Introduction
Desmopressin (1-desamino-8-D-arginine vasopressin,
also known as dDAVP) is a synthetic derivative of the arginine
vasopressin hormone, used clinically in the treatment of water
imbalance and certain hemostatic disorders. Vasopressin is a cyclic
nonapeptide with a disulfide bridge between residues
cysteine1 (Cys1) and Cys6, and a
tail comprising residues seven to nine. Vasopressin has been
extensively studied and modified in order to enhance its
specificity and half-life for designing agonists and antagonists as
potential therapeutic agents (1,2).
Vasopressin mediates its action through three known receptors:
V1a, found in the vasculature, which mediates the
pressor activity via the phospholipase C pathway; V1b in
the anterior pituitary, which mediates adrenocorticotropic hormone
release via the same pathway; and V2 in the renal collecting ducts,
which mediates antidiuretic action via the adenylate cyclase
pathway (2).
dDAVP contains two modifications with respect to
natural vasopressin, including deamination of Cys1,
which improves the half-life and enhances the antidiuretic
activity, and an L-arginine (L-Arg) to D-Arg substitution at
position 8, which abolishes the vasopressor V1a
receptor-dependent activity. The biological activity of dDAVP is
thus selectively mediated through its interaction with V2 receptors
in the kidneys, as well as in the microvasculature, inducing the
secretion of coagulation factors. The presence of vasopressin V2
receptors has been reported in different cell lines and tumors,
including breast cancer (3). In a
previous study we demonstrated modest but significant
antiproliferative effects of dDAVP on an experimental mammary
carcinoma by agonist-V2 receptor interaction in vivo
(4). Furthermore, perioperative
administration of dDAVP during cancer surgery has been found to
reduce the metastatic progression associated with the cytostatic
and hemostatic effects of the compound (reviewed in 5).
The alanine-scanning technique (Ala-Scan) involves
the sequential substitution of each amino acid in a lead peptide
with an Ala residue in order to identify the amino acids that are
critical for biological function (6). Ala is used in this technique since it
is considered to be the most neutral amino acid, it has a simple
side-chain, it is not highly hydrophobic and it has no charge.
These features enable the structure-activity relationship of the
peptide of interest to be determined (7). Ala-Scan has been used to investigate
the structure-activity relationship of a number of potential
therapeutic molecules and has been utilized to design analogs with
enhanced activity and/or selectivity (8–10).
The aim of the present study was to identify key
positions involved in the antiproliferative activity of dDAVP,
using Ala-Scan analysis of the synthetic peptide. The
identification of key residues involved in the antiproliferative
activity of dDAVP may contribute to the rational design of improved
antitumor compounds. Additionally, proline7
(Pro7) was substituted with a hydroxyproline (Hyp)
residue in order to analyze the effect of a polar amino acid near
the positive charge of the peptide tail (Arg8).
Materials and methods
Chemicals
Rink amide resin, fluorenylmethyloxycarbonyl
(Fmoc)-amino acids and coupling reagents were obtained from Iris
Biotech GmbH (Marktredwitz, Germany). Solvents for the peptide
synthesis and purification were obtained from Tedia Company Inc.
(Fairfield, OH, USA).
Analog design: dDAVP Ala-Scan
Positions 2–5 and 7–9 were sequentially substituted
with Ala. Positions 1 and 6, involved in the disulfide-bridge
formation, were not Ala-substituted in order to maintain the cyclic
feature of the analogs (Table I).
Additionally, the amino acid at position 7 was substituted by a Hyp
residue (Table II).
 | Table IdDAVP Ala-Scan: Name, sequence and
physicochemical features of dDAVP and its analogs. |
Table I
dDAVP Ala-Scan: Name, sequence and
physicochemical features of dDAVP and its analogs.
