Introduction
With the introduction of the serum prostate-specific
antigen (PSA) assay in combination with digital rectal examination
(DRE) to screen for prostate cancer (PCa), the number of patients
undergoing subsequent transrectal ultrasonography (TRUS)-guided
needle biopsy has markedly increased (1). In addition, the systematic sextant
biopsy technique has become the standard method for prostate
biopsy, which has resulted in a significant improvement in the
accuracy of PCa diagnosis (2).
However, a number of studies have suggested that sextant biopsy may
underestimate the presence of malignancy (3,4). Thus,
there has been increasing interest in defining more accurate
prostate biopsy strategies in order to improve the PCa detection
rate. The benefits of increasing the number of biopsy cores and/or
expanding the number of regions sampled have been demonstrated
previously (5,6); however, it has also been suggested that
a 10- to 12-core initial biopsy may miss PCa in almost a third of
cases (7,8). Saturation prostate biopsy, which
consists of ≥20 cores, has been developed to improve the detection
rate of PCa in the context of repeat biopsies, radically altering
the general concept of prostate biopsy (9–12).
The decision to conduct a prostate biopsy
predominantly depends on the PSA value, which often generates false
positive results as this value may be increased by inflammation
(13). Our group previously reported
that histological prostatic inflammation significantly correlated
with the serum PSA level and a negative initial biopsy result
(14). Consequently, we hypothesized
that asymptomatic prostatic inflammation may cause a persistent PSA
increase in patients with a negative initial biopsy, leading to
repeat biopsy.
The present study attempted to clarify the
association between the risk factors for PCa and PCa detection in a
repeat biopsy with a 24-core saturation protocol. Repeat biopsy
specimens were evaluated for evidence of histological inflammation
by examining the leukocytes as one of the risk factors for PCa.
Materials and methods
Patients
Commencing in December 2010, 24-core repeat biopsies
were performed in patients for whom findings aroused suspicion of
PCa despite previous negative prostate biopsies. Such patients
included those with any of the following indications for repeat
biopsy: PSA >10 ng/ml; free-to-total PSA (fPSA/tPSA) ratio
<15%; PSA velocity (PSAV) >0.75 ng/ml/year; abnormal findings
on DRE, magnetic resonance imaging (MRI) or TRUS; or atypical small
acinar proliferation (ASAP) in the initial biopsy specimens.
The analysis included 78 Japanese patients whose
initial biopsy was negative and who consecutively underwent 24-core
prostate transperineal needle biopsy as a repeat biopsy between
December 2010 and November 2013 at Toyama University Hospital
(Toyama, Japan). All patients underwent TRUS to calculate the whole
prostate volume. Prostate volume (ml) was calculated using the
prostate ellipsoid formula (width × length × height × 0.523). PSA
density (PSAD) was calculated by dividing the total PSA (ng/ml) by
the prostate volume. The data were retrospectively analyzed. The
institutional review board of the University of Toyama (Toyama,
