Introduction
In China, the third most common cause of
cancer-related mortality is hepatocellular carcinoma (HCC)
(1). HCC accounts for ~90% of the
total liver cancer burden globally (2). In clinical practice, surgical
resection is the most commonly used treatment procedure, followed
by adjuvant and systemic chemotherapy. However, the complex
aetiology and high metastatic potential of the disease renders
surgical treatment futile in the majority of cases. In recent
years, cellular immunotherapy has been increasingly applied in the
treatment of HCC (3). Following
the success of the new generation of targeted cell therapy
technologies represented by chimeric antigen receptor (CAR)-T
cytotherapy for the treatment of haematological tumours,
researchers have begun to explore the possibility of specific cell
therapies for the treatment of solid tumours (4). Currently, one of the major barriers
to CAR-T cell therapy is cytokine release syndrome (CRS), which is
mainly mediated by interleukin-6. CRS can lead to acute respiratory
distress syndrome and multiple organ failure (5). Unlike T cells, natural killer (NK)
cells mainly secrete interferon γ (IFN-γ) and
granulocyte-macrophage colony-stimulating factor (GM-CSF), which
are unlikely to cause CRS (6,7).
Therefore, CAR-expressing NK (CAR-NK) cells may be safer compared
with CAR-T cells in clinical application.
NK cells, which are a crucial component of the
innate immune system, are characterized as CD3−
CD56+ and serve important roles in the immune
surveillance and early control of tumorigenesis (8). NK cells can recognize and eradicate
tumour cells without prior antigenic exposure (9,10).
Their recognition of tumour cells depends on the imbalance of
activatory and inhibitory receptors on the cell surface (11). Following the identification of
tumour cells, NK cells can destroy tumour cells rapidly via
secretion of perforin and granzymes (12,13),
induction of apoptosis, expression of tumour necrosis factor family
molecules (14) or expression of
CD16, which leads to antibody-dependent cellular cytotoxicity
(15,16).
However, the anti-tumour activity of NK cells in
patients with tumours is limited due to the decline in the quantity
and quality of NK cells and tumour immune escape (9). Modifying NK cells through recombinant
CAR engineering may enhance their tumour targeting and killing
efficiency (17). CAR-NK cells
have been tested in vitro and in animal models (6), but clinical studies of CAR-NK cells
remain mostly in the preclinical stage. CAR-NK cells take advantage
of the CAR gene-modification strategy and present several unique
advantages over CAR-T cells (7).
First, long-term persistence of CAR-expressing cells increases the
risk of autoimmunity or malignant transformation; however, since NK
cells have relatively limited lifespans in vivo, infused
CAR-NK cells perish rapidly after mediating their antitumor
effects. Second, allogeneic NK cells do not induce
graft-versus-host disease (18).
In addition, even when tumour cells escape immune surveillance by
downregulating the expression of CAR-targeted antigens, CAR-NK
cells are still be able to mediate cytotoxic effects through their
natural receptor molecules (19).
Notably, the cytokines secreted by NK cells are mainly the
aforementioned IFN-γ and GM-CSF, which are unlikely to cause CRS
(6,7). Considering the multiple advantages of
CAR-NK cells, the potential applications of CAR-NK cells for the
treatment of various cancer types are being studied widely
(18).
A CAR construct consists of an extracellular
antigen- recognition domain, a transmembrane domain and an
intracellular signalling domain. The extracellular
antigen-recognition domain is generally composed of a single-chain
variable fragment (scFv) derived from the variable regions of the
heavy and light chains of a monoclonal antibody, which are fused
together via a flexible linker (19). The intracellular signalling region,
which contains an immunoreceptor tyrosine-based activation motif,
determines the intensity of CAR-NK activation (19).
Similar to T cells, NK cells can be gene-modified
with CARs and specifically combined tumour antigens to enhance
cytotoxicity (18). However, to
the best of our knowledge, only a few previous studies have
explored their therapeutic potential in HCC. Primary NK cells are
usually derived from autogenous peripheral blood mononuclear cells
(PBMCs) from patients (7). Primary
NK cells were also examined for a comprehensive insight into NK
cell-based modification (7). The
c-MET-specific CAR in the present study was based on the humanized
c-MET-specific antibody developed in our laboratory and the TYRO
protein tyrosine kinase-binding protein (DAP12) signalling
domains.
c-MET is the product of the proto-oncogene
MET, which is expressed by epithelial and endothelial cells,
neurons, hepatocytes and haematopoietic cells (20,21).
c-MET serves crucial roles in the development and progression of
cancer (22). c-MET is associated
with the proliferation, survival, invasion and metastasis of cancer
cells (23). The overexpression of
c-MET has been observed in various solid malignancies, such as
liver (24–26), breast (27), lung (28) and colorectal (29) cancer. Notably, c-MET aberrations
occur in ~50% of patients with HCC (30). c-MET has been identified as a
carcinogen in HCC, as increased c-MET activity can initiate, drive
or contribute to the development and progression of HCC (25). Thus, c-MET has been increasingly
used as an immunotherapeutic CAR-T cell target in progressive HCC.
However, the lack of specific tumor antigens and the limited
penetration of CAR-T cells into the tumour site reduce the effect
of CAR-T cells on hepatocellular carcinoma (31).
In the present study, the function and effectiveness
of c-MET-CAR-engineered NK cells against liver cancer cells was
determined with the aim of developing an effective and specific NK
cell-based therapeutic strategy for liver cancer treatment.
Materials and methods
Cell lines
The human liver cancer cell line HepG2 and lung
cancer cell line H1299 (Shanghai Zhong Qiao Xin Zhou Biotechnology
Co., Ltd.) were maintained in RPMI 1640 medium (Thermo Fisher
Scientific, Inc.) supplemented with 10% fetal bovine serum (FBS;
Thermo Fisher Scientific, Inc.). 293T cells (American Type Culture
Collection) were cultured in high-glucose DMEM (Thermo Fisher
Scientific, Inc.) containing 10% FBS. All cell lines were cultured
in a humidified atmosphere containing 5% CO2 at
37°C.
RNA interference of HepG2 cells
HepG2 cells were transfected with 100 nM
C-MET-homo-3918 and C-MET-homo-2659 short interfering (si) RNAs
(Shanghai GenePharma Co., Ltd.) using Lipofectamine®
3000 (Thermo Fisher Scientific, Inc.) according to the
manufacturer's instructions. Scrambled siRNA was used as a negative
control. Cells were cultured for 48 h prior to further experiments.
To avoid off-target effects, two pairs of siRNAs were used for RNA
interference: C-MET-homo-3918 forward, 5′-CCAGAGACAUGUAUGAUAATT-3′
and reverse, 5′-UUAUCAUACAUGUCUCUGGTT-3′; C-MET-homo-2659 forward,
5′-GCAACAGCUGAAUCUGCAATT-3′ and reverse,
5′-UUGCAGAUUCAGCUGUUGCTT-3′. The sequences of control siRNAs
(scrambled negative control) were forward,
5′-UUCUCCGAACGUGUCACGUTT-3′ and reverse,
5′-ACGUGACACGUUCGGAGAATT-3′.
Construction of the c-MET-CAR
lentiviral plasmid
The scFv nucleotide sequence of an anti-c-MET
antibody was fused with a sequence encoding truncated human
epidermal growth factor receptor (huEGFRt) immediately following
the V5 tag-encoding sequence. The fused DNA sequences were
incorporated with 41BB-DAP12. The entire
c-MET-scFv-CD8α-41BB–DAP12-EGFRt-V5 fragment was ligated into a
green fluorescent protein (GFP)-expressing lentiviral vector
Plv-Easy-GFP [previously constructed in our laboratory (32)] to construct the c-MET-CAR
lentiviral plasmid. The plasmid was digested with the KpnI
and NotI enzymes to confirm that the insert was the correct
size. Sequencing analysis of the plasmid was completed by Sangon
Biotech (Shanghai) Co., Ltd. An empty GFP lentiviral plasmid was
used as a negative control.
