Introduction
Previous studies focused on developing novel
reagents that exhibit anticarcinogenic and antimutagenic properties
in a number of types of cancer (1–3).
Capsaicin (trans-8- methyl-N-vanillyl-6-nonenamide) is an important
pungent ingredient with a spicy flavor that is widely used, and may
be extracted from chili peppers of the genus
Capsicum(4,5). It has been demonstrated that
capsaicin is able to inhibit the growth of various types of cancer
cells, such as human hepatoma carcinoma (6), human colon cancer (7), human breast cancer (8) and human neuroblastoma (9) cells.
Osteosarcoma is the most common malignant bone tumor
in children and adolescents (10).
This aggressive cancer mostly occurs in the long bones. For the
past two decades, chemotherapy and surgery have been commonly used
as therapies to improve the condition of patients with
osteosarcoma. However, the major problems associated with such
intense chemotherapies have increased, with a number of patients
showing no improvement in their condition, as a result of the
development of resistance against the treatment, and some even
presenting with serious side effects in other organs of the body
(11–14). Accordingly, novel therapeutic
approaches, such as biological therapies and gene therapy, are
required to efficiently treat osteosarcoma.
A number of studies have shown that osteosarcoma may
be vulnerable to biological therapies (15–17);
however, little is known with regard to the therapeutic effects of
capsaicin on osteosarcoma. This study examined the effects of
capsaicin on MG63 human osteosarcoma cells using
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay, flow cytometry, western blot analysis and terminal
deoxynucleotidyltransferase-mediated deoxyuridine triphosphate
(dUTP) nick end-labelling (TUNEL) assay. In addition, the study
explored the regulatory signaling pathway underlying the effects of
capsaicin, using a variety of inhibitors.
Materials and methods
Reagents
Capsaicin and MTT were purchased from Sigma-Aldrich
(St. Louis, MO, USA), while Dulbecco's modified Eagle's medium
(DMEM), phosphate-buffered saline (PBS) and fetal bovine serum
(FBS) were obtained from Invitrogen Life Technologies (Carlsbad,
CA, USA). The U0126, PD98053, SP600125 and Z-VAD-FMK used in the
study were purchased from Calbiochem® (Merck KGaA,
Darmstadt, Germany) and a chemiluminescence (ECL) kit was obtained
from Amersham Pharmacia Biotech (GE Healthcare, Amersham, UK).
Bcl-2, Bcl-2-associated X protein (Bax) and pro-caspase-3 were
obtained from Epitomics, Inc. (Burlingame, CA, USA), while
phosphorylated extracellular signal-regulated kinase (p-ERK),
phosphorylated p-38 (p-p38) and phosphorylated c-Jun N-terminal
kinase (p-JNK) were purchased from Cell Signaling Technology, Inc.
(Beverly, MA, USA).
Cell line and culture conditions
MG63 cells (human osteosarcoma cell line) were
purchased from the Korean Cell Line Bank (Seoul, Korea) and
cultured in DMEM containing 10% heat-inactivated FBS. The cells
were plated in tissue culture dishes at 37°C in a humidified 5%
CO2 incubator and cultured for 2–4 days until confluence
was reached. Subcultures were prepared using 0.05% trypsin solution
and seeded in 6- or 96-well tissue culture plates. Serum was
starved from the culture media at the time of adding various
agents.
Measurement of cell growth by MTT
Cell viability was assessed using an MTT assay,
based on the reduction of MTT into formazan dye by the action of
mitochondrial enzymes. MG63 cells were seeded in 96-well plates at
a density of 5×102 cells per well and indicated
concentrations of capsaicin were added for indicated time-periods.
Briefly, following treatment with capsaicin at various
concentrations (0, 50, 100, 150, 200, 250 and 400 μM) and various
time-periods (0, 3, 6, 12, 24 and 48 h) under 150 μM of capsaicin,
the cells were washed and 0.5 mg/ml MTT in DMEM solution was added
to each well, prior to incubation for 2 h at 37°C. The supernatant
was then removed and the cells were dissolved in dimethylsulfoxide
(DMSO). The absorbance of each well was measured at 570 nm with a
680 microplate enzyme-linked immunosorbent assay (ELISA) reader
(Bio-Rad Laboratories, Hemel Hempstead, UK).
Cell morphology
The untreated and treated cells were seeded in
6-well plates at a density of 5×104 cells per well and
incubated for 24 h with 50–400 μM capsaicin (0, 50, 100, 150, 200,
250 and 400 μM). Cell morphology was examined under a light
microscope.
Flow cytometric analysis
Cells were seeded in 6-well plates at a density of
5×104 cells per well and treated with the indicated
reagents for 24 h at 37°C. The suspended and adherent cells were
then harvested using 0.05% trypsin solution. The harvested cells
were centrifuged at 10,000 × g for 15 min at 4°C and the pellets
were then washed in PBS, prior to the addition of fixing solution
with ice-cold 100% ethanol containing 0.25% Triton X-100 for
treatment overnight at 4°C. Subsequent to fixation, the cells were
washed and stained with 50 μg/ml propidium iodide containing 100
μg/ml RNase, prior to being incubated for 20 min at 37°C and
analyzed using a FACSort flow cytometer (Becton Dickinson, Franklin
Lakes, NJ, USA).
TUNEL assay
The presence of DNA fragmentation was evaluated
using TUNEL assay with an in situ Cell Death Detection kit
(fluorescein) from Roche Applied Science (Indianapolis, IN, USA).
The cells were seeded in cover slides (5×102 cells per
slide) and then treated with capsaicin. Following this, the cells
were washed in PBS and freshly prepared 4% paraformaldehyde was
added for cell fixation for 1 h at 37°C in a humidified 5%
CO2 incubator. The cells were then washed again in PBS,
prior to being permeabilized in permeabilization solution (0.1%
Triton X-100 in 0.1% sodium citrate) for 2 min on ice. The cells
were then subjected to the TUNEL reaction at 37°C in a humidified
atmosphere in the dark for 60 min. The fluorescence signal emitted
by the fluorescein-labeled dUTP incorporated into the fragmented
DNA was detected using Leica confocal microscopy (Leica
Microsystems, Wetzlar, Germany).
Measurement of cell death using the
trypan blue dye exclusion assay
Capsaicin-treated cells were harvested using 0.05%
trypsin solution and then suspended with 0.4% trypan blue solution.