| Peptide | Sequence | Retention time
(min) | MW in Da,
experimental (theoretical) |
|---|
| dDAVP |
MpaYFQNCPrG-NH2 | 24.7 | 1068.45
(1068.24) |
| [Ala2]
dDAVP | MpaAFQNCPrG-NH2 | 20.3 | 976.40 (976.14) |
| [Ala3]
dDAVP | MpaYAQNCPrG-NH2 | 15.5 | 992.41 (992.14) |
| [Ala4]
dDAVP | MpaYFANCPrG-NH2 | 26.3 | 1011.54
(1011.18) |
| [Ala5]
dDAVP | MpaYFQACPrG-NH2 | 28.1 | 1025.45
(1025.21) |
| [Ala7]
dDAVP | MpaYFQNCArG-NH2 | 23.7 | 1042.58
(1042.20) |
| [Ala8]
dDAVP | MpaYFQNCPAG-NH2 | 24.9 | 983.40 (983.13) |
| [Ala9]
dDAVP |
MpaYFQNCPrA-NH2 | 25.3 | 1082.49
(1082.26) |
 | Table IIdDAVP Hyp analog: Name, sequence and
physicochemical features of [Hyp7] dDAVP. |
Table II
dDAVP Hyp analog: Name, sequence and
physicochemical features of [Hyp7] dDAVP.
| Name | Sequence | Retention time
(min) | MW in Da,
experimental (theoretical) |
|---|
| dDAVP |
MpaYFQNCPrG-NH2 | 24.7 | 1068.45
(1068.24) |
| [Hyp7]
dDAVP |
MpaYFQNCHyprG-NH2 | 22.7 | 1084.55
(1084.24) |
Peptide synthesis
Peptides were synthesized in solid phase, using
Nα-Fmoc protection, following the ‘tea-bag’ strategy as
previously described by Houghten (11). Amide resin was used as the solid
support in order to obtain the amide peptides. Cyclization of the
peptides was performed on solid phase, taking advantage of the
pseudo-dilution phenomenon, which favors intramolecular bridge
formation (12). Briefly,
(Trt)-3-mercaptopropionic acid and (Trt)-Cys were deprotected in a
trifluoroacetic acid (TFA), dichloromethane and triisopropylsilane
(TIS) (2:95:3) solution for 1 h at room temperature, following
oxidation with 10 eq I2 in N,N dimethylformamide for 30
min at room temperature. The peptides were then deprotected and
cleaved from the resin using a TFA, H2O and TIS
(95:2.5:2.5) solution for 3 h at room temperature.
Peptide purification and
quantification
Peptides were purified using reversed-phase
high-performance liquid chromatography on an Ultrasphere ODS C-18
column (Beckman Instruments, Palo Alto, CA, USA) using a linear
gradient of 10–40% acetonitrile in water containing 0.05% TFA for
30 min. Peptides were quantified using a commercial dDAVP standard
from BCN Peptides (San Quinti de Mediona, Barcelona, Spain).
Peptide characterization
Peptides were identified using electrospray
ionization-mass spectrometry in a LCQ-Duo (ion trap) mass
spectrometer (Thermo Fisher Scientific, Inc., San José, CA, USA).
Samples were introduced from a Surveyor pump (Thermo Fisher
Scientific, Inc.) in a 40-μl/min solvent flow. Peptide analysis was
performed by full scan spectra covering the mass range of 200–2,000
amu.
Theoretical molecular weights were calculated using
the ProtParam tool from the Expasy server (available at http://www.expasy.org/tools/protparam.html).
Cell lines and culture conditions
The aggressive MDA-MB-231 human breast carcinoma
cell line [American Type Culture Collection (ATCC) cat. no. HTB-26]
was grown in Dulbecco’s modified Eagle’s medium (DMEM; Gibco-BRL,
Grand Island, NY, USA) supplemented with 10% fetal bovine serum
(FBS), 2 mM glutamine and 80 μg/ml gentamycin, in a monolayer
culture at 37°C in a humidified atmosphere of 5% CO2.
The hormone-dependent MCF-7 cell line (ATCC cat. no. HTB-22), a
well-differentiated breast carcinoma, was also used as a reference
of V2 receptor-positive cells.
Vasopressin receptor expression
The expression of V2 receptor was investigated using
reverse transcription-polymerase chain reaction (RT-PCR). Primers
corresponding to the mRNA sequences (GenBank accession no.
NM000054.4; gi:225903383) were as follows: 867–886, forward 5′-GTG
GCC AAG ACT GTG AGG AT-3′ and 1,356–1,375 reverse, 5′-ATA CAG CTG
GGG ATG TGG AG-3′.