Lentivirus production
To produce lentiviruses for the infection of NK
cells, 293T cells (5×106 cells/dish) were seeded in 10
cm dishes and cultured overnight. The c-MET-CAR lentiviral plasmid
or GFP lentiviral plasmid was transfected with the psPAX2 and
pMD2.G packaging plasmids (gifts from Professor Hu Ying) using a
calcium chloride transfection reagent as previously described
(33), the cells were incubated at
37°C with 5% CO2 overnight and then the culture medium
was replaced with fresh cell culture medium. The c-MET-CAR and GFP
lentiviruses were harvested 48–72 h following transfection,
filtered through Millex-GP 0.45 µm filters (Merck KGaA), and
concentrated by ultracentrifugation (4°C; 72,000 × g; 2 h). The
pellet was resuspended with 1X PBS. The c-MET-CAR lentivirus titer
was determined by detecting GFP expression using a flow
cytometry-based method. Briefly, 293T cells were plated at
1×105 cells/well in a 24-well plate and incubated
overnight. The next day, the medium was removed and the cells were
infected with 1 ml of undiluted, 1:4-, 1:16-, 1:64-, 1:128- or
1:256-diluted c-MET-CAR lentivirus containing 8 µg/ml polybrene
(Sigma-Aldrich; Merck KGaA). Following incubation overnight at
37°C, the medium was replaced with fresh DMEM. At 48 h, the cells
were detached with trypsin in EDTA, resuspended in 1X PBS and
analysed for GFP expression by flow cytometry. The titer of the
c-MET-CAR lentivirus was calculated as follows: Titer (transducing
units/ml)=(percentage of GFP+ cells ×10,000)/dilution
ratio.
Transduction of NK cells
Human PBMCs were isolated from fresh blood.
Peripheral blood samples from donors were collected at Shenzhen
Luohu People's Hospital (from June 2018 to January 2019). Written
informed consent was collected and the study was approved by the
Ethics Committee of Shenzhen Luohu People's Hospital. A Human NK
Cell Culture kit (cat. no. MCF-004; Morecell Biomedical Co., Ltd.)
and Serum-free Medium for NK Cells (cat. no. MCM-002; Morecell
Biomedical Co., Ltd.) was used for the induction of NK cells,
according to the manufacturer's instructions. The cell number and
viability were determined under a microscope using trypan blue. The
purity of CD3−CD56+ NK cells was detected by
flow cytometry. NK cells were seeded at 1×105 cells/well
in a 24-well plate, infected with the lentivirus at a multiplicity
of infection (MOI) of 100 and supplemented with 8 µg/ml polybrene.
At 6 h, the NK cell culture medium was replaced with fresh
medium.
Flow cytometric analysis
A single cell suspension of primary NK cells was
prepared in Cell Staining Buffer (cat. no. 420201; BioLegend,
Inc.). Cells were pre-incubated with 5 µl of Human TruStain FcX™
(cat. no. 422301; BioLegend, Inc.) per 100 µl (1×106
cells) of cell suspension for 5–10 min at room temperature to block
Fc-receptors. The samples were centrifuged at 350 × g for 5 min at
room temperature and the supernatant was discarded. Cells were
incubated with the following fluorescently labelled antibodies:
Anti-CD3-FITC (5 µl/1×106 cells; cat. no. 300305;
BioLegend, Inc.) or anti-CD56-phycoerythrin (5 µl/1×106
cells; cat. no. 362507; BioLegend, Inc.) and incubated on ice for
15–20 min in the dark. Cells were washed two times with 2 ml of
Cell Staining Buffer and centrifuged at 350 × g for 5 min at room
temperature. Cells were analyzed by flow cytometry using a BD
FACSCalibur (Becton-Dickinson and Company) and data was analyzed
using FCS Express 6.06.0022 (De Novo Software).
Western blot analysis
Western blotting was performed to examine c-MET
expression in tumour cells and CAR expression in c-MET-CAR-NK
cells. Briefly, cells were harvested and lysed with RIPA buffer
(Beyotime Institute of Biotechnology) supplemented with a protease
inhibitor cocktail and phenylmethylsulphonyl fluoride. Protein
concentration was determined using the bicinchoninic acid method. A
total of 20 µg of the protein was loaded onto 7.5% SDS-PAGE at room
temperature (RT). The proteins were transferred to PVDF membranes
(Immobilon-P; EMD Millipore), blocked with 5% semi-skimmed milk for
1 h at RT and incubated overnight at 4°C with a primary anti-c-MET
antibody (1:1,000; cat. no. AF1432; Beyotime Institute of
Biotechnology) or anti-V5 antibody (1:1,000; cat. no. 4AA261811F;
Beijing 4A Biotech Co., Ltd.). The next day, the membranes were
thoroughly rinsed with TBS + 0.05% Tween-20 and incubated for 1 h
at RT with a horseradish peroxidase-conjugated secondary antibody
(1:4,000; cat. no. A0218; Beyotime Institute of Biotechnology).
Following mild stripping, the same membranes were incubated with a
mouse anti-β-actin antibody (1:2,000; cat. no. 3700T; Cell
Signalling Technology, Inc.) for 1 h at room temperature as a
loading control. Reaction products were detected using enhanced
chemiluminescence (ECL Plus; GE Healthcare). ImageJ version K1.45
(National Institutes of Health) was used for densitometric analysis
to quantify the relative expression of c-MET in the different cell
lines.
Immunofluorescence
Immunostaining was performed as previously described
(34). The staining reagents
included an anti-c-MET antibody (1:500; cat. no. AF1432; Beyotime
Institute of Biotechnology), Alexa Fluor 488-labelled goat
anti-rabbit IgG antibody (1:400; cat. no. A0428; Beyotime Institute
of Biotechnology), DAPI (cat. no. D9542; Sigma-Aldrich; Merck KGaA)
and TRITC-conjugated phalloidin (1:1,000; cat. no. P1951;
Sigma-Aldrich; Merck KGaA). The cells were imaged using an inverted
fluorescent microscope (magnification, ×20; Axio Observe 3; Carl
Zeiss AG). Images were processed using ImageJ version K1.45.
Cytokine release assay
c-MET-CAR-NK, GFP-NK and NK cells were co-incubated
with HepG2 or H1299 tumour cells at an effector-to-target (E:T)
ratio of 2.5:1. Following 24 h of incubation at 37°C, the
supernatant was harvested and the concentration of released IFN-γ
was measured using the Human IFN-γ ELISA kit (cat. no. SIF50;
R&D Systems, Inc.), according to the manufacturer's
protocol.
In vitro cytotoxicity assay
Lactate dehydrogenase (LDH) release cytotoxicity
assays were performed using the LDH Cytotoxicity Assay kit
(Beyotime Institute of Biotechnology), following the manufacturer's
protocol. c-MET-CAR-NK cells or NK cells and tumor cells (HEPG2 and
H1299) were co-cultured at an E:T ratio of 2.5:1 for 4 h at 37°C in
96-well plates in triplicate. Specific lysis was calculated as
follows: Percentage of specific lysis=(experimental
release-effector spontaneous release-target spontaneous
release)/(target maximal release-target spontaneous release)
×100%.
Apoptosis assay
For apoptosis quantification, c-MET-CAR- NK cells or
NK cells and tumor cells (HEPG2 and H1299) were co-cultured at an
E:T ratio of 2.5:1 for 4 h at 37°C in 6-well plates in triplicate.