The cells were counted using a hemocytometer under a light
microscope and cells that were observed to exclude the dye were
considered viable.
Western blot analysis
The cells were seeded in 6-well plates at a density
of 5×104 cells/cm2, cultured and incubated in
DMEM containing 10% FBS. Prior to treatment with the indicated
conditions, the cells were serum-starved overnight, treated with
the agent and then harvested. Using lysis buffer [20 mM Tris-HCl
(pH 7.4), 10 mM NaCl, 1 mM EDTA, 1 mM ethylene
glycol-O-O′-bis(2-amino-ethyl)-N,N,N′,N′-tetraacetic acid (EGTA),
0.1 mM phenylmethylsulfonyl fluoride, protease inhibitor cocktail
and 1% Triton X-100], the cells were lysed on ice. The lysates were
subsequently centrifuged at 10,000 × g for 20 min at 4°C and the
supernatants were loaded on 15% sodium dodecyl
sulphate-polyacrylamide gel electrophoresis gel and transferred to
a nitrocellulose membrane. The membranes were subsequently
immunoblotted with various primary antibodies and incubated with
the respective peroxidase-conjugated secondary antibodies. The
signals were visualized using an enhanced ECL kit from Amersham
Pharmacia Biotech.
Statistical analysis
Experiments were performed at least three times.
Statistical significance was analyzed using a Student's t-test
(two-tailed). P<0.05 was considered to indicate a statistically
significant difference.
Results
Inhibitory effects of capsaicin on the
cell viability of osteosarcoma cells
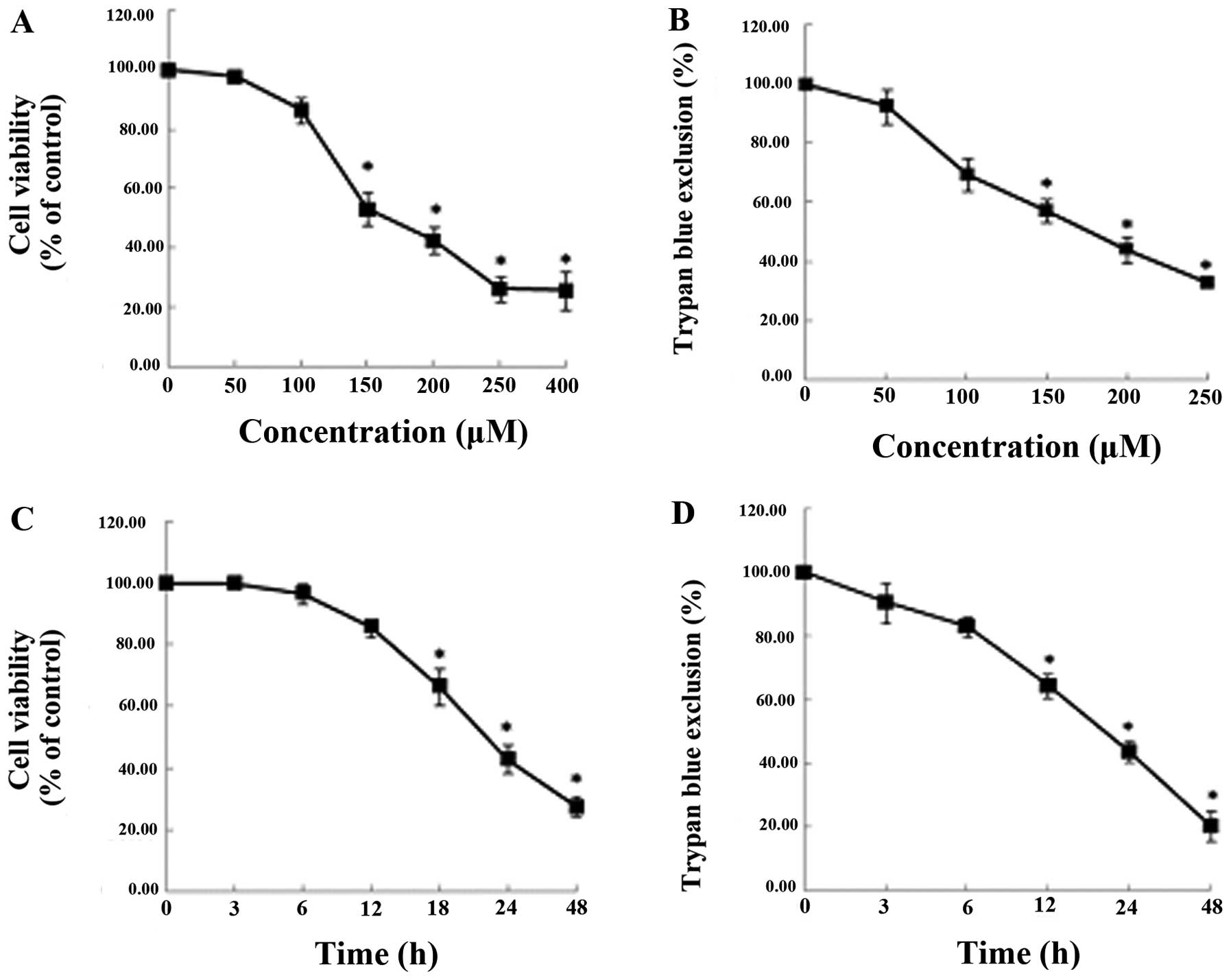
We examined the effects of capsaicin in the MG63
osteosarcoma cell lines, which had been treated with various
concentrations of capsaicin (0, 50, 100, 150, 200, 250 and 400 μM)
for various time-periods (0, 3, 6, 12, 24 and 48 h). As shown in
Fig. 1, capsaicin reduced the
viability of the MG63 cells in a dose- and time-dependent manner,
as demonstrated using MTT (Fig. 1A and
C) and trypan blue exclusion (Fig.
1B and D) assays. The viability of the cells treated with 150
μM capsaicin for 24 h was markedly reduced.
