1. Introduction
The regulation of lipid and carbohydrate metabolism
is of vital importance for homeostasis, involving the organization
and appropriate response to environmental variables, such as food
intake, stress, physical activities and temperature (1,2). In
addition, to achieve this goal, there are several levels of
metabolic controls, all of which require the involvement of
numerous metabolic mediators, hormones, growth factors and
ultimately transcription factors (3). Moreover, metabolic syndrome is a
condition that consists of a large number of symptoms that affect
the metabolism (4,5).
Metabolic syndrome apperars to have three potential
etiological categories: obesity and disorders of adipose tissue,
insulin resistance and a constellation of independent factors
(molecules of hepatic, vascular and immunologic origin) that
mediate specific components of the metabolic syndrome (6).
With respect to disorders of adipose tissue,
adipocytes are a critical component of metabolic control and
endocrine organs that have both positive and negative effects
(7).
Obesity is associated with a chronic inflammatory
response, characterized by abnormal adipokine production, and the
activation of certain pro-inflammatory signalling pathways,
resulting in the induction of several biological inflammation
markers (8). The main physical
consequence of obesity is atherosclerosis and CVD (9). Furthermore, obesity is accompanied by
other medical complications; these include fatty liver, cholesterol
gallstones, sleep apnea, osteoarthritis, and polycystic ovary
disease (10).
Inflammation is receiving an increasing amount of
attention for its potential role in the pathogenesis of a variety
of disorders, from insulin resistance and type 2 diabetes mellitus
(DM2) to fatty liver and CVD (11). The changes presented by adipose
tissue in MS favor the secretion of several molecular mediators
capable of activating, or suppressing, numerous transcription
factors, such as peroxisome proliferator-activated receptors
(PPARs) (12,13).
Expressed in three isoforms (α, δ and γ), PPARs are
nuclear hormone receptors, structurally similar to steroid hormone
receptors (14,15). Upon activation by a ligand,
including endogenous fatty acids and fatty acid derivatives, the
receptor forms a heterodimer with members of the retinoid X
receptor (RXR) family and may act as a transcription factor
(16). The activation of PPAR
pathways has a favorable effect on lipid synthesis and oxidation,
glucose uptake, inflammation and the expression of immunoregulatory
genes (17,18).
This review presents the principal molecular aspects
of the role of PPARs in adipose tissue inflammation in MS and
future therapeutics based on novel molecular pathways.
2. Adipose tissue in metabolic syndrome
The MS is characterized by a multiplex risk factor
that arises from insulin resistance accompanying abnormal adipose
deposition and function (19).
Patients with MS present with high blood pressure, a large waist
circumference and high levels of plasma triglycerides with an
increased risk of developing DM2 and CVD (20,21).
Physiopathological changes encountered in MS are varied, including
insulin resistance, dyslipemia and obesity (22,23).
Any metabolic changes related to obesity may be attributed to the
increased intra-abdominal fat mass, and are independent of the
total mass of the body (24).
Hypotheses have altered from the theory that adipose
tissue is used solely as a storage site for energy, to the theory
that adipose tissue has an active role in energy homeostasis and
various other processes (25,26).
The functional failure of adipose tissue occurs due to alterations
in the delivery of systemic energy, the impaired consumption of
glucose and the activation of self-regulatory mechanisms which
extend their influence to the body’s entire homeostasis system,
with elevated levels of adipokine secretion and vascular effects
(27,28). The progression of obesity is
accompanied by chronic inflammation which involves innate and
acquired immunity (29).
Inflammation is a key process which underlies a variety of
metabolic diseases and is often found in obese patients (30). Studies have shown that when mice
are provided with a high-fat diet, their weight gain correlates
with the induction of adipose tissue inflammatory pathways
(31).
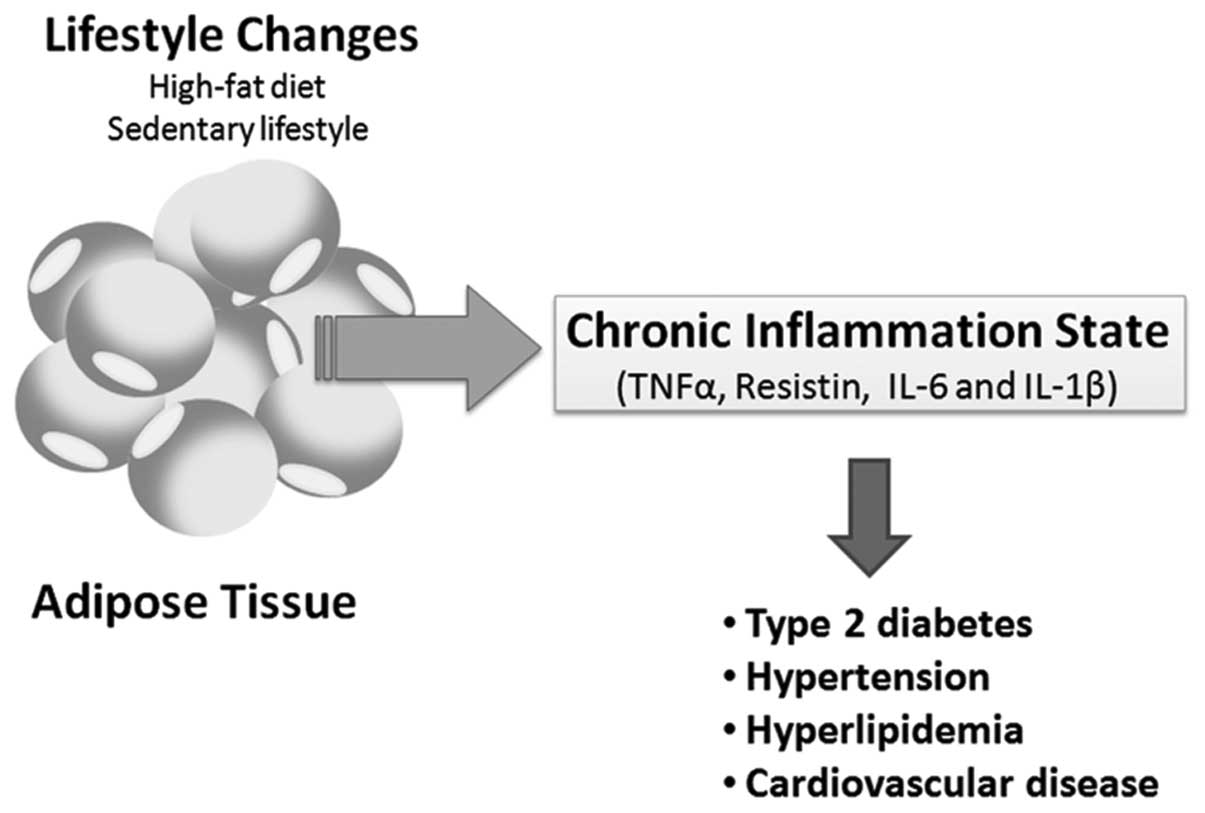
The production of proinflammatory molecules
[interleukin (IL)-6, tumor necrosis factor-α (TNF-α), plasminogen
activator inhibitor (PAI)-1, angiotensinogen, complement factor 3
(C3), tissue factor and other inflammatory cytokines] in the
adipose tissue during obesity contributes to a low degree of
systemic inflammation, which is observed in a variety of chronic
diseases associated with MS (Fig.