The cells were washed twice with cold PBS and resuspend in 1X
Binding Buffer (BD Biosciences) at a concentration of
1×105 cells/ml. A total of 5 µl FITC Annexin V (BD
Biosciences) and 5 µl propidium iodide (BD Biosciences) were added,
and the cells were gently vortexed and incubated for 15 min at 37°C
in the dark. Subsequently, 400 µl 1X Binding Buffer was added to
each tube, and the cells were analysed by flow cytometry using a BD
FACSCalibur (Becton-Dickinson and Company) within 1 h and data was
analyzed using FCS Express 6.06.0022 (De Novo Software). All data
are presented as the mean ± standard deviation.
Live-cell video microscopy
A HepG2-mCherry stable cell line expressing the
mCherry protein was created by transfection with the plasmid
FT106-mCherry (gifts from Professor Hu Ying) using
Lipofectamine® 3000 reagent (Thermo Fisher Scientific,
Inc.), according to the manufacturer's instructions. Stably
transfected cells were selected using 600 µg/ml of G418 sulfate
(Thermo Fisher Scientific, Inc.) for 3 weeks. The G418-resistant
clones were isolated, expanded and maintained on plates in complete
media with 400 µg/ml G418 at 37°C in a humidified incubator with 5%
CO2. For live-cell video experiments, HepG2-mCherry
cells (5×103 cells/well) were plated in a 96-well plate
and maintained at 37°C in a humidified incubator for 24 h to allow
for adherence. Subsequently, c-MET-CAR-NK cells were added at a
ratio of 1:1. The cells were imaged every 5 min for 36 h at 37°C
using a Cytation1 imaging reader (BioTek Instruments, Inc.). Images
were processed using ImageJ version K1.45.
Statistical analysis
Data are expressed as the mean ± SD.Statistical
analysis was performed with GraphPad Prism 5 software (GraphPad
Software, Inc.). Student's t-test or one-way ANOVA with Bonferroni
post hoc test were used to analyse the differences between groups.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Generation of the c-MET-CAR lentiviral
plasmid
The scFv nucleotide sequence of an anti-c-MET
antibody (Data S1A) was fused with a sequence encoding huEGFRt
immediately following the V5 tag-encoding sequence. To improve
signal transduction, the c-MET-CAR was designed with a CD8α hinge
and the intracellular signalling domains of 41BB and DAP12. The
entire c-MET-scFv-CD8α-41BB–DAP12-EGFRt-V5 fragment (Fig. 1A) was ligated into a GFP lentiviral
vector to construct the c-MET-CAR lentiviral plasmid. Fig. 1B shows bands at 6,034 and 2,432 bp
after agarose gel electrophoresis of the digested recombinant
c-MET-CAR lentiviral plasmid; the band sizes were consistent with
the expected results. The sequencing results were consistent with
