Introduction
Reactive oxygen species (ROS) are crucial cellular
secondary messengers involved in diverse biological processes in
cancer cells (1–7). ROS are oxygen-containing species,
including superoxide (O2•−), hydroxyl
(•OH), and hydrogen peroxide
(H2O2). The majority of ROS are produced as
side products of oxidative phosphorylation in mitochondria
(4,5,8,9), but
they are also induced by NADPH oxidase (NOX), growth factors, and
cytokines (2,4). ROS are increased in many types of
cancers and these elevated ROS are highly involved in tumor
development and progression (2,6,10).
High levels of ROS exert cytotoxic effects by inducing DNA damage
or cell death, thereby limiting cancer progression (2,4,5,7). On
the other hand, at low levels, ROS function as signaling molecules
that can induce nucleic acid and protein synthesis, cell cycle
progression, the expression of numerous genes involved in cell
proliferation and survival, the activation of redox-sensitive
transcription factors [such as p53, hypoxia inducible factor
(HIF)-1, and NF-κB], epigenetic alterations, and tumorigenesis
(2,4,5,7). In
addition, ROS have been shown to induce epithelial-mesenchymal
transition (EMT) in cancer cells (11–14).
However, the precise mechanism of ROS-induced EMT remains to be
elucidated.
EMT plays critical roles in embryogenesis as well as
in tumor invasion and metastasis. Epithelial cells undergoing EMT
lose their polarization and acquire mesenchymal-like morphology.
EMT is also characterized by decreased expression of epithelial
markers, such as E-cadherin and induction of mesenchymal markers,
such as vimentin. Loss of E-cadherin is considered a hallmark of
EMT (15–19). EMT is regulated by several
transcription factors, including Snail, Slug, Twist-related protein
(Twist)1, Twist2, zinc finger E-box binding homeobox (ZEB)1, ZEB2,
and E12/E47. Snail, a key transcription factor in EMT, promotes
invasion and metastasis in response to several oncogenic signaling
pathways (20–22). Recently, Snail has been implicated
in metabolic alterations, such as glycolytic switch (21,23).
Glycolytic switch is a phenomenon whereby cancer cells rely on
glycolysis more than mitochondrial oxidative phosphorylation for
ATP production, even when sufficient oxygen is available (24–28).
Recently, we showed that distal-less homeobox-2 (Dlx-2) induces EMT
and glycolytic switch by activating Snail (29,30).
Dlx-2 is one of the human distal-less (Dlx) gene family proteins
and is involved in embryonic development (31,32)
and tumor progression (33–36).
Dlx-2 is known to play an important role in shifting the activity
of TGF-β from tumor suppression to tumor promotion (34).
In this study, we show that ROS induce EMT through
Snail and Dlx-2 cascades. We also show that Dlx-2/Snail signaling
plays a critical role(s) in ROS-induced glycolytic switch,
inhibition of mitochondrial respiration, and cytochrome c oxidase
(COX) inhibition. These results clarify the mechanism by which ROS
promotes tumor progression through Dlx-2/Snail axis-dependent EMT
and glycolytic switch in MCF-7 cells.
Materials and methods
Cell culture and ROS treatment
MCF-7 cells were obtained from the American Type
Culture Collection (ATCC, Manassas, VA, USA; authenticated by short
tandem repeat profiling). MCF-7 cells were cultured in Eagle's
minimal essential medium (EMEM; Hyclone; GE Healthcare, Logan, UT,
USA) supplemented with 10% (v/v) heat-inactivated fetal bovine
serum (FBS, Hyclone; GE Healthcare) and 1% penicillin-streptomycin
(PS, Hyclone; GE Healthcare). Cells were cultured at 37°C in a
humidified incubator with 5% CO2 and were passaged two
times per week. Low-passage cultures (passage 5–25) were used for
all experiments. Cells were routinely checked for mycoplasma
contamination using the Mycoplasma PCR Detection kit (iNtRON
Biotechnology). MCF-7 cells were treated with ROS [200 µM
H2O2 (Sigma-Aldrich, St. Louis, MO, USA) and
10 µM menadione (an O2− generator;
Sigma)].
Transfection and short hairpin RNA
(shRNA) interference
pCR3.1-Snail-Flg (provided by J.I. Yook, Yonsei
University, Korea) was transfected into MCF-7 cells using jetPEI
(Polyplus-transfection SA, New York, NY, USA). pSUPER vectors with
control shRNA and shRNAs against Dlx-2 and Snail were produced and
transfected as described previously (23). shRNA target sequences have also
been described previously (23,29).
Immunoblotting and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Immunoblotting was performed using the following
antibodies: anti-Dlx-2 (EMD Millipore, Billerica, MA, USA);
anti-Snail (Abgent, San Diego, CA, USA); anti-E-cadherin,
anti-COXVIIc, anti-COX15, and anti-COX19 (Santa Cruz Biotechnology,
Dallas, TX, USA); anti-COXVIc, and anti-COXVIIa (MitoSciences,
Eugene, OR, USA); anti-COX18 (Abcam, Cambridge, UK); anti-α-tubulin
(Biogenex, Fermont, CA, USA).
