Introduction
Lung cancer is the main cause of cancer-related
mortality worldwide, and is pathologically classified as one of two
types, namely, small cell (15%) or non-small cell (85%) lung cancer
(1). The disease is notoriously fatal
at an advanced stage, with a 5-year survival rate of <15%
(1,2).
Therefore, an improved understanding of the molecular mechanisms
involved in the development of lung cancer is urgently required as
the basis of the identification of novel therapeutic targets and
the development of novel treatment strategies.
The metabolism of cancer cells is significantly
different from that of normally differentiated cells (3). Normally differentiated cells rely
primarily on the oxidation of pyruvate in the mitochondria to
generate energy for cellular physiological functions; however, even
with sufficient oxygen, rapidly growing cancer cells rely on
aerobic glycolysis to generate energy (4). This phenomenon is termed ‘the Warburg
effect’ (5). This metabolic shift
towards enhanced glycolysis is hypothesized to be the result of
adaptations to support the continuous proliferation of cancer
cells. The upregulation of specific glucose transporter 1 (GLUT1)
may represent a key mechanism by which malignant cells may achieve
increased glucose uptake to support the high rate of glycolysis.
There is a growing body of evidence indicating that reprogrammed
cancer metabolism is a potential therapeutic target (5).
microRNAs (miRs/miRNAs) are an evolutionarily
conserved group of small (18–24-nucleotide) non-coding RNAs, which
suppress gene expression in a sequence-specific manner (6). miRNAs negatively regulate the expression
of target genes through complementarity between the miRNA seed
sequence and the 3′-untranslated region (UTR) of the target mRNA
(6). Target mRNA is degraded when
miRNAs bind with exact complementarity to the protein-encoding
messenger RNA (mRNA), while mRNA translation is repressed when
miRNAs exhibit inexact complementarity to the 3′UTR of the target
mRNA (7).
miRNAs are now known as essential regulators of
development and physiological processes (7). By regulating oncogenic and tumor
suppressor signal pathways, miRNAs may also play significant roles
in cancer development. Recently, a large number of miRNAs were
identified as important natural regulators of metabolism (8). For example, miR-33a and miR-33b play a
key role in regulating fatty acid degradation and cholesterol
homeostasis (9), while numerous
miRNAs, such as miR-21, miR-143, miR-130 and miR-27a, are reported
to be involved in white-adipocyte differentiation. Furthermore, the
inhibition of miR-122 is believed to decrease plasma triglyceride
and cholesterol concentrations (10).
An increasing level of attention has been conferred
on the role of microRNA-144 in tumorigenesis and the treatment of
cancer. A number of studies have reported miR-144 downregulation in
a range of cancer types, including osteosarcoma and mesothelioma,
indicating that the miRNA is a potential tumor suppressor (11,12). In
particular, one study revealed an inverse correlation between the
level of miR-144 and the development of gastric cancer (13), while another study reported an
elevated miR-144 level in colorectal cancer (14). A study by Fu et al showed that
cell proliferation, migration and invasion is promoted by miR-144
in nasopharyngeal carcinoma by the suppression of PTEN expression
(15). Consequently, miR-144 appears
to have a complex and highly tissue-specific function in
tumorigenesis and cancer development.
The direct role of miR-144 in lung cancer cells has
not yet been reported. In the present study, it was found that
miR-144 is significantly decreased in lung cancer. The loss of
miR-144 function enhanced the proliferation of the tumor cells via
increased glycolysis through the direct targeting of the 3′-UTR of
GLUT1.
Materials and methods
Cell culture
A549 and HEK293T cells were purchased from the
American Type Culture Collection and cultured in Dulbecco' modified
Eagle's medium (DMEM) supplemented with 10% fetal bovine serum
(FBS; Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA,
USA), 4.5 g/l glucose and 100 U/ml penicillin/streptomycin in a
tissue incubator maintained at 37°C.
Transfections
All transfections were performed using Lipofectamine
2000 (Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocols. The miR-144 mimics or inhibitor and the
negative controls were purchased from GenePharma (Shanghai, China).
The miR-144 mimics and miR-144 inhibitor were used at a final
concentration of 50 nM.
Luciferase reporter assay
For the luciferase reporter assay, HEK293T cells
were seeded in a 24-well plate and were grown to 80–90% confluence.