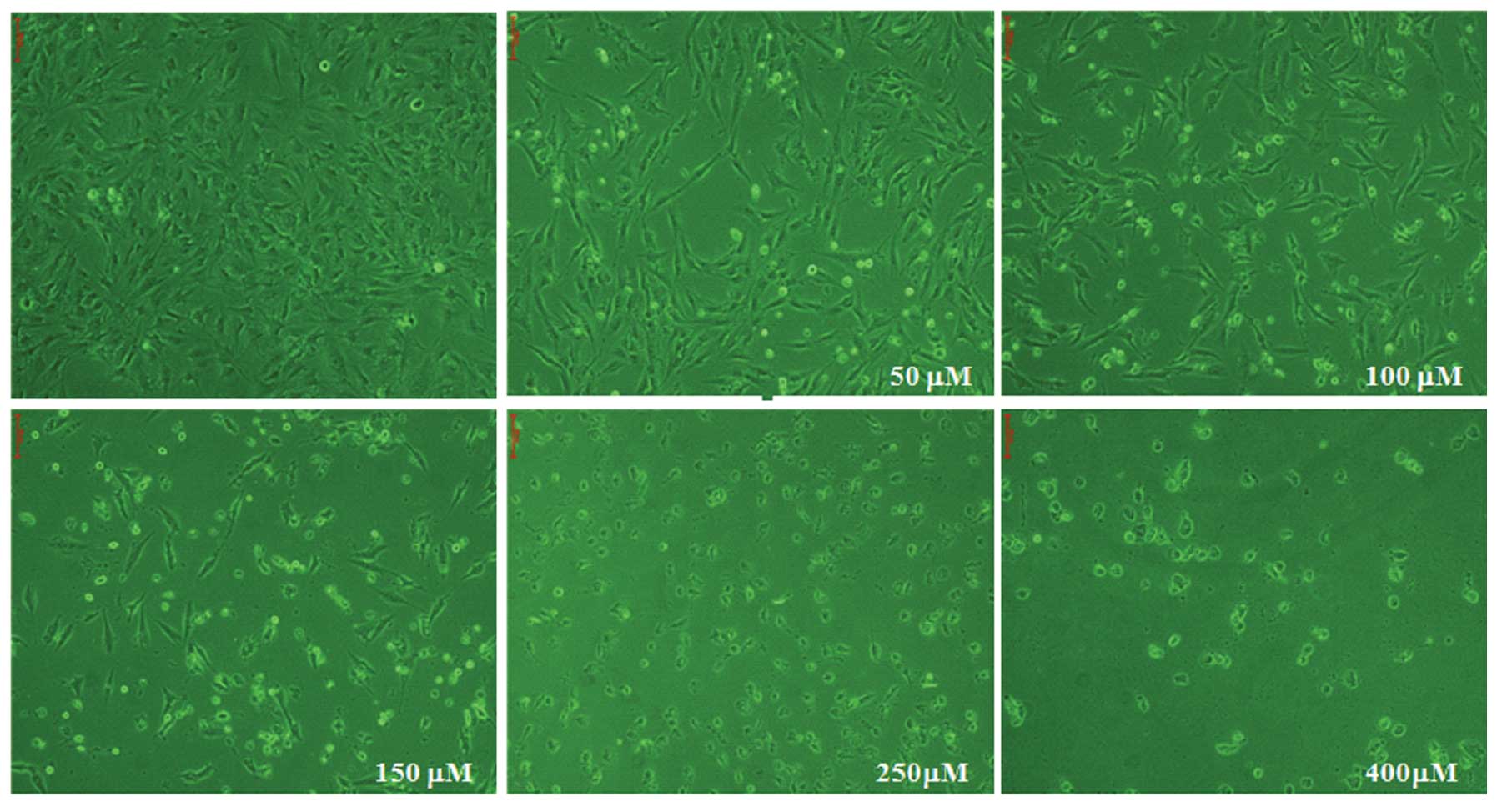
MG63 cell morphology observed using light
microscopy
MG63 cells were cultured for 24 h with different
concentrations of capsaicin (0, 50, 100, 150, 250 and 400 μM).
Following 24 h treatment with capsaicin, no significant
morphological changes were observed in the cells treated with
capsaicin at 50 and 100 μM. However, the cells exhibited the
morphological features of apoptosis when treated with 150 μM
capsaicin for 24 h (Fig. 2). These
morphological changes of the cells represented the apoptotic cell
death that occurred with 150 μM capsaicin at the end of the 24-h
exposure.
Capsaicin-induced apoptosis in MG63
cells
In order to characterize the type of cell death that
had been observed, we examined whether the cell death was
apoptosis. MG63 cells were treated with 150 μM capsaicin for 24 h
and the apoptotic DNA fragmentation of the MG63 cells was
visualized using TUNEL assay. TUNEL assay is a tool that is
frequently used for the detection of DNA fragmentation (18,19).
The significant increase in the number of TUNEL-positive cells
(green) showed that capsaicin induced apoptosis in the MG63 cells
(Fig. 3A). In addition, as shown
in Fig. 3B, capsaicin treatment
resulted in an increased proportion of cells in the G0–G1 phase,
from 0.26 to 24.8%. The G0–G1 phase is an indicator for cell
apoptosis when increased. These results suggested that capsaicin
induced apoptosis.