In vitro growth assays
The antiproliferative effects of dDAVP and the
peptide analogs were analyzed against rapidly growing breast cancer
cells. A range of concentrations between 100 nM and 1.5 μM was used
with a three-day exposure of log-phase growing cells. MDA-MB-231
cells were seeded on 96-well plates (2.5–5×103
cells/well) in DMEM plus 10% FBS. After 24 h, peptides were added
and the cells were cultured for a further 72 h, prior to being
analyzed using the MTT assay. Additionally, the cytostatic effects
of dDAVP were verified at low cell density using the colony
formation assay. Cells were plated at 0.8–1.2×103
cells/well in 24-well plates and grown for seven days in complete
medium in the presence of varying concentrations of dDAVP. Cultures
were then fixed with formalin and stained with crystal violet, and
colonies were counted. The 50% inhibitory concentration (IC50) was
determined by plotting the percentage of cell colonies versus drug
concentration.
Results
dDAVP analogs and their effect on
proliferation and colony formation
The features of the synthetic peptides are
summarized in Table I and II. The expression of vasopressin V2
receptor in the aggressive MDA-MB breast carcinoma cell line-231
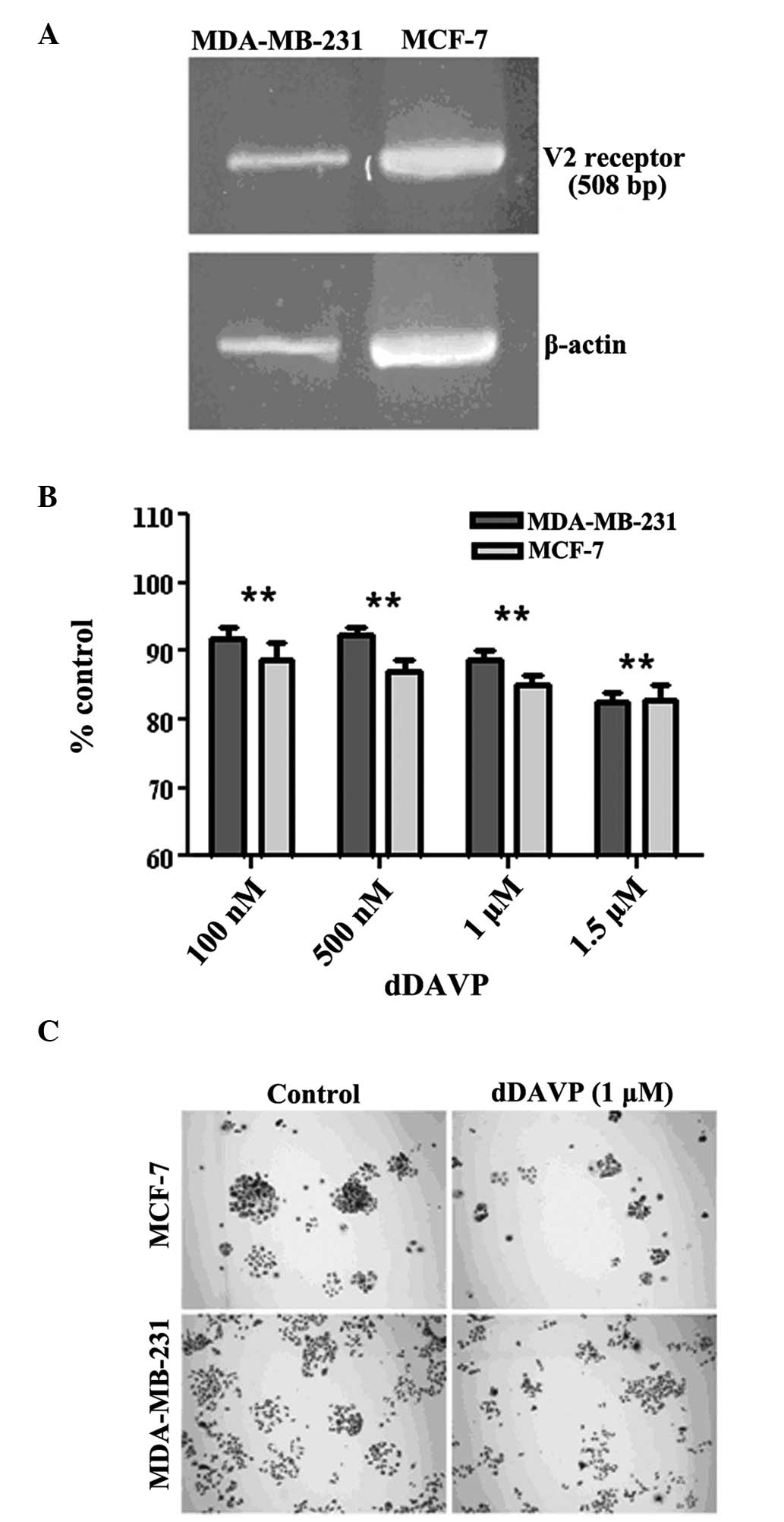
was first verified. As shown in Fig.
