Introduction
Chromosome 14 open reading frame 166 (C14orf166) is
a highly conserved gene that is located on chromosome 14, at the
cytogenetic band 14q22.1. It encodes a 28-kDa protein known as
C14orf166, CLE, hCLE or CGI-199 that localizes to the nucleus and
the cytoplasm. In the developing brain, C14orf166 is a core element
of cytosolic RNA granules containing ribosomes that transport
specific mRNAs from the cell body to the dendrites, including mRNAs
encoding RNA-binding proteins and microtubule-associated proteins,
serving a crucial role in local mRNA translation at sites away from
the nucleus in neuronal processes (1,2). As
the expression of C14orf166 is higher in fetal brain and lungs than
in these organs once they are fully developed, it is possible that
C14orf166 serves a role in brain and lung organogenesis (3). Furthermore, proteomic analysis of mice
brains has demonstrated that C14orf166 is downregulated after
embryonic day 15, suggesting its role in cell growth during
development (4). In addition,
proteomic analysis has demonstrated that C14orf166 is associated
with transcriptional-related functions as it is part of the human
spliceosome (5) and the
tRNA-splicing ligase complex (6),
and it interacts with the 7SK snRNA methylphosphate capping enzyme
(7). Furthermore, certain studies
have reported that C14orf166 may serve an immunogenic role and act
as a autoantigen, although this remains to be confirmed (8,9). The
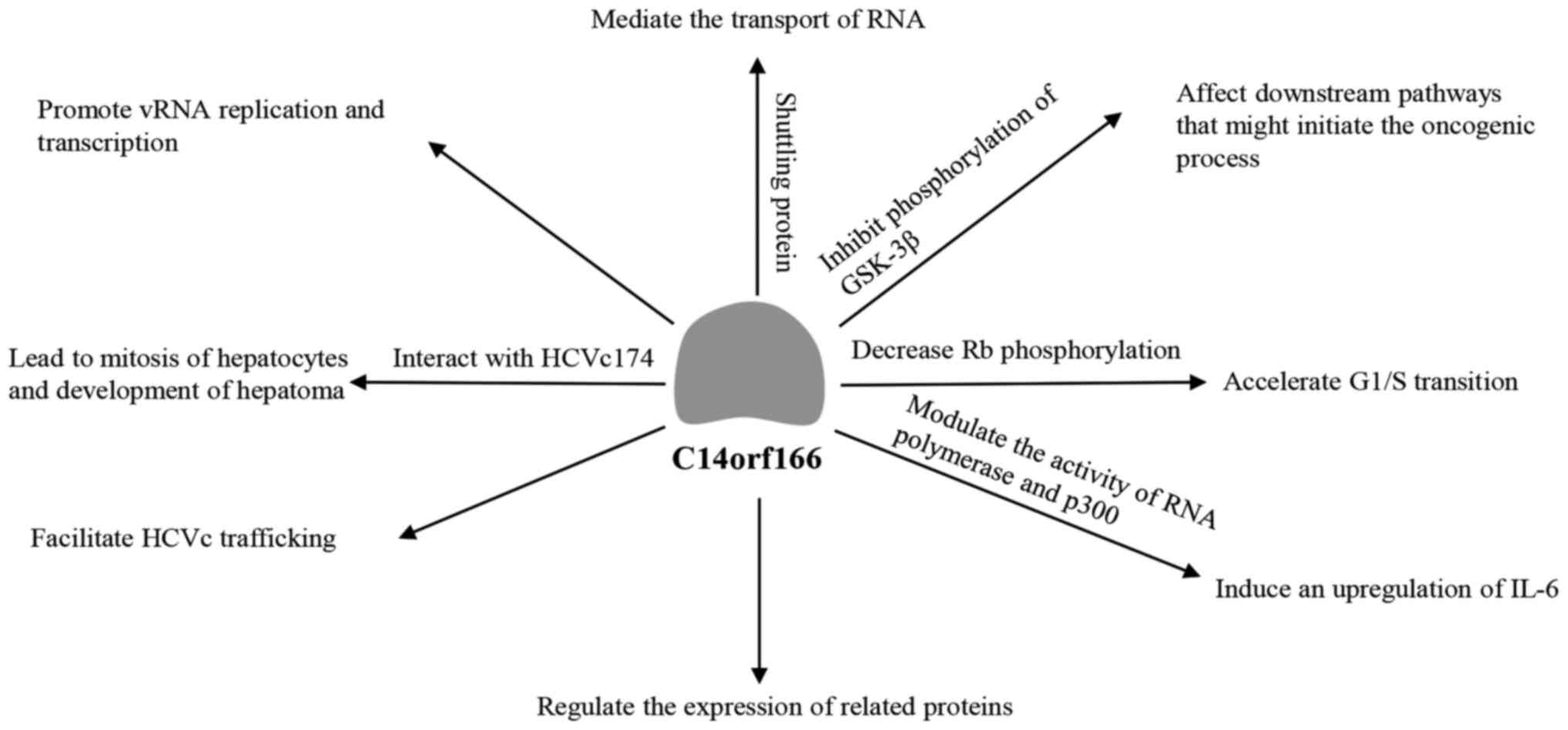
present review focuses on the main effects of C14orf166, including
its role during viral infections and RNA metabolism, and
investigates its potential pathogenic roles of C14orf166 in cancer
and the suggested underlying mechanism (Fig. 1).
