Introduction
The use of ascorbic acid (A) as an anti-cancer agent
has been analyzed in the last 50 years (1,2). Previous
epidemiological studies have demonstrated that dietary
administration of A exerts a preventive effect on multiple tumors
(1,2).
However, Cameron and Pauling (3) have
argued its role as a therapeutic anti-cancer agent, in agreement
with previous studies disproving its potential role as an
anti-cancer agent (4,5). These studies were performed
supplementing the diet with high doses of orally administered
vitamin C, which allows to reach saturation at plasma
concentrations of 1 g/day, while higher doses of vitamin C are
excreted (6,7). Blood concentrations of vitamin C at mM
levels are only possible to obtain via intravenous injections of
high doses of the compound (8,9). In recent
years, the interest on A and its effects on cancer growth has
increased (10). A has been recently
demonstrated to be highly effective in cancer therapy in
association with commonly used chemotherapeutic agents in ovarian
tumors (11).
Similarly to the case of A, the role of potassium
(K) in cancer has remained unclear since Cone (12) reported in 1971 higher levels of
Na+ and lower levels of K+, Ca2+
and Zn in cancer cells, compared with healthy cells. K+
is capable of acting as an anti-apoptotic and as a pro-apoptotic
agent (13,14), and is also a regulator of cell
proliferation (15–17). The intracellular homeostasis of
Na+ and K+ is disregulated in cancer cells
(18–20). Altered expression and activity of
Na+/K+ adenosine triphosphate (ATP)ase has
been observed in cancer cells, which may explain the differences in
concentration of Na+ and K+ observed in these
cells, compared with normal cells (21,22).
Furthermore, K+ is essential to fold and stabilize
G-quadruplexes (23). Agents that
stabilize or target G-quadruplexes may act as anti-tumor agents
(24); thus, physiological
concentrations of K+ are likely to be required for
normal cell behavior (25,26). K ascorbate has been proposed as an
anti-degenerative agent (27).
Previous studies reported a strong antioxidant effect of K
ascorbate on red blood cell oxidation (28,29). In
addition, it has been suggested that K ascorbate may act as a K
intracellular carrier and may be able to inhibit the cell cycle in
tumor cells (30).
In order to clarify the potential role of K
ascorbate as an anti-tumor agent, the effects of A, K and A+K on
different breast cancer cell lines were analyzed in the present
study.
Materials and methods
Reagents
K bicarbonate and A were obtained from AEIE
Biochemical Research (Oviedo, Spain). Sulforhodamine B (SRB) was
purchased from Sigma-Aldrich (St. Louis, MO, USA). Rabbit
anti-human polyclonal B-cell lymphoma-2 (Bcl-2)-associated X
protein (Bax; cat. no. 554104) and mouse anti-human monoclonal
Bcl-2 (cat. no. 610538) antibodies were obtained from Transduction
Laboratories (BD Biosciences, Franklin Lakes, NJ, USA). Anti-human
mouse monoclonal p53 (cat. no. sc-126), rabbit anti-rat polyclonal
extracellular signal-regulated kinase (ERK)1/2 (C-14; cat. no.
sc-154), mouse anti-human monoclonal phosphorylated (p)-ERK (E-4,
which recognizes the phosphorylated and active form of ERK1 and
ERK2; cat. no. sc-7383), nuclear factor (NF)-κB p65 (cat. no.
sc-8008) and mouse anti-human monoclonal poly(adenosine
diphosphate-ribose) polymerase-1 (PARP-1; F-2; cat. no. sc-8007)
antibodies were obtained from Santa Cruz Biotechnology, Inc.
(Dallas, TX, USA). Rabbit anti-human polyclonal antibody against
actin (cat. no. A5060) and peroxidase-conjugated goat anti-mouse
polyclonal (cat. no. A2554) or anti-rabbit polyclonal (cat. no.
A6154) immunoglobulin (Ig)G were obtained from Sigma-Aldrich.
Cell lines and treatments
Human breast cancer cell lines MDA-MB-231,
MDA-MB-453, MDA-MB-468, T47-D and MCF-7 were maintained in
Dulbecco's modified Eagle medium - high glucose containing 10%
fetal bovine serum, 100 U/ml penicillin and 100 µg/ml streptomycin
(complete medium) (all purchased from Aurogene, Rome, Italy). Cells
were cultured at 37°C in a humidified incubator with 5%
CO2.
For treatments, cells were incubated for the
indicated times in the presence of K and A, alone or in combination
(A+K), or in the presence of culture medium (CTRL). The stock
solutions (150 mM) of K, A and A+K were obtained by diluting K and
A, alone or in combination, in distilled water. Thus, K stock
solution was obtained by dissolving 300 mg K in 20 ml distilled
water; A stock solution was obtained by dissolving 150 mg A in 20
ml distilled water; and A+K stock solution was obtained combining
300 mg K and 150 mg A in 20 ml distilled water. The concentrations
used for cell treatment were reached by diluting the stock
solutions in complete CTRL.
SRB cell proliferation assay
Cells were seeded in 96-well plates at a density of
5×103 cells/well, and incubated at 37°C to allow cell attachment.
Following 24 h, the medium was changed, and the cells were treated
with K and A, alone or in combination (A+K), at a dose range of
1.5–15 mM, or with CTRL, and incubated for 24, 48 or 72 h.
Subsequently, cells were fixed with cold trichloroacetic acid
(final concentration, 10%; Sigma-Aldrich) for 1 h at 4°C. Following
4 washes with distilled water, the plates were air-dried and
stained for 30 min with 0.4% (w/v) SRB in 1% acetic acid
(Sigma-Aldrich). Following 4 washes with 1% acetic acid to remove
the unbound dye, the plates were air-dried, and cell-bound SRB was
dissolved with 10 mM unbuffered Tris base solution (100 µl/well;
Sigma-Aldrich). The optical density (OD) of the samples was
determined at 540 nm using a spectrophotometric plate reader
(Wallac 1420 Victor; Perkin Elmer Inc., Waltham, MA, USA). The
percentage survival of the cultures treated with the aforementioned
compounds was calculated by normalizing their OD values to those of
the control cultures (31,32). The experiments were performed in
triplicate and repeated three times.
Fluorescence-activated cell sorting
(FACS) analysis
Asynchronized, log-phase growing cells (60%
confluent, ~1.5×105 cells/well in 6-well plates) were treated with
10 mM K and A, alone or in combination (A+K), or with CTRL.
Following 24 h, adherent and suspended cells were harvested,
centrifuged (5417R centrifuge; Eppendorf, Hamburg, Germany) at 300
× g for 10 min and washed twice with cold phosphate-buffered saline
(PBS). Cell pellets were resuspended in 70% ethanol and incubated
for 1 h at −20°C. Cells were then washed twice with cold PBS,
centrifuged at 300 × g for 10 min, incubated for 1 h in the dark
with propidium iodide (Sigma-Aldrich) at a final concentration of
25 µg/ml in 0.1% citrate (Sigma-Aldrich) and 0.1% Triton X-100
(Sigma-Aldrich), and analyzed by flow cytometry using a
FACSCalibur™ cytometer (BD Biosciences) with CellQuest Pro 5.2
software (BD Biosciences) (31,32).
Preparation of cell lysates and
western blotting
Cells (~1×106 cells/plate) were seeded into 100-mm
tissue culture plates for 24 h prior to the addition of 10 mM K and
A, alone or in combination (A+K), or CTRL. MCF-7 and MDA-MB-231
cells were selected for western blotting analysis of signaling
pathway proteins as these two cell lines were most sensitive to A+K
treatment. Following incubation for 24-h, the MCF-7 and MDA-MB-231
cells were harvested, washed twice with cold PBS and lysed in
radioimmunoprecipitation assay lysis buffer [1% Triton X-100, 0.1%
sodium dodecyl sulfate (SDS), 200 mM NaCl, 50 mM Tris-HCl pH 7.5, 1
mM phenylmethylsulfonyl fluoride and 1 mM
Na3VO4]. Following 30-min incubation at 4°C,
the mixtures were centrifuged at 12,000 × g for 15 min, and the
supernatants were analyzed by western blotting (33,34).
Handcast gels were prepared from acrylamide and bisacrylamide
monomer solutions (cat. no. A3574; Sigma-Aldrich). SDS-PAGE and
western blot analysis were performed using Mini-PROTEAN Tetra Cell
apparatus (Bio-Rad, Milan, Italy) according to the manufacturer's
instructions. Electrophoresis (cat. no. 161-0732) and blot transfer
(cat. no. 161-0734) buffers were purchased from Bio-Rad. Hyper PAGE
prestained markers (cat. no. BIO-33066; Bioline, London, UK) were
used. For immunoblotting analysis, 50 µg cell lysates were resolved
in 10% SDS-polyacrylamide gel electrophoresis (150 V for 1 h), and
then transferred to nitrocellulose membranes (30 V for 90 min; GE
Healthcare, Piscataway, NJ, USA) (35). After blocking with 5% skimmed dry milk
in PBS overnight at 4°C, the membranes were incubated overnight at
4°C with specific primary antibodies at a concentration of 1–2
µg/ml. Upon washing, the membranes were incubated with
peroxidase-conjugated goat anti-mouse or anti-rabbit IgG antibodies
diluted in PBS for 1 h at room temperature, and developed by
chemiluminescence (LiteABlot Plus; Euroclone, Milan, Italy), as
previously described (36–38). Densitometric analysis of the
autoradiographic bands was performed using ImageJ 1.42q software
(National Institutes of Health, Bethesda, MD, USA) following blot
scanning (HP Scanjet 4890 Photo Scanner; Hewlett-Packard, Palo
Alto, CA, USA) (39).