Total mRNA was isolated from cells using TRIzol
(Invitrogen, Carlsbad, CA, USA) according to the supplier's
instructions. Transcript levels were assessed with reverse
transcription-quantitative polymerase chain reaction using primers
for EMT-inducing transcription factors (including Snail, Slug,
Twist1, Twist2, ZEB1, ZEB2, E12/E47, Dlx-2), E-cadherin,
N-cadherin, vimentin, fibronectin, cluster of differentiation 44
(CD44), COX subunits and assembly factors, and β-actin. Primer
sequences described previously were used (23,29,37),
along with the following additional primers: Twist1 sense,
5′-CAGACCCTCAAGCTGGCGGC-3′; Twist1 antisense,
5′-CCAGGCCCCCTCCATCCTCC-3′; Twist2 sense,
5′-TGGGCACCAGCGAGGAGGAG-3′; Twist2 antisense,
5′-CTGGGGCTGCCCTTCTTGCC-3′; ZEB1 sense,
5′-GGGAGGATGACAGAAAGGAAGG-3′; ZEB1 antisense,
5′-TGCCTCTGGTCCTCTTCAGGTGC-3′; ZEB2 sense,
5′-CAGAAGCCACGATCCAGACCGC-3′; ZEB2 antisense,
5′-GTGCCAAGGCGAGACAGCTCC-3′; E12/E47 sense,
5′-GCCGGGCACATGTGAAAGTAAACAA-3′; E12/E47 antisense,
5′-CAGGTTTCCACAGCATCCCCCTT-3′; N-cadherin sense,
5′-AGCCAACCTTAACTGAGGAGT-3′; N-cadherin antisense,
5′-GGCAAGTTGATTGGAGGGATG-3′; vimentin sense,
5′-TGAAGGAGGAAATGGCTCGTC-3′; vimentin antisense,
5′-GTTTGGAAGAGGCAGAGAAATCC-3′; fibronectin sense,
5′-CAGTGGGAGACCTCGAGAAG-3′; fibronectin antisense,
5′-TCCCTCGGAACATCAGAAAC-3′; CD44 sense, 5′-TGCCGCTTTGCAGGTGTATT-3′;
CD44 antisense, 5′-CCGATGCTCAGAGCTTTCTCC-3′. Values were normalized
to those of β-actin.
Immunofluorescence (IF) staining
Cells were fixed with 3.7% formaldehyde,
permeabilized in 0.2% Triton X-100, and blocked with 2% BSA. Cells
were then incubated overnight with anti-E-cadherin antibody and
immunostained with AlexaFluor 488-conjugated goat anti-mouse
secondary antibody (Invitrogen). Hoechst 33342 (Invitrogen) was
used to stain cell nuclei. Cells were viewed under a fluorescence
microscope.
Chromatin immunoprecipitation (ChIP)
assay
ChIP assays were performed using a ChIP assay kit
(EMD Millipore) according to the manufacturer's instructions.
Isotype control IgG, anti-Dlx-2, and anti-Snail (Santa Cruz
Biotechnology) were used to immunoprecipitate DNA-containing
complexes. ChIP-enriched DNA was analyzed by PCR using primers
complementary to promoter regions containing Dlx-2 or Snail binding
sites, as described previously (23,29).
Assays for mitochondrial respiration,
COX activity, glucose consumption, lactate production, and ATP
production
Mitochondrial respiration and COX activity were
measured as described previously (23,38).
For the mitochondrial respiration assay, exponentially growing
cells (1.5×106) were washed with TD buffer (137 mM NaCl,
5 mM KCl, 0.7 mM Na2HPO4, 25 mM Tris-HCl; pH
7.4), collected, and resuspended in complete medium without phenol
red. Cells (5×105) were transferred to a Mitocell
chamber equipped with a Clark O2 electrode (782
O2 Meter; Strathkelvin Instruments, Glasgow, UK).
O2 consumption rates were measured after adding 30 µM
DNP to obtain the maximum respiration rate and specificity for
mitochondrial respiration was confirmed by adding 5 mM KCN. COX
activity was determined by measuring the KCN-sensitive,
COX-dependent O2 consumption rate by adding 3 mM TMPD in
the presence of 30 µM DNP and 20 µM antimycin A.
Glucose, lactate, and intracellular ATP levels in
the media were determined using a glucose oxidation assay kit
(Sigma-Aldrich), a colorimetric and fluorescence-based lactate
assay kit (BioVision, Inc., Milpitas, CA, USA), and an ATP
Bioluminescence Assay kit (Roche Diagnostics, Basel, Switzerland),
respectively, according to the manufacturers' instructions. Levels
of glucose, lactate, and intracellular ATP were normalized to
protein concentrations. Levels of ATP produced by aerobic
respiration and glycolysis were determined by measuring lactate
production and O2 consumption (23,39).
Measurement of circularity
Circularity assays were performed as described
previously (29). Briefly,
microscopic images were analyzed with AxioVision LE software
(version 4.8). Circularity was calculated using the formula, 4π
(area/perimeter2). Values closer to 1.0 indicate a more
circular cell morphology.
Statistical analysis
Reverse transcription-quantitative polymerase chain
reaction, mitochondrial respiration, glucose consumption, lactate
production, and ATP production assays were performed at least in
triplicate. Most experiments were repeated more than twice. Data
were analyzed by Student's t-test (unpaired, two-tailed) for
comparison between two groups, one-way ANOVA with Tukey's
multiple-comparisons test for comparison between three groups, and
two-way ANOVA for comparison between three groups with multiple
factors and results were expressed as mean ± SE. P<0.05 was
considered to indicate a statistically significant difference.
Results and Discussion
Dlx-2/Snail cascade is implicated in
ROS-induced EMT
It has been shown that ROS induces EMT by increasing
Snail expression (11–14). We examined the EMT-inducing effects
of H2O2 and O2− in
MCF-7 cells, a non-invasive luminal A subtype breast cancer cell
line. MCF-7 cells have low levels of Snail, but various stimuli
including TGF and Wnt can induce Snail expression, and
subsequently, EMT in MCF-7 cells; thus, MCF-7 cells are widely used
for studying breast cancer, particularly EMT (40–42).