Interaction with influenza A virus
(IAV)
IAVs cause an annually recurrent epidemic of acute
respiratory disease that poses a major public health problem
worldwide. The World Health Organization estimates that the
influenza epidemic leads to 3–5 million cases of severe illness and
up to 650,000 deaths each year (10). The genome of IAVs consists of eight
single-stranded negative-sense viral RNA (vRNA) segments that range
between 2,341 and 890 nucleotides in length, and are named after
the main protein they encode (11).
Regardless of the length of the segment, the 3′ and 5′termini of
each vRNA are bound to the RNA-dependent RNA polymerase (RdRp), and
the remaining RNA is encapsulated by a nucleoprotein (NP).
Therefore, the viral genetic material is packed in a vRNA-NP-RdRp
complex (12), also termed viral
ribonucleoprotein (vRNP) complex (13). The RdRp from influenza virus is a
heterotrimer composed of the polymerase basic proteins 1 (PB1) and
2 (PB2), and polymerase acidic (PA) protein. PB1 is the core
subunit of RdRp and harbors the polymerase activity (10). PB2 contains a cap-binding domain
that recognizes the capped cellular mRNAs. Following binding to
PB2, the cellular mRNAs are cleaved by the endonuclease activity of
the PA subunit at ~12 nucleotides away from the 5′-cap. This
process, referred to as ‘cap-snatching’, produces the primers
necessary for the viral transcription (14). Therefore, it is an essential step
for the transcription of viral RNA in the nucleus of a host cell
during influenza virus infection (15,16).
The vRNP complex is hypothesized to be a powerful
factor during the invasion of IAV into the host cell. A
considerable amount of literature has demonstrated that the nuclear
export process depends not only on the formation of a protein
complex comprising vRNPs, the viral nuclear export protein (NEP)
and the viral matrix protein 1 (M1), but also requires the
phosphorylation of NP, and to a minor extent, NEP, as well as the
SUMOylation of M1 (17–19). C14orf166, despite its cellular
origin, is also a key factor in the IAV life cycle, promoting vRNA
replication and transcription (20). C14orf166 interacts with and
activates the cellular RNA polymerase II and the PA subunit of the
RdRp (21). Silencing C14orf166
expression results in a decrease in vRNA transcription, replication
and production of the infectious virus (22). In addition to function as a
transcriptional modulator, C14orf166 interacts with several
proteins engaged in pre-mRNA processing, such as DDX1, suggesting
it also serves a role in RNA maturation (23). Furthermore, it has also been
demonstrated that C14orf166 binds to progeny vRNP in the cytoplasm,
suggesting that it accompanies the newly-generated vRNP molecules
during their export to the cytoplasm (24). In addition, it has been demonstrated
that C14orf166 may be incorporated into IAV particles, tightly
bound to vRNP, which promotes viral and cellular polymerase
interaction for viral transcription (24).