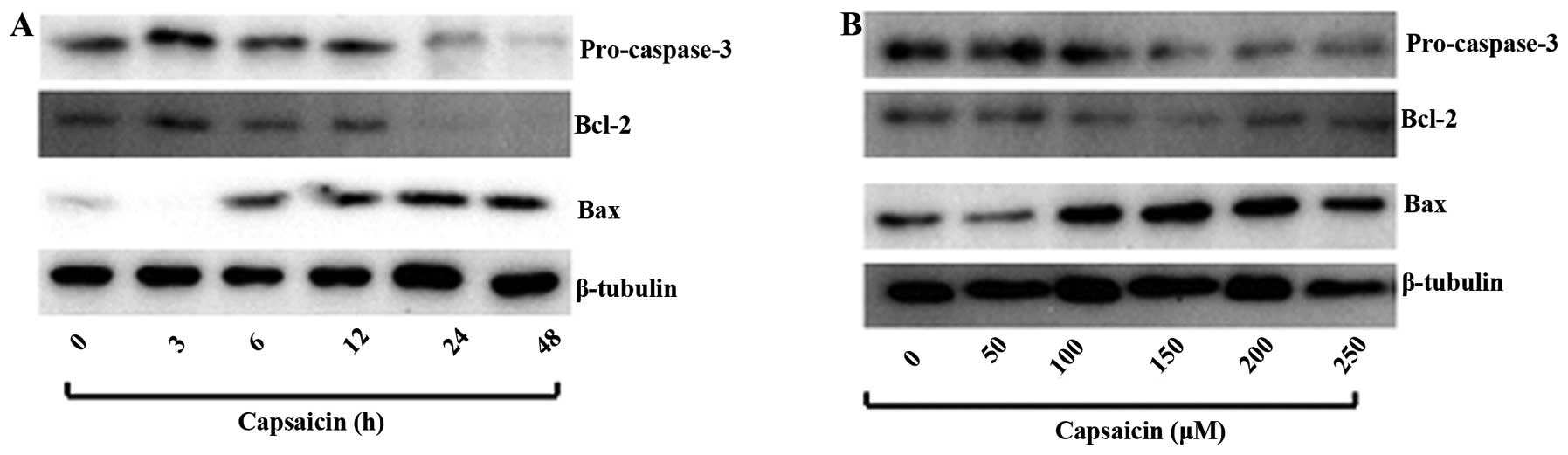
To investigate the effect of capsaicin on protein
molecules that are involved in apoptosis, western blot analysis was
used to test for the presence of the anti-apoptotic proteins Bcl-2
and cleaved caspase 3 (pro-caspase-3) and the pro-apoptotic protein
Bax. MG63 cells were treated with various concentrations of
capsaicin for 24 h and with 150 μM capsaicin for different
time-periods. Capsaicin decreased the expression of pro-caspase-3
and Bcl-2, while the expression of Bax was increased in a dose- and
time-dependent manner (Fig. 4A and
B).
Identifying the signaling pathway that
regulates the capsaicin-induced cell death
It has been demonstrated that apoptosis leads to
various signaling processes and, among them, the mitogen-activated
protein kinases (MAPKs) (20), the
caspase cascade (21) and the
antioxidant enzyme system (22)
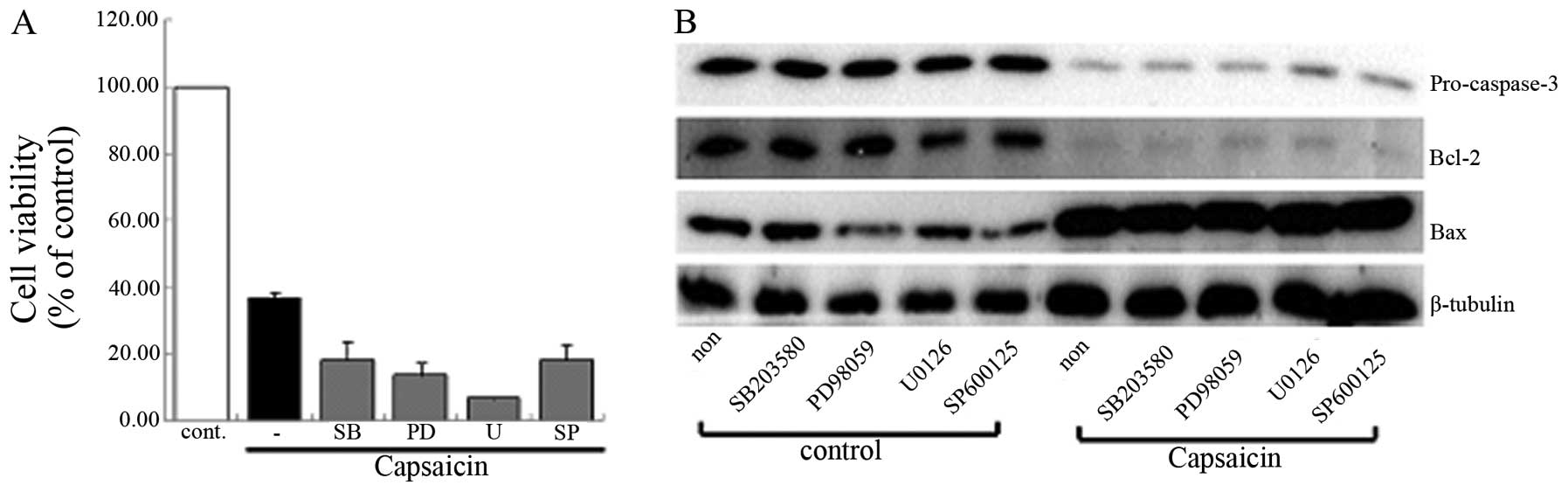
are the major executors of the process of apoptosis. We initially
suggested that the MAPK signaling pathway was involved in the
capsaicin-induced apoptosis. However, the group that was pretreated
with MAPK inhibitor did not show any differences when compared with
the group treated only with capsaicin using MTT assay and western