1) (31–33). Resistin and TNF-α, are adipokines
associated with insulin resistance in the skeletal muscle (34,35).
Furthermore, increasing adiposity and insulin resistance may
interact, thus raising the levels of C-reactive protein (36).
The association between insulin resistance, chronic
inflammation, hypertension, endothelial dysfunction and
dyslipidemia may be due to the activation of NF-κB (37). TNF-α is elevated in the adipose
tissues of obese rodents and humans and is implicated in the
induction of atherogenic adipokines, such as PAI-1 and IL-6, as
well as the inhibition of the anti-atherogenic adipokine,
adiponectin (38). Even, obese
individuals (with hyperinsulinemia) expresses 2.5-fold more TNF-α
mRNA in fat tissue compared with normal controls (39). The transcription factor NF-κB and
the TNF-α gene promoter have been activated by hypoxia in
adipocytes and fibroblasts. In turn, NF-κB signaling represses E2F
transcription factors, therefore inhibiting adipogenesis and
maintaining a chronic inflammatory condition (40).
3. Molecular interaction and gene expression
in adipose tissue
In order to reduce the risk factors of obesity,
patients are required to alter their lifestyle and food habits
(41). Factors dependant upon
transcription factors, which are able to change the levels of
relevant gene expression by adapting to signals from the
surrounding environment are used to regulate MS (42,43).
Observations regarding alterations in gene expression found in
adipose tissue have led to the theory that the modification of
carbohydrates may affect the risk to the patient of CVD and DM2
(44).
A series of transcription factors, the majority of
which are PPARs, regulate the maturation of adipocytes and hundreds
of other proteins that participate in the metabolism and storage of
lipids (45). In adipose tissues,
chronic inflammation is evident from the differential expression of
genes involved in inflammatory responses and natural immunity
(46).
PPARs are connected to the nuclear membrane, and
their main function is storage regulation and the catabolization of
fatty acids (47), when activated
by their ligands (synthetic or endogenous), PPARs control several
genes which are involved in intermediate metabolism (48). To date, three isoforms have been
identified: PPAR-α, PPAR-β/δ and PPAR-γ (49). Each PPAR forms a heterodimer with
RXR. This heterodimer joins the PPAR response elements (PPREs),
which regulate target gene domains. The activation of PPARs by an
appropriate ligand results in the recruitment of co-activators and
the loss of co-repressors that remodel chromatin and activate
transcription (50).
A major target for PPAR-γ agonists are adipocytes
(51). PPAR-γ is crucial in
adipogenesis, as it acts as a regulator of the differentiation and
function of adipocytes and the absorption of stored fatty acids
(52–54). However, PPAR-γ is also a key
regulator of inflammatory and immune responses (55).
The activation of PPAR-γ does not affect the
expression of M1 or M2 markers in resting macrophages, which
indicates that only native monocytes may be stimulated by PPAR-γ
activators to a M2 phenotype (56). Furthermore, PPAR-γ transcriptional
signaling is required for the maturation of the anti-inflammatory
M2 phenotype, whereas PPAR-β/δ controls the expression of
alternative phenotypes in the Kupffer cells of obese mice (57).
Dominant mutations may cause a loss of function of
PPAR-γ, which in turn leads to an increase in insulin resistance
and the early onset of severe hypertension (58,59).
The loss of PPAR-γ function in immune cells reduces the ability of
abscisic acid to increase insulin sensitivity by suppressing the
expression of MCP-1 and the infiltration of macrophages into white
adipose tissue (60).
Furthermore, PPAR-α belongs to a subfamily of
nuclear receptors which control the expression of proteins and
enzymes that participate in inflammation and metabolism (48). Therefore, the activation of PPAR-α
prevents inflammation in adipose tissue and the dual activation of
PPAR-α and PPAR-γ enhances the action of adiponectin by increasing
the adiponectin and adiponectin receptors, which may result in the
amelioration of obesity-induced insulin resistance (61).
Contrary to PPAR-α and PPAR-γ, PPAR-β/δ is expressed
ubiquitously, yet its pharmacology is understood less compared with
other subtypes. PPAR-β/δ knockout mice demonstrate an obese
phenotype when administered with a high-fat diet (62).