Role in other viral infections
C14orf166 is also involved in other viral
infections, where it serves similar roles as those described
previously in IAVs (25–27). A previous study reported that
C14orf166 interacts with a core protein of the hepatitis C virus
(HCV), HCVc174 (25). This
interaction appears to be relevant in acute and chronic HCV
infection. In the nucleus, it has been suggested that the
C14orf166/HCVc174 complex may lead to aberrant mitosis of infected
hepatocytes, and result in hepatic carcinoma (25). In addition, the C14orf166/HCVc174
complex also interacts with cytoplasmic ninein molecules, essential
for microtubule assembly and organization (25). This may facilitate viral entry and
assembly, contributing toward more efficient establishment of the
infection. In addition, C14orf166 also interacts with the
nucleocapsid protein of infection with the bronchitis virus
(26) and is involved in the
nuclear steps of HIV-1 RNA metabolism (27).
Regulation of RNA metabolism
RNA metabolism is modulated by the interaction
between RNA molecules and RNA-binding proteins. C14orf166 is
involved in several steps of RNA metabolism, including RNA
transcription, maturation and translation. C14orf166 interacts with
several factors essential for RNA synthesis and processing,
including transcription factor 4, heterogeneous nuclear
ribonucleoprotein R, poly A binding protein 1 and the nuclear pore
complex Nup153 (28–30). In addition, it has been demonstrated
that C14orf166 acts as a shuttling protein for DDX1, HSPC117 and
FAM98B (31). DDX1 is an RNA
helicase that binds to homopolymeric poly(A) RNA and regulates
HIV-1 replication (32,33), HSPC117 is an essential subunit of a
tRNA splicing ligase complex (6),
and FAM98B has been associated with colorectal cancer malignancy,
but its physiological function remains unknown. As a shuttling
protein, C14orf166 mediates the transport of the RNA molecules
encoding these proteins between the nucleus and the cytoplasm.
Notably, C14orf166 has demonstrated asymmetric kinetics in its
nucleo-cytoplasmic movement, as it leaves the nucleus faster than
it enters it (34). Reimportation
of C14orf166, DDX1, HSPC117 and FAM98B requires active
transcription, that is, initiation of the
C14orf166-DDX1-HSPC117-FAM98B complex requires the synthesis of new
RNA cargos (34). In addition,
C14orf166 regulates the expression of these C14orf166-interacting
proteins, as C14orf166-silencing leads to their nuclear and
cytosolic downregulation (34).
Further research is required to unravel how this complex is formed
and its role in the regulation of the nuclear and cytosolic RNA
fate.
The 5′end of mRNA molecules contains a
7-methyguanilate molecule connected to the RNA through a 5′ to
5′triphosphate linkage. This structure protects mRNA molecules from
degradation by ribonucleases and binds to initiation factors,
including eIF4E triggering the translation of the messenger
molecule. Recently, it has been reported that the complex
C14orf166-HSPC117-DDX1-FAM98B may also bind to the cap structure
independently to eIF4E (35).
C14orf166 retained the ability to bind to the cap structure without
its complex partners, although the binding affinity was markedly
lower, suggesting that HSPC117, DDX1 and FAM98B enhance the
cap-binding ability of C14orf166 (35). In addition, the same study reported
that the C14orf166 complex may positively regulate the translation
of specific mRNAs (35). Finally,
in addition to the previously described roles in transcription and
translation, C14orf166 is also involved in RNA maturation (36).
Role of C14orf166 in cancer
Cancer is a disease involving uncontrolled cell
proliferation due to the cells' ability to escape the body's
natural mechanism of cell death (37). The cancer mortality rate has
markedly increased in recent years (37). Although great efforts have been made
to decrease mortality and prolong the survival time of patients
with cancer, this disorder remains a major threat to human health.
The lack of specific and sensitive markers for early diagnosis is
one of the major causes of a poor prognosis (37). Conventional treatments, including
surgical resection of the tumor, chemotherapy or radiotherapy,
often have serious associated side effects, and reoccurrence of the
cancer following the treatment is a common concern (37). In addition, tumors often develop
resistance to chemotherapy drugs (31). Therefore, there is an urgent
requirement to develop novel approaches for the diagnosis and
treatment of cancer. Over the past 20 years, a large number of
studies have reported a role for C14orf166 in cancer (Table I). C14orf166 is overexpressed in
cancer tissue, compared with healthy tissue (Table I). In addition, high expression of
C14orf166 is associated with shorter overall survival and
disease-free survival times in various types of cancer (31,38–40).