Statistical analysis
Data distribution of cell survival and FACS analyses
were initially verified by the Kolmogorov-Smirnov test, and data
sets were analyzed by one-way analysis of variance, followed by
Newman-Keuls test. Differences between the intensity of
immunoreactive bands were evaluated by two-tailed Student's t test.
P≤0.05 was considered to indicate a statistically significant
difference.
Results
Inhibition of breast cancer cell lines
survival by K and A, alone or in combination
Survival of breast cancer cell lines (MDA-MB-231,
MDA-MB-453, MDA-MB-468, T47-D and MCF-7) was evaluated by SRB assay
following exposure to increasing doses (1.5, 5, 10 and 15 mM) of K
and A, alone or in combination (A+K), for 24, 48 and 72 h (Table I). The effect of the compounds on cell
growth was determined and compared with the growth of cells
incubated with CTRL. The effect of A was dose- and time-dependent
on all cell lines, with the exception of MDA-MB-231. K
significantly inhibited cell growth of MCF-7 cells only at the
highest concentration tested following 48-h incubation. The effect
obtained with equimolar combinations of A+K was significantly
higher than the effect of treatment with the highest concentration
tested of A on MCF7 (P<0.01), MDA-MB-231 (P<0.05 at 10 mM for
48 h; P<0.001 at 15 mM for 48 h and 10–15 mM for 72 h) and
MDA-MB-453 (P<0.05 at 10–15 mM for 48 h; P<0.01 at 10 mM for
72 h; P<0.001 at 15 mM for 72 h) cells following incubation for
48 or 72 h. The effect obtained with equimolar combinations of A+K
was significantly higher than the effect obtained with 10 mM
(P<0.01) and 15 mM (P<0.001) A on T47-D cells following
incubation for 24 or 72 h, respectively. Conversely, the effects
with A+K were not significantly different from those obtained with
A in MDA-MB-468 cells. In addition, treatment with A was more
effective than treatment with A+K on MDA-MB-468 cells at the lowest
concentration tested. Overall, these results indicate that there is
a heterogeneous response of the different cell lines toward the
treatment with A and/or K, and revealed that the maximum effect was
achieved with the combined treatment A+K.
 | Table I.Effects of K and A, alone or in
combination, on the survival of breast cancer cells. |
Table I.
Effects of K and A, alone or in
combination, on the survival of breast cancer cells.
|
|
| 15 mM | 10 mM | 5 mM | 1.5 mM |
|---|
|
|
|
|
|
|
|
|---|
| Cell line | Treatment | Mean % cell growth
(±SD) | P-value | Mean % cell growth
(±SD) | P-value | Mean % cell growth
(±SD) | P-value | Mean % cell growth
(±SD) | P-value |
|---|
| MCF-7 | K | 24 h | 90±7 | – |
96±1 | – |
98±4 | – | 100±0 | – |
|
| K | 48 h | 85±1 | 1a |
91±5 | – |
90±0 | – |
93±2 | – |
|
| K | 72 h | 99±2 | – | 100±0 | – | 100±0 | – | 100±0 | – |
|
| A | 24 h | 70±5 | 1a |
85±1 | – |
93±9 | – |
96±6 | – |
|
| A | 48 h | 55±4 | 1c, 2c |
69±9 | 1b, 2a |
84±6 | – |
92±3 | – |
|
| A | 72 h | 30±4 | 1c, 2c |
70±2 | 1c, 2c |
99±2 | – | 100±0 | – |
|
| A+K | 24 h | 52±6 |
1c, 2b |
78±3 | 1a |
95±4 | – |
97±5 | – |
|
| A+K | 48 h | 37±5 | 1c, 2c, 3b |
64±8 | 1c, 2b |
84±2 | – |
87±1 | – |
|
| A+K | 72 h | 25±1 | 1c, 2c |
48±3 | 1c, 2c, 3b |
90±4 | – | 100±1 | – |
| T47-D | K | 24 h | 95±5 | – | 100±0 | – | 100±1 | – |
99±1 | – |
|
| K | 48 h | 96±0 | – |
96±6 | – |
98±1 | – |
99±2 | – |
|
| K | 72 h | 95±0 | – |
99±2 | – | 100±0 | – | 100±0 | – |
|
| A | 24 h | 75±6 | 1b, 2a |
87±1 | – |
90±4 | 1c, 2b |
97±5 | – |
|
| A | 48 h | 30±7 | 1c, 2c |
68±7 | 1c, 2c |
92±0 |
| 100±1 | – |
|
| A | 72 h | 13±0 | 1c, 2c, |
28±4 | 1c, 2c |
87±4 | – |
96±6 | – |
|
| A+K | 24 h | 36±4 | 1c, 3c |
77±7 | 1c, 2b |
89±4 | 1c, 2b |
96±3 | – |
|
| A+K | 48 h | 25±3 | 1c, 2c |
61±6 | 1c 2c |
83±4 | 1c, 2a |
98±2 | – |
|
| A+K | 72 h | 11±1 | 1c, 2c |
15±2 | 1c, 2c, 3b |
98±4 | – |
95±7 | – |
| MDA-MB-231 | K | 24 h | 93±10 | – |
92±4 | – |
95±5 | – |
95±7 | – |
|
| K | 48 h | 91±8 | – |
92±8 | – |
93±6 | – |
90±8 | – |
|
| K | 72 h | 94±1 | – |
96±6 | – |
99±2 | – |
93±0 | – |
|
| A | 24 h | 80±5 | – |
95±6 | – |
97±1 | – |
93±7 | – |
|
| A | 48 h | 67±7 | 1c, 2b |
92±6 | – |
96±7 | – |
98±2 | – |
|
| A | 72 h | 60±2 | 1c, 2c |
92±6 | – |
94±1 | – |
97±1 | – |
|
| A+K | 24 h | 46±10 | 1c, 3c |
76±11 | 1c, 3b, 2a |
93±3 | – |
94±7 | – |
|
| A+K | 48 h | 29±8 | 1c, 3c |
73±7 | 1c, 2b, 3a |
92±9 | – |
90±10 | – |
|
| A+K | 72 h | 18±8 | 1c, 3c |
59±3 | 1c, 3c |
93±10 | – |
95±7 | – |
| MDA-MB-453 | K | 24 h |
95±7 | – | 100±1 | – | 100±1 | – | 100±0 | – |
|
| K | 48 h |
99±2 | – | 100±0 | – |
96±6 | – | 100±0 | – |
|
| K | 72 h | 100±0 | – | 100±0 | – | 100±0 | – | 100±0 | – |
|
| A | 24 h |
74±4 | 1a |
85±9 | – |
92±7 | – |
96±6 | – |
| MDA-MB-453 | A | 48 h |
47±4 | 1c, 2c |
56±4 | 1c, 2c |
74±3 | 1a |
90±14 | – |
|
| A | 72 h |
37±1 | 1c, 2c |
47±9 | 1c, 2c |
74±4 | 1c, 2a |
98±3 | – |
|
| A+K | 24 h |
65±5 | 1c, 2a |
72±13 | – |
94±7 | – |
95±7 | – |
|
| A+K | 48 h |
39±2 | 1c, 2c, 3a |
46±4 | 1c, 2c, 3a |
68±6 | 1c, 2a |
92±12 | – |
|
| A+K | 72 h |
26±1 | 1c, 3c |
33±4 | 1c, 2c, 3b |
67±11 | 1c, 2a |
82±12 | – |
| MDA-MB-468 | K | 24 h | 100±0 | – |
98±4 | – |
96±5 | – | 100±0 | – |
|
| K | 48 h |
94±9 | – | 100±0 | – | 100±0 | – | 100±0 | – |
|
| K | 72 h |
84±9 | – |
83±7 | – |
82±11 | – |
82±12 | – |
|
| A | 24 h |
41±1 | 1c, 2c |
43±6 | 1c, 2b |
56±16 | 1c, 2b |
95±8 | – |
|
| A | 48 h |
40±2 | 1c, 2c |
41±0 | 1c, 2c |
39±3 | 1c, 2c |
88±17 | – |
|
| A | 72 h |
38±2 | 1c, 2c |
35±3 | 1c, 2b |
35±2 | 1c, 2c |
58±1 | 1c, 2b |
|
| A+K | 24 h |
46±8 | 1c, 2c |
53±14 | 1c, 2b |
63±6 | 1c, 2a | 100±0 | – |
|
| A+K | 48 h |
43±2 | 1c, 2c |
56±9 | 1c, 2c | 100±0 | – | 100±0 | – |
|
| A+K | 72 h |
39±1 | 1c, 2c |
50±10 | 1c, 2b | 100±0 | – | 100±0 | – |
The concentration of compound that inhibits 50% of
the cell growth (IC50) was also determined (Table II). The IC50 of A+K was
significantly reduced, compared with that of A, in MCF-7 (P<0.05
for 72 h), MDA-MB-231 (P<0.01 for 24 h; P<0.001 for 48 and 72
h) and MDA-MB-453 (P<0.05 for 24 and 48 h) cells. This effect
was observed only upon 24-h incubation in T47-D cells (P<0.001).