1A, the expected fragment of 508 bp was observed in the cells
using RT-PCR. Well-differentiated MCF-7 cells also expressed the
receptor, as previously reported (3). The antiproliferative effects of dDAVP
on log-phase growing cells were then investigated. Following
exposure to dDAVP for 72 h, a modest but significant inhibition of
cell growth was observed at doses of ≥100 nM (Fig. 1B). Higher doses (1 or 1.5 μM)
reduced proliferation in breast cancer cell cultures by ~20%. By
contrast, dDAVP had a stronger effect on colony formation at low
cell density (Fig. 1C), with IC50
values of 1.44 μM (R2=0.96; P=0.0033) and 0.97 μM
(R2=0.98; P=0.0012) against MDA-MB-231 and MCF-7 cells,
respectively.
Identification of key residues in
dDAVP
The structure antiproliferative activity
relationship of dDAVP was performed on the aggressive MDA-MB-231
cell line. The peptides concentration was 1 μM, which exerts a
significant antiproliferative effect of dDAVP on log-phase growing
cells. The results from the Ala-Scan demonstrated the importance of
the amino acids located at the loop of dDAVP for antiproliferative
activity. The activity was reduced by up to 60% when amino acids
2–5 were substituted (Fig. 2). A
similar profile was observed at lower doses of dDAVP (250 nM) in
the colony formation assay (data not shown). The cytostatic effect
on log-phase growing cells was conserved when amino acids in
positions 7 and 8 were substituted and partially reduced when the
substitution occurred at position 9 (Fig. 2). Similarly, a polar substitution
at position 7 by Hyp had no effect on the antiproliferative
activity of the resultant analog (data not shown).
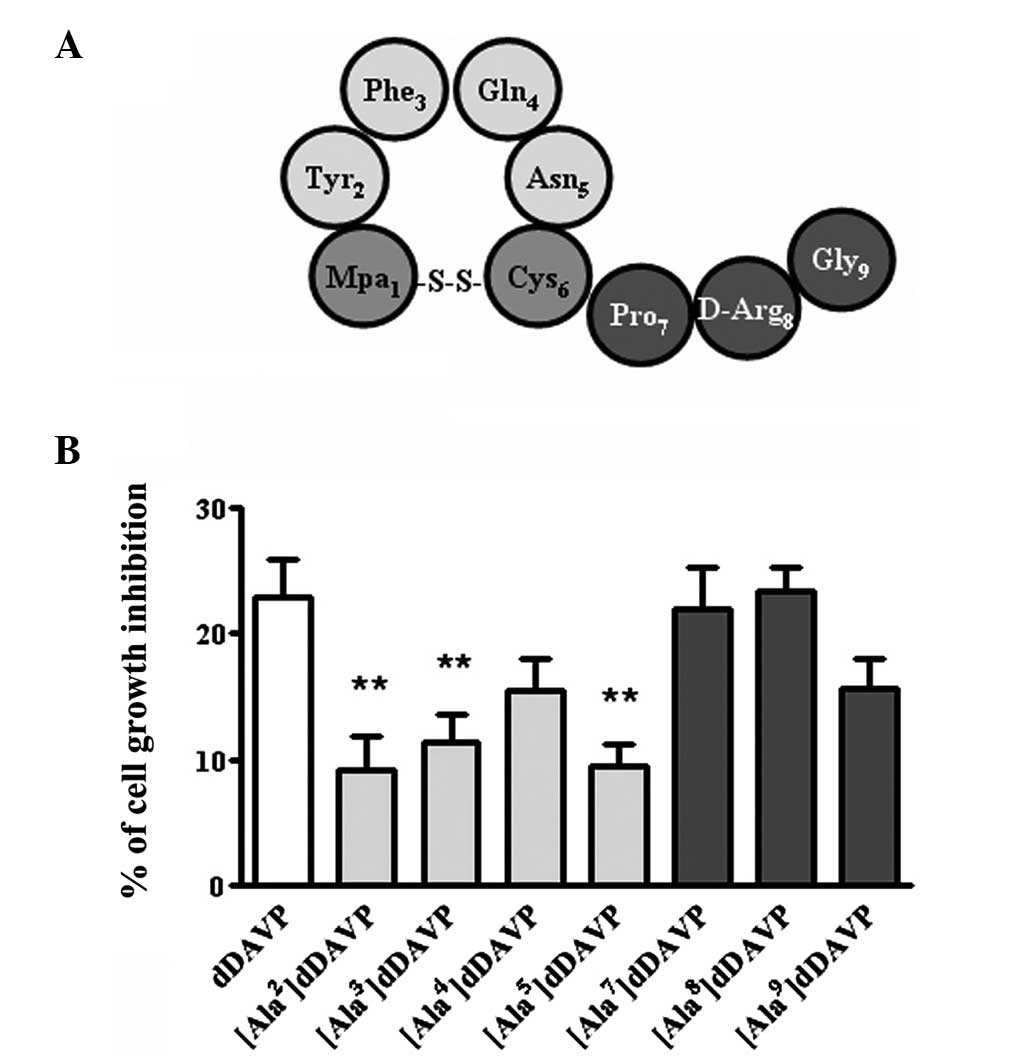 | Figure 2Ala-Scan analysis of dDAVP in
MDA-MB-231 breast cancer cells. (A) Schematic representation of
dDAVP. (B) Growth inhibition following a 72-h exposure to dDAVP on
log-phase growing cells at a peptide concentration of 1 μM. Data
are presented as the mean ± standard error. **P<0.01,
versus dDAVP; analysis of variance plus Dunnett’s test. Mpa,
3-mercaptopropionic acid; Tyr, tyrosine; Phe, phenylalanine; Gln,