blot analysis (Fig. 5). The data
demonstrated that capsaicin-induced apoptosis was not regulated by
the MAPK signaling pathway in MG63 cells. The involvement of the
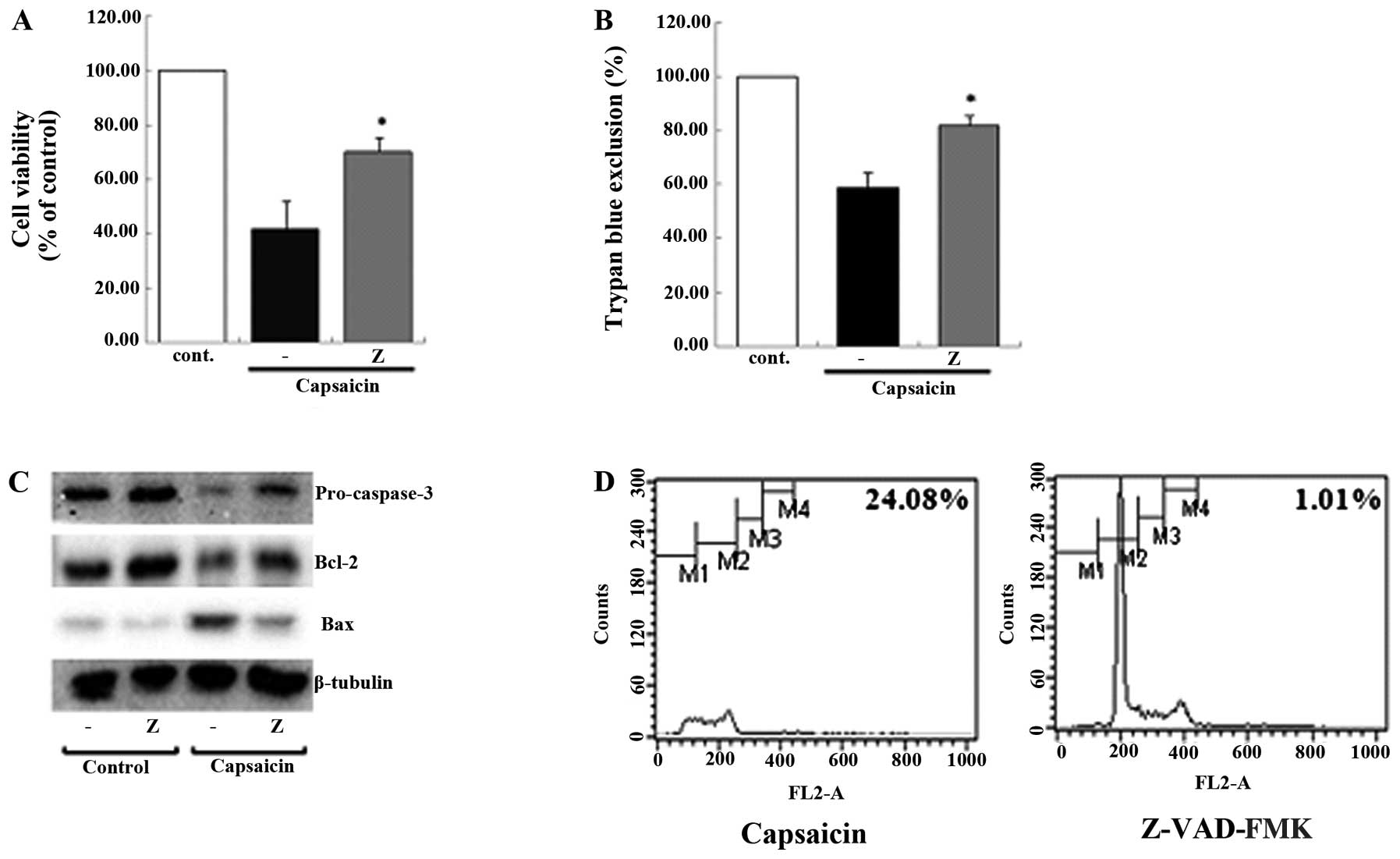
caspase cascade in the capsaicin-induced apoptosis using MTT assay,
trypan blue exclusion, western blot analysis and flow cytometry was
then examined. Consistently, it was shown that the general caspase
cascade inhibitor, Z-VAD-FMK, had some effect when the results were
compared with those from the group treated only with capsaicin
(Fig. 6). These results suggested
that the caspase cascade was involved in capsaicin-induced
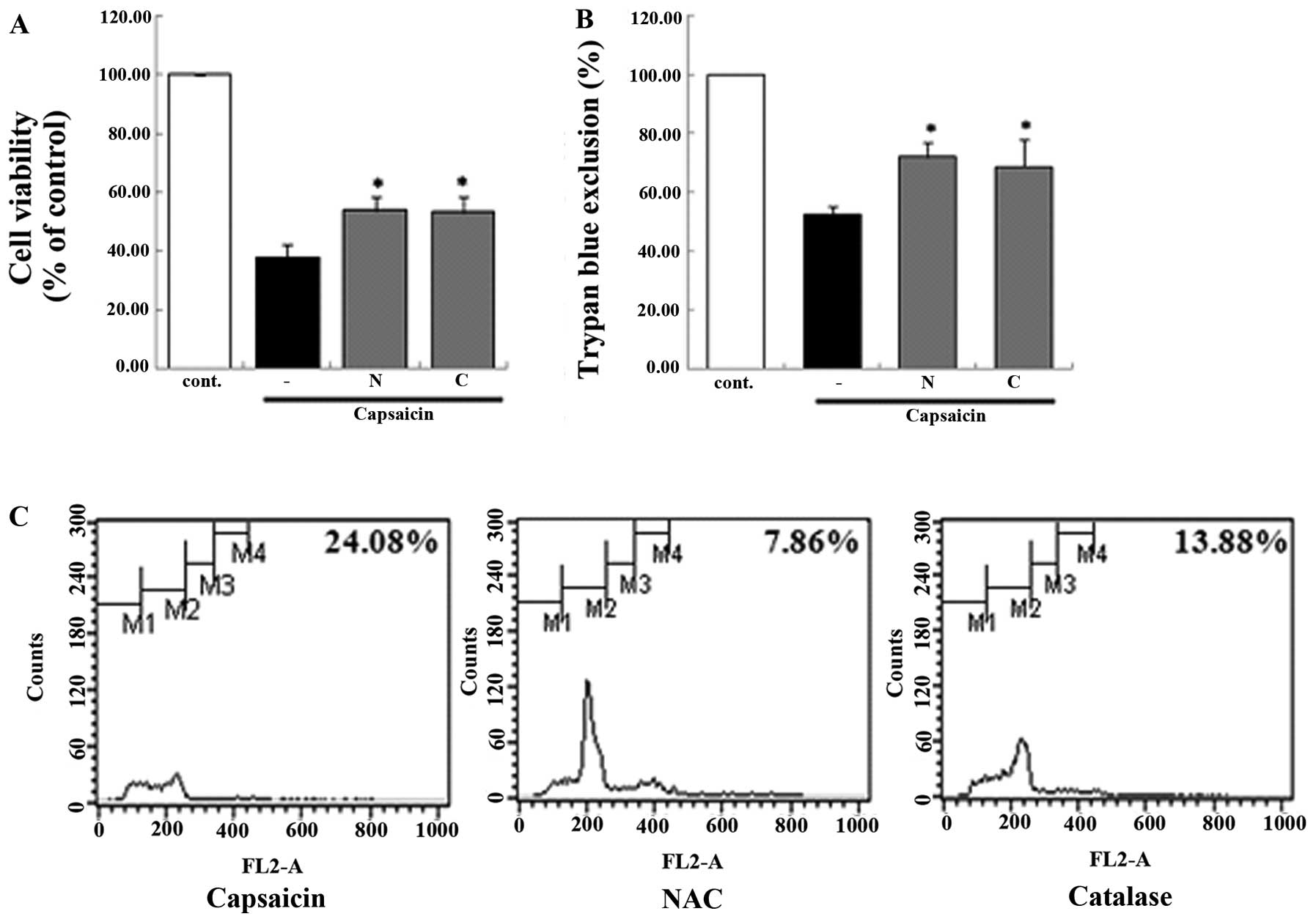
apoptosis. In addition, the antioxidant enzyme system was
demonstrated to be involved in the capsaicin-induced apoptosis. In
the groups that were pretreated with antioxidant enzyme inhibitor,
the viability of the MG63 cells decreased from 24.08 to 7.86%
[N-acetyl-L-cysteine (NAC)] and 13.88% (catalase) compared with the
group treated only with capsaicin. This result showed that
antioxidant enzyme inhibitors affected the apoptosis using a
variety of methods (Fig. 7). We
demonstrated that antioxidant enzymes were involved in the
capsaicin-induced apoptosis in MG63 cells.