By contrast, a lower IC50 value was observed in
MDA-MB-468 cells following treatment with A alone, compared with
A+K (P<0.001 for 48 and 72 h).
 | Table II.IC50 values of A, alone or
in combination with K, in breast cancer cells. |
Table II.
IC50 values of A, alone or
in combination with K, in breast cancer cells.
| Cell line | Treatment | IC50 ±
SD (mM) | P-value |
|---|
| MCF-7 | A | 24 h | 25.79±1.23 | – |
|
| A | 48 h | 18.23±1.17 | – |
|
| A | 72 h | 12.22±1.01 | – |
|
| A+K | 24 h | 15.51±1.04 | – |
|
| A+K | 48 h | 12.16±1.09 | – |
|
| A+K | 72 h |
9.96±1.02 | <0.050 |
| MDA-MB-231 | A | 24 h | 22.65±1.23 | – |
|
| A | 48 h | 18.00±1.07 | – |
|
| A | 72 h | 16.25±1.04 | – |
|
| A+K | 24 h | 14.37±1.06 | <0.010 |
|
| A+K | 48 h | 12.30±1.05 | <0.001 |
|
| A+K | 72 h | 10.69±1.04 | <0.001 |
| MDA-MB-453 | A | 24 h | 36.87±1.55 | – |
|
| A | 48 h | 12.88±1.12 | – |
|
| A | 72 h |
9.91±1.04 | – |
|
| A+K | 24 h | 21.28±1.27 | <0.050 |
|
| A+K | 48 h |
9.37±1.09 | <0.050 |
|
| A+K | 72 h |
6.71±1.12 | – |
| T47-D | A | 24 h | 47.84±1.44 | – |
|
| A | 48 h | 12.01±1.03 | – |
|
| A | 72 h |
7.99±1.02 | – |
|
| A+K | 24 h | 13.14±1.04 | <0.001 |
|
| A+K | 48 h | 10.78±1.05 | – |
|
| A+K | 72 h |
7.86±1.07 | – |
| MDA-MB-468 | A | 24 h |
8.40±1.17 | – |
|
| A | 48 h |
5.47±1.23 | <0.001 |
|
| A | 72 h |
2.29±1.38 | <0.001 |
|
| A+K | 24 h | 11.09±1.17 | – |
|
| A+K | 48 h | 12.39±1.07 | – |
|
| A+K | 72 h | 10.79±1.06 | – |
The model of interaction between A and K when used
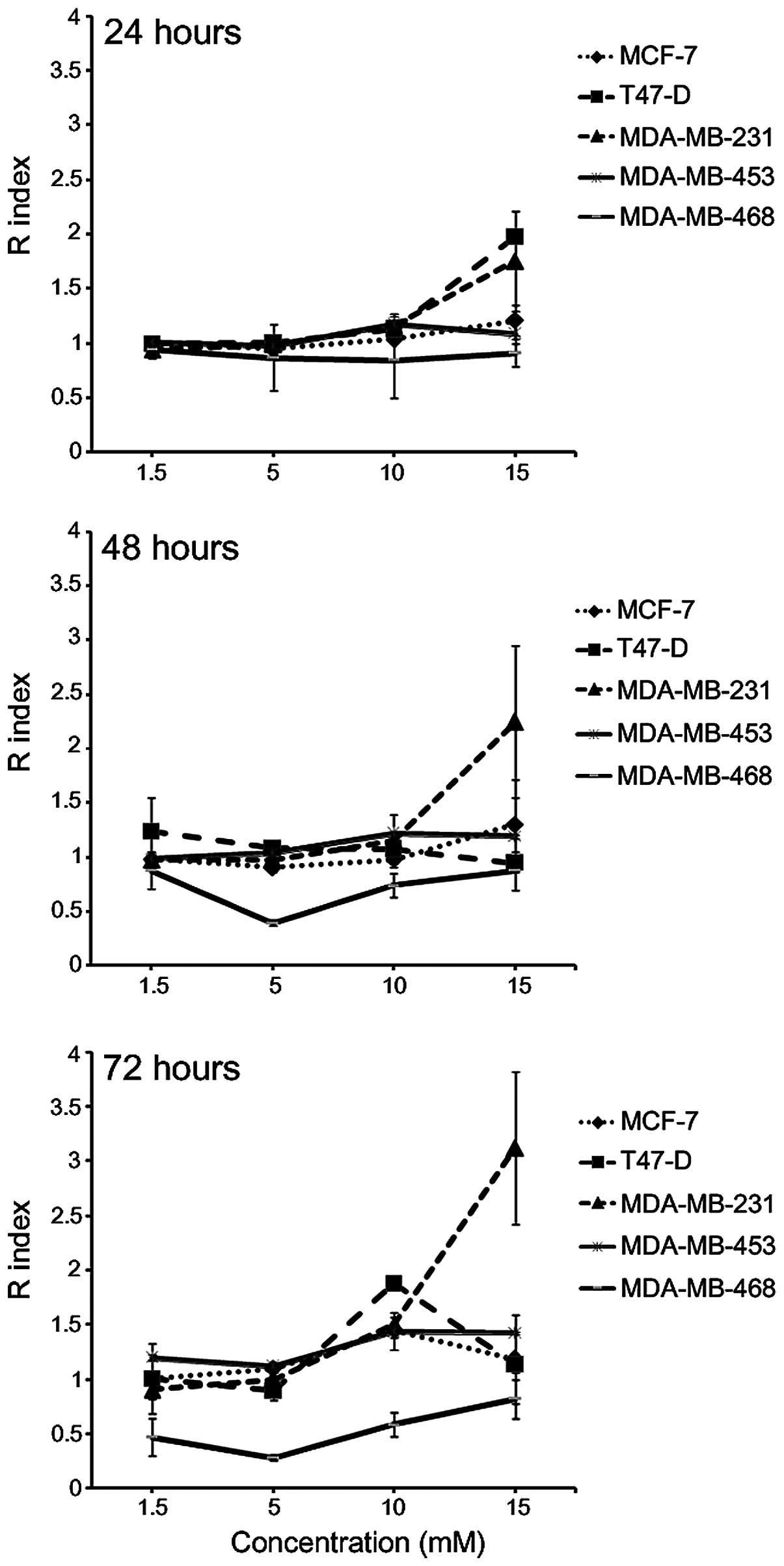
in combination was determined using the Kern's method (40) (Fig. 1).
The results indicated that the interaction between A+K was lower
than the additive effect in MDA-MB-468 cells following incubation
for 24, 48 and 72 h at all the concentrations tested, whereas it
was the result of additive effect in MCF-7 and MDA-MB-453 cells at
all the concentrations tested following 24 and 48-h incubation. In
addition, the R index >1 obtained indicated the onset of a
synergistic effect of the two compounds, compared with the
corresponding single treatment, at a concentration of 15 mM in
T47-D cells following 24-h incubation (P=0.0003), and in MDA-MB-231
cells following 24 and 48-h incubation (P<0.05). The combination
of A+K resulted in a synergistic effect in MDA-MB-231 and
MDA-MB-453 cells at concentrations of 10–15 mM (P<0.01), and in
MCF-7 and T47-D cells at 10 mM concentration (P<0.001),
following 72-h incubation.
K potentiates the apoptotic effect of
A in breast cancer cell lines
In order to determine the effect of K and A, alone
or in combination, on the apoptosis and cell cycle distribution of
breast cancer cells, a FACS analysis of their DNA content was
performed. Cells were incubated for 24 h with the aforementioned
compounds, alone or in combination, at a concentration of 10 mM, or
with CTRL. Compared with CTRL, K treatment did not affect the cell
cycle distribution in any of the cell lines evaluated (Table III). Compared with CTRL, treatment
with 10 mM A induced a significant increase in the percentage of
cells in the sub-G1 phase of the cell cycle in all the cell lines
tested (P<0.001), except in T47-D and MDA-MB-468. This effect
was associated with a significant decrease in the percentage of
cells in G0/G1, S and G2/M phases in MDA-MB-231 (P<0.001), while
a significant decrease in the percentage of cells in the G2/M phase
was observed in MCF-7 cells (P<0.001). Treatment with A+K
significantly increased the percentage of cells in the sub-G1 phase
in MCF-7, MDA-MB-231, MDA-MB-453 and MDA-MB-468 cells, compared
with treatment with A alone (P<0.001).
 | Table III.Effects of 24-h treatment with 10 mM
K and A, alone or in combination, on the cell cycle of breast
cancer cell lines. |
Table III.