4. Future therapeutics based on novel
molecular pathways
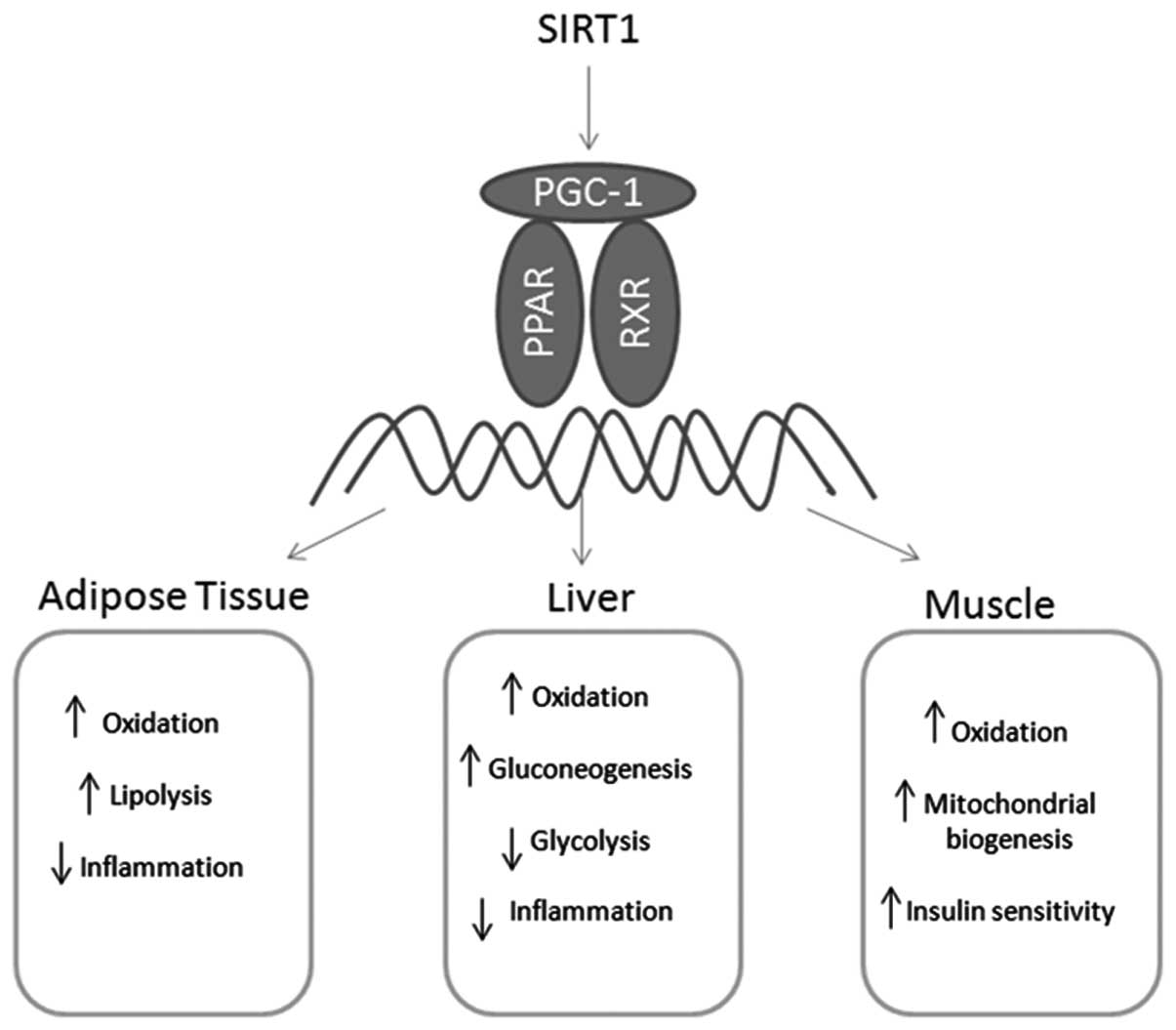
PPARs are potential targets for the treatment of
metabolic diseases and their cardiovascular complications. PPARs
regulate gene expression by binding with RXR as a heterodimeric
partner to specific DNA sequences and modulating other
transcription factor pathways in an independent manner (Fig. 2) (63,64).
Although, PPAR-γ is widely expressed in tissues, it
is highly concentrated in adipose tissue. PPAR-γ is essential for
the differentiation of adipocytes and promotes the accumulation of
lipids in adipocytes (65).
Furthermore, adipocyte-specific knockout mice for PPAR-γ
demonstrated adipocytic hypocellularity, developing only hepatic
insulin resistance. Anti-diabetic thiazolidinediones (TZDs)
suppress insulin resistance in adipose tissue, but also affect the
liver and muscles, despite exhibiting low concentrations of PPAR-γ
in these tissues. There are two well-identified PPAR-γ isoforms
which are derived from the same gene due to the use of alternative
promoters. PPAR-γ2 is expressed specifically in adipose tissue, and
differs from PPAR-γ1 by the presence of 30 additional amino acids
in the N-terminal region. PPAR-γ is not only involved in the
metabolism of lipids and carbohydrates, but also in inflammation
(66,67) and is key in neoplasic growth
(68,69).
Studies regarding genetic expression have revealed
that insulin sensibilizing TZDs alter the expression of genes
involved in recapturing lipids, lipid metabolism and in the action
of insulin in adipocytes (70).
This leads to an increased accumulation of lipids in the adipose
tissue and a decrease in the release of free fatty acids. The
effect on lipid metabolism by TZDs is greater than that of
adipokine secretion, thus, they reduce the secretion of
inflammatory cytokines (71) and
chemokines which promote insulin resistance, such as TNF-α
(72). This action occurs in
adipocytes and associated macrophages. Other adipokines are
over-regulated, particularly adiponectin (73), which is known to be a potential
sensitizer of insulin for the liver and skeletal muscle. These
insulin sensibilizing effects on the skeletal muscle and liver are
controlled by alterations in the gene expression of adipokines due
to the activation of the PPAR-γ receptor. Furthermore, the
activation of PPAR-γ increases the expression and translocation
towards the cell surface of glucose transporters GLUT 1 and 4
(74). This also increases the
capture of hepatic and muscular glucose, thereby lowering glucose
plasma levels. PPAR-γ agonists restore sensibility to insulin,
lowering the expression of TNF-α and increasing the expression of
adiponectin (52).