This suggested that C14orf166 levels in serum may be used as a
prognostic factor and therapeutic target, encouraging further
investigation to elucidate the pathological role that C14orf166 may
serve (31,41,42).
Howng et al (3) demonstrated
that C14orf166 interacts with the C-terminal coiled-coil of
centrosomal ninein suppressing the N-terminal phosphorylation by
glycogen synthase kinase 3β (GSK-3β). GSK-3β is associated with
cancer, as high GSK-3β expression levels are associated with the
development of cancer (38).
Additionally, GSK-3β may phosphorylate several substrates from the
JAK2/STAT3, Wnt/β-catenin and PI3K-AKT-mTOR signaling pathways that
mediate cancer initiation, progression and drug resistance
(39,43,44).
In addition, C14orf166 is a JKA2-interacting protein that activates
JKA2/STAT3 signaling, which may lead to esophageal and cervical
cancer (40,45). Although speculative, we hypothesize
that C14orf166-ninein binding inhibits the ninein-GSK-3β and the
phosphorylation of GSK-3β, which affects downstream pathways that
may initiate the oncogenic process. C14orf166 has also been
reported to decrease retinoblastoma protein phosphorylation,
accelerating G1/S transition in bladder and breast cancer cells and
contributing toward uncontrolled proliferation, although the
details of this mechanism have not yet been fully elucidated
(31,41). Finally, C14orf166 has been
demonstrated to modulate the activity of RNA polymerase and p300,
inducing an upregulation of IL-6 (46). High levels of this cytokine are
associated with poor prognosis (46) and promote continuous, unregulated
signaling through STAT3 (47).
Taken together, the results of these studies demonstrated that
C14orf166 serves a critical role in the initiation and progression
of cancer. Therefore, C14orf166 stands as a promising biomarker
candidate and actionable drug target, and further research should
be conducted to broaden our knowledge regarding its functions.
 | Table I.Studies of C14orf166 in various types
of carcinoma. |
Table I.
Studies of C14orf166 in various types
of carcinoma.
| Carcinoma | Changing trend | Associated
clinicopathological characteristics | Prognosis | (Refs.) |
|---|
| Brain tumor | Upregulated | – | – | (3) |
| Esophageal squamous
cell carcinoma | Upregulated | T, N and M
stage | Negative | (20) |
| Breast cancer | Upregulated | T, N and M stage,
PR, survival time, vital status | Negative | (31) |
| Bladder cancer | Upregulated | T and N stage,
histological differentiation, vital status | Negative | (38) |
| Nasopharyngeal
carcinoma | Upregulated | Sex, clinical
stage, T, N and M stage, vital status, treatment method | Negative | (39) |
| Uterine cervical
cancer | Upregulated | FIGO stage, vital
status, tumor size, M stage, serum squamous cell carcinoma antigen
level | Negative | (40) |
| Pancreatic
adenocarcinoma | Upregulated | N stage | Negative | (48) |
| Hepatocellular
carcinoma | Upregulated | T, N and M stage,
tumor size, serum AFP level, tumor recurrence | Negative | (49) |
Conclusion
C14orf166 has been identified as a crucial protein
during several virus infections, including IAV and HCV. In a
physiological context, C14orf166 is a key factor for RNA
transcription, maturation and translation. In cancer tissue, it is
overexpressed and appears to contribute toward the uncontrolled
cell proliferation. Therefore, it may be used as a diagnostic and
prognostic biomarker for various types of cancer in the future,
although there remain a number of questions that require
addressing.
Acknowledgements
Not applicable.
Funding
The present study was supported by Natural Science
Foundation of Hunan Province (grant no. S2020JJQNJJ1802).
Availability of data and materials
Not applicable.