Effects of 24-h treatment with 10 mM
K and A, alone or in combination, on the cell cycle of breast
cancer cell lines.
|
|
| Sub-G1 | G0/G1 | S | G2/M |
|---|
|
|
|
|
|
|
|
|---|
| Cell line | Treatment | Cells (%) ±SD | P-value | Cells (%) ±SD | P-value | Cells (%) ±SD | P-value | Cells (%) ±SD | P-value |
|---|
| MCF-7 | CTRL | 6.32±2.09 | – | 38.42±2.35 | – | 16.77±1.85 | – | 37.13±0.15 | – |
|
| K | 5.85±1.81 | – | 36.57±2.76 | – | 17.07±2.74 | – | 38.56±0.52 | – |
|
| A | 27.28±0.66 | 1c, 2c | 41.26±2.91 | – | 13.72±0.49 | – | 18.32±4.20 | 1c, 2c |
|
| A+K | 51.05±0.91 | 1c, 3c | 36.76±0.86 | – | 3.97±0.16 | 1c, 2c, 3b | 8.42±2.38 | 1c, 2c, 3b |
| T47-D | CTRL | 7.40±1.55 | – | 42.43±1.18 | – | 11.74±0.54 | – | 38.29±0.68 | – |
|
| K | 6.82±0.05 | – | 41.34±5.18 | – | 12.67±1.16 | – | 38.97±5.11 | – |
|
| A | 17.86±5.12 | – | 39.26±5.27 | – | 12.43±1.68 | – | 30.36±1.86 | – |
|
| A+K | 26.12±7.19 | 1c, 2a | 33.98±3.22 | – | 13.96±0.98 | – | 25.54±3.59 | – |
| MDA-MB-231 | CTRL | 18.89±1.47 | – | 39.83±0.23 | – | 16.49±0.28 | – | 24.61±0.76 | – |
|
| K | 16.59±0.18 | – | 39.22± 0.48 | – | 17.23±0.09 | – | 26.17±0.64 | – |
|
| A | 68.58±3.87 | 1c, 2c | 14.14±3.08 | 1c, 2c | 7.92±0.79 | 1c, 2c | 8.98±0.01 | 1c, 2c |
|
| A+K | 91.07±3.45 | 1c, 3c | 5.39±1.81 | 1c, 2c, 3b | 2.58±1.22 | 1c, 2c, 3b | 0.91±0.35 | 1c, 2c, 3a |
| MDA-MB-453 | CTRL | 7.14±1.09 | – | 50.98±3.79 | – | 12.03±1.45 | – | 28.43±3.15 | – |
|
| K | 6.85±1.28 | – | 51.59±1.76 | – | 11.55±1.06 | – | 29.17±1.75 | – |
|
| A | 24.12±3.14 | 1c, 2a | 41.38±1.06 | 1c, 2a | 11.24±0.30 | – | 22.46±3.24 | – |
|
| A+K | 43.36±11.03 | 1c, 3c | 30.30±4.76 | 1c, 2c, 3b | 8.83±0.90 | 1c, 2a | 16.96±4.33 | 1b, 2c |
| MDA-MB-468 | CTRL | 6.74±0.73 | – | 52.04±4.75 | – | 15.86±2.70 | – | 25.66±3.12 | – |
|
| K | 7.65±1.07 | – | 51.12±5.13 | – | 16.81±3.35 | – | 24.74±4.83 | – |
|
| A | 13.95±2.57 | – | 43.31±5.91 | – | 16.85±4.59 | – | 26.18±3.85 | – |
|
| A+K | 37.30±6.10 | 1c, 3c | 34.18±2.60 | 1c, 2b | 14.23±2.66 | – | 14.55±5.55 | 1c, 3a |
In particular, the apoptotic rate obtained with the
combined treatment was 1.87, 1.46, 1.33, 1.80 and 2.67 times higher
than that obtained following treatment with A in MCF-7, T47-D,
MDA-MB-231, MDA-MB-453 and MDA-MB-468 cells, respectively.
In addition, a significant decrease in the
percentage of MCF-7 cells in S and G2/M phases was observed
following treatment with A+K, compared with A alone (P<0.01).
Treatment with A+K reduced the percentage of MDA-MB-231 cells in
G0/G1 (P<0.01), S (P<0.01) and G2/M (P<0.05) phases,
compared with A. Treatment with A+K resulted in a significant
decrease in the percentage of cells in G0/G1 phase, compared with
treatment with A, in MDA-MB-453 (P<0.01) and MDA-MB-468
(P<0.05) cells. Overall, these results indicated an
heterogeneous response of different cell lines to treatment with A
and/or K, with the maximum effect achieved following combined
treatment with A and K.
Effect of K and A, alone or in
combination, on signaling proteins associated with apoptosis
The expression levels of signaling proteins
associated with apoptosis were investigated by western blotting in
MCF-7, MDA-MB-231 and MDA-MD-435 cells treated for 24 h with 10 mM
A and K, alone or in combination. A representative experiment is
illustrated in Fig. 2.
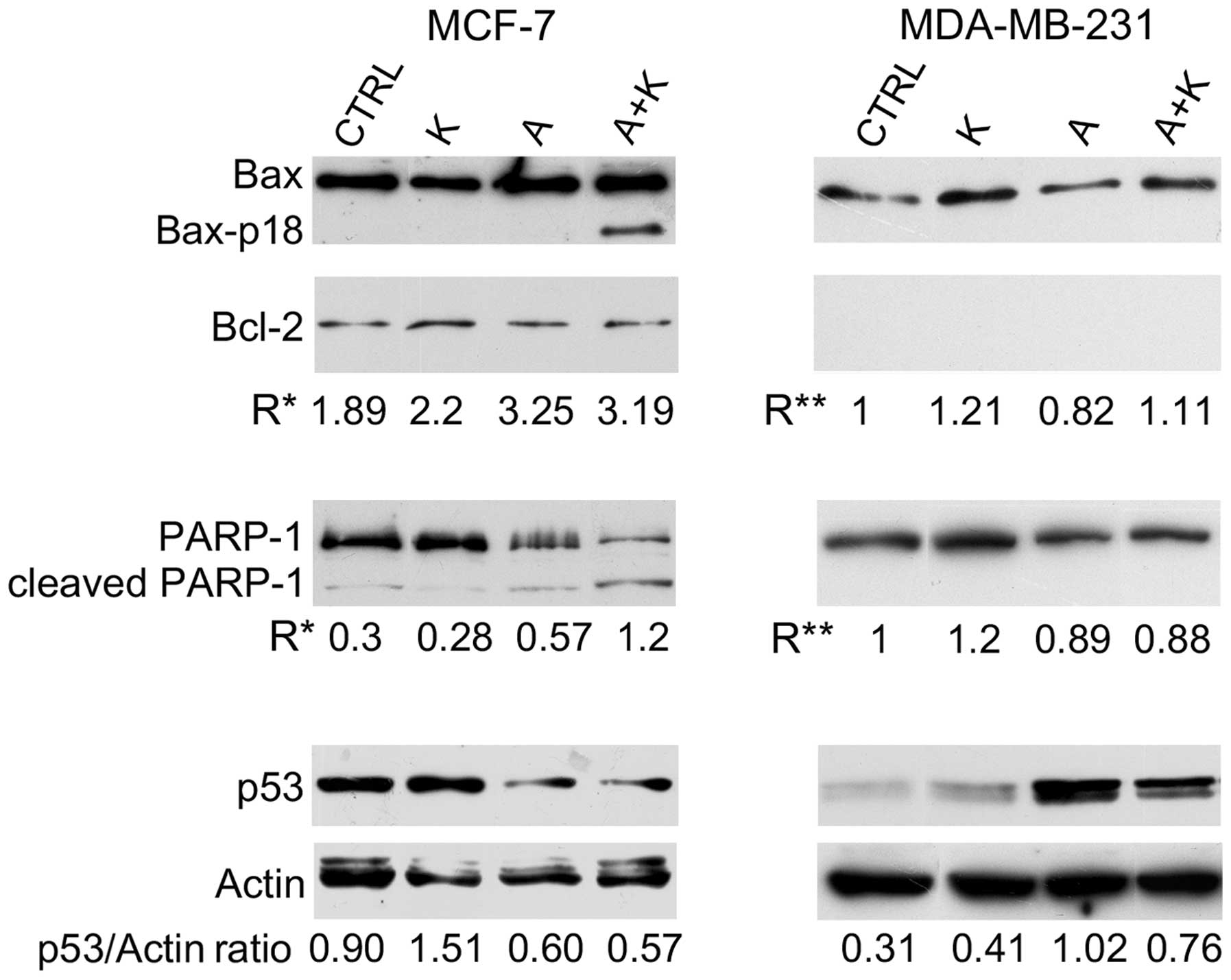 | Figure 2.Effect of A and K, alone or in
combination, on signaling proteins associated with apoptosis. The
expression levels of Bax, Bax-p18 (molecular weight, 18 kDa),
Bcl-2, cleaved PARP-1 and p53 were assessed by western blotting in
MCF-7 and MDA-MB-231 cells treated for 24 h with 10 mM A and K,
alone or in combination, or with CTRL. Actin was used as an
internal control. The intensity of the bands obtained in two
independent experiments was quantified using ImageJ software
following blot scanning. R* represents the densitometry ratios
between the expression levels of Bax and Bcl-2 or between the
levels of cleaved vs. full-length PARP-1. R** represents the
increase in the expression levels of Bax following treatment with A
and/or K respect to CTRL, or the decrease in the expression levels
of full-length PARP-1 following treatment with A and/or K, compared
with CTRL. The expression levels of p53 vs. actin are also
reported. The faint higher molecular weight products observed with
the anti-actin antibody and the lower molecular weight product
observed with the anti-p53 antibody may be due to non-specific
antibody reactions in these cell lines. CTRL, culture medium; K,
potassium; A, ascorbic acid; Bcl-2, B-cell lymphoma-2; Bax,
Bcl-2-associated X protein; PARP-1, poly(adenosine
diphosphate-ribose) polymerase-1. |
Treatment with A alone (P=0.0028), and in
combination with K (P=0.0025) increased the Bax/Bcl-2 ratio (R*),
compared with CTRL, in MCF-7 cells. Notably, A+K induced the
appearance of the 18 kDa Bax isoform (Bax-p18) in MCF-7 cells,
which is known to be a more potent inducer of apoptotic cell death
than the full-length Bax-p21 (41).