Japan) approved the study (#26–34). Due to the retrospective nature
of the study, written informed consent was waived. The study
conformed to the principles outlined in the Declaration of Helsinki
(15).
TRUS-guided systematic biopsy
Biopsies were performed with the patient in a dorsal
lithotomy position under spinal anesthesia. TRUS was performed
using the Aloka Prosound Alpha 5 SV Ultrasound system and a 5.0/7.5
MHz biplanar probe (Hitachi Aloka Medical, Ltd., Tokyo, Japan).
Transperineal biopsies of the prostate were obtained with an
18-gauge spring-loaded biopsy gun (Bard®
Max-Core® Disposable Core Biopsy Instrument; C. R. Bard,
Inc., Tempe, AZ, USA) under TRUS guidance. Biopsy cores were taken
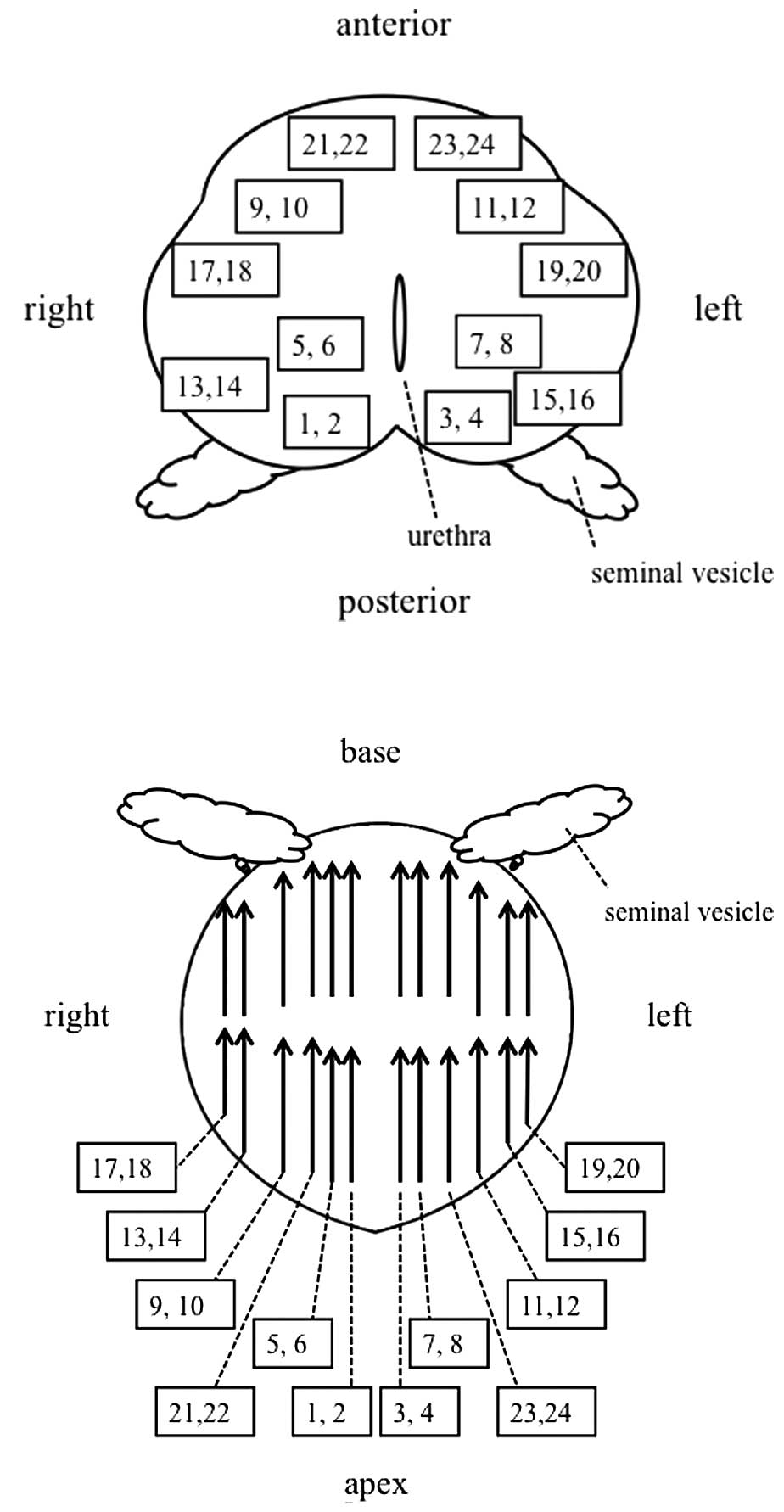
from 24 biopsy locations based on a standardized biopsy scheme
(Fig. 1) described by Abdollah et
al (9), with some modification.
Biopsy cores of odd numbers were taken from the apex, whereas cores
of even numbers were taken from the base of the prostate. Urethral
catheters were routinely inserted following the procedures. In the
majority of cases without severe adverse effects, the urethral
catheters were removed on the first day and patients were
discharged within three days subsequent to the procedure. All
biopsy samples were evaluated by the Department of Pathology at
Toyama University Hospital using the Gleason scoring system
(16). These findings were
morphologically evaluated on hematoxylin and eosin stained tissue
sections. Histological inflammation of the prostate was defined as
infiltration of prostatic biopsy specimens by inflammatory cells,
lymphocytes, plasma cells and/or histiocytes (17).
Statistical analysis
Data are presented as the mean ± standard deviation
(SD). Variables from the different groups were compared by the
Mann-Whitney U-test or Student's t-test using StatView
version 5 software (SAS Institute, Cary, NC, USA). P<0.05 was
considered to indicate a statistically significant difference.
Independent factors predicting a positive repeat biopsy were
identified by a logistic regression analysis.
Results
Patient characteristics
Tables I and II list the characteristics of all 78
patients who underwent repeat prostate biopsies following previous
negative biopsies according to the indications described. The
indications for biopsies were as follows: a PSA value >10 ng/ml
in 43.6% of cases; a fPSA/tPSA ratio <15% in 51.3%; a PSAV
>0.75 ng/ml/year in 53.8%; abnormal findings on DRE in 10.2%,
TRUS in 26.9% or MRI in 44.1%; and ASAP in previous biopsy
specimens in 18.4%. Of the included patients, 19 (24.4%) had a
single indication, whilst the remaining 59 (75.6%) had multiple
indications. The mean age at the time of repeat biopsy was 68.5±7.0
years and the mean PSA level prior to repeat biopsy was 12.4±10.7
ng/ml. The mean number of previous TRUS-guided needle biopsies was
1.6±1.0 (range, 1–6). Collectively, the 78 patients had experienced
a total of 124 previous biopsies, with mean of 10.7±3.5 cores per
patient (range, 6–24 cores), sampled via 82 transrectal (66.1%), 13
transperineal (10.4%) and 29 unknown (23.3%) routes.
 | Table I.Characteristics of 78 patients who
underwent repeat prostate biopsies following previous negative
biopsies. |
Table I.