To detect the interaction between miR-497 and GLUT1 3′UTR, the
cells were cotransfected with 50 nM of either scramble or miR-497
mimics and 40 ng of either pmirGLO-GLUT1-3′UTR-wild-type (WT) or
pmirGLO-GLUT1-3′UTR-mutant (MUT) using Lipofectamine 2000 according
to the manufacturer's protocols. The cells were collected 48 h
after transfection and analyzed using the
Dual-Luciferase® Reporter Assay system (Promega
Corporation, Madison, WI, USA). A plasmid constitutively expressing
Renilla luciferase was cotransfected as an internal control to
correct for differences in transfection and harvesting
efficiencies. The transfections were performed in duplicate, and at
least three independent experiments were performed.
Western blotting
Protein was harvested with radioimmunoprecipitation
assay buffer (Sigma-Aldrich, St. Louis, MO, USA), quantified and
then resolved on a 1% sodium dodecyl sulfate (SDS)-polyacrylamide
gel electrophoresis gel. The protein was then transferred onto
nitrocellulose membrane, which was blocked in 5% non-fat milk and
incubated with the following primary antibodies: Rabbit monoclonal
anti-β-actin (1:5,000; catalog no. 4970), rabbit monoclonal
anti-GLUT1 (1:1,000; catalog no. 12939) (Cell Signaling Technology,
Inc., Danvers, MA, USA). After being washed, the membranes were
incubated with hydrogen peroxide and alkaline
phosphatase-conjugated secondary antibodies (Abcam, Cambridge, UK).
The proteins were detected by chemiluminescence using Bio-Rad Gel
Imaging system (ChemiDoc MP System; Bio-Rad Laboratories, Hercules,
CA, USA) and Pierce™ ECL Western Blotting Substrate (Thermo Fisher
Scientific, Inc.).
Cell viability assays
A total of 5,000 cells/well were seeded into a
96-well plate and transfected with miR-144 mimics or miR-144
inhibitor (final concentration, 50 nm). Following incubation for 48
h at 37°C, the cells were labeled with Cell Counting kit-8 (CCK8)
(Dojindo, Tabaru, Japan) for 1 h. Cell viability was measured at an
absorbance of 450 nm using Synergy™ H4 Hybrid Multi-mode Microplate
Reader (BioTek Instruments, Inc., Winooski, VT, USA).
Measurement of extracellular
lactate
In total, 5×105 cells were seeded into
60-nm dishes and transfected with miR-144 mimics or miR-144
inhibitor, then incubated in DMEM with 10% FBS overnight. Next, the
media was removed, and the cells were incubated in DMEM without
FBS. Subsequent tot incubation for 1 h, the supernatant was
collected. The concentration of lactate in the supernatant was
measured using a colorimetric assay according to the manufacturer's
protocols (Lactate Assay kit; BioVision Inc., Milpitas, CA,
USA).
RNA extraction and reverse
transcription quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from A549 cells using TRIzol
Reagent (Invitrogen; Thermo Fisher Scientific, Inc.) in accordance
with the manufacturer's protocol. miR expression was measured using
a TaqMan MicroRNA Assay (Invitrogen; Thermo Fisher Scientific,
Inc.), according to the manufacturer's protocol and treated with
DNase (1 µg RNA per 5 U DNase) (Invitrogen; Thermo Fisher
Scientific, Inc.). cDNA was synthesized from 200 ng of total RNA
using the Universal cDNA Synthesis kit (Exiqon, Vedbaek, Denmark)
in a 15 µl reaction. miRNA expression was measured using a
TaqMan® MicroRNA Assay (Invitrogen; Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol. cDNAs
were then amplified using qPCR with TaqMan miRNA Assays Human Panel
sequence-specific primers. PCR was performed in an Applied
Biosystems 7500 Fast Real-Time PCR System (Invitrogen; Thermo
Fisher Scientific, Inc.) under the following conditions: 95°C for
60 sec, followed by 40 cycles of 95°C for 5 sec and 60°C for 40
sec. All samples were normalized to the internal control and fold
changes were calculated using the 2−ΔΔCq quantification
method (16). The expression of RNU48
served as an internal control. The primers used for miR-144 and
RNU48 (Sigma-Aldrich) were as follows: miR-144, forward
5′-TACAGTATAGATGAT-3′ and reverse 5′-GTGCAGGGTCCGAGGT-3′; RNU48
forward 5′-TGATGATGACCCCAGGTAACTC-3′ and reverse
5′-GAGCGCTGCGGTGATG-3′ (2,17).