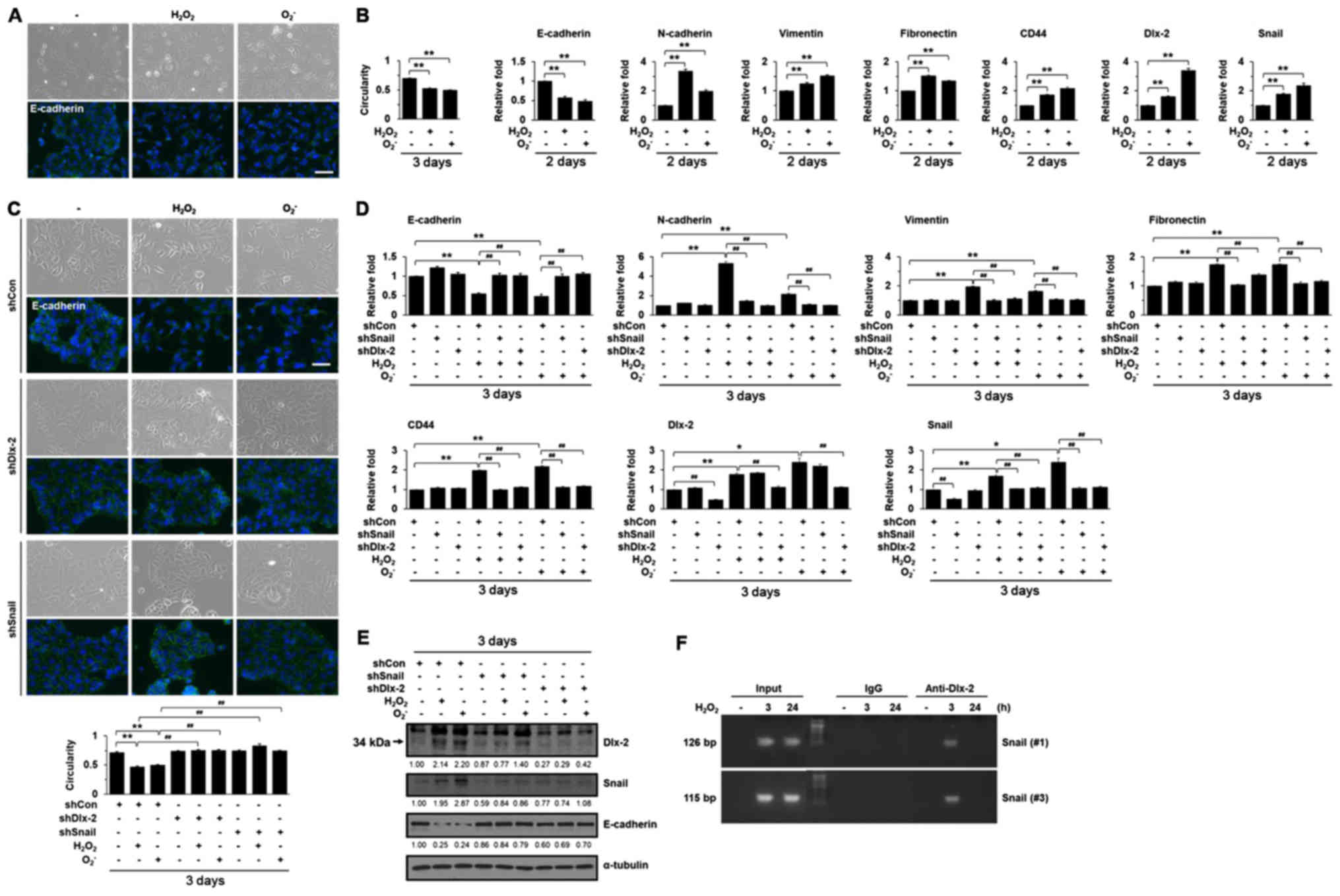
ROS induced a phenotypical change to an elongated morphology with
pseudopodia, which is a characteristic of mesenchymal cells, and
decreased the expression of an epithelial marker, E-cadherin,
indicating that ROS induce EMT (Fig.
1A and B). We further examined the effects of ROS on the
expression of mesenchymal markers, such as N-cadherin, vimentin,
and fibronectin. In addition, we also examined the expression of
CD44, a glycoprotein receptor for various extracellular matrix
(ECM) ligands, such as hyaluronic acid (HA); HA-CD44 interaction
promotes EMT and CSC self-renewal through Nanog expression
(43,44). ROS were shown to increase the
expression of mesenchymal markers including N-cadherin, vimentin,
and fibronectin (Table I and
Fig. 1B). ROS also upregulated the
CD44 expression (Table I and
Fig. 1B). We investigated which
EMT-inducing transcription factors (Snail, Slug, Twist1, Twist2,
ZEB1, ZEB2, E12/E47, and Dlx-2) were involved in ROS
treatment-induced EMT using reverse transcription-quantitative
polymerase chain reaction (Table
I). ROS induced Snail and Dlx-2 expression, but had no effect
on the other EMT-inducing transcription factors (Table I and Fig. 1B). Therefore, we examined if the
Dlx-2/Snail cascade was involved in ROS-induced EMT. Dlx-2 and
Snail shRNAs appeared to block the EMT phenotype and downregulation
of E-cadherin induced by ROS (Fig.