Discussion
In this study, we used a variety of techniques and
demonstrated that the cell viability of MG63 cells was able to be
reduced by capsaicin in a dose- and time-dependent manner. In
addition, the capsaicin-treated group showed an increased level of
positivity with the TUNEL assay and an increase in Bax protein.
Moreover, the experiments with the signaling pathway inhibitors
showed that the groups pretreated with Z-VAD-FMK, NAC and catalase,
respectively, had different results compared with those from the
group treated only with capsaicin. These results indicated that the
capsaicin-induced apoptosis in MG63 cells may have been mediated by
the caspase cascade and the antioxidant enzyme system, among
various signaling pathways.
Osteosarcoma is the most frequently occurring
primary malignant neoplasms of the long bones, including the distal
femur and the proximal humerus, and mainly affects children and
adolescents (10,23). The prognosis for osteosarcoma, for
which conventional treatments include surgery, chemotherapy and
radiotherapy, is poor due to the early pulmonary metastasis and
limited improvements. Chemotherapy has become a foundation for the
basic treatment of osteosarcoma. A number of studies have focused
on the development of new effective therapeutic strategies for
osteosarcoma, using novel materials extracted from natural food
substances that exhibit an anticancer effect, despite the
successful use of neoadjuvant chemotherapy in the treatment of
osteosarcoma (11,12,15,24).
It has been demonstrated that a number of reagents
are able to induce apoptosis on MG63 human osteosarcoma cells
(25–27); however, the effect of capsaicin on
MG63 cells has remained unclear. Capsaicin, the main pungent
ingredient in the genus Capsicum, has long been used in
drugs for weight loss and has been studied as an attractive drug
for cancer treatment, as an agent that induces apoptosis in various
cell types in vitro(28–31).
Moreover, the compound has been indicated to promote apoptosis
in vivo as the mechanism of tumor cell elimination in animal
models for carcinogenesis (32,33).
These observations have continued to draw attention to capsaicin as
a possible anticancer agent.
Apoptosis has been suggested as a promising target
for cancer chemotherapy (34–36).
Apoptosis is a form of self-regulated cell death and occurs during
normal cell turnover, development and immune regulation (37,38).
The characteristic morphological changes involved in apoptosis
include cytoplasmic shrinkage, plasma membrane blebbing, chromatin
condensation and the formation of apoptotic bodies containing
well-preserved organelles. In addition, during apoptosis cells
undergo double-strand cleavage of nuclear DNA (34,39–41).
This study was designed to investigate
capsaicin-induced apoptosis in MG63 human osteosarcoma cells and
its underlying molecular mechanisms. Using TUNEL assay, flow
cytometric assay and western blot analysis, we demonstrated that
the anticancer effect of capsaicin resulted in morphological
changes, decreased cell viability and apoptosis in the MG63 cells
(Figs. 1–4). These results showed that capsaicin
was able to inhibit cell viability and growth and induce
apoptosis.
We investigated the molecular factors that were
involved in the apoptosis of capsaicin-treated MG63 cells. The
MAPKs are expressed in all mammalian cell types and have
individually different functions in the regulation of specific cell
responses. MAPKs have been demonstrated to be composed of three
parallel kinase modules, including ERK, JNK and p-38-MAPK (42–44).
As shown in numerous studies, the MAPK signaling pathway is
important in the regulation of cellular growth, differentiation,
survival, angiogenesis and apoptosis (20,45,46–48).
Accordingly, we initially suggested that the MAPK signaling pathway
was involved in the cellular response of capsaicin-induced
apoptosis. Using groups pretreated with MAPK inhibitors, it was
revealed that MAPKs exerted no specific effect in capsaicin-induced
apoptosis in the MG63 cells (Fig.
5).
It has been revealed that caspase, or
cysteine-aspartic protease, belongs to the group of enzymes known
as cysteine proteases, which are homologous to the
Caenorhabditis elegans, the cell death gene, CED-3 (21,49).
Cysteine proteases have multi-faceted functions in virtually every
aspect of physiology, such as in growth and development, senescence
and apoptosis (50,51). Moreover, the components of the
caspase cascade are present in various cells in the form of
inactive zymogens, which are then activated to convey the apoptotic
signal (52). Furthermore, it has
been suggested that the caspase cascade may induce the apoptotic
reaction (53). Our results showed
that the caspase cascade regulated capsaicin-induced apoptosis,
observed through cell viability, western blot analysis and flow
cytometry (Fig. 6).
In present study it was demonstrated that the
antioxidant enzyme system was also involved in the
capsaicin-induced apoptosis. The antioxidant enzyme system has been
indicated to be important in the control of apoptosis (54,55).
In addition, antioxidant enzymes defend cells from oxidative
damage, such as reactive oxygen species (ROS) production (56–58).
ROS interact with a wide range of cell components and cause damage
to cell structures, including the membrane, and are regulated with
apoptosis (30,59–61).
As such, antioxidant enzymes have the potential to protect the
cells from oxidative damage. Based on the results of our study, we
verified that the antioxidant enzyme system was particularly
effective in capsaicin-induced apoptosis in the MG63 cells, as
demonstrated using a variety of methods (Fig. 7). Therefore, it was indicated that
ROS were part of the capsaicin-induced apoptosis pathway in the
MG63 cells.
The present study provided distinct results
describing the effect of capsaicin on MG63 cells, in addition to
elucidating the molecular mechanisms that were implicated in the
indution of apoptosis. In combination, the results showed that
capsaicin induced apoptosis in the MG63 cells and that the caspase
cascade and antioxidant enzyme system were the underlying
regulatory signaling pathways involved in the capsaicin-induced
apoptosis. In a previous study, we demonstrated the effect of
capsaicin on human glioblastoma U87MG cells and concluded that
capsaicin induced apoptosis in the U87MG cells (62). The present results indicated that
capsaicin exhibited an anticancer effect in osteosarcoma cells.