Bcl-2 was not detected in MDA-MB-231 cells; thus, only the
expression of Bax following treatment with A and/or K vs. CTRL was
evaluated. Treatment with A decreased Bax expression in MDA-MB-231
cells, compared with CTRL (R=0.82 vs. R=1.00, P=0.0017).
Conversely, in this cell line, treatment with K increased Bax
expression, and A+K combination was more effective than A (R=1.11
vs. R=0.82; P=0.0015).
To further corroborate that the effect of the
aforementioned compounds on the increased number of cells in the
sub-G1 phase of the cell cycle was due to the induction of
apoptosis, the cleavage of PARP-1 was analyzed by western blotting.
As represented in Fig. 2, treatment
with A, alone or in combination with K, resulted in considerable
proteolytic cleavage of PARP-1 or in decreased expression of
full-length PARP-1, compared with CTRL, in MCF-7 cells. A+K was the
most effective treatment in activating the degradation of PARP-1,
compared with CTRL (R=1.20 vs. R=0.30, P=0.0045) and A (R=1.20 vs.
R=0.57, P=0.0072). Decreased expression of PARP-1, possibly due to
degradation of the protein, was observed in MDA-MB-231 cells
following treatment with A, alone or in combination with K.
Treatment with K alone did not decrease the expression of PARP-1 or
induced its degradation.
To determine whether the apoptosis induced by A in
MCF-7 and MDA-MB-231 cells was p53-dependent, the expression levels
of p53 were analyzed. A (R=0.60) and A+K (R=0.57) reduced the
expression of p53 in MCF-7 cells, compared with CTRL (R=0.90,
P<0.01) and K (R=1.51, P<0.001). In addition, K and A
increased p53 expression in MDA-MB-231 cells, compared with CTRL
(R=0.41 vs. R=0.31, P=0.014 and R=1.02 vs. R=0.31, P=0.001,
respectively). However, the combination of the two compounds was
less effective than treatment with A alone in increasing the
expression of p53 (R=0.76 vs. R=1.02, P=0.014).
Effect of K and A, alone or in
combination, on ERK1/ERK2 and NF-κB signaling proteins
The effect of A and K on ERK phosphorylation was
investigated. The expression levels of p-ERK1 and p-ERK2 were
compared with those of total ERK. The results are presented in
Fig. 3. The expression of ERK was not
altered following different treatments in any of the cell lines
assessed. K increased the phosphorylation of ERK2 in MCF-7 cells.
However, when A was administered in combination with K, it was able
to counteract the phosphorylation of ERK1/ERK2 observed upon
treatment with A or K alone (R=0.28 vs. R=0.93, P=0.0008 for K and
R=0.28 vs. R=0.96, P=0.0005 for A, in the case of ERK1; and R=0.26
vs. R=1.01, P=0.0001 for K and R=0.26 vs. R=0.84, P=0.0004 for A,
in the case of ERK2). Similarly, while A did not affect ERK1/ERK2
phosphorylation, K increased ERK1 and ERK2 phosphorylation,
compared with CTRL (R=0.34 vs. R=0.18, P=0.024 for ERK1 and R=0.78
vs. R=0.32, P=0.006 for ERK2) in MDA-MB-231 cells.
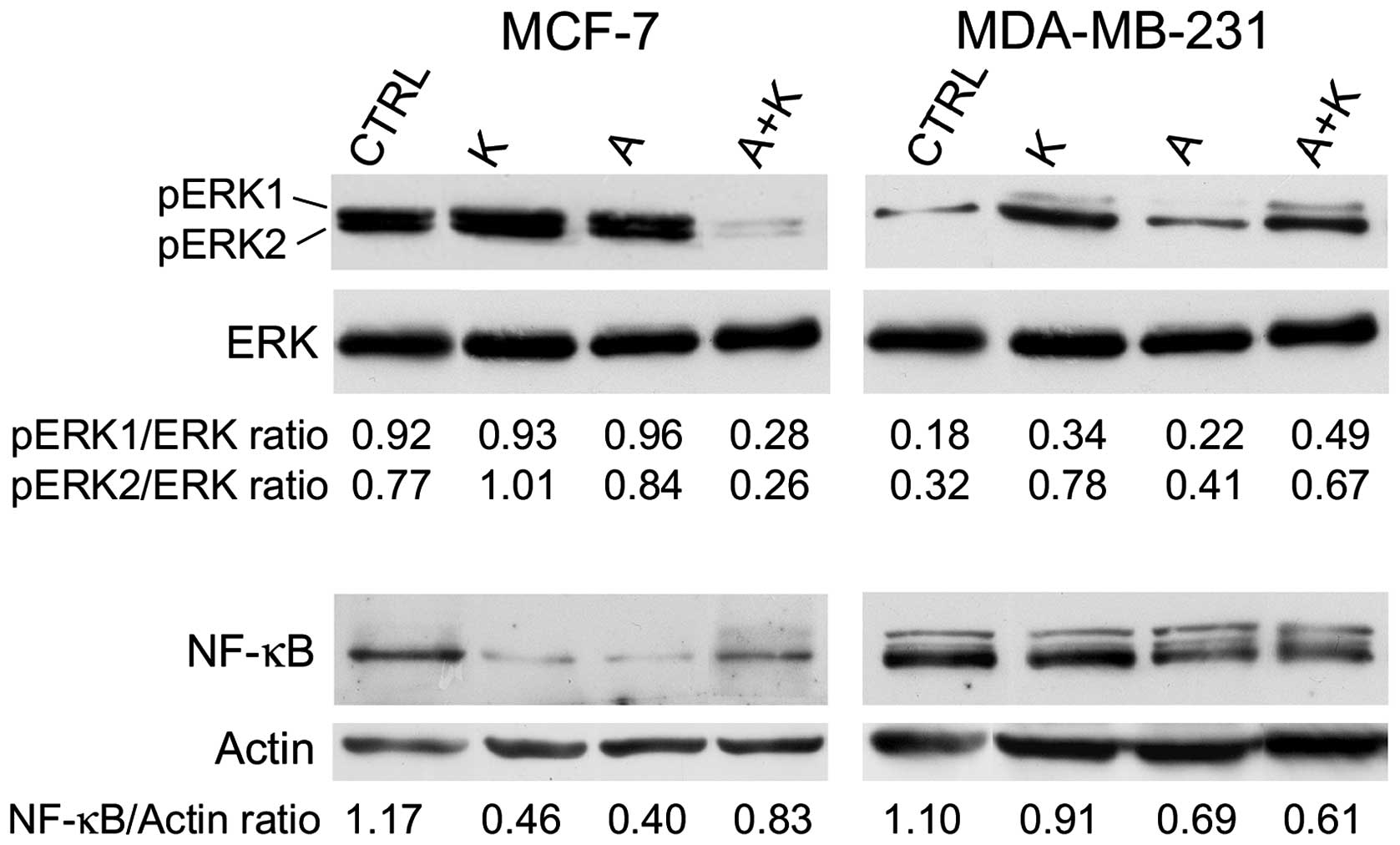 | Figure 3.Effect of treatment with A and K,
alone or in combination, on pro-survival signaling proteins.