1C-E). We also found that Dlx-2 and Snail shRNAs prevented the
ROS-induced expression of N-cadherin, vimentin, fibronectin, and
CD44 (Fig. 1D). Because Dlx-2 has
been shown to be an upstream regulator of Snail (29,30),
we examined the effects of Dlx-2 shRNA on ROS-induced Snail
expression. In the immunoblotting of Dlx-2, anti-Dlx-2 antibody
recognized 2 bands (33). The
lower band (indicated by arrowhead, 34 kDa) is Dlx-2; the higher
band is non-specific band. Expectedly, Snail shRNA suppressed
ROS-induced Snail expression and Dlx-2 shRNA suppressed ROS-induced
Dlx-2 expression. In addition, Dlx-2 shRNA also prevented Snail
induction by ROS, whereas Snail shRNA did not affect Dlx-2
induction by ROS (Fig. 1D and E),
indicating that Dlx-2 consistently acts upstream of ROS-induced
Snail. A ChIP assay showed that ROS increased Dlx-2 binding at the
Snail promoter, which was detected at an early time point (3 h)
after ROS treatment (Fig. 1F).
Dlx-2 appeared to act as a mediator for ROS-Snail-induced EMT.
These results suggest that the Dlx-2/Snail axis are implicated in
ROS-induced EMT.
 | Table I.Regulation of EMT markers and
EMT-inducing transcription factors by ROS. |
Table I.
Regulation of EMT markers and
EMT-inducing transcription factors by ROS.
|
|
H2O2 |
O2− |
|---|
|
|
|
|
|---|
| Genes | 24 h | 48 h | 72 h | 24 h | 48 h | 72 h |
|---|
| EMT markers |
|
|
|
|
|
|
|
E-cadherin | 0.980 | 0.670b | 0.665b | 0.963 | 0.496b | 0.430b |
|
N-cadherin | 1.612a | 3.382b | 5.719b | 1.644b | 2.043b | 2.135b |
|
Vimentin | 1.028 | 1.226 | 1.894b | 0.975 | 1.537b | 1.628b |
|
Fibronectin | 1.390 | 1.527b | 1.945b | 1.249 | 1.353 | 1.651b |
|
CD44 | 1.551b | 1.738b | 2.034b | 1.129 | 2.208b | 2.068b |
| EMT-inducing
transcription factors |
|
|
|
|
|
|
|
Snail | 1.254 | 1.243 | 1.724b | 1.209 | 2.183b | 2.553a |
|
Slug | 1.210 | 0.835 | 0.940 | 0.935 | 0.705 | 1.080 |
|
Dlx-2 | 1.573b | 1.564b | 1.573b | 1.615b | 3.457b | 2.383b |
|
Twist1 | 1.240 | 1.080 | 1.255 | 1.150 | 1.025 | 1.270 |
|
Twist2 | 1.065 | 1.070 | 1.155 | 0.895 | 1.110 | 1.360 |
|
ZEB1 | 0.955 | 0.940 | 1.100 | 1.315 | 1.260 | 1.245 |
|
ZEB2 | 0.950 | 1.050 | 1.500 | 0.835 | 0.960 | 1.435 |
|
E12/E47 | 0.834 | 0.960 | 0.934 | 1.061 | 0.877 | 0.937 |
Dlx-2/Snail signaling is implicated in
ROS-induced glycolytic switch and inhibition of mitochondrial
respiration
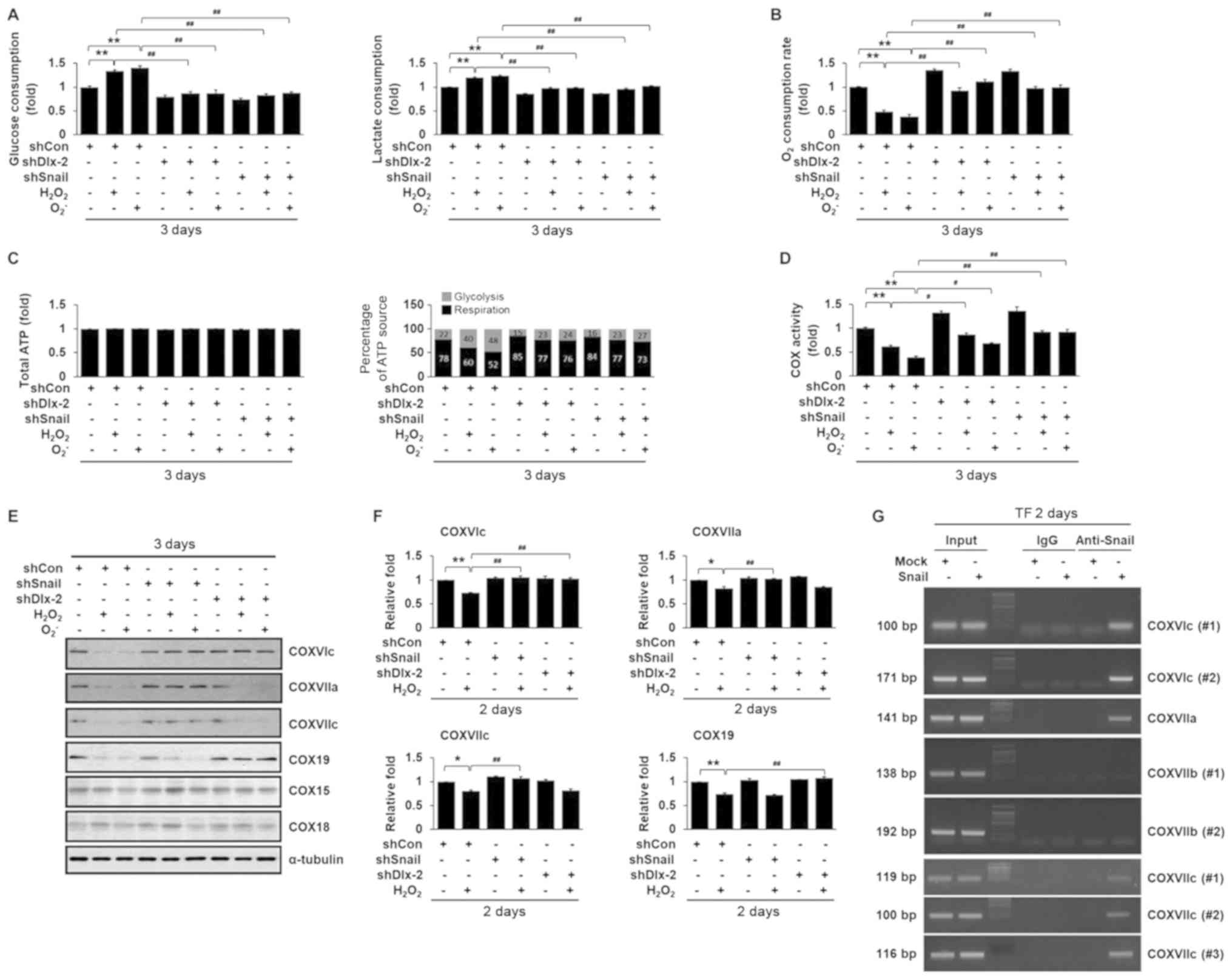
Cancer cells exhibit glycolytic switch as well as
EMT during tumor development and progression (24–28).
Thus, we examined the effects of ROS on glycolytic switch and
mitochondrial respiration. ROS significantly increased glucose