Further in vitro and in vivo studies are required
before capasaicin is able to be ultimately applied to treat human
patients with osteosarcoma.
Acknowledgements
This study was supported by a grant from the SNUBH
Research Fund (grant no. 02-2012-036).
References
|
1
|
Firdous AP, Sindhu ER, Ramnath V and
Kuttan R: Anti-mutagenic and anti-carcinogenic potential of the
carotenoid meso-zeaxanthin. Asian Pac J Cancer Prev. 11:1795–1800.
2010.PubMed/NCBI
|
|
2
|
Patel D, Shukla S and Gupta S: Apigenin
and cancer chemoprevention: Progress potential and promise
(Review). Int J Oncol. 30:233–245. 2007.PubMed/NCBI
|
|
3
|
Weisburger JH: Antimutagens,
anticarcinogens, and effective worldwide cancer prevention. J
Environ Pathol Toxicol Oncol. 18:85–93. 1999.PubMed/NCBI
|
|
4
|
Cordell GA and Araujo OE: Capsaicin:
identification, nomenclature, and pharmacotherapy. Ann
Pharmacother. 27:330–336. 1993.PubMed/NCBI
|
|
5
|
Stavric B: Role of chemopreventers in
human diet. Clin Biochem. 27:319–332. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Huang SP, Chen JC, Wu CC, et al:
Capsaicin-induced apoptosis in human hepatoma HepG2 cells.
Anticancer Res. 29:165–174. 2009.PubMed/NCBI
|
|
7
|
Kim CS, Park WH, Park JY, et al:
Capsaicin, a spicy component of hot pepper, induces apoptosis by
activation of the peroxisome proliferator-activated receptor gamma
in HT-29 human colon cancer cells. J Med Food. 7:267–273. 2004.
View Article : Google Scholar
|
|
8
|
Chang HC, Chen ST, Chien SY, Kuo SJ, Tsai
HT and Chen DR: Capsaicin may induce breast cancer cell death
through apoptosis-inducing factor involving mitochondrial
dysfunction. Hum Exp Toxicol. 30:1657–1665. 2011. View Article : Google Scholar
|
|
9
|
Baek YM, Hwang HJ, Kim SW, et al: A
comparative proteomic analysis for capsaicin-induced apoptosis
between human hepatocarcinoma (HepG2) and human neuroblastoma
(SK-N-SH) cells. Proteomics. 8:4748–4767. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Enneking WF and Springfield DS:
Osteosarcoma. Orthop Clin North Am. 8:785–803. 1977.
|
|
11
|
Ferguson WS and Goorin AM: Current
treatment of osteosarcoma. Cancer Invest. 19:292–315. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bacci G and Lari S: Adjuvant and
neoadjuvant chemotherapy in osteosarcoma. Chir Organi Mov.
86:253–268. 2001.PubMed/NCBI
|
|
13
|
Biermann JS and Baker LH: The future of
sarcoma treatment. Semin Oncol. 24:592–597. 1997.PubMed/NCBI
|
|
14
|
La Quaglia MP: Osteosarcoma. Specific
tumor management and results. Chest Surg Clin N Am. 8:77–95.
1998.PubMed/NCBI
|
|
15
|
Marina N, Gebhardt M, Teot L and Gorlick
R: Biology and therapeutic advances for pediatric osteosarcoma.
Oncologist. 9:422–441. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Papagelopoulos PJ, Galanis EC, Vlastou C,
et al: Current concepts in the evaluation and treatment of
osteosarcoma. Orthopedics. 23:858–867; quiz 868–869.
2000.PubMed/NCBI
|
|
17
|
Yang C, Ji D, Weinstein EJ, et al: The
kinase Mirk is a potential therapeutic target in osteosarcoma.
Carcinogenesis. 31:552–558. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wieder R: TUNEL assay as a measure of
chemotherapy-induced apoptosis. Methods Mol Med. 111:43–54.
2005.PubMed/NCBI
|
|
19
|
Rohwer F and Azam F: Detection of DNA
damage in prokaryotes by terminal deoxyribonucleotide
transferase-mediated dUTP nick end labeling. Appl Environ
Microbiol. 66:1001–1006. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dhanasekaran DN and Johnson GL: MAPKs:
function, regulation, role in cancer and therapeutic targeting.
Oncogene. 26:3097–3099. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cohen GM: Caspases: the executioners of
apoptosis. Biochem J. 326:1–16. 1997.
|
|
22
|
Kong Q and Lillehei KO: Antioxidant
inhibitors for cancer therapy. Med Hypotheses. 51:405–409. 1998.
View Article : Google Scholar
|
|
23
|
Delling G: Diagnosis of bone tumors. Verh
Dtsch Ges Pathol. 82:121–132. 1998.(In German).
|
|
24
|
Federman N, Bernthal N, Eilber FC and Tap
WD: The multidisciplinary management of osteosarcoma. Curr Treat
Options Oncol. 10:82–93. 2009. View Article : Google Scholar
|
|
25
|
De Luna-Bertos E, Ramos-Torrecillas J,
Garcia-Martinez O, Diaz-Rodriguez L and Ruiz C: Effect of aspirin
on cell growth of human MG-63 osteosarcoma line. Scientific World
Journal. 2012:8342462012.PubMed/NCBI
|
|
26
|
Lin KL, Chi CC, Lu T, et al: Effect of
sertraline on [Ca2+]i and viability of human MG63
osteosarcoma cells. Drug Chem Toxicol. 36:231–240. 2013.
|
|
27
|
Jin S, Shen JN, Wang J, Huang G and Zhou
JG: Oridonin induced apoptosis through Akt and MAPKs signaling
pathways in human osteosarcoma cells. Cancer Biol Ther. 6:261–268.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wu CC, Lin JP, Yang JS, et al: Capsaicin
induced cell cycle arrest and apoptosis in human esophagus
epidermoid carcinoma CE 81T/VGH cells through the elevation of
intracellular reactive oxygen species and
Ca2+productions and caspase-3 activation. Mutat Res.