Authors' contributions
QC conducted the literature search. RL proofread the
manuscript. Both authors wrote, read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
IAVs
|
influenza A viruses
|
|
NEP
|
nuclear export protein
|
|
PA
|
polymerase acidic
|
References
|
1
|
Elvira G, Wasiak S, Blandford V, Tong XK,
Serrano A, Fan X, del Rayo Sánchez-Carbente M, Servant F, Bell AW,
Boismenu D, et al: Characterization of an RNA granule from
developing brain. Mol Cell Proteomics. 5:635–651. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kanai Y, Dohmae N and Hirokawa N: Kinesin
transports RNA: Isolation and characterization of an
RNA-transporting granule. Neuron. 43:513–525. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Howng SL, Hsu HC, Cheng TS, Lee YL, Chang
LK, Lu PJ and Hong YR: A novel ninein-interaction protein, CGI-99,
blocks ninein phosphorylation by GSK3beta and is highly expressed
in brain tumors. FEBS Lett. 566:162–168. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang J, Gu Y, Wang L, Hang X, Gao Y, Wang
H and Zhang C: HUPO BPP pilot study: A proteomics analysis of the
mouse brain of different developmental stages. Proteomics.
7:4008–4015. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rappsilber J, Ryder U, Lamond AI and Mann
M: Large-scale proteomic analysis of the human spliceosome. Genome
Res. 12:1231–1245. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Popow J, Englert M, Weitzer S, Schleiffer
A, Mierzwa B, Mechtler K, Trowitzsch S, Will CL, Lührmann R, Söll D
and Martinez J: HSPC117 is the essential subunit of a human tRNA
splicing ligase complex. Science. 331:760–764. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jeronimo C, Forget D, Bouchard A, Li Q,
Chua G, Poitras C, Thérien C, Bergeron D, Bourassa S, Greenblatt J,
et al: Systematic analysis of the protein interaction network for
the human transcription machinery reveals the identity of the 7SK
capping enzyme. Mol Cell. 27:262–274. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lupi I, Broman KW, Tzou SC, Gutenberg A,
Martino E and Caturegli P: Novel autoantigens in autoimmune
hypophysitis. Clin Endocrinol (Oxf). 69:269–278. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Uemura M, Nouso K, Kobayashi Y, Tanaka H,
Nakamura S, Higashi T, Ono T, Nakayama E, Hanafusa T and Shiratori
Y: Identification of the antigens predominantly reacted with serum
from patients with hepatocellular carcinoma. Cancer. 97:2474–2479.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhao X, Wang Y, Cui Q, Li P, Wang L, Chen
Z, Rong L and Du R: A parallel phenotypic versus Target-Based
screening strategy for RNA-Dependent RNA polymerase inhibitors of
the influenza A Virus. Viruses. 11:8262019. View Article : Google Scholar
|
|
11
|
Isel C, Munier S and Naffakh N:
Experimental approaches to study genome packaging of influenza A
Viruses. Viruses. 8:2182016. View
Article : Google Scholar
|
|
12
|
Ghorbani A, Ngunjiri JM and Lee CW:
Influenza A Virus subpopulations and their implication in
pathogenesis and vaccine development. Annu Rev Anim Biosci.
8:247–267. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Eisfeld AJ, Neumann G and Kawaoka Y: At
the centre: Influenza A virus ribonucleoproteins. Nat Rev
Microbiol. 13:28–41. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ma X, Xie L, Wartchow C, Warne R, Xu Y,
Rivkin A, Tully D, Shia S, Uehara K, Baldwin DM, et al: Structural
basis for therapeutic inhibition of influenza A polymerase PB2
subunit. Sci Rep. 7:93852017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tomescu AI, Robb NC, Hengrung N, Fodor E
and Kapanidis AN: Single-molecule FRET reveals a corkscrew RNA
structure for the polymerase-bound influenza virus promoter. Proc
Natl Acad Sci USA. 111:E3335–E3342. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Noshi T, Kitano M, Taniguchi K, Yamamoto
A, Omoto S, Baba K, Hashimoto T, Ishida K, Kushima Y, Hattori K, et
al: In vitro characterization of baloxavir acid, a first-in-class
cap-dependent endonuclease inhibitor of the influenza virus
polymerase PA subunit. Antiviral Res. 160:109–117. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zheng W, Li J, Wang S, Cao S, Jiang J,
Chen C, Ding C, Qin C, Ye X, Gao GF and Liu W: Phosphorylation
controls the nuclear-cytoplasmic shuttling of influenza A virus
nucleoprotein. J Virol. 89:5822–5834. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wu CY, Jeng KS and Lai MM: The SUMOylation
of matrix protein M1 modulates the assembly and morphogenesis of
influenza A virus. J Virol. 85:6618–6628. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Reuther P, Giese S, Götz V, Riegger D and
Schwemmle M: Phosphorylation of highly conserved serine residues in
the influenza A virus nuclear export protein NEP plays a minor role
in viral growth in human cells and mice. J Virol. 88:7668–7673.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhou YW, Li R, Duan CJ, Gao Y, Cheng YD,
He ZW, Zeng JX and Zhang CF: Expression and clinical significance
of C14orf166 in esophageal squamous cell carcinoma. Mol Med Rep.