consumption and lactate production (Fig. 2A). In addition, ROS reduced
O2 consumption (Fig.
2B). Although total ATP concentrations remained the same in all
cells, ROS increased the ATP ratio produced by glycolysis versus
aerobic respiration (Fig. 2C),
indicating that ROS induced glycolytic switch. Previously, we
showed that the Dlx-2/Snail cascade induces glycolytic switch and
inhibits mitochondrial respiration (29,30).
We further examined whether the Dlx-2/Snail cascade affected
ROS-induced glycolytic switch and inhibition of mitochondrial
respiration. Dlx-2 and Snail shRNAs impaired the effects of ROS on
glucose consumption, lactate production, and O2
consumption (Fig. 2A-C),
indicating that the Dlx-2-Snail axis is involved in ROS-induced
glycolytic switch and inhibition of mitochondrial respiration.
Dlx-2/Snail signaling is implicated in
ROS-induced COX activity repression
Inhibition of mitochondrial respiratory activity is
closely related to changes in the activity of COX, the terminal
enzyme of the mitochondrial respiratory chain. We found that ROS
repressed COX activity (Fig. 2D).
Thus, we further examined the involvement of the Dlx-2/Snail
cascade in ROS-induced downregulation of COX activity. Dlx-2 and
Snail shRNAs suppressed ROS-induced downregulation of COX activity
(Fig. 2D), implicating the
Dlx-2/Snail cascade in ROS-induced COX activity repression.
Dlx-2/Snail signaling is implicated in
ROS-induced COX activity repression by downregulating multiple COX
subunits and assembly factors
Eukaryotic COX is composed of 13 different subunits
in the inner mitochondrial membrane and the sequential action of
several assembly factors regulates its assembly (Table II). We further examined the
effects of ROS on the expression of COX subunits and assembly
factors. ROS downregulated the expression of COXVIc, COXVIIa,
COXVIIc, COX15, COX18, and COX19 (Table II). Note that ROS decreased the
mRNA levels of COX15 and COX18, but not their protein levels
(Fig. 2E and F; Table II). We previously found that Snail
decreased expression of COXVIc, COXVIIa, and COXVIIc (23) and Dlx-2 decreased expression of
COXVIc and COX19 (29). Snail
shRNA suppressed the ROS-induced reduction in the levels of COXVIc,
COXVIIa, and COXVIIc, but not COX19 (Fig. 2E and F). Dlx-2 shRNA suppressed the
ROS-induced reduction in the levels of COXVIc and COX19, but not
COXVIIa and COXVIIc (Fig. 2E and
F).
 | Table II.Regulation of gene expression of COX
subunits and assembly factors by ROS. |
Table II.
Regulation of gene expression of COX
subunits and assembly factors by ROS.