Measurement of glucose uptake
Following 12 h of serum starvation, the cells were
cultured in DMEM containing 25 mM glucose. To measure the rate of
glucose uptake, the cells were washed three times with
phosphate-buffered saline (PBS) and then incubated for 3 h in DMEM
containing 1 mCi/ml 2-deoxy-D-[1,2-3H]-glucose (PerkinElmer Inc.,
Waltham, MA, USA). The cells were washed three times with ice-cold
PBS and solubilized in 1% SDS. A scintillation counter (Beckman LS
6000SC Scintillation Counter; Beckman Coulter, Brea, CA, USA) was
used to determined the radioactivity of each aliquot. Each assay
was performed in triplicate.
Statistical analysis
Statistical analysis was performed with SPSS version
17 software (SPSS Inc., Chicago, IL, USA). Analysis of variance
were used to determined statistical significance. P<0.05 was
used to indicate a statistically significant difference.
Results
miR-144 expression is decreased in
lung cancer tissue
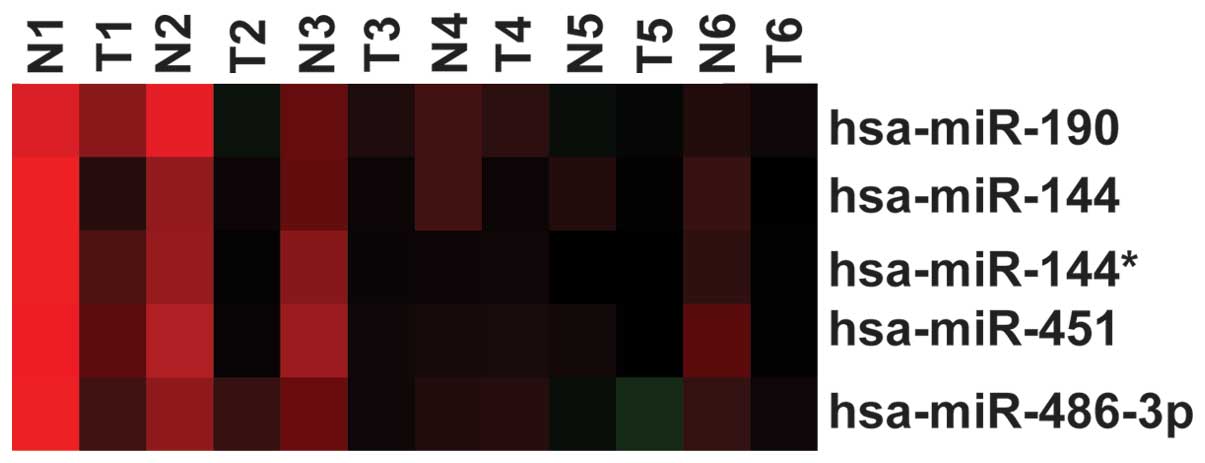
First, a previously published dataset (ArrayExpress
Accession no. E-MTAB-113; www.ebi.ac.uk/arrayexpress/) was downloaded, and the
expression of miR-144 was analyzed in lung cancer tissue; the
result showed that miR-144 was decreased in the lung cancer tissues
compared with the normal lung tissues (P=0.0075) (Fig. 1). This result was consistent with that
of the study by Zha et al (2).