Western blotting was performed on MCF-7 and MDA-MB-231 cells
treated with A or K, alone or in combination, for 24 h. The levels
of phosphorylated ERK1/ERK2 were compared with the total protein
levels of ERK, and the ratio values are reported. Quantitative
densitometric analysis of the expression levels of nuclear
factor-κB, compared with the levels of actin, is provided. The
faint higher molecular weight product observed with the NF-κB
antibody in the MDA-MB-231 cells may be due to non-specific
reactions in this cell line. CTRL, culture medium; K, potassium; A,
ascorbic acid; ERK, extracellular signal-regulated kinase; p,
phosphorylated; NF-κB, nuclear factor-κB. |
Next, the effect of A and K, alone or in
combination, on NF-κB expression was investigated. Treatment with
K, A and A+K inhibited NF-κB expression, compared with CTRL, in all
cell lines (P=0.0017 for K, P=0.0017 for A and P=0.016 for A+K in
MCF-7 cells; P=0.0084 for K, P=0.0015 for A and P=0.001 for A+K in
MDA-MB-231 cells). A+K inhibited the expression of NF-κB in
MDA-MB-231 cells, compared with A or K alone (A+K vs. K, R=0.61 vs.
0.91, P=0.0014; A+K vs. A, R=0.61 vs. 0.69, P=0.008).
Discussion
The use of A as an anti-cancer agent has been
extensively analyzed during the last 50 years (1,2). Previous
epidemiological studies have demonstrated the preventive effect of
A in several human tumors when A was ingested through the diet
(1,2).
The intake of A in the diet was associated with lower mortality and
lower incidence of numerous human malignancies, including cancer of
the esophagus, oral cavity, stomach, pancreas, cervix, rectum,
breast and lung (1,2).
A possesses both pro-oxidant and anti-oxidant
properties (42–50). Although the preventive anti-cancer
effect of A results from its anti-oxidant properties (42), previous in vitro studies and
mouse models have demonstrated that A is able to inhibit cell
proliferation in various types of cancer due to its ability to
induce the production of H2O2 (43–49)
without being toxic to non-cancerous cells (43,50). A
also possesses anti-metastatic (51),
anti-angiogenic (42) and
immuno-stimulatory properties (52).
In addition, previous epidemiological studies have confirmed that A
in combination with chemotherapy or radiation does not cause side
effects in patients with breast cancer (53), and is able to extend survival
(54) and improve the quality of life
(55) of cancer patients.
Similarly, it has been previously demonstrated that
K is both an anti-apoptotic and a pro-apoptotic agent (13,14), as
well as a regulator of cell proliferation (15–17). The
intracellular homeostasis of Na+ and K+ is
disregulated in cancer cells (27).
This is due to an alteration in the expression and activity of
Na+/K+ ATPase in tumor cells, which modifies
the active transport of Na+ and K+, leading
to a diffusion of intracellular K+ outside the membrane
and a consequent increase of the intracellular levels of
Na+ (27,56,57). This
mechanism causes the release of calcium from its intracellular
deposits and a simultaneous increase in glucose uptake, thus
enhancing mitogenic stimulation (27,56,57). It
has been previously demonstrated that the administration of K
ascorbate produced anticancer effects in vitro (30,58),
possibly due to the carrier properties of A, which allows a rapid
diffusion of K into the cells, leading to the inhibition of tumor
cell proliferation (27,30).
The results of the present study confirm that A
exerts an inhibitory effect on the survival of various breast
cancer cell lines. K alone exhibited an inhibitory effect only at
the highest concentration tested and following 48-h incubation in
MCF-7 cells. The effect of A was dose- and time-dependent in all
the cell lines evaluated, with the exception of MDA-MB-231. K
ascorbate (formed by combining A+K) significantly increased the
apoptotic rate of all cell lines, with the exception of MDA-MB-468,
whose apoptotic rate did not significantly differ from that of
cells treated with A. The combination of A+K resulted in a
synergistic effect in MDA-MB-231 and MDA-MB-453 cells at 10–15 mM
concentration (P<0.01), and in MCF-7 and T47-D cells at 10 mM
concentration (P<0.001), following 72-h incubation.
The results of FACS analysis further supported a
synergic effect of A+K, since treatment with A+K significantly
increased the percentage of cells in the sub-G1 phase of the cell
cycle, compared with A alone, in MCF-7, MDA-MB-231, MDA-MB-453 and
MDA-MB-468 cells (P<0.001). The increase in the apoptotic rate
observed upon treatment with A+K indicated an anti-tumoral effect
of the compound K in the majority of the cell lines tested. A+K was
the most effective treatment in activating the degradation of
PARP-1, compared with CTRL and A alone, thus corroborating the
activation of apoptosis caused by A+K. The mechanisms responsible
for the markedly positive but heterogeneous effects observed in the
different cell lines analyzed in the present study, coupled with
the variable results obtained upon different exposure times,
require further investigation, possibly by evaluating the effect of
the aforementioned treatments at longer times. A possible
explanation for the heterogeneous effect of the compounds A and K
on the different cell lines tested in the present study may be the
intrinsic biological characteristics of the breast cancer cell
lines used, since all cell lines, with the exception of MCF-7,
exhibit a mutated p53 (59), while
MCF-7 and T47-D cell lines are positive for estrogen and
progesterone receptors, whereas MDA-MB-453 overexpresses ErbB2
(60).
Another potential explanation for the heterogeneous
effect observed in different breast cancer cell lines upon
treatment with A and K in the present study may be the cell length
and conformation of telomeres, which may be affected by the
concentration of K+ in the different cell lines tested
(61). In healthy cells, each cell
replication results in 50–200 bp loss of the telomere (62). When a critical shortening of the
telomeric DNA is reached, the cell undergoes apoptosis (62). Telomeres of cancer cells do not
shorten following replication, due to the activation of a reverse
transcriptase telomerase, which is activated in 80–85% of human
cancer cells and extends the telomeric sequence at the chromosome
ends (63). G-rich telomeres may fold
into G-quadruplexes, which are DNA secondary structures consisting
of stacked G-tetrad planes connected by a network of Hoogsteen
hydrogen bonds and stabilized by monovalent cations such as
Na+ and K+ (64). The formation of G-quadruplexes by
single-stranded human telomeric DNA inhibits the activity of the
aforementioned reverse transcriptase telomerase (64). Compounds that stabilize the
intramolecular DNA G-quadruplexes formed in the human telomeric
sequence have been demonstrated to inhibit the activity of this
telomerase, thus disrupting the capping and maintenance of the
telomeres. Therefore, intramolecular human telomeric DNA
G-quadruplexes are considered an attractive target for cancer
therapeutic intervention (65–67).
G-quadruplexes may adopt different shapes depending on the type of
mineral content that they are exposed to (23). The K+ structure of
G-quadruplexes is considered to be biologically more relevant than
Na+ structure, due to the higher physiological
intracellular concentration of K+ (68–71).
Previous studies have revealed that hybrid-type intramolecular
G-quadruplexes appear to be the predominant conformation formed in
human telomeric sequences in solution in the presence of
K+ (72,73). It has been reported that the increase
of K+ transported into the cells alters the shape of
G-quadruplexes (30). Therefore, the
time-dependent effect observed in the present study upon treatment
with A and K may reflect the time required for G-quadruplexes in
tumor cells to shift from the Na+ to the K+
form. This would allow the generation of reactive oxygen species
leading to oxidative DNA damage (74).
Overall, the present results indicated that K
potentiated the anti-tumor effects of A in certain breast cancer
cell lines. However, further in vitro and in vivo
analyses are required to understand the mechanisms of action of K
ascorbate, a natural compound with promising potential as an
anti-cancer drug.
Acknowledgements
The present study was partly funded by a grant from
the University of Rome ‘Sapienza’ (Rome, Italy; grant no.
C26A14T57T).
References
|
1.
|
Khaw KT, Bingham S, Welch A, Luben R,
Wareham N, Oakes S and Day N: Relation between plasma ascorbic acid
and mortality in men and women in EPIC-Norfolk prospective study: A
prospective population study. European Prospective Investigation
into Cancer and Nutrition. Lancet. 357:657–663. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Block G: Epidemiologic evidence regarding
vitamin C and cancer. Am J Clin Nutr. 54(Suppl 6): 1310S–1314S.
1991.PubMed/NCBI
|
|
3.
|
Cameron E and Pauling L: The
orthomolecular treatment of cancer. I. The role of ascorbic acid in
host resistance. Chem Biol Interact. 9:273–283. 1974. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Creagan ET, Moertel CG, O'Fallon JR,
Schutt AJ, O'Connell MJ, Rubin J and Frytak S: Failure of high-dose
vitamin C (ascorbic acid) therapy to benefit patients with advanced
cancer. A controlled trial. N Engl J Med. 301:687–690. 1979.
View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Ohno S, Ohno Y, Suzuki N, Soma G and Inoue
M: High-dose vitamin C (ascorbic acid) therapy in the treatment of
patients with advanced cancer. Anticancer Res. 29:809–815.