|
|
H2O2 (n=2-3) |
O2− (n=4) |
|---|
|
|
|
|
|---|
| Genes | 48 h | 48 h |
|---|
| COX subunits |
|
COXIV | 1.067 | 1.138 |
|
COXVa | 1.047 | 1.018 |
|
COXVb | 0.978 | 1.214 |
|
COXVIa | 1.021 | 1.047 |
|
COXVIb | 0.853 | 1.425 |
|
COXVIc | 0.728b | 0.704b |
|
COXVIIa | 0.826b | 0.431b |
|
COXVIIb | 1.188 | 1.045 |
|
COXVIIc | 0.804a | 0.778b |
|
COXVIII | 1.072 | 1.053 |
| Assembly
factors |
|
COX10 | 0.942 | 0.918 |
|
COX11 | 1.103 | 1.028 |
|
COX15 | 0.776a | 0.653b |
|
COX17 | 1.016 | 0.939 |
|
COX18 | 0.836a | 0.865b |
|
COX19 | 0.742b | 0.849b |
|
LRPPRC | 1.139 | 1.051 |
|
SURF1 | 0.773 | 1.274 |
|
SCO1 | 1.036 | 1.170 |
|
SCO2 | 1.058 | 1.055 |
Several Snail-binding sites (E-box)
were previously found in the promoters of COX subunits (23)
To examine whether the expression of the COX
subunits was regulated by Snail, we conducted a ChIP assay. Snail
bound to the COXVIc, COXVIIa, and COXVIIc promoters (Fig. 2G), which is consistent with
previous observations (23). Among
the COX subunits, COXVIc acted as a common target of ROS, Dlx-2,
and Snail. Therefore, COXVIc may play an important role in the
repression of COX activity via the ROS-Dlx-2/Snail-mediated
pathway. For judging the transformation of the proteome accurately,
the proteome needs to be analyzed by mass spectrometry-based
methods, such as liquid chromatography time-of-flight mass
spectrometry (LC-TOF-MS), ultra-performance liquid chromatography
triple quadrupole mass spectrometry (UPLC-TQ-MS), and gas
chromatography time-of-flight mass spectrometry (GC-TOF-MS),
together with bioinformatics analyses (45,46).
Thus, proteome transformation is yet to be examined in further
studies, but cumulatively, our findings suggest that the
ROS-Dlx-2/Snail axis plays a crucial role in breast tumor
progression by regulating EMT, mitochondrial repression, and
glycolytic switch.
NF-κB has been shown to be involved in
ROS-induced EMT (13)
NF-κB induces EMT-inducing transcription factors
such as Snail, Slug, ZEB1, ZEB2, and Twist; NF-κB/p65
transcriptionally regulates the expression of these transcription
factors, which in turn represses the epithelial marker E-cadherin
and activates the mesenchymal marker N-cadherin, thereby resulting
in the induction of EMT (47–49).
Additionally, NF-κB stimulates the expression of HIF-1α, also
contributing to EMT (48).
Furthermore, NF-κB activation induces matrix-degrading enzymes such
as MMP9, thus contributing to EMT (50). NF-κB is known to be involved in
ROS-induced EMT via Snail induction (13). Because we found that ROS induce EMT
through the Dlx-2/Snail cascade, we think that an interplay between
NF-κB and Dlx-2 may possibly exist for Snail activation in
ROS-induced EMT; this interplay between NF-κB and Dlx-2 is yet to
be elucidated in further studies.
It has been shown that ROS are
involved in many aspects of cellular signaling
TGF-β1 has been shown to activate the ROS-NFκB
pathway, which plays an important role in TGF-β1-induced EMT, cell
migration, and invasion (51). We
have also shown that Dlx-2/Snail signaling is involved in
TGF-β-induced EMT (29,30). Thus, the ROS-Dlx-2/Snail cascade
may be involved in TGF-β-induced EMT. In addition, it was recently
reported that ROS are involved in EMT and cancer metastasis induced
by chemotherapeutics, such as 5-fluorouracil (5-FU), gemcitabine
(GEM), and oxaliplatin (52–54).
EMT contributes to chemoresistance in cancer cells. Dual oxidase 2
(DUOX2)-induced ROS promote the induction of EMT in 5-FU-resistant
colon cancer cells (52). In
addition, in GEM-treated pancreatic ductal adenocarcinoma (PDAC)
patients, the levels of glutathione peroxidase-1 (GPx1), an
antioxidant enzyme, are negatively regulated. GPx1 inhibits
ROS-mediated Akt/GSK3β/Snail signaling, thereby suppressing EMT and
chemoresistance in PDAC (53). ROS
have been shown to mediate oxaliplatin-induced EMT and invasive
potential in colon cancer (54).
Our results suggest that ROS-induced Dlx-2/Snail signaling may be
involved in EMT and may be induced by these chemotherapeutics. Note
that we used only one cell line in this study, thus our results are
limited to MCF-7 cells. Therefore, the Dlx-2/Snail axis is a
potential therapeutic target for the prevention of metastasis and
tumor progression in MCF-7 cells; this mechanism may be the case
for breast cancer in general.
Acknowledgements
Not applicable.
Funding
This study was supported by the National Research
Foundation of Korea (NRF) grant funded by the Korean government
(MSIP) (grant nos. 2015M2B2A9028108, 2015R1A2A2A01004468,
2017R1A2B4010411 and 2017R1A6A3A11030673).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
SYL, MKJ, HMJ and HSK conceived and designed the
project. SYL, MKJ and HMJ performed the experiments. SYL, MKJ, HMJ,
CHK, HGP, SIH, YJL and HSK analyzed and interpreted the data. SYL,
MKJ, HMJ, YJL and HSK wrote the manuscript. HSK supervised the
project. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Storz P: Reactive oxygen species in tumor
progression. Front Biosci. 10:1881–1896. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liou GY and Storz P: Reactive oxygen
species in cancer. Free Radic Res. 44:479–496. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Schieber M and Chandel NS: ROS function in
redox signaling and oxidative stress. Curr Biol. 24:R453–R462.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mittler R: ROS Are Good. Trends Plant Sci.
22:11–19. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Di Meo S, Reed TT, Venditti P and Victor
VM: Harmful and beneficial role of ROS. Oxid Med Cell Longev.