the c-MET-CAR gene sequence, which suggested that the recombinant
c-MET-CAR lentiviral plasmid was successfully constructed (Data
S1B).
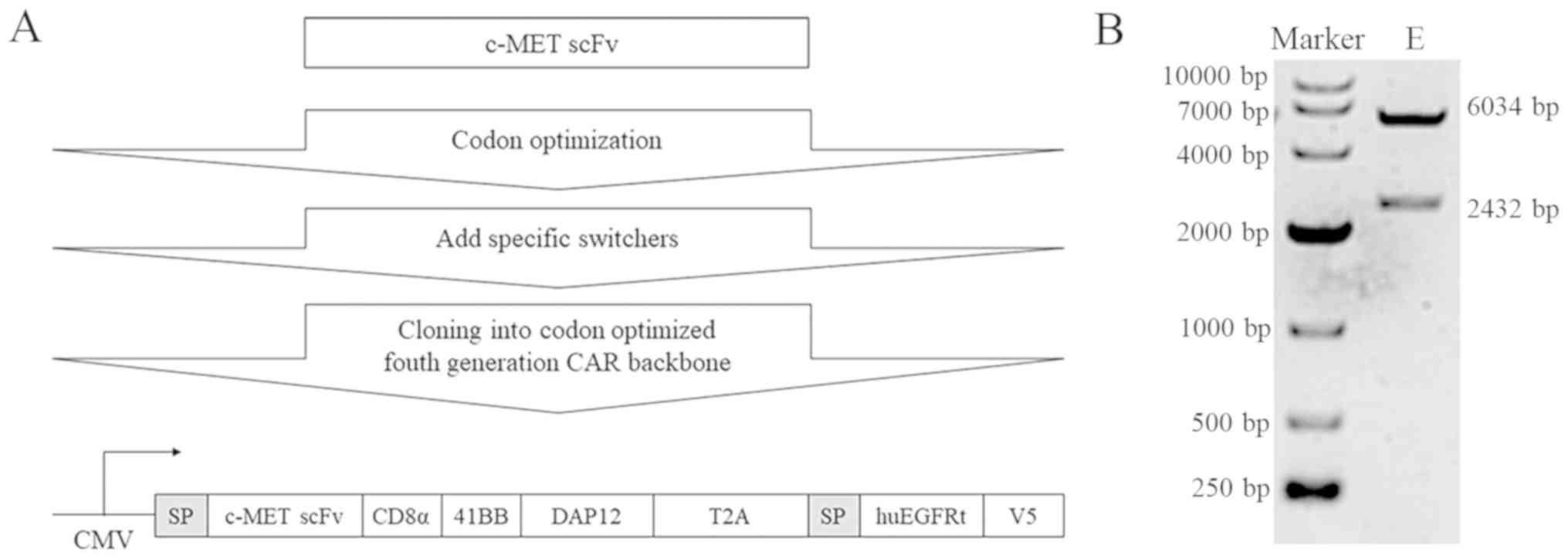 | Figure 1.Construction of the c-MET-CAR
lentiviral plasmid. (A) Schematic representation of the c-MET-CAR
lentiviral construct, which consisted of an SP, C-MET-specific scFv
antibody fragment and a CD8α hinge region, a T2A self-cleaving
peptide sequence followed by the intracellular domains of 41BB and
DAP12, a signaling adaptor molecule involved in the signal
transduction of the activating NK cell receptor. as well as a
safety switch from huEGFRt. CAR expression was driven by the CMV
promoter. (B) The constructed c-MET-CAR plasmid was digested with
the restriction endonucleases KpnI and NotI and
identified by agarose gel electrophoresis. The lengths of the two
electrophoresis bands were 6,034 and 2,432 bp. SP, signal peptide;
c-MET, c type proto-oncogene receptor tyrosine kinase; CAR,
chimeric antigen receptor; scFv, single-chain variable fragment;
huEGFRt, truncated human epidermal growth factor receptor; CMV,
cytomegalovirus; DAP12, TYRO protein tyrosine kinase-binding
protein; E, experimental. |
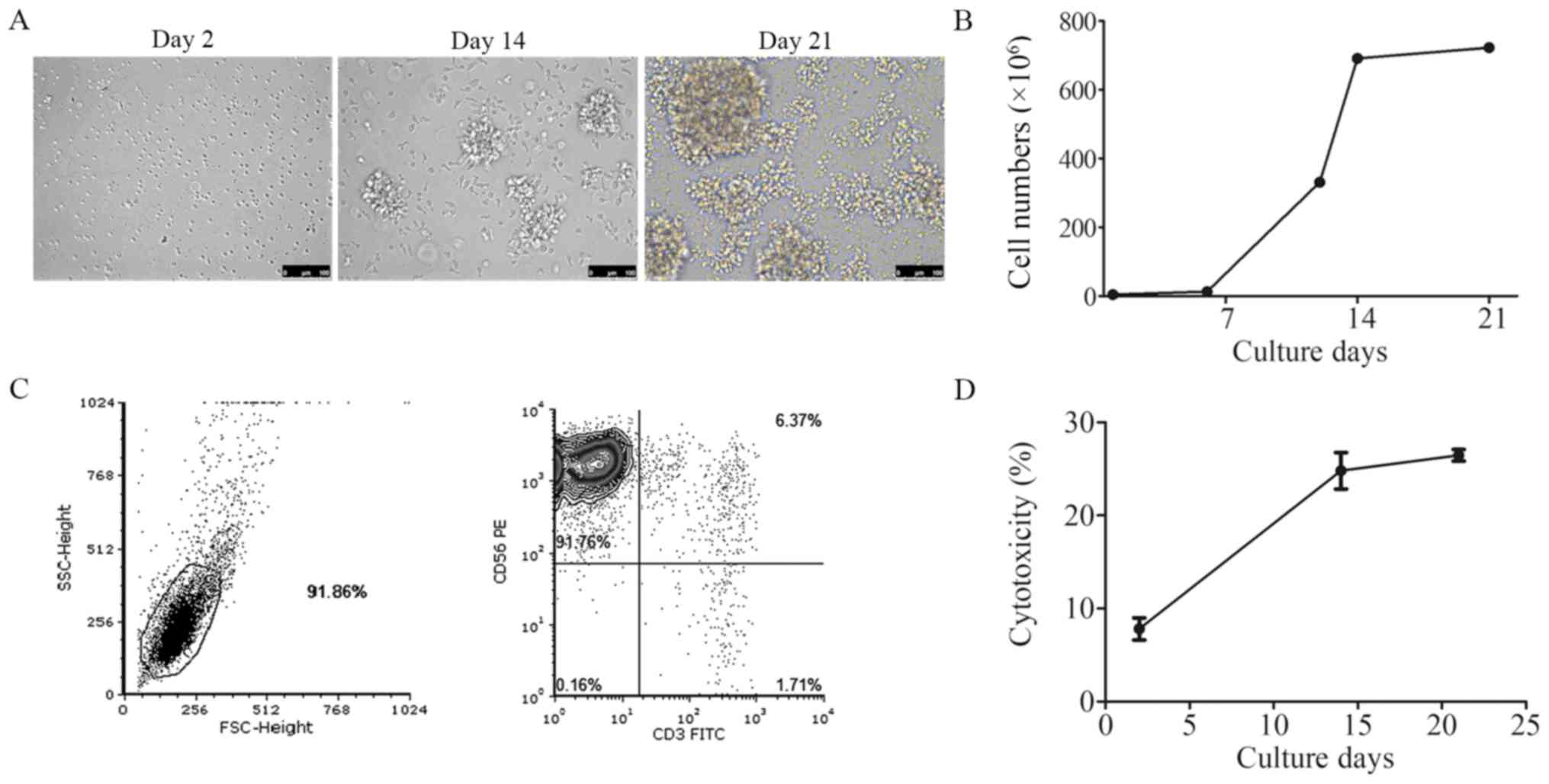
In vitro culture of human NK cells
induced from PBMCs
Human NK cells were induced from PBMCs isolated from
fresh blood from healthy donors. Images of the cultured NK cells
were taken on days 2, 14 and 21 under a microscope. Cell morphology
changed from single small cells to large cell clusters (Fig. 2A). The growth curve demonstrated
that the NK cells entered the rapid proliferation period on day 6
(Fig. 2B). The purity of the
CD3−CD56+ NK cells was detected by flow
cytometry following 21 days of culture. In Fig. 2C, the upper left quadrant of the
scatter diagram demonstrated that >90% of the cells were
CD3−CD56+ NK cells. The cytotoxicity of
cultured NK cells was also tested on days 2, 14 and 21; the
percentage of the NK cytotoxicity was 7.47, 25.93 and 27.40%,
respectively (Fig. 2D).
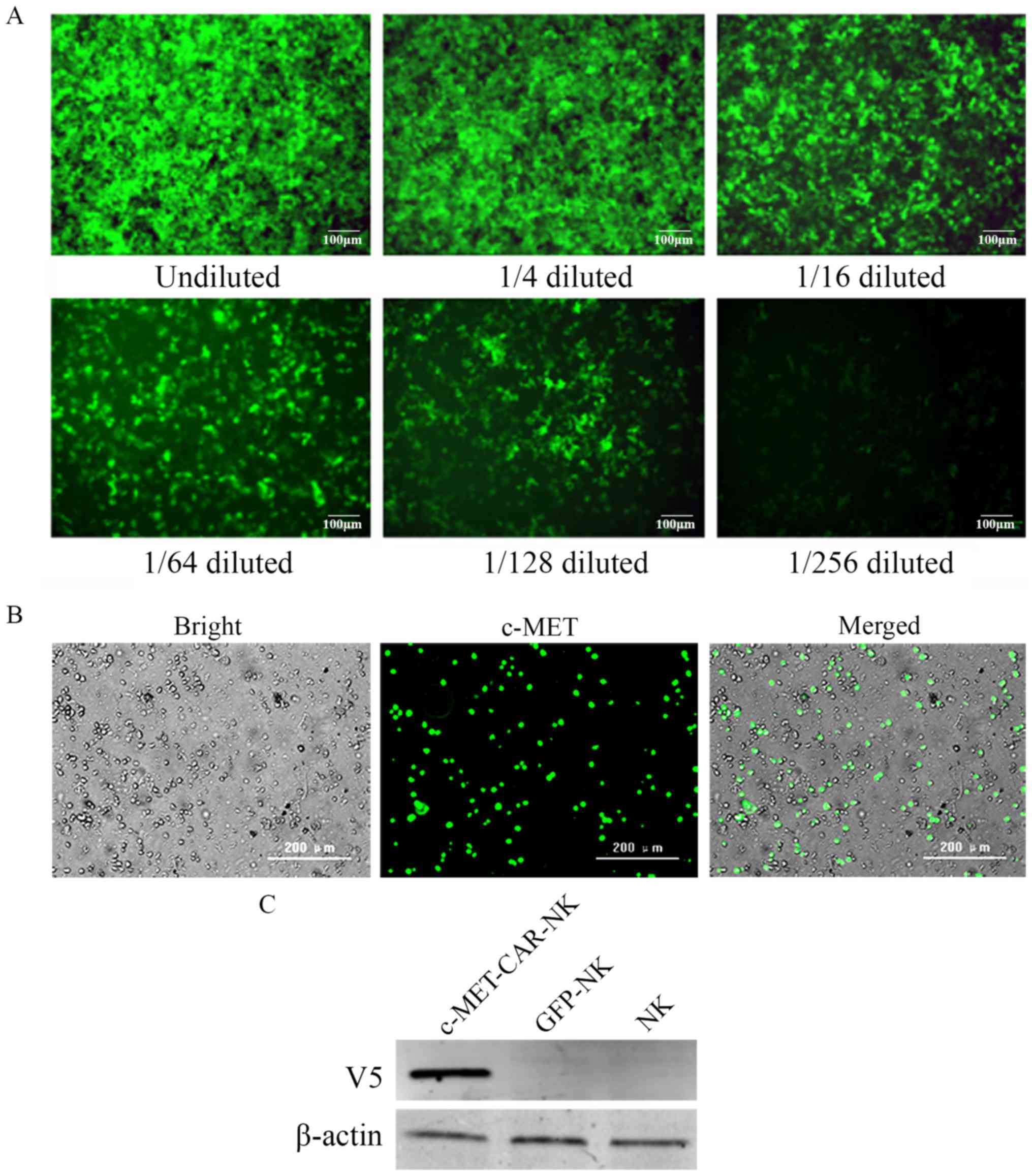
Generation of c-MET-CAR-NK cells
The c-MET-CAR lentiviral plasmid or control GFP
lentiviral plasmid was co-transfected with the psPAX2 and pMD2.G
packaging plasmids to produce the c-MET-CAR and GFP lentiviruses.
To confirm the titre, the c-MET-CAR lentivirus was diluted into six
gradient concentrations and used to infect 293T cells. After 48 h
of infection, the infected 293T cells were observed using
fluorescence microscopy (Fig. 3A)
and analysed for GFP expression using fluorescence-activated cell
sorting (data not shown).