glutamine; Asn, asparagine; Cys, cysteine; Pro, proline; D-Arg,
D-arginine; Gly, glycine; dDAVP, 1-desamino-8-D-arginine
vasopressin. |
As mentioned, the results from the Ala-Scan
demonstrated the importance of the amino acids located at the loop
of dDAVP for the antiproliferative activity (Fig. 2). The antiproliferative activity
was reduced by 30–60% when amino acids 2–5 were substituted, while
the activity was conserved when the amino acids Pro7 and
D-Arg8 were substituted. The activity was also reduced
30% when glycine9 was substituted. Consistent with these
results, Table III shows the
classification of the residues of dDAVP into three groups according
to the reduction in antiproliferative activity of the resultant
analog.
 | Table IIIClassification of dDAVP residues
according to their role in the antiproliferative activity of the
compound. |
Table III
Classification of dDAVP residues
according to their role in the antiproliferative activity of the
compound.
| Group | Residues | Antiproliferative
activity reduction (%) | Feature |
|---|
| I | 2, 3, 5 | 50–60 | Crucial for
antiproliferative activity |
| II | 4, 9 | 30 | Tolerant to
substitution |
| III | 7, 8 | 0–5 | Unrelated to
antiproliferative activity |
Discussion
The structure-antiproliferative activity
relationship of dDAVP was assessed in the present study using
Ala-Scan on MDA-MB-231 breast carcinoma cells. The analysis
highlighted the important role of the amino acids located in the
peptide loop (Table III), and
suggested that amino acids 2, 3 and 5 were crucial for the
agonistic interaction of dDAVP with the vasopressin V2 receptor, as
proposed in a previous molecular modeling study (13). This interaction led to the
antitumor effects on V2 receptor-expressing breast cancer cells.
Substitution of residues 4 and 9 only partially reduced the
antiproliferative activity of dDAVP. By contrast, the
antiproliferative activity of the peptide was unaffected by the
substitution of residues 7 and 8, located at the tail of the
peptide, and the same was observed when residue 7 was substituted
by a Hyp residue.