2016:79091862016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wu WS: The signaling mechanism of ROS in
tumor progression. Cancer Metastasis Rev. 25:695–705. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
de Sá, Junior PL, Câmara DAD, Porcacchia
AS, Fonseca PMM, Jorge SD, Araldi RP and Ferreira AK: The roles of
ROS in cancer heterogeneity and therapy. Oxid Med Cell Longev.
2017:24679402017.PubMed/NCBI
|
|
8
|
Zorov DB, Juhaszova M and Sollott SJ:
Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS
release. Physiol Rev. 94:909–950. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Han D, Williams E and Cadenas E:
Mitochondrial respiratory chain-dependent generation of superoxide
anion and its release into the intermembrane space. Biochem J.
353:411–416. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Burdon RH: Superoxide and hydrogen
peroxide in relation to mammalian cell proliferation. Free Radic
Biol Med. 18:775–794. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cannito S, Novo E, di Bonzo LV, Busletta
C, Colombatto S and Parola M: Epithelial-mesenchymal transition:
from molecular mechanisms, redox regulation to implications in
human health and disease. Antioxid Redox Signal. 12:1383–1430.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pani G, Giannoni E, Galeotti T and
Chiarugi P: Redox-based escape mechanism from death: the cancer
lesson. Antioxid Redox Signal. 11:2791–2806. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cichon MA and Radisky DC: ROS-induced
epithelial-mesenchymal transition in mammary epithelial cells is
mediated by NF-kB-dependent activation of Snail. Oncotarget.
5:2827–2838. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lim SO, Gu JM, Kim MS, Kim HS, Park YN,
Park CK, Cho JW, Park YM and Jung G: Epigenetic changes induced by
reactive oxygen species in hepatocellular carcinoma: methylation of
the E-cadherin promoter. Gastroenterology. 135:2128–2140, 2140
e2121-2128. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
De Craene B and Berx G: Regulatory
networks defining EMT during cancer initiation and progression. Nat
Rev Cancer. 13:97–110. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tsai JH and Yang J: Epithelial-mesenchymal
plasticity in carcinoma metastasis. Genes Dev. 27:2192–2206. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Puisieux A, Brabletz T and Caramel J:
Oncogenic roles of EMT-inducing transcription factors. Nat Cell
Biol. 16:488–494. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Micalizzi DS, Farabaugh SM and Ford HL:
Epithelial-mesenchymal transition in cancer: Parallels between
normal development and tumor progression. J Mammary Gland Biol
Neoplasia. 15:117–134. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Iwatsuki M, Mimori K, Yokobori T, Ishi H,
Beppu T, Nakamori S, Baba H and Mori M: Epithelial-mesenchymal
transition in cancer development and its clinical significance.
Cancer Sci. 101:293–299. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Peinado H, Olmeda D and Cano A: Snail, Zeb
and bHLH factors in tumour progression: an alliance against the
epithelial phenotype? Nat Rev Cancer. 7:415–428. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang Y, Shi J, Chai K, Ying X and Zhou BP:
The role of snail in EMT and tumorigenesis. Curr Cancer Drug
Targets. 13:963–972. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
De Craene B, Denecker G, Vermassen P,
Taminau J, Mauch C, Derore A, Jonkers J, Fuchs E and Berx G:
Epidermal snail expression drives skin cancer initiation and
progression through enhanced cytoprotection, epidermal
stem/progenitor cell expansion and enhanced metastatic potential.
Cell Death Differ. 21:310–320. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lee SY, Jeon HM, Ju MK, Kim CH, Yoon G,
Han SI, Park HG and Kang HS: Wnt/Snail signaling regulates
cytochrome C oxidase and glucose metabolism. Cancer Res.
72:3607–3617. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Warburg O: On the origin of cancer cells.
Science. 123:309–314. 1956. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hsu PP and Sabatini DM: Cancer cell
metabolism: Warburg and beyond. Cell. 134:703–707. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Vander Heiden MG, Cantley LC and Thompson
CB: Understanding the Warburg effect: the metabolic requirements of
cell proliferation. Science. 324:1029–1033. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cairns RA, Harris IS and Mak TW:
Regulation of cancer cell metabolism. Nat Rev Cancer. 11:85–95.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Finley LW, Zhang J, Ye J, Ward PS and
Thompson CB: SnapShot: cancer metabolism pathways. Cell Metab.
17:466, e462. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lee SY, Jeon HM, Ju MK, Jeong EK, Kim CH,
Yoo MA, Park HG, Han SI and Kang HS: Dlx-2 is implicated in TGF-β-
and Wnt-induced epithelial-mesenchymal, glycolytic switch, and
mitochondrial repression by Snail activation. Int J Oncol.
46:1768–1780. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lee SY, Jeon HM, Ju MK, Jeong EK, Kim CH,
Park HG, Han SI and Kang HS: Dlx-2 and glutaminase upregulate
epithelial-mesenchymal transition and glycolytic switch.
Oncotarget. 7:7925–7939. 2016.PubMed/NCBI
|
|
31
|
Merlo GR, Zerega B, Paleari L, Trombino S,
Mantero S and Levi G: Multiple functions of Dlx genes. Int J Dev
Biol. 44:619–626. 2000.PubMed/NCBI
|
|
32
|
Panganiban G and Rubenstein JL:
Developmental functions of the Distal-less/Dlx homeobox genes.
Development. 129:4371–4386. 2002.PubMed/NCBI
|
|
33
|
Lee SY, Jeon HM, Kim CH, Ju MK, Bae HS,
Park HG, Lim SC, Han SI and Kang HS: Homeobox gene Dlx-2 is
implicated in metabolic stress-induced necrosis. Mol Cancer.