601:71–82. 2006. View Article : Google Scholar
|
|
29
|
Ip SW, Lan SH, Huang AC, et al: Capsaicin
induces apoptosis in SCC-4 human tongue cancer cells through
mitochondria-dependent and -independent pathways. Environ Toxicol.
27:332–341. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yang ZH, Wang XH, Wang HP, Hu LQ, Zheng XM
and Li SW: Capsaicin mediates cell death in bladder cancer T24
cells through reactive oxygen species production and mitochondrial
depolarization. Urology. 75:735–741. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang R, Humphreys I, Sahu RP, Shi Y and
Srivastava SK: In vitro and in vivo induction of apoptosis by
capsaicin in pancreatic cancer cells is mediated through ROS
generation and mitochondrial death pathway. Apoptosis.
13:1465–1478. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mori A, Lehmann S, O'Kelly J, et al:
Capsaicin, a component of red peppers, inhibits the growth of
androgen-independent, p53 mutant prostate cancer cells. Cancer Res.
66:3222–3229. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bai H, Li H, Zhang W, et al: Inhibition of
chronic pancreatitis and pancreatic intraepithelial neoplasia
(PanIN) by capsaicin in LSL-KrasG12D/Pdx1-Cre mice.
Carcinogenesis. 32:1689–1696. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wong RS: Apoptosis in cancer: from
pathogenesis to treatment. J Exp Clin Cancer Res. 30:872011.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yue TL, Ohlstein EH and Ruffolo RR Jr:
Apoptosis: a potential target for discovering novel therapies for
cardiovascular diseases. Curr Opin Chem Biol. 3:474–480. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kerr JF, Wyllie AH and Currie AR:
Apoptosis: a basic biological phenomenon with wide-ranging
implications in tissue kinetics. Br J Cancer. 26:239–257. 1972.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Elmore S: Apoptosis: a review of
programmed cell death. Toxicol Pathol. 35:495–516. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lawen A: Apoptosis - an introduction.
Bioessays. 25:888–896. 2003. View Article : Google Scholar
|
|
39
|
Nagata S: DNA degradation in development
and programmed cell death. Annu Rev Immunol. 23:853–875. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhivotosky B and Orrenius S: Assessment of
apoptosis and necrosis by DNA fragmentation and morphological
criteria. Curr Protoc Cell Biol. Chapter 18(Unit 18):
132001.PubMed/NCBI
|
|
41
|
Negoescu A, Guillermet C, Lorimier P,
Brambilla E and Labat-Moleur F: Importance of DNA fragmentation in
apoptosis with regard to TUNEL specificity. Biomed Pharmacother.
52:252–258. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Seger R and Krebs EG: The MAPK signaling
cascade. FASEB J. 9:726–735. 1995.PubMed/NCBI
|
|
43
|
Raman M, Chen W and Cobb MH: Differential
regulation and properties of MAPKs. Oncogene. 26:3100–3112. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lee S, Lee HS, Baek M, et al: MAPK
signaling is involved in camptothecin-induced cell death. Mol
Cells. 14:348–354. 2002.PubMed/NCBI
|
|
45
|
Ren D, Yang H and Zhang S: Cell death
mediated by MAPK is associated with hydrogen peroxide production in
Arabidopsis. J Biol Chem. 277:559–565. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zohrabian VM, Forzani B, Chau Z, Murali R
and Jhanwar-Uniyal M: Rho/ROCK and MAPK signaling pathways are
involved in glioblastoma cell migration and proliferation.
Anticancer Res. 29:119–123. 2009.PubMed/NCBI
|
|
47
|
Mavria G, Vercoulen Y, Yeo M, et al:
ERK-MAPK signaling opposes Rho-kinase to promote endothelial cell
survival and sprouting during angiogenesis. Cancer Cell. 9:33–44.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Roux PP and Blenis J: ERK and p38
MAPK-activated protein kinases: a family of protein kinases with
diverse biological functions. Microbiol Mol Biol Rev. 68:320–344.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Thornberry NA: The caspase family of
cysteine proteases. Br Med Bull. 53:478–490. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Li X, Su B, Liu R, Wu D and He D:
Tetrandrine induces apoptosis and triggers caspase cascade in human
bladder cancer cells. J Surg Res. 166:e45–e51. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Fan TJ, Han LH, Cong RS and Liang J:
Caspase family proteases and apoptosis. Acta Biochim Biophys Sin
(Shanghai). 37:719–727. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Denault JB and Salvesen GS: Caspases: keys
in the ignition of cell death. Chem Rev. 102:4489–4500. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Desouza M, Gunning PW and Stehn JR: The
actin cytoskeleton as a sensor and mediator of apoptosis.
Bioarchitecture. 2:75–87. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Kannan K and Jain SK: Oxidative stress and
apoptosis. Pathophysiology. 7:153–163. 2000. View Article : Google Scholar
|
|
55
|
Mates JM, Perez-Gomez C and Nunez de
Castro I: Antioxidant enzymes and human diseases. Clin Biochem.
32:595–603. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Bostwick DG, Alexander EE, Singh R, et al:
Antioxidant enzyme expression and reactive oxygen species damage in
prostatic intraepithelial neoplasia and cancer. Cancer. 89:123–134.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Circu ML and Aw TY: Reactive oxygen
species, cellular redox systems, and apoptosis. Free Radic Biol
Med. 48:749–762. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Stadtman ER: Role of oxidant species in
aging. Curr Med Chem. 11:1105–1112. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Bechtel W and Bauer G: Catalase protects
tumor cells from apoptosis induction by intercellular ROS
signaling. Anticancer Res. 29:4541–4557. 2009.PubMed/NCBI
|
|
60
|
Inoue M, Sakaguchi N, Isuzugawa K, Tani H
and Ogihara Y: Role of reactive oxygen species in gallic
acid-induced apoptosis. Biol Pharm Bull. 23:1153–1157. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Gao F, Yi J, Yuan JQ, Shi GY and Tang XM:
The cell cycle related apoptotic susceptibility to arsenic trioxide
is associated with the level of reactive oxygen species. Cell Res.
14:81–85. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Cha JH, Choi YJ, Cha SH, Choi CH and Cho
WH: Allicin inhibits cell growth and induces apoptosis in U87MG
human glioblastoma cells through an ERK-dependent pathway. Oncol
Rep. 28:41–48. 2012.PubMed/NCBI
|