miR-144 inhibits the proliferation of
A549 cells
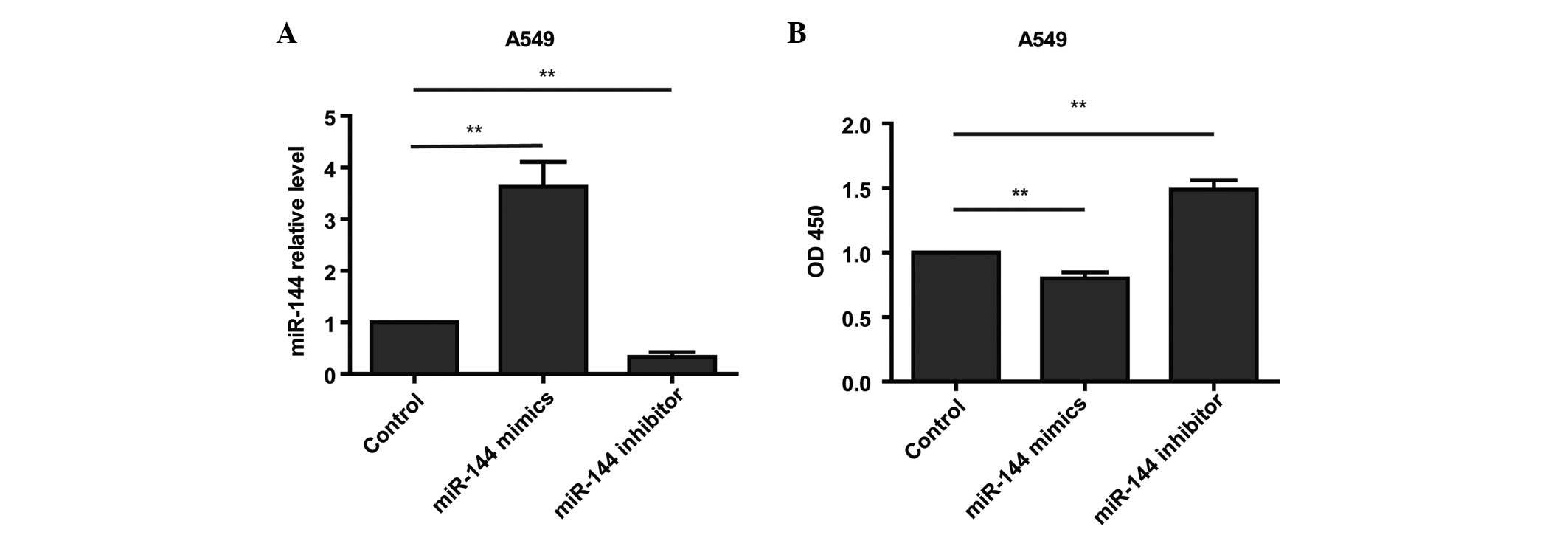
To assess the role of miR-144 in the growth of
glioma cells, miR-144 mimic or miR-144 inhibitor was transfected
into A549 cells at a final concentration of 50 nM. The RT-qPCR
analysis revealed that miR-144 expression was markedly and
specifically increased in the mimic-transfected cells and decreased
in the inhibitor-transfected cells (Fig.
2A). A CCK8 assay showed that cell proliferation was decreased
in the miR-144-overexpressing A549 cells compared with the control
cells (Fig. 2B). The study then
investigated whether reducing miR-144 in the A549 cells enhanced
cell proliferation. As expected, the loss of miR-144 function in
the A549 cells treated with miR-144 inhibitor increased cell
proliferation (Fig. 2B). Taken
together, these results suggested that miR-144 plays a role as a
tumor suppressor in A549 cell proliferation.