In vitro cultured human NK cells were
transduced with the c-MET-CAR lentivirus or GFP lentivirus at the
MOI of 100 to generate c-MET-CAR-NK cells or GFP-NK cells,
respectively. The c-MET-CAR-NK cells were observed under
brightfield (Fig. 3B, left) and a
green channel filter (Fig. 3B,
middle). According to the merged picture (Fig. 3B, right), ~50% of the NK cells were
GFP-positive. This subpopulation represented the c-MET-CAR-NK
cells.
To validate the expression of c-MET-CAR in the
transduced NK cells, western blot analysis was performed using an
anti-V5 antibody that recognized the V5 tag within the c-MET-CAR
structure. c-MET-CAR (V5) was expressed in the c-MET-CAR-NK cells,
but not in the GFP-NK cells or normal NK cells (Fig. 3C).
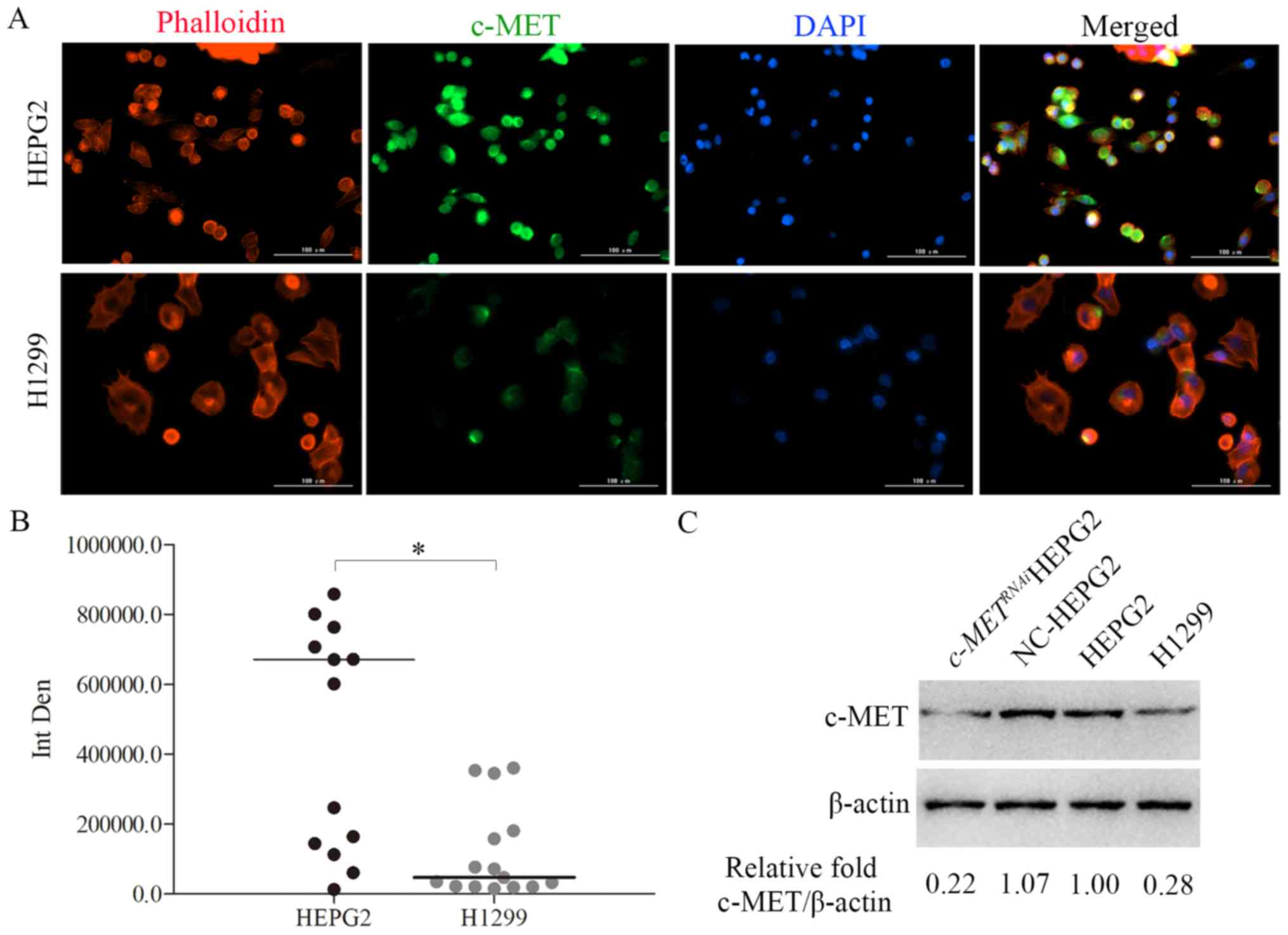
c-MET is highly expressed in HepG2
cells but not H1299 cells
To assess the expression of c-MET in HepG2 and H1299
cells, cells were stained with a c-MET-specific antibody in an
immunofluorescence assay (Fig.
4A). Fig. 4B demonstrates the
statistical results of the fluorescence intensity in the green
(c-MET) channel in Fig. 4A,
indicating that the c-MET expression level in HepG2 cells was
higher compared with that in the H1299 cells. The western blotting
results further revealed that c-MET was highly expressed in the
HepG2 cells, but expressed at a low level in the H1299 cells
(Fig. 4C). HepG2 cell transfection
with siRNA interfering with c-MET (c-METRNAi)
revealed that the level of c-MET protein had been knocked down
successfully (Fig. 4C, far left
lane).
c-MET-CAR-NK cells kill HepG2 cells
through c-MET CAR
Two cell lines were used to test the specificity of
c-MET-CAR-NK cells: The c-MET low-expression cell line H1299 and
c-MET high-expression cell line HepG2 (Fig. 5A). c-METRNAi
HepG2 cells were used as a negative control. The E:T ratio was
2.5:1. The results demonstrated that the ratio of apoptotic cells
in the H1299 group was 18.57, 18.87 and 19.54% for c-MET-CAR-NK,
GFP-NK and NK, respectively. The ratio of apoptotic cells in the
HepG2 group was 45.64, 20.74 and 16.86% for c-MET-CAR-NK, GFP-NK
and NK, respectively. The ratio of apoptotic cells in
c-METRNAi HepG2 cells group was 22.31, 21.95 and
18.31% for c-MET-CAR-NK, GFP-NK and NK, respectively. The
cytotoxicity of c-MET-CAR-NK against the c-MET high-expression cell
line HepG2 was significantly increased compared with the other two
groups (P<0.01; Fig.
5B).