An ideal dual PPAR-α/γ agonist would provide
glycemic control and enhance the lipid profile with well-tolerated
therapeutic doses (75).
PPAR-α agonists have been proven to lower the
production of certain inflammatory cytokines (76), such as TNF-α, in a dependent
mechanism, involving NF-κB and AP-1 (77). Furthermore, the PPAR-α WY14643
agonist may directly increase the expression of adiponectin
expression and it may also exert anti-diabetic,
anti-atherosclerotic and anti-inflammatory effects. PPAR-α is the
molecular target for fibrate-type hypolipemiant agents, such as
fenofibrate and gemfibrozil. PPAR-α is highly expressed in the
liver and the activation results in an increase of hepatic
recapture and oxidation (12).
During fasting, PPAR-α knockout mice present with hypoglycemia,
hypoketonemia, hypertriglyceridemia and hepatic steatosis (12,78,79).
The treatment of DM2 patients with metformin reduces
the production of hepatic glucose, by lowering gluconeogenesis. It
has been suggested that metformin exerts its action by using
incretins, which raises the levels of glucagon-like peptide-1
(GLP-1) (80) and those receptors
for incretins in pancreatic β-cells by mechanisms that are both
independent and dependent upon PPAR-α (81).
PPAR-α activators are used for the treatment of
dyslipidemia. They lower the plasma levels of triglycerides and
increase the plasma levels of HDL-c. These effects take place due
to an increase in the production of the major component of HDL-c,
apolipoprotein AI (82) and AII
(83).
Lipid peroxidation and its subsequent production of
4-hydroxynonenal (4-HNE) in β-cells have been described as triggers
of insulin secretion by a mechanism dependent on PPAR-β/δ as an
antagonist of this nuclear factor, thereby blocking its effect
(84). Research has demonstrated
that PPAR-β/δ has a protective function in metabolic diseases that
presents with chronic inflammatory conditions (85).
Treatment with PPAR-β/δ agonist, L-165041, decreases
IL-1, IL-6 and TNF-α levels in mice with streptozotocin-induced
diabetes (86). It was also
demonstrated that PPAR-β/δ agonists may prevent renal alterations
for the same type of diabetes. As far as the latter aspect, the
PPAR-β/δ agonist GW0742, reduces the excretion of albumin, the
infiltration of macrophages and the accumulation of type VI
collagen amongst other effects that help to heal renal alterations
related to diabetes (87).
Animals that are administered a high-fat diet
develop metabolic alterations, such as glycemia, muscle glucose
storage, alterations in the enzymes involved in carbohydrate
metabolism and fat accumulation in the liver. All these alterations
are reversible by treatment with NNC61-5920, a PPAR-β/δ agonist
(88). The same agonist, causes a
differential response in the treatment of metabolic alterations
related to MS and diabetes. This evidence demonstrates that PPAR
agonists may have outstanding metabolic effects, yet this is not
always optimal, as the response to treatment may be too dependent
on the etiology of the base. In this sense, an association has been
made amongst brain-vascular accidents, weight gain and
carcinogenesis along with other unwanted effects of the treatment
with PPAR agonists (89).
5. Other targets
Peroxisome proliferator-activated
receptor γ coactivator-1-α PGC-1
PPARs are important in regulating metabolism, there
are molecules which may exert a co-stimulatory or co-repressor
effect on the activity of these nuclear receptors, such as PGC-1α.
This metabolic regulatory molecule was first described in 1998, as
a key molecule in the regulation of the thermogenesis of brown
adipose tissue (90,91). Various regulatory mechanisms have
been described, which not only involves PPAR receptors, but also
estrogen-related receptors (ERRs), thyroid hormone receptors,
glucocorticoid receptors and non-nuclear receptors, such as myocyte
enhancer factor-2 (MEF-2), among others. By modulating all these
nuclear and non-nuclear receptors, PGC-1 is capable of regulating
energy metabolism (92). A number
of mechanisms have been described in which PGC-1 participates and
regulates, and acts as a therapeutic target for cancer (93), DM2 (94) and heart failure (95). It has been hypothesized that PGC-1
is capable of inhibiting proinflammatory cytokine production
through the inhibition of NF-κB, by inhibiting the phosphorylation
of the p65 subunit (96).
Silent information regulator T1
(SIRT1)
SIRT1 was the first gene of the sirtuin genes to be
located, which is also capable of metabolism regulation and has
been proposed as a new therapeutic target in metabolic diseases
(97) and aging (98). Sirtuins are a class of enzymes,
NAD-dependent histone deacetylases, found in prokaryotic and
eukaryotic cells, which affect the regulation of cellular
metabolism and the expression of certain genes. It is a cellular
regulator of the balance between NADH and NAD+. SIRT1
has been postulated as a sensor which is connected to metabolic
homeostasis (99), and directly
regulates the activity of the acetyl-CoA synthetases through
deacetylation (100).
Furthermore, SIRT1 may directly interact with PGC-1 (101), suggesting that SIRT1 is capable
of regulating the transcriptional activity of PGC-1 and thereby
regulating the energy balance and metabolism (92).
6. Conclusion
MS represents a clustering of cardiometabolic risk
factors that are considered to be a direct consequence of
overnutrition, sedentary lifestyles and the resultant obesity.
Inflammation is receiving increased attention for its potential
role in the pathogenesis of a range of disorders from insulin
resistance and DM2 to fatty liver and CVD, the unexpected overlap
between inflammatory and metabolic sensors and their downstream
tissue responses indicates that inflammation plays a crucial role
in the numerous complications of obesity.