2009.PubMed/NCBI
|
|
6.
|
Levine M, Conry-Cantilena C, Wang Y, Welch
RW, Washko PW, Dhariwal KR, Park JB, Lazarev A, Graumlich JF, King
J and Cantilena LR: Vitamin C pharmacokinetics in healthy
volunteers: Evidence for a recommended dietary allowance. Proc Natl
Acad Sci USA. 93:3704–3709. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Levine M, Wang Y, Padayatty SJ and Morrow
J: A new recommended dietary allowance of vitamin C for healthy
young women. Proc Natl Acad Sci USA. 98:9842–9846. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Padayatty SJ, Sun H, Wang Y, Riordan HD,
Hewitt SM, Katz A, Wesley RA and Levine M: Vitamin C
pharmacokinetics: Implications for oral and intravenous use. Ann
Intern Med. 140:533–537. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Hoffer LJ, Levine M, Assouline S,
Melnychuk D, Padayatty SJ, Rosadiuk K, Rousseau C, Robitaille L and
Miller WH Jr: Phase I clinical trial of i.v. ascorbic acid in
advanced malignancy. Ann Oncol. 19:1969–1974. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Parrow NL, Leshin JA and Levine M:
Parenteral ascorbate as a cancer therapeutic: A reassessment based
on pharmacokinetics. Antioxid Redox Signal. 19:2141–2156. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Ma Y, Chapman J, Levine M, Polireddy K,
Drisko J and Chen Q: High-dose parenteral ascorbate enhanced
chemosensitivity of ovarian cancer and reduced toxicity of
chemotherapy. Sci Transl Med. 6:222ra182014. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Cone CD Jr: Unified theory on the basic
mechanism of normal mitotic control and oncogenesis. J Theor Biol.
30:151–181. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Hughes FM Jr and Cidlowski JA: Potassium
is a critical regulator of apoptotic enzymes in vitro and in vivo.
Adv Enzyme Regul. 39:157–171. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Wang Z: Roles of K+ channels in
regulating tumour cell proliferation and apoptosis. Pflugers Arch.
448:274–286. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
DeCoursey TE, Chandy KG, Gupta S and
Cahalan MD: Voltage-gated K+ channels in human T
lymphocytes: A role in mitogenesis? Nature. 307:465–468. 1984.
View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Pardo LA: Voltage-gated potassium channels
in cell proliferation. Physiology (Bethesda). 19:285–292. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Wonderlin WF and Strobl JS: Potassium
channels, proliferation and G1 progression. J Membr Biol.
154:91–107. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Boros LG, Lee PW, Brandes JL, Cascante M,
Muscarella P, Schirmer WJ, Melvin WS and Ellison EC: Nonoxidative
pentose phosphate pathways and their direct role in ribose
synthesis in tumors: Is cancer a disease of cellular glucose
metabolism? Med Hypotheses. 50:55–59. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Smith NK, Stabler SB, Cameron IL and
Medina D: X-Ray microanalysis of electrolyte content of normal,
preneoplastic, and neoplastic mouse mammary tissue. Cancer Res.
41:3877–3880. 1981.PubMed/NCBI
|
|
20.
|
Arrebola F, Fernández-Segura E, Campos A,
Crespo PV, Skepper JN and Warley A: Changes in intracellular
electrolyte concentrations during apoptosis induced by UV
irradiation of human myeloblastic cells. Am J Physiol Cell Physiol.
290:C638–C649. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Mobasheri A, Fox R, Evans I, Cullingham F,
Martín-Vasallo P and Foster CS: Epithelial Na, K-ATPase expression
is down-regulated in canine prostate cancer; a possible consequence
of metabolic transformation in the process of prostate malignancy.
Cancer Cell Int. 3:82003. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Chen JQ, Contreras RG, Wang R, Fernandez
SV, Shoshani L, Russo IH, Cereijido M and Russo J: Sodium/potassium
ATPase (Na+, K+-ATPase) and ouabain/related
cardiac glycosides: A new paradigm for development of anti-breast
cancer drugs? Breast Cancer Res Treat. 96:1–15. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Parkinson GN, Lee MP and Neidle S: Crystal
structure of parallel quadruplexes from human telomeric DNA.
Nature. 417:876–880. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Salvati E, Rizzo A, Iachettini S, Zizza P,
Cingolani C, D'Angelo C, Porru M, Mondello C, Aiello A, Farsetti A,
et al: A basal level of DNA damage and telomere deprotection
increases the sensitivity of cancer cells to G-quadruplex
interactive compounds. Nucleic Acids Res. 43:1759–1769. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Dai J, Carver M and Yang D: Polymorphism
of human telomeric quadruplex structures. Biochimie. 90:1172–1183.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Kelland LR: Overcoming the immortality of
tumour cells by telomere and telomerase based cancer therapeutics -
current status and future prospects. Eur J Cancer. 41:971–979.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Paoli G: The Bio-magnetic nature of cancer
and the role of potassium ascorbate and ribose against cellar
degeneration. J Nucl Energy. 7:114–119. 2003.
|
|
28.
|
Croci S, Pedrazzi G, Passeri G, Delsignore
R and Ortalli I: Red cell Hb oxidation of healthy subjects compared
to breast cancer patients. Anticancer Res. 22:2903–2906.
2002.PubMed/NCBI
|
|
29.
|
Croci S, Pedrazzi G, Passeri G, Piccolo P
and Ortalli I: Acetylphenylhydrazine induced haemoglobin oxidation
in erythrocytes studied by Mössbauer spectroscopy. Biochim Biophys
Acta. 1568:99–104. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Croci S, Bruni L, Bussolati S, Castaldo M
and Dondi M: Potassium bicarbonate and D-ribose effects on A72
canine and HTB-126 human cancer cell line proliferation in vitro.
Cancer Cell Int. 11:302011. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Masuelli L, Marzocchella L, Quaranta A,
Palumbo C, Pompa G, Izzi V, Canini A, Modesti A, Galvano F and Bei
R: Apigenin induces apoptosis and impairs head and neck carcinomas
EGFR/ErbB2 signaling. Front Biosci (Landmark Ed). 16:1060–1068.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Masuelli L, Marzocchella L, Focaccetti C,
Tresoldi I, Palumbo C, Izzi V, Benvenuto M, Fantini M, Lista F,
Tarantino U, et al: Resveratrol and diallyl disulfide enhance
curcumin-induced sarcoma cell apoptosis. Front Biosci (Landmark
Ed). 17:498–508. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Bei R, Masuelli L, Moriconi E, Visco V,
Moretti A, Kraus MH and Muraro R: Immune responses to all ErbB
family receptors detectable in serum of cancer patients. Oncogene.
18:1267–1275. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Bei R, Budillon A, Masuelli L, Cereda V,
Vitolo D, Di Gennaro E, Ripavecchia V, Palumbo C, Ionna F, Losito
S, et al: Frequent overexpression of multiple ErbB receptors by
head and neck squamous cell carcinoma contrasts with rare antibody
immunity in patients. J Pathol. 204:317–325. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Faggioni G, Pomponi A, De Santis R,
Masuelli L, Ciammaruconi A, Monaco F, Di Gennaro A, Marzocchella L,
Sambri V, Lelli R, et al: West Nile alternative open reading frame
(N-NS4B/WARF4) is produced in infected West Nile Virus (WNV) cells
and induces humoral response in WNV infected individuals. Virol J.
9:2832012. View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Masuelli L, Budillon A, Marzocchella L,
Mrozek MA, Vitolo D, Di Gennaro E, Losito S, Sale P, Longo F, Ionna
F, et al: Caveolin-1 overexpression is associated with simultaneous
abnormal expression of the E-cadherin/α-β catenins complex and
multiple ErbB receptors and with lymph nodes metastasis in head and
neck squamous cell carcinomas. J Cell Physiol. 227:3344–3353. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Masuelli L, Bei R, Sacchetti P,
Scappaticci I, Francalanci P, Albonici L, Coletti A, Palumbo C,
Minieri M, Fiaccavento R, et al: Beta-catenin accumulates in
intercalated disks of hypertrophic cardiomyopathic hearts.
Cardiovasc Res. 60:376–387. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Masuelli L, Pompa G, Fabrizi M, Quaranta
A, Vozza I, Piccoli L, Antonelli A, Marzocchella L, Di Carlo S,
Perrotti V, et al: Patients with peri-implantitis, unlike those
with a healthy periimplant microenvironment, display antibodies to
more than one heat shock protein (HSP 27, HSP 65 and HSP 90) linear
epitope. Eur J Inflamm. 9:257–267. 2011.
|
|
39.
|
Ingrosso G, Fantini M, Nardi A, Benvenuto
M, Sacchetti P, Masuelli L, Ponti E, Frajese GV, Lista F, Schillaci
O, et al: Local radiotherapy increases the level of autoantibodies
to ribosomal P0 protein but not to heat shock proteins,
extracellular matrix molecules and EGFR/ErbB2 receptors in prostate
cancer patients. Oncol Rep. 29:1167–1174. 2013.PubMed/NCBI
|
|
40.
|
Palumbo C, Albonici L, Bei R, Bocci C,
Scarpa S, Di Nardo P and Modesti A: HMBA induces cell death and
potentiates doxorubicin toxicity in malignant mesothelioma cells.
Cancer Chemother Pharmacol. 54:398–406. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
41.
|
Toyota H, Yanase N, Yoshimoto T, Moriyama
M, Sudo T and Mizuguchi J: Calpain-induced Bax-cleavage product is
a more potent inducer of apoptotic cell death than wild-type Bax.