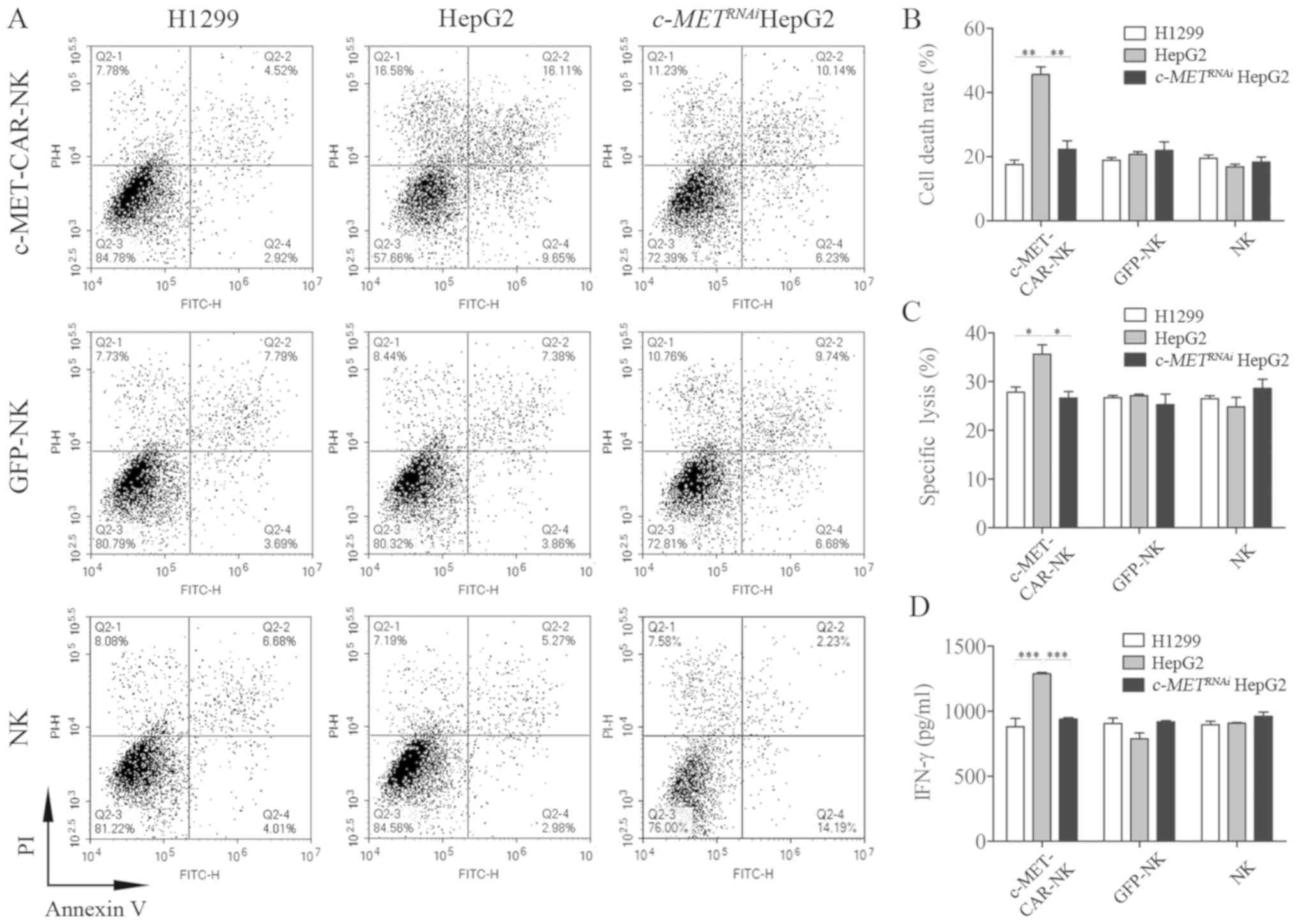 | Figure 5.c-MET-CAR-NK cells specifically kill
HepG2 cells. c-MET-CAR-NK, GFP-NK and NK cells were co-incubated
with H1299, HepG2 or c-METRNAi HepG2 cells at an
effector-to-target ratio of 2.5:1. After 4 h of incubation,
apoptosis was (A) measured by flow cytometry and (B) quantified.
(C) After 4 h of incubation, the specific lysis percentage was
detected with a lactate dehydrogenase release assay. (D) After 24 h
of incubation, the supernatant was harvested and the concentration
of the released IFN-γ was measured with a sandwich ELISA. The data
are presented as the mean ± SEM from three independent experiments.
*P<0.05, **P<0.01 and ***P<0.001. NK, natural killer;
c-MET, c type proto-oncogene receptor tyrosine kinase; CAR,
chimeric antigen receptor; GFP, green fluorescent protein; IFN-γ,
interferon-γ; c-METRNAi, cells treated with short
interfering RNA targeting c-MET; PI, propidium iodide. |
The specific lysis of the two tumour cell lines
induced by c-MET-CAR-NK cells was evaluated using the GFP-NK and
normal NK cells as controls. The E:T ratio was 2.5:1. The
cytotoxicity assay results demonstrated that c-MET-CAR-NK cells
exhibited a significant increase in cytotoxicity against HepG2
cells with a lysis ratio of 35.64±3.28% compared with the H1299 and
c-METRNAi HepG2 groups (Fig. 5C), however, there was no
significant difference when the E:T ratio was 5:1 (Fig. S1A).
The level of IFN-γ secreted by c-MET-CAR-NK, GFP-NK
and NK cells was determined by ELISA at an E:T ratio of 2.5:1; the
concentration of IFN-γ in the H1299 group was 882.36, 906.32 and
897.32 pg/ml in c-MET-CAR-NK, GFP-NK and NK, respectively. The
concentration of IFN-γ in the HepG2 group was 1,288.35, 787.67 and
908.25 pg/ml for c-MET-CAR-NK, GFP-NK and NK, respectively. The
concentration of IFN-γ in c-METRNAi HepG2 group
was 940.17, 918.25 and 962.23 pg/ml for c-MET-CAR-NK, GFP-NK and
NK, respectively. The level of IFN-γ secreted by c-MET-CAR-NK
against the c-MET high-expression cell line HepG2 was significantly
increased compared with the other two groups (P<0.001; Fig. 5D), however, there was no
significant difference when the E:T ratio was 5:1 (Fig. S1B).
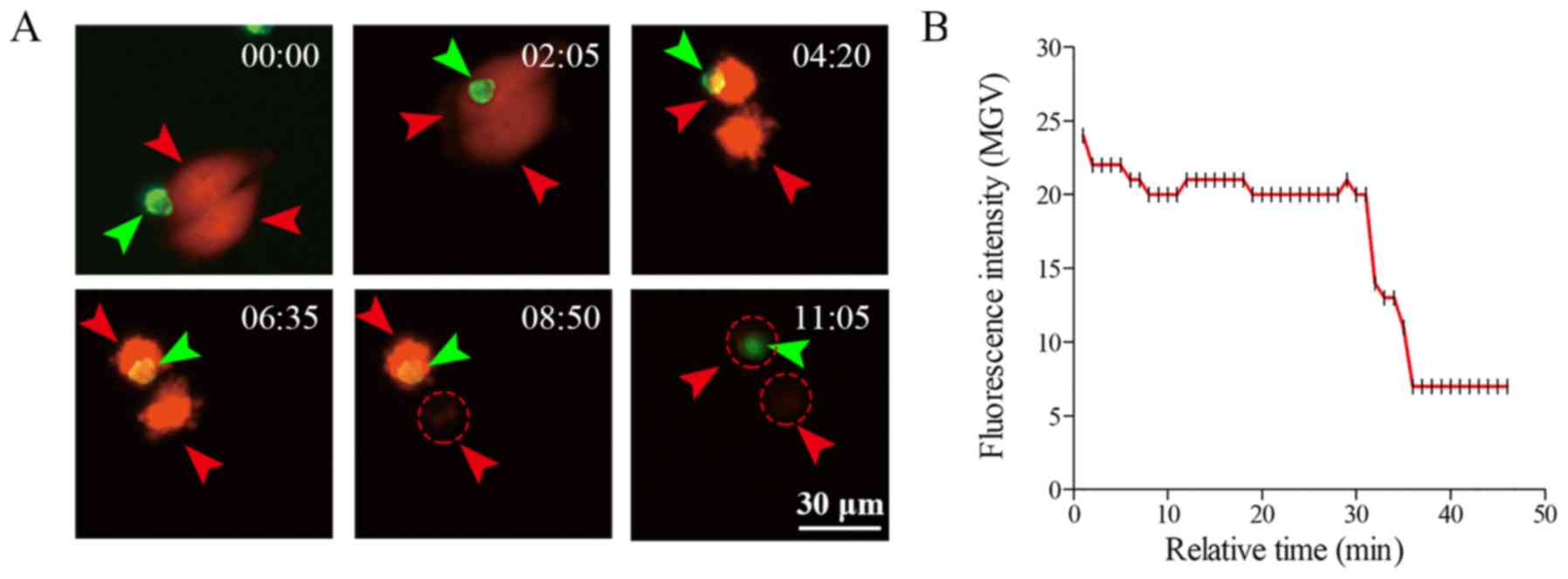
Observation of c-MET-CAR-NK cell
cytotoxicity in vitro using live imaging
The cytotoxic activity of c-MET-CAR-NK cells against
HepG2 cells stably expressing mCherry (HepG2-mCherry) was analysed
using live imaging. The integrated process of a mitotic HepG2 cell
being attacked by a c-MET-CAR-NK cell was recorded and analysed
(Fig. 6). The mCherry fluorescence
gradually disappeared, representing the apoptosis of a
c-MET-positive HepG2 cell, which was due to the attack from
c-MET-CAR-NK cells (Fig. 6A). The
quantification of the live imaging results is presented in Fig. 6B. Following 30 min of c-MET-CAR-NK
and HepG2 cell co-culture, the fluorescence intensity of HepG2 cell
reduced from 25 to 6.5 mean grey value, which indicated the death
of the target cells.