The ability of PPARs to serve as master regulators
of various metabolic processes, including lipid, glucose and energy
homeostasis, inflammation and cardiovascular events, has made them
the ideal target for the development of new pharmacological tools
by which to treat individual risk factors. However, as TZDs and
fibrates only have an impact on individual components of MS, they
exhibit undesirable side effects, particularly with the use of
TZDs, and are ineffective against CVD.
Further studies are required in order to approach
the role of the innate immune system in maintaining obesity, and
the teleological reasons for obesity-dependent inflammation.
Acknowledgements
This study was funded by the CONICYT REGIONAL/GORE
MAULE/CEAP/R09I2001, Programa de Investigación de Excelencia
Interdisciplinaria en Envejecimiento Saludable (PIEI-ES), and
supported by grant no. 1130216 (I.P., M.G., R.M., M.A., J.C.) from
Fondecyt, Chile.
References
|
1
|
Bastard JP, Maachi M, Lagathu C, et al:
Recent advances in the relationship between obesity, inflammation,
and insulin resistance. Eur Cytokine Netw. 17:4–12. 2006.PubMed/NCBI
|
|
2
|
Cnop M, Havel PJ, Utzschneider KM, et al:
Relationship of adiponectin to body fat distribution, insulin
sensitivity and plasma lipoproteins: evidence for independent roles
of age and sex. Diabetologia. 46:459–469. 2003.PubMed/NCBI
|
|
3
|
Kersten S, Desvergne B and Wahli W: Roles
of PPARs in health and disease. Nature. 405:421–424. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lakka HM, Laaksonen DE, Lakka TA, et al:
The metabolic syndrome and total and cardiovascular disease
mortality in middle-aged men. JAMA. 288:2709–2716. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sattar N, Gaw A, Scherbakova O, et al:
Metabolic syndrome with and without C-reactive protein as a
predictor of coronary heart disease and diabetes in the West of
Scotland Coronary Prevention Study. Circulation. 108:414–419. 2003.
View Article : Google Scholar
|
|
6
|
Grundy SM, Brewer HB Jr, Cleeman JI, et
al: Definition of metabolic syndrome. Report of the National Heart,
Lung, and Blood Institute/American Heart Association conference on
scientific issues related to definition. Arterioscler Thromb Vasc
Biol. 24:e13–e18. 2004. View Article : Google Scholar
|
|
7
|
Greenberg AS and Obin MS: Obesity and the
role of adipose tissue in inflammation and metabolism. Am J Clin
Nutr. 83:461S–465S. 2006.PubMed/NCBI
|
|
8
|
Hotamisligil GS, Shargill NS and
Spiegelman BM: Adipose expression of tumor necrosis factor-alpha:
direct role in obesity-linked insulin resistance. Science.
259:87–91. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Orio F Jr, Palomba S, Cascella T,
Savastano S, Lombardi G and Colao A: Cardiovascular complications
of obesity in adolescents. J Endocrinol Invest. 30:70–80. 2007.
View Article : Google Scholar
|
|
10
|
Grundy SM: Obesity, metabolic syndrome,
and cardiovascular disease. J Clin Endocrinol Metab. 89:2595–2600.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shoelson SE and Goldfine AB: Getting away
from glucose: fanning the flames of obesity-induced inflammation.
Nat Med. 15:373–374. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Leone TC, Weinheimer CJ and Kelly DP: A
critical role for the peroxisome proliferator-activated receptor
alpha (PPARalpha) in the cellular fasting response: the
PPARalpha-null mouse as a model of fatty acid oxidation disorders.
Proc Natl Acad Sci USA. 96:7473–7478. 1999. View Article : Google Scholar
|
|
13
|
Iizuka K and Horikawa Y: ChREBP: a
glucose-activated transcription factor involved in the development
of metabolic syndrome. Endocr J. 55:617–624. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Berger J and Moller DE: The mechanisms of
action of PPARs. Annu Rev Med. 53:409–435. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Viana Abranches M, Esteves de Oliveira FC
and Bressan J: Peroxisome proliferator-activated receptor: effects
on nutritional homeostasis, obesity and diabetes mellitus. Nutr
Hosp. 26:271–279. 2011.
|
|
16
|
Adeghate E, Adem A, Hasan MY, Tekes K and
Kalasz H: Medicinal chemistry and actions of dual and pan PPAR
modulators. Open Med Chem J. 5:93–98. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Israelian-Konaraki Z and Reaven PD:
Peroxisome proliferator-activated receptor-alpha and
atherosclerosis: from basic mechanisms to clinical implications.
Cardiology. 103:1–9. 2005. View Article : Google Scholar
|
|
18
|
Nicholls SJ and Uno K: Peroxisome
proliferator-activated receptor (PPAR alpha/gamma) agonists as a
potential target to reduce cardiovascular risk in diabetes. Diab
Vasc Dis Res. 9:89–94. 2012. View Article : Google Scholar
|
|
19
|
Salmenniemi U, Ruotsalainen E, Pihlajamäki
J, et al: Multiple abnormalities in glucose and energy metabolism
and coordinated changes in levels of adiponectin, cytokines, and
adhesion molecules in subjects with metabolic syndrome.
Circulation. 110:3842–3848. 2004. View Article : Google Scholar
|
|
20
|
Mujica V, Leiva E, Icaza G, et al:
Evaluation of metabolic syndrome in adults of Talca city, Chile.
Nutr J. 7:142008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Palomo I, Contreras A, Alarcon LM, et al:
Elevated concentration of asymmetric dimethylarginine (ADMA) in
individuals with metabolic syndrome. Nitric Oxide. 24:224–228.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Palomo I, Moore-Carrasco R, Alarcon M, et
al: Pathophysiology of the proatherothrombotic state in the
metabolic syndrome. Front Biosci (Schol Ed). 2:194–208. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Palomo I, Alarcón M, Moore-Carrasco R and
Argilés JM: Hemostasis alterations in metabolic syndrome (review).