15:605–612. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rodriguez A, Pérez-González A and Nieto A:
Cellular human CLE/C14orf166 protein interacts with influenza virus
polymerase and is required for viral replication. J Virol.
85:12062–12066. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tafforeau L, Chantier T, Pradezynski F,
Pellet J, Mangeot PE, Vidalain PO, Andre P, Rabourdin-Combe C and
Lotteau V: Generation and comprehensive analysis of an influenza
virus polymerase cellular interaction network. J Virol.
85:13010–13018. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pérez-González A, Rodriguez A, Huarte M,
Salanueva IJ and Nieto A: hCLE/CGI-99, a human protein that
interacts with the influenza virus polymerase, is a mRNA
transcription modulator. J Mol Biol. 362:887–900. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rodriguez-Frandsen A, de Lucas S,
Pérez-González A, Pérez-Cidoncha M, Roldan-Gomendio A, Pazo A,
Marcos-Villar L, Landeras-Bueno S, Ortín J and Nieto A:
hCLE/C14orf166, a cellular protein required for viral replication,
is incorporated into influenza virus particles. Sci Rep.
6:207442016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lee JW, Liao PC, Young KC, Chang CL, Chen
SS, Chang TT, Lai MD and Wang SW: Identification of hnRNPH1, NF45,
and C14orf166 as novel host interacting partners of the mature
hepatitis C virus core protein. J Proteome Res. 10:4522–4534. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Emmott E, Munday D, Bickerton E, Britton
P, Rodgers MA, Whitehouse A, Zhou EM and Hiscox JA: The cellular
interactome of the coronavirus infectious bronchitis virus
nucleocapsid protein and functional implications for virus biology.
J Virol. 87:9486–9500. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kula A, Guerra J, Knezevich A, Kleva D,
Myers MP and Marcello A: Characterization of the HIV-1 RNA
associated proteome identifies Matrin 3 as a nuclear cofactor of
Rev function. Retrovirology. 8:602011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Amiel J, Rio M, de Pontual L, Redon R,
Malan V, Boddaert N, Plouin P, Carter NP, Lyonnet S, Munnich A and
Colleaux L: Mutations in TCF4, encoding a class I basic
helix-loop-helix transcription factor, are responsible for
Pitt-Hopkins syndrome, a severe epileptic encephalopathy associated
with autonomic dysfunction. Am J Hum Genet. 80:988–993. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fukuda A, Shimada M, Nakadai T, Nishimura
K and Hisatake K: Heterogeneous nuclear ribonucleoprotein R
cooperates with mediator to facilitate transcription reinitiation
on the c-Fos gene. PLoS One. 8:e724962013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Makise M, Mackay DR, Elgort S, Shankaran
SS, Adam SA and Ullman KS: The Nup153-Nup50 protein interface and
its role in nuclear import. J Biol Chem. 287:38515–38522. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cheang TY, Zhou HY, Chen W, Zhang B, Liu
L, Yang J, Wang S and Li H: C14orf166 overexpression correlates
with tumor progression and poor prognosis of breast cancer. J
Transl Med. 14:542016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen HC, Lin WC, Tsay YG, Lee SC and Chang
CJ: An RNA helicase, DDX1, interacting with poly(A) RNA and
heterogeneous nuclear ribonucleoprotein K. J Biol Chem.