Cancer Lett. 189:221–230. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
42.
|
Du J, Cullen JJ and Buettner GR: Ascorbic
acid: Chemistry, biology and the treatment of cancer. Biochim
Biophys Acta. 1826:443–457. 2012.PubMed/NCBI
|
|
43.
|
Chen Q, Espey MG, Krishna MC, Mitchell JB,
Corpe CP, Buettner GR, Shacter E and Levine M: Pharmacologic
ascorbic acid concentrations selectively kill cancer cells: Action
as a pro-drug to deliver hydrogen peroxide to tissues. Proc Natl
Acad Sci USA. 102:13604–13609. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
44.
|
Du J, Martin SM, Levine M, Wagner BA,
Buettner GR, Wang SH, Taghiyev AF, Du C, Knudson CM and Cullen JJ:
Mechanisms of ascorbate-induced cytotoxicity in pancreatic cancer.
Clin Cancer Res. 16:509–520. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
45.
|
Chen P, Yu J, Chalmers B, Drisko J, Yang
J, Li B and Chen Q: Pharmacological ascorbate induces cytotoxicity
in prostate cancer cells through ATP depletion and induction of
autophagy. Anticancer Drugs. 23:437–444. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
46.
|
Martinovich GG, Golubeva EN, Martinovich
IV and Cherenkevich SN: Redox regulation of calcium signaling in
cancer cells by ascorbic acid involving the mitochondrial electron
transport chain. J Biophys. 2012:9216532012. View Article : Google Scholar : PubMed/NCBI
|
|
47.
|
Han SS, Kim K, Hahm ER, Lee SJ, Surh YJ,
Park HK, Kim WS, Jung CW, Lee MH, Park K, et al: L-ascorbic acid
represses constitutive activation of NF-kappaB and COX-2 expression
in human acute myeloid leukemia, HL-60. J Cell Biochem. 93:257–270.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
48.
|
Park S, Ahn ES, Lee S, Jung M, Park JH, Yi
SY and Yeom CH: Proteomic analysis reveals upregulation of RKIP in
S-180 implanted BALB/C mouse after treatment with ascorbic acid. J
Cell Biochem. 106:1136–1145. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
49.
|
Ranzato E, Biffo S and Burlando B:
Selective ascorbate toxicity in malignant mesothelioma: A redox
Trojan mechanism. Am J Respir Cell Mol Biol. 44:108–117. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
50.
|
Chen Q, Espey MG, Sun AY, Lee JH, Krishna
MC, Shacter E, Choyke PL, Pooput C, Kirk KL, Buettner GR and Levine
M: Ascorbate in pharmacologic concentrations selectively generates
ascorbate radical and hydrogen peroxide in extracellular fluid in
vivo. Proc Natl Acad Sci USA. 104:8749–8754. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
51.
|
Cha J, Roomi MW, Ivanov V, Kalinovsky T,
Niedzwiecki A and Rath M: Ascorbate supplementation inhibits growth
and metastasis of B16FO melanoma and 4T1 breast cancer cells in
vitamin C-deficient mice. Int J Oncol. 42:55–64. 2013.PubMed/NCBI
|
|
52.
|
Kim JE, Cho HS, Yang HS, Jung DJ, Hong SW,
Hung CF, Lee WJ and Kim D: Depletion of ascorbic acid impairs NK
cell activity against ovarian cancer in a mouse model.
Immunobiology. 217:873–881. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
53.
|
Riordan HD, Riordan NH, Jackson JA,
Casciari JJ, Hunninghake R, González MJ, Mora EM, Miranda-Massari
JR, Rosario N and Rivera A: Intravenous vitamin C as a chemotherapy
agent: A report on clinical cases. P R Health Sci J. 23:115–118.
2004.PubMed/NCBI
|
|
54.
|
Shimpo K, Nagatsu T, Yamada K, Sato T,
Niimi H, Shamoto M, Takeuchi T, Umezawa H and Fujita K: Ascorbic
acid and adriamycin toxicity. Am J Clin Nutr. 54(Suppl 6):
1298S–1301S. 1991.PubMed/NCBI
|
|
55.
|
Vollbracht C, Schneider B, Leendert V,
Weiss G, Auerbach L and Beuth J: Intravenous vitamin C
administration improves quality of life in breast cancer patients
during chemo-/radiotherapy and aftercare: Results of a
retrospective, multicentre, epidemiological cohort study in
Germany. In Vivo. 25:983–990. 2011.PubMed/NCBI
|
|
56.
|
L'Allemain G, Paris S and Pouysségur J:
Growth factor action and intracellular pH regulation in
fibroblasts. Evidence for a major role of the
Na+/H+ antiport. J Biol Chem. 259:5809–5815.
1984.PubMed/NCBI
|
|
57.
|
Gerson DF, Kiefer H and Eufe W:
Intracellular pH of mitogen-stimulated lymphocytes. Science.
216:1009–1010. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
58.
|
Bruni L, Babarinde AA, Ortalli I and Croci
S: K-D:rib dampens Hs 578T cancer cell chemoinvasion and
proliferation. Cancer Cell Int. 14:772014. View Article : Google Scholar : PubMed/NCBI
|
|
59.
|
Lacroix M, Toillon RA and Leclercq G: p53
and breast cancer, an update. Endocr Relat Cancer. 13:293–325.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
60.
|
Subik K, Lee JF, Baxter L, Strzepek T,
Costello D, Crowley P, Xing L, Hung MC, Bonfiglio T, Hicks DG and
Tang P: The expression patterns of ER, PR, HER2, CK5/6, EGFR, Ki-67
and AR by immunohistochemical analysis in breast cancer cell lines.
Breast Cancer (Auckl). 4:35–41. 2010.PubMed/NCBI
|
|
61.
|
Sun D, Lopez-Guajardo CC, Quada J, Hurley
LH and Von Hoff DD: Regulation of catalytic activity and
processivity of human telomerase. Biochemistry. 38:4037–4044. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
62.
|
Harley CB, Futcher AB and Greider CW:
Telomeres shorten during ageing of human fibroblasts. Nature.
345:458–460. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
63.
|
Kim NW, Piatyszek MA, Prowse KR, Harley
CB, West MD, Ho PL, Coviello GM, Wright WE, Weinrich SL and Shay
JW: Specific association of human telomerase activity with immortal
cells and cancer. Science. 266:2011–2015. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
64.
|
Zahler AM, Williamson JR, Cech TR and
Prescott DM: Inhibition of telomerase by G-quartet DNA structures.
Nature. 350:718–720. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
65.
|
Hurley LH: DNA and its associated
processes as targets for cancer therapy. Nat Rev Cancer. 2:188–200.
2002. View
Article : Google Scholar : PubMed/NCBI
|
|
66.
|
Neidle S and Parkinson G: Telomere
maintenance as a target for anticancer drug discovery. Nat Rev Drug
Discov. 1:383–393. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
67.
|
Hurley LH, Wheelhouse RT, Sun D, Kerwin
SM, Salazar M, Fedoroff OY, Han FX, Han H, Izbicka E and Von Hoff
DD: G-quadruplexes as targets for drug design. Pharmacol Ther.
85:141–158. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
68.
|
Redon S, Bombard S, Elizondo-Riojas MA and
Chottard JC: Platinum cross-linking of adenines and guanines on the
quadruplex structures of the
AG3(T2AG3)3 and
(T2AG3)4 human telomere sequences
in Na+ and K+ solutions. Nucleic Acids Res.
31:1605–1613. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
69.
|
Phan AT and Patel DJ: Two-repeat human
telomeric d(TAGGGTTAGGGT) sequence forms interconverting parallel
and antiparallel G-quadruplexes in solution: Distinct topologies,
thermodynamic properties, and folding/unfolding kinetics. J Am Chem
Soc. 125:15021–15027. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
70.
|
Li J, Correia JJ, Wang L, Trent JO and
Chaires JB: Not so crystal clear: The structure of the human
telomere G-quadruplex in solution differs from that present in a
crystal. Nucleic Acids Res. 33:4649–4659. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
71.
|
Włodarczyk A, Grzybowski P, Patkowski A
and Dobek A: Effect of ions on the polymorphism, effective charge,
and stability of human telomeric DNA. Photon correlation
spectroscopy and circular dichroism studies. J Phys Chem B.
109:3594–3605. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
72.
|
Ambrus A, Chen D, Dai J, Bialis T, Jones
RA and Yang D: Human telomeric sequence forms a hybrid-type
intramolecular G-quadruplex structure with mixed
parallel/antiparallel strands in potassium solution. Nucleic Acids
Res. 34:2723–2735. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
73.
|
Dai J, Carver M, Punchihewa C, Jones RA
and Yang D: Structure of the Hybrid-2 type intramolecular human
telomeric G-quadruplex in K+ solution: Insights into
structure polymorphism of the human telomeric sequence. Nucleic
Acids Res. 35:4927–4940. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
74.
|
Hadi SM, Ullah MF, Shamim U, Bhatt SH and
Azmi AS: Catalytic therapy of cancer by ascorbic acid involves
redox cycling of exogenous/endogenous copper ions and generation of
reactive oxygen species. Chemotherapy. 56:280–284. 2010. View Article : Google Scholar : PubMed/NCBI
|