Int J Mol Med. 18:969–974. 2006.PubMed/NCBI
|
|
24
|
Salmenniemi U, Ruotsalainen E, Vänttinen
M, et al: High amount of visceral fat mass is associated with
multiple metabolic changes in offspring of type 2 diabetic
patients. Int J Obes (Lond). 29:1464–1470. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Flier JS: Obesity wars: molecular progress
confronts an expanding epidemic. Cell. 116:337–350. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ahima RS: Adipose tissue as an endocrine
organ. Obesity (Silver Spring). 14:242S–249S. 2006. View Article : Google Scholar
|
|
27
|
Barreda R and Ros PR: Diagnostic imaging
of liver abscess. Crit Rev Diagn Imaging. 33:29–58. 1992.PubMed/NCBI
|
|
28
|
Pittas AG, Joseph NA and Greenberg AS:
Adipocytokines and insulin resistance. J Clin Endocrinol Metab.
89:447–452. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Satoh M, Andoh Y, Clingan CS, et al: Type
II NKT cells stimulate diet-induced obesity by mediating adipose
tissue inflammation, steatohepatitis and insulin resistance. PLoS
One. 7:e305682012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lumeng CN and Saltiel AR: Inflammatory
links between obesity and metabolic disease. J Clin Invest.
121:2111–2117. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xu H, Barnes GT, Yang Q, et al: Chronic
inflammation in fat plays a crucial role in the development of
obesity-related insulin resistance. J Clin Invest. 112:1821–1830.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kern PA, Ranganathan S, Li C, Wood L and
Ranganathan G: Adipose tissue tumor necrosis factor and
interleukin-6 expression in human obesity and insulin resistance.
Am J Physiol Endocrinol Metab. 280:E745–751. 2001.PubMed/NCBI
|
|
33
|
Shimomura I, Funahashi T, Takahashi M, et
al: Enhanced expression of PAI-1 in visceral fat: possible
contributor to vascular disease in obesity. Nat Med. 2:800–803.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bruce CR and Dyck DJ: Cytokine regulation
of skeletal muscle fatty acid metabolism: effect of interleukin-6
and tumor necrosis factor-alpha. Am J Physiol Endocrinol Metab.
287:E616–E621. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Dyck DJ: Adipokines as regulators of
muscle metabolism and insulin sensitivity. Appl Physiol Nutr Metab.
34:396–402. 2009.PubMed/NCBI
|
|
36
|
Kriketos AD, Greenfield JR, Peake PW, et
al: Inflammation, insulin resistance, and adiposity: a study of
first-degree relatives of type 2 diabetic subjects. Diabetes Care.
27:2033–2040. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kaidashev IP: NF-kB activation as a
molecular basis of pathological process by metabolic syndrome.
Fiziol Zh. 58:93–101. 2012.(In Ukranian).
|
|
38
|
Ahn J, Lee H, Kim S and Ha T: Resveratrol
inhibits TNF-alpha-induced changes of adipokines in 3T3-L1
adipocytes. Biochem Biophys Res Commun. 364:972–977. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hotamisligil GS, Arner P, Caro JF,
Atkinson RL and Spiegelman BM: Increased adipose tissue expression
of tumor necrosis factor-alpha in human obesity and insulin
resistance. J Clin Invest. 95:2409–2415. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Araki K, Kawauchi K and Tanaka N:
IKK/NF-kappaB signaling pathway inhibits cell-cycle progression by
a novel Rb-independent suppression system for E2F transcription
factors. Oncogene. 27:5696–5705. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Gupta S and Gupta BM: Metabolic syndrome:
diabetes and cardiovascular disease. Indian Heart J. 58:149–152.
2006.PubMed/NCBI
|
|
42
|
Mujica V, Urzua A, Leiva E, et al:
Intervention with education and exercise reverses the metabolic
syndrome in adults. J Am Soc Hypertens. 4:148–153. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Klimcakova E, Roussel B, Kovacova Z, et
al: Macrophage gene expression is related to obesity and the
metabolic syndrome in human subcutaneous fat as well as in visceral
fat. Diabetologia. 54:876–887. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kallio P, Kolehmainen M, Laaksonen DE, et
al: Dietary carbohydrate modification induces alterations in gene
expression in abdominal subcutaneous adipose tissue in persons with
the metabolic syndrome: the FUNGENUT Study. Am J Clin Nutr.
85:1417–1427. 2007.
|
|
45
|
Vernochet C, Peres SB, Davis KE, et al:
C/EBPalpha and the corepressors CtBP1 and CtBP2 regulate repression
of select visceral white adipose genes during induction of the
brown phenotype in white adipocytes by peroxisome
proliferator-activated receptor gamma agonists. Mol Cell Biol.
29:4714–4728. 2009. View Article : Google Scholar
|
|
46
|
Xue B, Sukumaran S, Nie J, Jusko WJ,
Dubois DC and Almon RR: Adipose tissue deficiency and chronic
inflammation in diabetic Goto-Kakizaki rats. PLoS One.
6:e173862011. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wahli W, Braissant O and Desvergne B:
Peroxisome proliferator activated receptors: transcriptional
regulators of adipogenesis, lipid metabolism and more. Chem Biol.
2:261–266. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Motojima K: Peroxisome
proliferator-activated receptor (PPAR): structure, mechanisms of
activation and diverse functions. Cell Struct Funct. 18:267–277.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Jay MA and Ren J: Peroxisome
proliferator-activated receptor (PPAR) in metabolic syndrome and
type 2 diabetes mellitus. Curr Diabetes Rev. 3:33–39. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Keller H, Mahfoudi A, Dreyer C, et al:
Peroxisome proliferator-activated receptors and lipid metabolism.
Ann N Y Acad Sci. 684:157–173. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Lowell BB: PPARgamma: an essential
regulator of adipogenesis and modulator of fat cell function. Cell.
99:239–242. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Sugii S and Evans RM: Epigenetic codes of
PPARgamma in metabolic disease. FEBS Lett. 585:2121–2128. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Heikkinen S, Auwerx J and Argmann CA:
PPARgamma in human and mouse physiology. Biochim Biophys Acta.