Decreased expression of miR-144
induces a metabolic shift in A549 cells
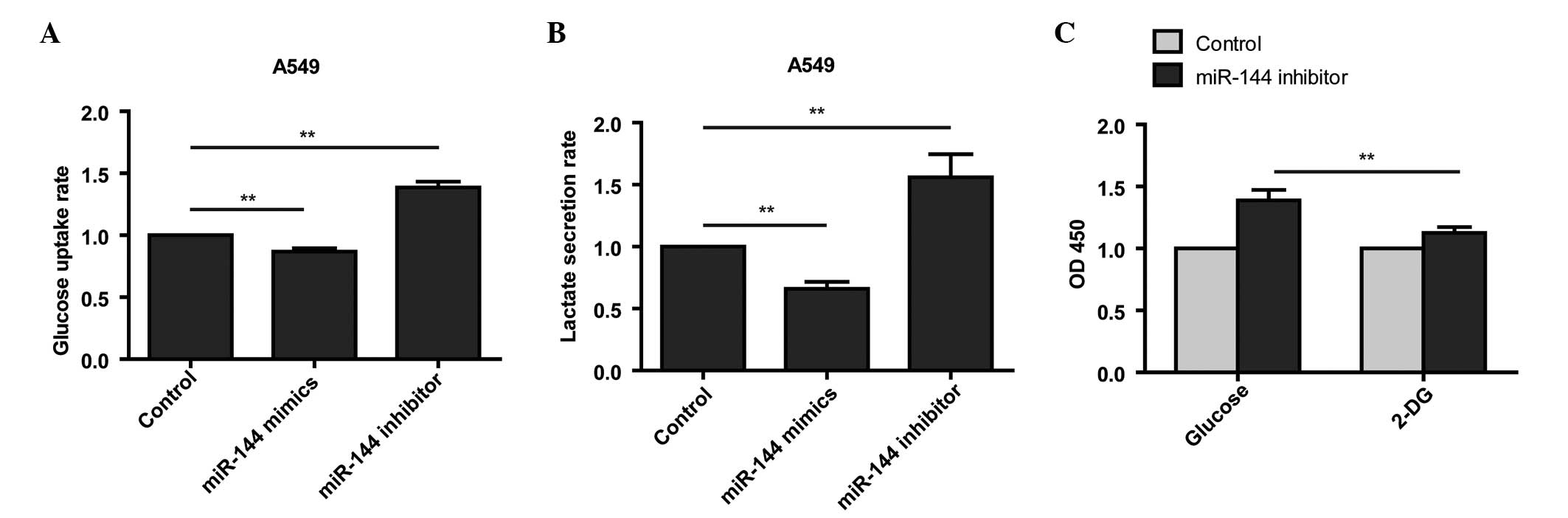
The present study next sought to investigate whether
miR-144 was capable of inducing a metabolic shift in the A549
cells. Loss of miR-144 function in the A549 cells was found to
increase glucose uptake and lactate secretion (Fig. 3A and B). Conversely, gain of miR-144
function in A549 cells treated with mimics reduced glucose uptake
and lactate secretion (Fig. 3A and
B).
To detect whether the miR-144-induced metabolic
switch is required for miR-144-dependent cell proliferation,
miR-144 inhibitor-transfected A549 cells were administered
2-deoxyglucose (2-DG), a glucose analog that inhibits glycolysis by
its action on hexokinase, and analyzed cell proliferation with a
CCK8 assay. The results demonstrated that inhibition of glycolysis
induced by loss of miR-144 function is sufficient to block cell
proliferation (Fig. 3C). Taken
together, these results suggested that miR-144-induced glycolysis
is responsible for the enhanced cell proliferation in
miR-144-knockdown cells.
miR-144 suppresses the expression of
GLUT1 by targeting its 3′-UTR
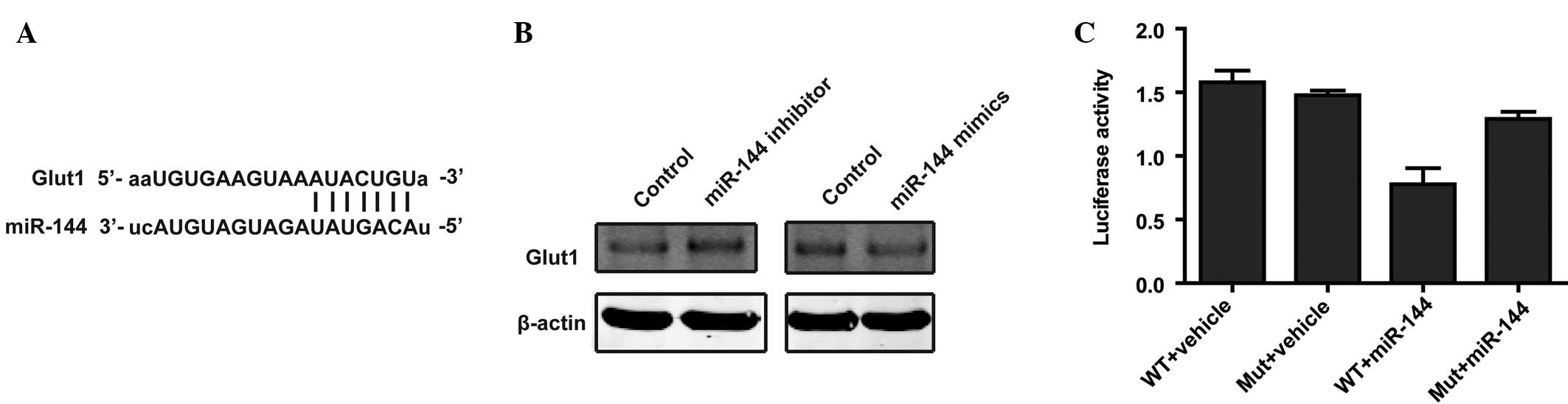
Two different mRNA target-predicting algorithms
(TargetScan and miRanda (18,19) were used to identify potential direct
targets of miR-144 based on binding sites in the 3′UTR. Candidate
genes that are involved in glycolysis were selected. mRNA
target-predicting algorithms identified an area within the 3′-UTR
of GLUT1 as a potential target of miR-144 (Fig. 4A). Western blot analysis confirmed
that the overexpression of miR-144 decreased the protein level of
GLUT1 (Fig. 4B), while knockdown of
miR-144 increased the protein level of GLUT1 (Fig. 4B). To further determine whether GLUT1
is a direct target of miR-144, a luciferase assay was performed.