Discussion
The present study focused on the possibility of
developing a specific cell therapy using NK cells as effector cells
in HCC. Based on previous in vitro data, c-MET was been
identified as a carcinogen in liver cancer (17–19).
Consequently, a CAR structure that can guide NK cells targeting the
c-MET antigen expressed on the HCC cell surface was constructed in
the present study. The results demonstrated a high affinity between
c-MET-CAR-positive NK cells and HepG2 liver cancer cells. Based on
these results, CAR-NK cell-based immunotherapy may provide a safe
and specific approach for liver cancer therapy. However, the exact
curative effect of CAR-NK cell immunotherapy still needs to be
demonstrated in animal models and clinical trials. Apart from NK
cell cytotoxicity, cytokines such as IFN-γ are involved in the
effect of NK cell-based immunotherapy. NK cells are an early
producer of IFN-γ, which exerts multiple effects on the immune
response, such as the induction of major histocompatibility complex
class II molecules on antigen-presenting cells, activation of
myeloid cells and induction of T helper 1 cells, as well as
angiogenesis. Macrophage activation by NK cell-derived IFN-γ has
been demonstrated to be essential for the resistance to chemical
carcinogenesis in a mouse model of primary tumorigenesis. Thus,
IFN-γ is an important anti-tumour cytokine (35).
In the majority of published studies, the NK92 cell
line was used as effective cells against tumours (3,4,36).
In the present study, a method to produce autologous NK cells from
PBMCs was developed. The advantage of this method is that it
guarantees safety for potential future clinical applications. In
addition, a lentivirus system was adapted to transduce the
c-MET-CAR structure into effective NK cells, which largely
increased the ratio of infected NK cells from the 30% reported by
previous studies (19,37,38)
to >50%. In addition, c-Met-CAR-engineered NK cells were more
specific and cytotoxic against the liver cancer cell line HepG2
with high c-MET expression compared with the lung cancer cell line
H1299 with low c-MET expression. These results suggest that CAR-NK
cells may be a promising, specific and safe approach for liver
cancer treatment.
Although the preliminary data of the present study
demonstrated that c-MET-CAR-NK had potential application prospects
in liver cancer, several problems emerged during the development
process. Firstly, it has been reported in another study that the
lifespan of CAR-NK cell is only 28 days in vivo (39), which may result in a reduction of
persistence of the curative effect due to the high cost of CAR-NK
cells. Secondly, the dose of CAR-NK cells may be difficult to apply
in vivo. Previous studies have reported the optimal
anti-tumour activity for CAR-NK cells in vivo, which is
1×107 cells/mouse (40,41).
However, the titer of the lentivirus in the present study was
MOI=100. Lentiviruses are used as a gene-modification tool in gene
therapy. The first lentiviral therapy, Tisagenlecleucel, was
approved in the United States in 2017 for the treatment of
pediatric and young adult patients with acute lymphoblastic
leukemia (42). The safety of
lentiviruses has been accepted worldwide (43).
There are also limitations to solid tumour treatment
using CAR-NK cells. The most concerning problem is the off-target
effect caused by tumour-associated antigen mutation post-CAR-NK
treatment, as similar problems have been reported in CAR-T therapy
(36,38). Recently, Zhang et al
(37) reported that the checkpoint
receptor T cell immunoreceptor with immunoglobulin and
immunoreceptor tyrosine-based inhibitory motif domains was
associated with NK cell exhaustion in tumour-bearing mice and
patients with colon cancer. Thus, the microenvironment of solid
tumours may inhibit the anti-tumour activity of CAR-NK cells.
However, potential NK cell immune checkpoint blockers such as
anti-programmed cell death protein 1 antibody for T cells may be
used as a combined treatment in future studies.
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to thank Professor Hu Ying of
The Harbin Institute of Technology for providing the psPAX2 and
pMD2.G plasmids.
Funding
The present study was supported by the Shenzhen
Science and Technology Innovation Committee (grant nos.
KQJSCX20170331160008397, JCYJ20170412155231633,
JCYJ20170307171034705, JCYJ20170816105345191 and
GGFW2016030117123665) and the Science and Technology Planning
Project of Shenzhen, China (grant no. JCYJ20170816105345191).
Availability of data and materials
The datasets used and analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
BL and ZZL performed the experiments. MLZ, JWL, XMC,
WBG, ZL and ZDY assisted with the experiments. TL designed the
project and wrote the manuscript.
Ethics approval and consent to
participate
The generation of human NK cells was approved by the
Ethics Committee of Shenzhen Luohu People's Hospital. All blood
donors provided written informed consent for the collection of
samples and subsequent analyses.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
NK
|
natural killer
|
|
c-MET
|
c type proto-oncogene receptor
tyrosine kinase
|
|
CAR
|
chimeric antigen receptor
|
|
LDH
|
lactate dehydrogenase
|
|
HCC
|
hepatocellular carcinoma
|
|
PBMCs
|
peripheral blood mononuclear cells
|
References
|
1
|
Yuan P, Chen TH, Chen ZW and Lin XQ:
Calculation of life-time death probability due malignant tumors
based on a sampling survey area in China. Asian Pac J Cancer Prev.
15:4307–4309. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Omata M, Cheng AL, Kokudo N, Kudo M, Lee
JM, Jia J, Tateishi R, Han KH, Chawla YK, Shiina S, et al:
Asia-Pacific clinical practice guidelines on the management of
hepatocellular carcinoma: A 2017 update. Hepatol Int. 11:317–370.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ma W, Wu L, Zhou F, Hong Z, Yuan Y and Liu
Z: T cell-associated immunotherapy for hepatocellular carcinoma.
Cell Physiol Biochem. 41:609–622. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang Z, Wu Z, Liu Y and Han W: New
development in CAR-T cell therapy. J Hematol Oncol. 10:532017.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
DeFrancesco L: CAR-T cell therapy seeks
strategies to harness cytokine storm. Nat Biotechnol. 32:6042014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Klingemann H: Are natural killer cells
superior CAR drivers? Oncoimmunology. 3:e281472014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Glienke W, Esser R, Priesner C, Suerth JD,
Schambach A, Wels WS, Grez M, Kloess S, Arseniev L and Koehl U:
Advantages and applications of CAR-expressing natural killer cells.
Front Pharmacol. 6:212015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bordon Y: Tumour immunology: Natural
killer cells spy greedy tumours. Nat Rev Immunol. 18:772018.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rosenberg EB, Herberman RB, Levine PH,
Halterman RH, McCoy JL and Wunderlich JR: Lymphocyte cytotoxicity
reactions to leukemia-associated antigens in identical twins. Int J
Cancer. 9:648–658. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Qian X, Wang X and Jin H: Cell transfer
therapy for cancer: Past, present, and future. J Immunol Res.
2014:5259132014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Vivier E, Raulet DH, Moretta A, Caligiuri
MA, Zitvogel L, Lanier LL, Yokoyama WM and Ugolini S: Innate or
adaptive immunity? The example of natural killer cells. Science.