1771:999–1013. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Fujiki K, Kano F, Shiota K and Murata M:
Expression of the peroxisome proliferator activated receptor gamma
gene is repressed by DNA methylation in visceral adipose tissue of
mouse models of diabetes. BMC Biol. 7:382009. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Luconi M, Cantini G and Serio M:
Peroxisome proliferator-activated receptor gamma (PPARgamma): Is
the genomic activity the only answer? Steroids. 75:585–594. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Bouhlel MA, Derudas B, Rigamonti E, et al:
PPARgamma activation primes human monocytes into alternative M2
macrophages with anti-inflammatory properties. Cell Metab.
6:137–143. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Odegaard JI, Ricardo-Gonzalez RR, Red
Eagle A, et al: Alternative M2 activation of Kupffer cells by
PPARdelta ameliorates obesity-induced insulin resistance. Cell
Metab. 7:496–507. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Ketsawatsomkron P, Pelham CJ, Groh S, Keen
HL, Faraci FM and Sigmund CD: Does peroxisome
proliferator-activated receptor-gamma (PPAR gamma) protect from
hypertension directly through effects in the vasculature? J Biol
Chem. 285:9311–9316. 2010. View Article : Google Scholar
|
|
59
|
Halabi CM, Beyer AM, de Lange WJ, et al:
Interference with PPAR gamma function in smooth muscle causes
vascular dysfunction and hypertension. Cell Metab. 7:215–226. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Guri AJ, Hontecillas R, Ferrer G, et al:
Loss of PPAR gamma in immune cells impairs the ability of abscisic
acid to improve insulin sensitivity by suppressing monocyte
chemoattractant protein-1 expression and macrophage infiltration
into white adipose tissue. J Nutr Biochem. 19:216–228. 2008.
View Article : Google Scholar
|
|
61
|
Tsuchida A, Yamauchi T, Takekawa S, et al:
Peroxisome proliferator-activated receptor (PPAR)alpha activation
increases adiponectin receptors and reduces obesity-related
inflammation in adipose tissue: comparison of activation of
PPARalpha, PPARgamma, and their combination. Diabetes.
54:3358–3370. 2005. View Article : Google Scholar
|
|
62
|
Li Y, Cheng L, Qin Q, et al: High-fat
feeding in cardiomyocyte-restricted PPARdelta knockout mice leads
to cardiac overexpression of lipid metabolic genes but fails to
rescue cardiac phenotypes. J Mol Cell Cardiol. 47:536–543. 2009.
View Article : Google Scholar
|
|
63
|
Juge-Aubry C, Pernin A, Favez T, et al:
DNA binding properties of peroxisome proliferator-activated
receptor subtypes on various natural peroxisome proliferator
response elements. Importance of the 5′-flanking region. J Biol
Chem. 272:25252–25259. 1997.PubMed/NCBI
|
|
64
|
Delerive P, De Bosscher K, Vanden Berghe
W, Fruchart JC, Haegeman G and Staels B: DNA binding-independent
induction of IkappaBalpha gene transcription by PPARalpha. Mol
Endocrinol. 16:1029–1039. 2002.PubMed/NCBI
|
|
65
|
Tontonoz P and Spiegelman BM: Fat and
beyond: the diverse biology of PPARgamma. Annu Rev Biochem.
77:289–312. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Cortez M, Carmo LS, Rogero MM, Borelli P
and Fock RA: A high-fat diet increases IL-1, IL-6, and TNF-alpha
production by increasing NF-kappaB and attenuating PPAR-gamma
expression in bone marrow mesenchymal stem cells. Inflammation.
36:379–386. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Chinetti G, Fruchart JC and Staels B:
Peroxisome proliferator-activated receptors and inflammation: from
basic science to clinical applications. Int J Obes Relat Metab
Disord. 27:S41–S45. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Skelhorne-Gross G and Nicol CJ: The key to
unlocking the chemotherapeutic potential of PPARgamma ligands:
Having the right combination. PPAR Res. 2012:9469432012. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Moore-Carrasco R, Figueras M, Ametller E,
López-Soriano FJ, Argilés JM and Busquets S: Effects of the
PPARgamma agonist GW1929 on muscle wasting in tumour-bearing mice.
Oncol Rep. 19:253–256. 2008.PubMed/NCBI
|
|
70
|
Scheen AJ: Combined
thiazolidinedione-insulin therapy: should we be concerned about
safety? Drug Saf. 27:841–586. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Jiang C, Ting AT and Seed B: PPAR-gamma
agonists inhibit production of monocyte inflammatory cytokines.
Nature. 391:82–86. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
72
|
Hong G, Davis B, Khatoon N, Baker SF and
Brown J: PPAR gamma-dependent anti-inflammatory action of
rosiglitazone in human monocytes: suppression of TNF alpha
secretion is not mediated by PTEN regulation. Biochem Biophys Res
Commun. 303:782–787. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Kusminski CM and Scherer PE: The road from
discovery to clinic: adiponectin as a biomarker of metabolic
status. Clin Pharmacol Ther. 86:592–595. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Furukawa H, Mawatari K, Koyama K, et al:
Telmisartan increases localization of glucose transporter 4 to the
plasma membrane and increases glucose uptake via peroxisome
proliferator-activated receptor gamma in 3T3-L1 adipocytes. Eur J
Pharmacol. 660:485–491. 2011. View Article : Google Scholar
|
|
75
|
Charbonnel B: PPAR-alpha and PPAR-gamma
agonists for type 2 diabetes. Lancet. 374:96–98. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Clockaerts S, Bastiaansen-Jenniskens YM,
Feijt C, et al: Cytokine production by infrapatellar fat pad can be
stimulated by interleukin 1beta and inhibited by peroxisome
proliferator activated receptor alpha agonist. Ann Rheum Dis.