The results showed that miR-144 effectively suppressed the
luciferase reporter activity of the WT vector, but that this
decreased luciferase activity was reversed when cells were
transfected with the MUT vector (Fig.
4C). Taken together, these results demonstrated that miR-144
directly regulates GLUT1 expression via binding sites in the
3′-UTR.
Discussion
miRNAs have attracted an increasing level of
attention due to their key role in a number of biological events,
including cell proliferation, cell motility, differentiation,
metabolic switch and apoptosis (7).
In recent years, the dysregulation of miR-144 has been associated
with several types of human cancer. Significant miR-144
downregulation has been reported in osteosarcoma and mesothelioma
(11,12). miR-144 acts as a tumor suppressor in
uveal melanoma by inhibiting cell proliferation and migration
(20). Chen et al reported
that downregulation of miR-144 in lung cancer inhibits
proliferation and induces apoptosis and autophagy by targeting
TP53-inducible glycolysis and apoptosis regulator (21). However, the function of miR-144 in
lung tumorigenesis remains unclear.
In the present study, it was found that miR-144
expression was decreased in lung cancer compared with adjacent
normal tissues. Previous studies have demonstrated that miR-144
acts as a tumor suppressor to inhibit cell proliferation in
osteosarcoma and mesothelioma (11,12). The
results of the CCK8 assay in the present study also indicated that
miR-144 decreased cell proliferation.
The molecular mechanisms responsible for shifting
energy metabolism to the advantage of cancer cells are complicated
and only partially understood (22).
In the present study, it was found that miR-144 plays a significant
role in regulating the metabolism of lung cancer cells. Knockdown
of miR-144 increased glucose uptake rate and lactate secretion,
while overexpression of miR-144 decreased glucose uptake and
lactate secretion. The altered metabolism induced by miR-144 was
required for lung cancer cell proliferation. Moreover, it was found
that GLUT1 is a direct target of miR-144. The induction of GLUT1
enhanced aerobic glycolysis in the cancer cells. Dysregulation of
miR-144 in lung cancer cells requires extensive investigation
concerning its role in cancer cell metabolism.
In summary, the present study provides direct
evidence that the decrease of miR-144 in lung cancer cells promotes
cell glycolysis by increasing GLUT1 expression, thus enhancing cell
growth. These results identify miR-144 as a molecular switch
involved in the orchestration of the Warburg effect in lung cancer
cells and as a potential therapeutic target for lung cancer.
References
|
1
|
Mehan MR, Ayers D, Thirstrup D, Xiong W,
Ostroff RM, Brody EN, Walker JJ, Gold L, Jarvis TC, Janjic N, et
al: Protein signature of lung cancer tissues. PLoS One.