331:44–49. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bradley M, Zeytun A, Rafi-Janajreh A,
Nagarkatti PS and Nagarkatti M: Role of spontaneous and
interleukin-2-induced natural killer cell activity in the
cytotoxicity and rejection of Fas+ and Fas- tumor cells. Blood.
92:4248–4255. 1998.PubMed/NCBI
|
|
13
|
Screpanti V, Wallin RP, Ljunggren HG and
Grandien A: A central role for death receptor-mediated apoptosis in
the rejection of tumors by NK cells. J Immunol. 167:2068–2073.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kayagaki N, Yamaguchi N, Nakayama M,
Takeda K, Akiba H, Tsutsui H, Okamura H, Nakanishi K, Okumura K and
Yagita H: Expression and function of TNF-related apoptosis-inducing
ligand on murine activated NK cells. J Immunol. 163:1906–1913.
1999.PubMed/NCBI
|
|
15
|
Brehm C, Huenecke S, Esser R, Kloess S,
Quaiser A, Betz S, Zimmermann O, Soerensen J, Passweg JR,
Klingebiel T, et al: Interleukin-2-stimulated natural killer cells
are less susceptible to mycophenolate mofetil than non-activated NK
cells: possible consequences for immunotherapy. Cancer Immunol
Immunother. 63:821–833. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Campbell KS and Hasegawa J: Natural killer
cell biology: An update and future directions. J Allergy Clin
Immunol. 132:536–544. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Guillerey C, Huntington ND and Smyth MJ:
Targeting natural killer cells in cancer immunotherapy. Nat
Immunol. 17:1025–1036. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rezvani K, Rouce R, Liu E and Shpall E:
Engineering natural killer cells for cancer immunotherapy. Mol
Ther. 25:1769–1781. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mehta RS and Rezvani K: Chimeric antigen
receptor expressing natural killer cells for the immunotherapy of
cancer. Front Immunol. 9:2832018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fasolo A, Sessa C, Gianni L and Broggini
M: Seminars in clinical pharmacology: An introduction to MET
inhibitors for the medical oncologist. Ann Oncol. 24:14–20. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Furge KA, Zhang YW and Vande Woude GF: Met
receptor tyrosine kinase: Enhanced signaling through adapter
proteins. Oncogene. 19:5582–5589. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ma PC, Maulik G, Christensen J and Salgia
R: c-Met: Structure, functions and potential for therapeutic
inhibition. Cancer Metastasis Rev. 22:309–325. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang YW, Su Y, Volpert OV and Vande Woude
GF: Hepatocyte growth factor/scatter factor mediates angiogenesis
through positive VEGF and negative thrombospondin 1 regulation.
Proc Natl Acad Sci USA. 100:12718–12723. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhuang PH, Xu L, Gao L, Lu W, Ruan LT and
Yang J: Correlations of microvascular blood flow of
contrast-enhanced ultrasound and HGF/c-Met signaling pathway with
clinicopathological features and prognosis of patients with
hepatocellular carcinoma. Onco Targets Ther. 10:847–857. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bouattour M, Raymond E, Qin S, Cheng AL,
Stammberger U, Locatelli G and Faivre S: Recent developments of
c-Met as a therapeutic target in hepatocellular carcinoma.
Hepatology. 67:1132–1149. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kim JH, Kim HS, Kim BJ, Jang HJ and Lee J:
Prognostic value of c-Met overexpression in hepatocellular
carcinoma: A meta-analysis and review. Oncotarget. 8:90351–90357.
2017.PubMed/NCBI
|
|
27
|
Yan S, Jiao X, Zou H and Li K: Prognostic
significance of c-Met in breast cancer: A meta-analysis of 6010
cases. Diagn Pathol. 10:622015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pyo JS, Kang G, Cho WJ and Choi SB:
Clinicopathological significance and concordance analysis of c-MET
immunohistochemistry in non-small cell lung cancers: A
meta-analysis. Pathol Res Pract. 212:710–716. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu Y, Yu XF, Zou J and Luo ZH: Prognostic
value of c-Met in colorectal cancer: A meta-analysis. World J
Gastroenterol. 21:3706–3710. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xin Y, Jin D, Eppler S, Damico-Beyer LA,
Joshi A, Davis JD, Kaur S, Nijem I, Bothos J, Peterson A, et al:
Population pharmacokinetic analysis from phase I and phase II
studies of the humanized monovalent antibody, onartuzumab (MetMAb),
in patients with advanced solid tumors. J Clin Pharmacol.
53:1103–1111. 2013.PubMed/NCBI
|
|
31
|
Lohitesh K, Chowdhury R and Mukherjee S:
Resistance a major hindrance to chemotherapy in hepatocellular
carcinoma: An insight. Cancer Cell Int. 18:442018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang Q, Zhou M, Wu X, Li Z, Liu B, Gao W,
Yue J and Liu T: Promoting therapeutic angiogenesis of focal
cerebral ischemia using thrombospondin-4 (TSP4) gene-modified bone
marrow stromal cells (BMSCs) in a rat model. J Trans Med.
17:1112019. View Article : Google Scholar
|
|
33
|
Gandara C, Affleck V and Stoll EA:
Manufacture of third- generation lentivirus for preclinical use,
with process development considerations for translation to good
manufacturing practice. Hum Gene Ther Methods. 29:1–15. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang YF, Kunda PE, Lin JW, Wang H, Chen
XM, Liu QL and Liu T: Cytokine-induced killer cells co-cultured
with complete tumor antigen-loaded dendritic cells, have enhanced
selective cytotoxicity on carboplatin-resistant retinoblastoma
cells. Oncol Rep. 29:1841–1850. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jackson HJ and Brentjens RJ: Overcoming
antigen escape with CAR T-cell therapy. Cancer Discov. 5:1238–1240.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Harjes U: CAR antigens beyond recognition.
Nat Rev Cancer. 18:7232018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang Q, Bi J, Zheng X, Chen Y, Wang H, Wu
W, Wang Z, Wu Q, Peng H, Wei H, et al: Blockade of the checkpoint
receptor TIGIT prevents NK cell exhaustion and elicits potent
anti-tumor immunity. Nature immunology. 19:723–732. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Orlando EJ, Han X, Tribouley C, Wood PA,
Leary RJ, Riester M, Levine JE, Qayed M, Grupp SA, Boyer M, et al:
Genetic mechanisms of target antigen loss in CAR19 therapy of acute
lymphoblastic leukemia. Nat Med. 24:1504–1506. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Li Y, Hermanson DL, Moriarity BS and
Kaufman DS: Human iPSC-derived natural killer cells engineered with
chimeric antigen receptors enhance anti-tumor activity. Cell Stem
Cell. 23:181–192, e185. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Oelsner S, Waldmann A, Billmeier A, Röder
J, Lindner A, Ullrich E, Marschalek R, Dotti G, Jung G,
Grosse-Hovest L, et al: Genetically engineered CAR NK cells display
selective cytotoxicity against FLT3-positive B-ALL and inhibit in
vivo leukemia growth. Int J Cancer. Mar 13–2019.(Epub ahead of
print). View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Shiozawa M, Chang CH, Huang YC, Chen YC,
Chi MS, Hao HC, Chang YC, Takeda S, Chi KH and Wang YS:
Pharmacologically upregulated carcinoembryonic antigen-expression
enhances the cytolytic activity of genetically-modified chimeric
antigen receptor NK-92MI against colorectal cancer cells. BMC
Immunol. 19:272018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Escors D and Breckpot K: Lentiviral
vectors in gene therapy: Their current status and future potential.
Arch Immunol Ther Exp (Warsz). 58:107–119. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Schambach A, Zychlinski D, Ehrnstroem B
and Baum C: Biosafety features of lentiviral vectors. Hum Gene
Ther. 24:132–142. 2013. View Article : Google Scholar : PubMed/NCBI
|