277:40403–40409. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Robertson-Anderson RM, Wang J, Edgcomb SP,
Carmel AB, Williamson JR and Millar DP: Single-molecule studies
reveal that DEAD box protein DDX1 promotes oligomerization of HIV-1
Rev on the Rev response element. J Mol Biol. 410:959–971. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Pérez-González A, Pazo A, Navajas R,
Ciordia S, Rodriguez-Frandsen A and Nieto A: hCLE/C14orf166
associates with DDX1-HSPC117-FAM98B in a novel
transcription-dependent shuttling RNA-transporting complex. PLoS
One. 9:e909572014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Pazo A, Pérez-González A, Oliveros JC,
Huarte M, Chavez JP and Nieto A: hCLE/RTRAF-HSPC117-DDX1-FAM98B: A
new Cap-Binding complex that activates mRNA Translation. Front
Physiol. 10:922019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Freibaum BD, Chitta RK, High AA and Taylor
JP: Global analysis of TDP-43 interacting proteins reveals strong
association with RNA splicing and translation machinery. J Proteome
Res. 9:1104–1120. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Thakkar S, Sharma D, Kalia K and Tekade
RK: Tumor microenvironment targeted nanotherapeutics for cancer
therapy and diagnosis: A review. Acta Biomater. 101:43–68. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Jacobs KM, Bhave SR, Ferraro DJ, Jaboin
JJ, Hallahan DE and Thotala D: GSK-3β: A bifunctional role in cell
death pathways. Int J Cell Biol. 2012:9307102012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yu H, Pardoll D and Jove R: STATs in
cancer inflammation and immunity: A leading role for STAT3. Nat Rev
Cancer. 9:798–809. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhang W, Ou J, Lei F, Hou T, Wu S, Niu C,
Xu L and Zhang Y: C14ORF166 overexpression is associated with
pelvic lymph node metastasis and poor prognosis in uterine cervical
cancer. Tumour Biol. 37:369–379. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chen M, Ye Y, Zou B, Guo S, Zhou F, Lu K,
Liu J, Xu Z, Han H, Liu Z, et al: C14orf166 is a high-risk
biomarker for bladder cancer and promotes bladder cancer cell
proliferation. J Transl Med. 14:552016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yang L, Li F, Lei F, Wang Y, Wu S, Song L
and Chen Y: Overexpression of chromosome 14 open reading frame 166
correlates with disease progression and poorer prognosis in human
NPC. Tumour Biol. 36:7977–7986. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wang H, Sun L, Jiang J, Yu S and Zhou Q:
Suppression of the proliferation and invasion of breast cancer
cells by ST7L occurs through inhibition of activation of
Wnt/GSK-3β/β-catenin signalling. Clin Exp Pharmacol Physiol.
47:119–126. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Jain S, Ghanghas P, Rana C and Sanyal SN:
Role of GSK-3β in Regulation of Canonical Wnt/β-catenin Signaling
and PI3-K/Akt oncogenic pathway in colon cancer. Cancer Invest.
35:473–483. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Chen X, Ying Z, Lin X, Lin H, Wu J, Li M
and Song L: Acylglycerol kinase augments JAK2/STAT3 signaling in
esophageal squamous cells. J Clin Invest. 123:2576–2589. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Aryappalli P, Shabbiri K, Masad RJ,
Al-Marri RH, Haneefa SM, Mohamed YA, Arafat K, Attoub S,
Cabral-Marques O, Ramadi KB, et al: Inhibition of
Tyrosine-Phosphorylated STAT3 in human breast and lung cancer cells
by Manuka Honey is mediated by selective antagonism of the IL-6
Receptor. Int J Mol Sci. 20:43402019. View Article : Google Scholar
|
|
47
|
Lin C, Liao W, Jian Y, Peng Y, Zhang X, Ye
L, Cui Y, Wang B, Wu X, Xiong Z, et al: CGI-99 promotes breast
cancer metastasis via autocrine interleukin-6 signaling. Oncogene.
36:3695–3705. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Cui Y, Wu J, Zong M, Song G, Jia Q, Jiang
J and Han J: Proteomic profiling in pancreatic cancer with and
without lymph node metastasis. Int J Cancer. 124:1614–1621. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Chen J, Chen J, Gong Y, Zou B, Liu X, Ding
L, Huang J, Zhang B and Li J: C14orf166 Is a biomarker for
predicting hepatocellular carcinoma recurrence. J Invest Surgery.
33:914–923. 2020. View Article : Google Scholar
|