7:e351572012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zha W, Cao L, Shen Y and Huang M: Roles of
Mir-144-ZFX pathway in growth regulation of non-small-cell lung
cancer. PloS One. 8:e741752013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kroemer G and Pouyssegur J: Tumor cell
metabolism: Cancer's Achilles' heel. Cancer Cell. 13:472–482. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Muñoz-Pinedo C, El Mjiyad N and Ricci JE:
Cancer metabolism: Current perspectives and future directions. Cell
Death Dis. 3:e2482012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Vander Heiden MG, Cantley LC and Thompson
CB: Understanding the Warburg effect: the metabolic requirements of
cell proliferation. Science. 324:1029–1033. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bartel DP: MicroRNAs: Target recognition
and regulatory functions. Cell. 136:215–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jansson MD and Lund AH: MicroRNA and
cancer. Mol Oncol. 6:590–610. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rottiers V and Näär AM: MicroRNAs in
metabolism and metabolic disorders. Nat Rev Mol Cell Biol.
13:239–250. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rayner KJ, Suárez Y, Dávalos A, Parathath
S, Fitzgerald ML, Tamehiro N, Fisher EA, Moore KJ and
Fernández-Hernando C: MiR-33 contributes to the regulation of
cholesterol homeostasis. Science. 328:1570–1573. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wilfred BR, Wang WX and Nelson PT:
Energizing miRNA research: A review of the role of miRNAs in lipid
metabolism, with a prediction that miR-103/107 regulates human
metabolic pathways. Mol Genet Metab. 91:209–217. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Namløs HM, Meza-Zepeda LA, Barøy T,
Østensen IH, Kresse SH, Kuijjer ML, Serra M, Bürger H,
Cleton-Jansen AM and Myklebost O: Modulation of the osteosarcoma
expression phenotype by microRNAs. PloS One. 7:e480862012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Guled M, Lahti L, Lindholm PM, Salmenkivi
K, Bagwan I, Nicholson AG and Knuutila S: CDKN2A, NF2 and JUN are
dysregulated among other genes by miRNAs in malignant
mesothelioma-A miRNA microarray analysis. Genes Chromosomes Cancer.
48:615–623. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Akiyoshi S, Fukagawa T, Ueo H, Ishibashi
M, Takahashi Y, Fabbri M, Sasako M, Maehara Y, Mimori K and Mori M:
Clinical significance of miR-144-ZFX axis in disseminated tumour
cells in bone marrow in gastric cancer cases. Br J Cancer.
107:1345–1353. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kalimutho M, Del Vecchio Blanco G, Di
Cecilia S, Sileri P, Cretella M, Pallone F, Federici G and
Bernardini S: Differential expression of miR-144* as a novel
fecal-based diagnostic marker for colorectal cancer. J
Gastroenterol. 46:1391–1402. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang LY, Ho-Fun Lee V, Wong AM, Kwong DL,
Zhu YH, Dong SS, Kong KL, Chen J, Tsao SW, Guan XY and Fu L:
MicroRNA-144 promotes cell proliferation, migration and invasion in
nasopharyngeal carcinoma through repression of PTEN.
Carcinogenesis. 34:454–463. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pfaffl MW: A new mathematical model for
relative quantification in real-time RT-PCR. Nucleic Acids Res.
29:e452001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen C, Ridzon DA, Broomer AJ, Zhou Z, Lee
DH, Nguyen JT, Barbisin M, Xu NL, Mahuvakar VR, Andersen MR, et al:
Real-time quantification of microRNAs by stem-loop RT-PCR. Nucleic
Acids Res. 33:e1792005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lewis BP, Burge CB and Bartel DP:
Conserved seed pairing, often flanked by adenosines, indicates that
thousands of human genes are microRNA targets. Cell. 120:15–20.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Betel D, Wilson M, Gabow A, Marks DS and
Sander C: The microRNA.org resource: Targets and expression.
Nucleic Acids Res. 36(Database issue): D149–D153. 2008.PubMed/NCBI
|
|
20
|
Sun L, Bian G, Meng Z, Dang G, Shi D and
Mi S: MiR-144 inhibits uveal melanoma cell proliferation and
invasion by regulating c-Met expression. PLoS One. 10:e01244282015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen S, Li P, Li J, Wang Y, Du Y, Chen X,
Zang W, Wang H, Chu H, Zhao G and Zhang G: MiR-144 inhibits
proliferation and induces apoptosis and autophagy in lung cancer
cells by targeting TIGAR. Cell Physiol Biochem. 35:997–1007. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhao Y, Butler EB and Tan M: Targeting
cellular metabolism to improve cancer therapeutics. Cell Death Dis.
4:e5322013. View Article : Google Scholar : PubMed/NCBI
|