In a recent study, we reported that the analog
4-valine-5-glutamine-dDAVP (known as
[Val4Gln5]dDAVP) was a potent agonist for the
vasopressin V2 receptor in MCF-7 cell cultures (14). Glutamine4
(Gln4) is located in the loop of the dDAVP, and is the
best candidate to be substituted by a hydrophobic residue. As
hypothesized by Manning et al (1), enhancing hydrophobicity at position 4
improves the interaction of vasopressin analogs with the V2
receptor; thus, a Gln by valine (Val) substitution was introduced.
In a separate study, Manning et al (2) also found that [Val4]dDAVP
has a 10-fold higher affinity for the human V2 receptor than dDAVP
(2). In order to improve the
stability of the analog, we therefore introduced a conservative
substitution at position 5, replacing asparagine with Gln, based on
its distinctive susceptibility to the deamidation process, which
resulted in the cytostatic analog
[Val4Gln5]dDAVP (14).
The results from the present study are in accordance
with those from previous studies with regard to the key role of the
N-terminal region of the molecule (loop) in the physiological
activities of the neurohypophyseal hormone vasopressin (1,2,15).
In the present study, it was demonstrated that there is a close
relationship between the loop of dDAVP and its antiproliferative
activity, as assayed on MDA-MB-231 cells. This knowledge may aid
the development of novel strategies for the design of dDAVP analogs
with improved antitumor properties. V2 receptor expression has been
found in a number of types of human cancer, including breast
(16) gastrointestinal (17) and small cell lung (18) cancer. Furthermore, it is known that
dDAVP exerts a specific effect on V2 receptors present in
microvascular endothelial cells, and therefore induces a rapid
release of multimeric forms of von Willebrand factors in
vivo. Such hemostatic factors have a protective role against
tumor cell dissemination, by causing the death of metastatic cells
early after their arrest at the target organ (19,20).
The key roles of the loop of dDAVP and its improved
analog [Val4Gln5]dDAVP warrant further
investigation, including the synthesis and analysis of the cyclic
fragments of both peptides (residues 1–6) to determine the minimal
active sequences. These active sequences may be introduced in
highly stable scaffolds, such as cyclotides, which are cyclic and
knotted peptides with extreme thermal, chemical and enzymatic
stability (21,22). This hypothesis is likely to be the
starting point to encourage the design of cytostatic peptide
compounds with oral bioavailability.
Acknowledgements
The authors would like to thank Chemo-Romikin SA
(Argentina) for their support and the use of their
peptide-synthesis facilities. This study was partially funded by
the National Agency of Science and Technology and Quilmes National
University.
References
|
1
|
Manning M, Stoev S, Chini B, Durroux T,
Mouillac B and Guillon G: Peptide and non-peptide agonists and
antagonists for the vasopressin and oxytocin V1a, V1b, V2 ant OT
receptors: research tools and potential therapeutic agents. Prog
Brain Res. 170:473–512. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Manning M, Misicka A, Olma A, Bankowski K,
Stoev S, Chini B, Durroux T, Mouillac B, Corbani M and Guillon G:
Oxytocin and vasopressin agonists and antagonists as research tools
and potential therapeutics. J Neuroendocrinol. 24:609–628. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
North WG, Fay MJ and Du J: MCF-7 breast
cancer cells express normal forms of all vasopressin receptors plus
an abnormal V2R. Peptides. 20:837–842. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ripoll GV, Giron S, Krzymuski MJ, Hermo
GA, Gomez DE and Alonso DF: Antitumor effects of desmopressin in
combination with chemotherapeutic agents in a mouse model of breast
cancer. Anticancer Res. 28:2607–2611. 2008.PubMed/NCBI
|
|
5
|
Alonso DF, Ripoll GV, Garona J, Iannucci
NB and Gomez DE: Metastasis: recent discoveries and novel
perioperative treatment strategies with particular interest in the
hemostatic compound desmopressin. Curr Pharm Biotech. 12:1974–1980.
