, Cho Y, Singh G, Stapfer M,
Donovan J, Kahn J, Fong TL, Sher L, Jabbour N, Aswad S, et al: Risk
factors for graft survival after liver transplantation from
donation after cardiac death donors: An analysis of OPTN/UNOS data.
Am J Transplant. 6:791–796. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Eltzschig HK and Eckle T: Ischemia and
reperfusion-from mechanism to translation. Nat Med. 17:1391–401.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nace GW, Huang H, Klune JR, Eid RE,
Rosborough BR, Korff S, Li S, Shapiro RA, Stolz DB, Sodhi CP, et
al: Cellular specific role of toll-like receptor 4 in hepatic
ischemia-reperfusion injury. Hepatology. 58:374–387. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Takashima K, Matsunaga N, Yoshimatsu M,
Hazeki K, Kaisho T, Uekata M, Hazeki O, Akira S, Iizawa Y and Ii M:
Analysis of binding site for the novel small-molecule TLR4 signal
transduction inhibitor TAK242 and its therapeutic effect on mouse
sepsis model. Br J Pharmacol. 157:1250–1262. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Granger DN and Kvietys PR: Reperfusion
injury and reactive oxygen species: The evolution of a concept.
Redox Biol. 6:524–551. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kalogeris T, Baines CP, Krenz M and
Korthuis RJ: Cell biology of ischemia/reperfusion injury. Int Rev
Cell Mol Biol. 298:229–317. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Papanicolaou KN, Phillippo MM and Walsh K:
Mitofusins and the mitochondrial permeability transition: The
potential downside of mitochondrial fusion. Am J Physiol Heart Circ
Physiol. 303:H243–H255. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Baines CP and Molkentin JD: Adenine
nucleotide translocase-1 induces cardiomyocyte death through
upregulation of the pro-apoptotic protein Bax. J Mol Cell Cardiol.
46:969–977. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Luedde T, Kaplowitz N and Schwabe RF: Cell
death and cell death responses in liver disease: Mechanisms and
clinical relevance. Gastroenterology. 147:765–783.e4. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chen L, Ren F, Zhang H, Wen T, Piao Z,
Zhou L, Zheng S, Zhang J, Chen Y, Han Y, et al: Inhibition of
glycogen synthase kinase 3β ameliorates D-GalN/LPS-induced liver
injury by reducing endoplasmic reticulum stress-triggered
apoptosis. PLoS One. 7:e452022012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hamada T, Duarte S, Tsuchihashi S,
Busuttil RW and Coito AJ: Inducible nitric oxide synthase
deficiency impairs matrix metalloproteinase-9 activity and disrupts
leukocyte migration in hepatic ischemia/reperfusion injury. Am J
Pathol. 174:2265–2277. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Tian Y, Wang J, Wang W, Ding Y, Sun Z,
Zhang Q, Wang Y, Xie H, Yan S and Zheng S: Mesenchymal stem cells
improve mouse non-heart-beating liver graft survival by inhibiting
Kupffer cell apoptosis via TLR4-ERK1/2-Fas/FasL-caspase3 pathway
regulation. Stem Cell Res Ther. 7:1572016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Konishi T, Schuster RM and Lentsch AB:
Liver repair and regeneration after ischemia-reperfusion injury is
associated with prolonged fibrosis. Am J Physiol Gastrointest Liver
Physiol. 316:G323–G331. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Marrone G, Shah VH and Gracia-Sancho J:
Sinusoidal communication in liver fibrosis and regeneration. J
Hepatol. 65:608–617. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Barton GM and Kagan JC: A cell biological
view of Toll-like receptor function: Regulation through
compartmentalization. Nat Rev Immunol. 9:535–542. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhai Y, Shen XD, O'Connell R, Gao F,
Lassman C, Busuttil RW, Cheng G and Kupiec-Weglinski JW: Cutting
edge: TLR4 activation mediates liver ischemia/reperfusion
inflammatory response via ifn regulatory factor 3-dependent
MyD88-independent pathway. J Immunol. 12:7115–7119. 2004.
View Article : Google Scholar
|
|
44
|
Wittebole X, Castanares-Zapatero D and
Laterre PF: Toll-like receptor 4 modulation as a strategy to treat
sepsis. Mediators Inflamm. 2010:5683962010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
de Mingo Á, de Gregorio E, Moles A,
Tarrats N, Tutusaus A, Colell A, Fernandez-Checa JC, Morales A and
Marí M: Cysteine cathepsins control hepatic NF-κB-dependent
inflammation via sirtuin-1 regulation. Cell Death Dis. 7:e24642016.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Tsung A, Klune JR, Zhang X, Jeyabalan G,
Cao Z, Peng X, Stolz DB, Geller DA, Rosengart MR and Billiar TR:
HMGB1 release induced by liver ischemia involves Toll-like receptor
4-dependent reactive oxygen species production and calcium-mediated
signaling. J Exp Med. 204:2913–2923. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zhao G, Fu C, Wang L, Zhu L, Yan Y, Xiang
Y, Zheng F, Gong F, Chen S and Chen G: Down-regulation of nuclear
HMGB1 reduces ischemia-induced HMGB1 translocation and release and
protects against liver ischemia-reperfusion injury. Sci Rep.
7:462722017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zhang GY, Lu D, Duan SF, Gao YR, Liu SY,
Hong Y, Dong PZ, Chen YG, Li T, Wang DY, et al: Hydrogen Sulfide
alleviates lipopolysaccharide-induced diaphragm dysfunction in rats
by reducing apoptosis and inflammation through ROS/MAPK and
TLR4/NF-κB signaling pathways. Oxid Med Cell Longev.
2018:96478092018. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wang L, Li N, Lin D and Zang Y: Curcumin
protects against hepatic ischemia/reperfusion induced injury
through inhibiting TLR4/NF-κB pathway. Oncotarget. 8:65414–65420.
2017.PubMed/NCBI
|
|
50
|
Yu Y, Tang D and Kang R: Oxidative
stress-mediated HMGB1 biology. Front Physiol. 6:932015. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Yang H, Wang H, Chavan SS and Andersson U:
High mobility group box protein 1 (HMGB1): The prototypical
endogenous danger molecule. Mol Med. 21 (Suppl):S6–S12. 2015.
View Article : Google Scholar : PubMed/NCBI
|