71:1012–1018. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Delerive P, De Bosscher K, Besnard S, et
al: Peroxisome proliferator-activated receptor alpha negatively
regulates the vascular inflammatory gene response by negative
cross-talk with transcription factors NF-kappaB and AP-1. J Biol
Chem. 274:32048–32054. 1999. View Article : Google Scholar
|
|
78
|
Guerre-Millo M, Rouault C, Poulain P, et
al: PPAR-alpha-null mice are protected from high-fat diet-induced
insulin resistance. Diabetes. 50:2809–2814. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Tordjman K, Bernal-Mizrachi C, Zemany L,
et al: PPARalpha deficiency reduces insulin resistance and
atherosclerosis in apoE-null mice. J Clin Invest. 107:1025–1034.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Holst JJ and McGill MA: Potential new
approaches to modifying intestinal GLP-1 secretion in patients with
type 2 diabetes mellitus: focus on bile acid sequestrants. Clin
Drug Investig. 32:1–14. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Maida A, Lamont BJ, Cao X and Drucker DJ:
Metformin regulates the incretin receptor axis via a pathway
dependent on peroxisome proliferator-activated receptor-alpha in
mice. Diabetologia. 54:339–349. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Vu-Dac N, Schoonjans K, Laine B, Fruchart
JC, Auwerx J and Staels B: Negative regulation of the human
apolipoprotein A-I promoter by fibrates can be attenuated by the
interaction of the peroxisome proliferator-activated receptor with
its response element. J Biol Chem. 269:31012–31018. 1994.PubMed/NCBI
|
|
83
|
Vu-Dac N, Schoonjans K, Kosykh V, et al:
Fibrates increase human apolipoprotein A-II expression through
activation of the peroxisome proliferator-activated receptor. J
Clin Invest. 96:741–750. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Coleman JD, Prabhu KS, Thompson JT, et al:
The oxidative stress mediator 4-hydroxynonenal is an intracellular
agonist of the nuclear receptor peroxisome proliferator-activated
receptor-beta/delta (PPARbeta/delta). Free Radic Biol Med.
42:1155–1164. 2007. View Article : Google Scholar
|
|
85
|
Barish GD, Atkins AR, Downes M, et al:
PPARdelta regulates multiple proinflammatory pathways to suppress
atherosclerosis. Proc Natl Acad Sci USA. 105:4271–4276. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Schnegg CI, Kooshki M, Hsu FC, Sui G and
Robbins ME: PPARdelta prevents radiation-induced proinflammatory
responses in microglia via transrepression of NF-kappaB and
inhibition of the PKCalpha/MEK1/2/ERK1/2/AP-1 pathway. Free Radic
Biol Med. 52:1734–1743. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Matsushita Y, Ogawa D, Wada J, et al:
Activation of peroxisome proliferator-activated receptor delta
inhibits streptozotocin-induced diabetic nephropathy through
anti-inflammatory mechanisms in mice. Diabetes. 60:960–968. 2011.
View Article : Google Scholar
|
|
88
|
Ye JM, Tid-Ang J, Turner N, et al:
PPARdelta agonists have opposing effects on insulin resistance in
high fat-fed rats and mice due to different metabolic responses in
muscle. Br J Pharmacol. 163:556–566. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Moore-Carrasco R, Poblete Bustamante M,
González Guerra O, et al: Peroxisome proliferator-activated
receptors: Targets for the treatment of metabolic illnesses
(Review). Mol Med Report. 1:317–324. 2008.PubMed/NCBI
|
|
90
|
Puigserver P, Wu Z, Park CW, Graves R,
Wright M and Spiegelman BM: A cold-inducible coactivator of nuclear
receptors linked to adaptive thermogenesis. Cell. 92:829–839. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Wu Z, Puigserver P, Andersson U, et al:
Mechanisms controlling mitochondrial biogenesis and respiration
through the thermogenic coactivator PGC-1. Cell. 98:115–124. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Canto C and Auwerx J: PGC-1alpha, SIRT1
and AMPK, an energy sensing network that controls energy
expenditure. Curr Opin Lipidol. 20:98–105. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Girnun GD: The diverse role of the
PPARgamma coactivator 1 family of transcriptional coactivators in
cancer. Semin Cell Dev Biol. 23:381–388. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Buechler C and Schäffler A: Does global
gene expression analysis in type 2 diabetes provide an opportunity
to identify highly promising drug targets? Endocr Metab Immune
Disord Drug Targets. 7:250–258. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Schilling J and Kelly DP: The PGC-1
cascade as a therapeutic target for heart failure. J Mol Cell
Cardiol. 51:578–583. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Eisele PS, Salatino S, Sobek J, Hottiger
MO and Handschin C: The peroxisome proliferator-activated receptor
gamma coactivator 1alpha/beta (PGC-1) coactivators repress the
transcriptional activity of NF-kappaB in skeletal muscle cells. J
Biol Chem. 288:2246–2260. 2013. View Article : Google Scholar
|
|
97
|
Wang Y, Xu C, Liang Y and Vanhoutte PM:
SIRT1 in metabolic syndrome: where to target matters. Pharmacol
Ther. 136:305–318. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Porcu M and Chiarugi A: The emerging
therapeutic potential of sirtuin-interacting drugs: from cell death
to lifespan extension. Trends Pharmacol Sci. 26:94–103. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Canto C and Auwerx J: Targeting sirtuin 1
to improve metabolism: all you need is NAD(+)? Pharmacol Rev.
64:166–187. 2012.PubMed/NCBI
|
|
100
|
Hallows WC, Lee S and Denu JM: Sirtuins
deacetylate and activate mammalian acetyl-CoA synthetases. Proc
Natl Acad Sci USA. 103:10230–10235. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Rodgers JT, Lerin C, Haas W, Gygi SP,
Spiegelman BM and Puigserver P: Nutrient control of glucose
homeostasis through a complex of PGC-1alpha and SIRT1. Nature.
434:113–118. 2005. View Article : Google Scholar : PubMed/NCBI
|