2011. View Article : Google Scholar
|
|
6
|
Cunningham BC and Wells JA:
High-resolution epitope mapping of hGH receptor interactions by
alanine-scanning mutagenesis. Science. 244:1081–1085. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Beck-Sickinger AG, Wieland HA, Wittneben
H, Willim KD, Rudolf K and Jung G: Complete L-alanine scan of
neuropeptide Y reveals ligands binding to Y1 and Y2 receptors with
distinguished conformations. Eur J Biochem. 225:947–958. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nicole P, Lins L, Rouyer-Fessard C, Drouot
C, Fulcrand P, Thomas A, Couvineau A, Martínez J, Brasseur R and
Laburthe M: Identification of key residues for interaction of
vasoactive intestinal peptide with human VPAC1 and VPAC2 receptors
and development of a highly selective VPAC1 receptor agonist.
Alanine scanning and molecular modeling of the peptide. J Biol
Chem. 275:24003–24012. 2000. View Article : Google Scholar
|
|
9
|
Quartara L, Ricci R, Meini S, et al: Ala
scan analogues of HOE 140. Synthesis and biological activities. Eur
J Med Chem. 35:1001–1010. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nam J, Shin D, Rew Y and Boger DL: Alanine
scan of [L-Dap(2)]ramoplanin A2 aglycon: assessment of the
importance of each residue. J Am Chem Soc. 129:8747–8755. 2007.
|
|
11
|
Houghten RA: General method for the rapid
solid-phase synthesis of large numbers of peptides: specificity of
antigen-antibody interaction at the level of individual amino
acids. Proc Natl Acad Sci USA. 82:5131–5135. 1985. View Article : Google Scholar
|
|
12
|
Albericio F, Annis I, Royo M and Barany G:
Preparation and handling of peptides containing methionine and
cysteine. Fmoc Solid Phase Peptide Synthesis. A Practical Approach.
Chan WC and White PD: Oxford University Press Inc; New York, NY:
pp. 77–114. 2000
|
|
13
|
Czaplewski C, Kázmierkiewicz R and
Ciarkowski J: Molecular modeling of the human vasopressin V2
receptor/agonist complex. J Comp Aided Mol Des. 12:275–287. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Iannucci NB, Ripoll GV, Garona J, Cascone
O, Ciccia GN, Gomez DE and Alonso DF: Antiproliferative effect of
1-deamino-8-D-arginine vasopressin analogs on human breast cancer
cells. Future Med Chem. 3:1987–1993. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kowalczyk W, Prahl A, Derdowska I,
Sobolewski D, Olejnik J, Zabrocki J, Borovicková L, Slaninová J and
Lammek B: Analogues of neurohypophyseal hormones, oxytocin and
arginine vasopressin, conformationally restricted in the N-terminal
part of the molecule. J Med Chem. 49:2016–2021. 2006. View Article : Google Scholar
|
|
16
|
North WG, Pai S, Friedmann A, Yu X, Fay M
and Memoli V: Vasopressin gene related products are markers of
human breast cancer. Breast Cancer Res Treat. 34:229–235. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Monstein HJ, Truedsson M, Ryberg A and
Ohlsson B: Vasopressin receptor mRNA expression in the human
gastrointestinal tract. Eur Surg Res. 40:34–40. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
North WG, Fay MJ, Longo KA and Du J:
Expression of all known vasopressin receptor subtypes by small cell
tumors implies a multifaceted role for this neuropeptide. Cancer
Res. 58:1866–1871. 1998.PubMed/NCBI
|
|
19
|
Terraube V, Pendu R, Baruch D, Gebbink MF,
Meyer D, Lenting PJ and Denis CV: Increased metastatic potential of
tumor cells in von Willebrand factor-deficient mice. J Thromb
Haemost. 4:519–526. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mochizuki S, Soejima K, Shimoda M, Abe H,
Sasaki A, Okano HJ, Okano H and Okada Y: Effect of ADAM28 on
carcinoma cell metastasis by cleavage of von Willebrand factor. J
Natl Cancer Inst. 104:906–922. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Colgrave ML and Craik DJ: Thermal,
chemical and enzymatic stability of the cyclotide kalata B1: the
importance of the cyclic cystine knot. Biochemistry. 43:5965–5975.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Craik DJ, Cemazar M and Daly NL: The
cyclotides and related macrocyclic peptides as scaffolds in drug
design. Curr Opin Drug Discovery Devel. 9:251–260. 2006.PubMed/NCBI
|