10:1132011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yilmaz M, Maass D, Tiwari N, Waldmeier L,
Schmidt P, Lehembre F and Christofori G: Transcription factor Dlx2
protects from TGFβ-induced cell-cycle arrest and apoptosis. EMBO J.
30:4489–4499. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tang P, Huang H, Chang J, Zhao GF, Lu ML
and Wang Y: Increased expression of DLX2 correlates with advanced
stage of gastric adenocarcinoma. World J Gastroenterol.
19:2697–2703. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yan ZH, Bao ZS, Yan W, Liu YW, Zhang CB,
Wang HJ, Feng Y, Wang YZ, Zhang W, You G, et al: Upregulation of
DLX2 confers a poor prognosis in glioblastoma patients by inducing
a proliferative phenotype. Curr Mol Med. 13:438–445. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kim CH, Jeon HM, Lee SY, Ju MK, Moon JY,
Park HG, Yoo MA, Choi BT, Yook JI, Lim SC, et al: Implication of
snail in metabolic stress-induced necrosis. PLoS One. 6:e180002011.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yoon YS, Lee JH, Hwang SC, Choi KS and
Yoon G: TGF beta1 induces prolonged mitochondrial ROS generation
through decreased complex IV activity with senescent arrest in
Mv1Lu cells. Oncogene. 24:1895–1903. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sariban-Sohraby S, Magrath IT and Balaban
RS: Comparison of energy metabolism in human normal and neoplastic
(Burkitt's lymphoma) lymphoid cells. Cancer Res. 43:4662–4664.
1983.PubMed/NCBI
|
|
40
|
Dong C, Yuan T, Wu Y, Wang Y, Fan TW,
Miriyala S, Lin Y, Yao J, Shi J, Kang T, et al: Loss of FBP1 by
Snail-mediated repression provides metabolic advantages in
basal-like breast cancer. Cancer Cell. 23:316–331. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Jin L, Zhu C, Wang X, Li C, Cao C, Yuan J
and Li S: Urocortin attenuates TGFβ1-induced Snail1 and slug
expressions: inhibitory role of Smad7 in Smad2/3 signaling in
breast cancer cells. J Cell Biochem. 116:2494–2503. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Rios Garcia M, Steinbauer B, Srivastava K,
Singhal M, Mattijssen F, Maida A, Christian S, Hess-Stumpp H,
Augustin HG, et al: Acetyl-CoA carboxylase 1-dependent protein
acetylation controls breast cancer metastasis and recurrence. Cell
Metab. 26:842–855, e845. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Xu H, Tian Y, Yuan X, Wu H, Liu Q, Pestell
RG and Wu K: The role of CD44 in epithelial-mesenchymal transition
and cancer development. Onco Targets Ther. 8:3783–3792.
2015.PubMed/NCBI
|
|
44
|
Chanmee T, Ontong P, Kimata K and Itano N:
Key roles of hyaluronan and Its CD44 receptor in the stemness and
survival of cancer stem cells. Front Oncol. 5:1802015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Han X, Aslanian A and Yates JR III: Mass
spectrometry for proteomics. Curr Opin Chem Biol. 12:483–490. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Dettmer K, Aronov PA and Hammock BD: Mass
spectrometry-based metabolomics. Mass Spectrom Rev. 26:51–78. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Min C, Eddy SF, Sherr DH and Sonenshein
GE: NF-kappaB and epithelial to mesenchymal transition of cancer. J
Cell Biochem. 104:733–744. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Taniguchi K and Karin M: NF-κB,
inflammation, immunity and cancer: Coming of age. Nat Rev Immunol.
18:309–324. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Pires BR, Mencalha AL, Ferreira GM, de
Souza WF, Morgado-Díaz JA, Maia AM, Corrêa S and Abdelhay ES:
NF-kappaB is involved in the regulation of EMT genes in breast
cancer cells. PLoS One. 12:e01696222017. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Huang S, Pettaway CA, Uehara H, Bucana CD
and Fidler IJ: Blockade of NF-kappaB activity in human prostate
cancer cells is associated with suppression of angiogenesis,
invasion, and metastasis. Oncogene. 20:4188–4197. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Tobar N, Villar V and Santibanez JF:
ROS-NFkappaB mediates TGF-beta1-induced expression of
urokinase-type plasminogen activator, matrix metalloproteinase-9
and cell invasion. Mol Cell Biochem. 340:195–202. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Kang KA, Ryu YS, Piao MJ, Shilnikova K,
Kang HK, Yi JM, Boulanger M, Paolillo R, Bossis G, Yoon SY, et al:
DUOX2-mediated production of reactive oxygen species induces
epithelial mesenchymal transition in 5-fluorouracil resistant human
colon cancer cells. Redox Biol. 17:224–235. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Meng Q, Shi S, Liang C, Liang D, Hua J,
Zhang B, Xu J and Yu X: Abrogation of glutathione peroxidase-1
drives EMT and chemoresistance in pancreatic cancer by activating
ROS-mediated Akt/GSK3β/Snail signaling. Oncogene. 37:5843–5857.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Jiao L, Li DD, Yang CL, Peng RQ, Guo YQ,
Zhang XS and Zhu XF: Reactive oxygen species mediate
oxaliplatin-induced epithelial-mesenchymal transition and invasive
potential in colon cancer. Tumour Biol. 37:8413–8423. 2016.
View Article : Google Scholar : PubMed/NCBI
|