Characteristics of 78 patients who
underwent repeat prostate biopsies following previous negative
biopsies.
| Parameter | n | mean ± SD |
|---|
| Age at diagnosis,
years |
| 68.5±7.0 |
|
50–59 | 8 |
|
|
60–69 | 33 |
|
|
70–79 | 33 |
|
|
80+ | 4 |
|
| Number of previous
negative biopsies |
| 1.60±1.03 |
| 1 | 50 |
|
| 2 | 17 |
|
| 3 | 7 |
|
| ≥4 | 4 |
|
| Serum PSA value at
repeat biopsy, ng/ml |
| 12.4±10.7 |
|
3.0–10.0 | 44 |
|
|
10.1–20.0 | 24 |
|
|
20.1–50.0 | 9 |
|
|
50.1–100.0 | 1 |
|
| fPSA/tPSA ratio,
% |
| 15.6±9.3 |
|
5.0–15.0 | 40 |
|
|
15.1–30.0 | 22 |
|
|
30.1–50.0 | 4 |
|
| Not
measured | 12 |
|
| Prostate volume,
ml |
| 47.3±19.0 |
|
20.0–40.0 | 35 |
|
|
40.1–60.0 | 27 |
|
|
60.1–100.0 | 14 |
|
|
>100.0 | 2 |
|
| PSA density,
ng/ml/ml |
| 0.32±0.41 |
|
0.050–0.150 | 23 |
|
|
0.151–0.50 | 46 |
|
|
>0.50 | 9 |
|
| PSA velocity,
ng/ml/year |
| 0.63±16.27 |
|
<0 | 11 |
|
|
0–0.750 | 25 |
|
|
0.751–5.000 | 28 |
|
|
>5.000 | 14 |
|
| Abnormal findings
on DRE |
|
|
|
Yes | 8 |
|
| No | 70 |
|
| Abnormal findings
on TRUS |
|
|
|
Yes | 21 |
|
| No | 57 |
|
| Abnormal findings
on MRI |
|
|
|
Yes | 34 |
|
| No | 43 |
|
| Not
assessed | 1 |
|
| ASAP in initial
biopsy specimens |
|
|
|
Yes | 11 |
|
| No | 67 |
|
 | Table II.Clinical parameters of the PCa and
BPD patients. |
Table II.
Clinical parameters of the PCa and
BPD patients.
| Parameters | All (n=78) | PCa (n=16) | BPD (n=62) | P-value |
|---|
| Age at diagnosis,
yearsa | 68.5±7.0 | 69.5±7.7 | 68.2±6.8 | 0.54 |
| Number of previous
biopsiesa | 1.60±1.03 | 1.62±1.31 | 1.59±0.96 | 0.92 |
| Serum PSA value at
repeat biopsy, ng/mla | 12.4±10.7 | 18.9±18.2 | 10.8±7.0 | <0.01 |
| fPSA/tPSA ratio,
%a | 15.6±9.3 | 11.1±5.2 | 16.8±9.9 | 0.04 |
| Prostate volume,
mla | 47.3±19.0 | 36.5±17.3 | 50.0±18.5 | 0.01 |
| PSA density,
ng/ml/mla | 0.32±0.41 | 0.62±0.71 | 0.24±0.23 | <0.01 |
| PSA velocity,
ng/ml/yeara | 0.63±16.27 | 4.62±5.81 | 50.0±17.9 | 0.27 |
| Abnormal findings
on DREb | 10.2% (8/78) | 12.5% (2/16) | 9.6% (6/62) | 0.76 |
| Abnormal findings
on TRUSb | 26.9% (21/78) | 31.2% (5/16) | 25.8% (16/62) | 0.66 |
| Abnormal findings
on MRIb | 44.1% (34/77) | 56.3% (9/16) | 40.9% (25/61) | 0.28 |
| ASAP in initial
biopsy specimensb | 14.1% (11/76) | 18.8% (3/16) | 12.9% (8/62) | 0.55 |
| Histological
inflammationb | 47.4% (37/78) | 6.3% (1/16) | 58.0% (36/62) | <0.01 |
Cancer detection
PCa was confirmed histologically in 16 of the 78
patients (20.5%), and benign prostatic disease (BPD) was diagnosed
in the remaining 62 patients (79.5%) based on the repeat biopsies.
Table II shows the clinical
parameters of the PCa and BPD patients. A univariate analysis
revealed that the PSA value at repeat biopsy, the fPSA/tPSA ratio,
the prostate volume and the PSAD were significantly associated with
the repeat biopsy outcome. PSA at repeat biopsy was higher
(P<0.01), fPSA/tPSA ratio was lower (P=0.04), total prostate
volume was smaller (P=0.01) and PSAD was higher (P<0.01) in
patients with PCa compared with BPD patients. Furthermore,
histological inflammation was more frequently observed in BPD
patients than PCa patients (P<0.01). Unexpectedly, the
differences between the PCa and BPD patients were not significant
for abnormal findings on DRE, MRI or TRUS. The presence of ASAP in
the initial biopsy specimens was also not associated with the
outcome of the repeat biopsy.
Table III
demonstrates results of the multivariate analysis of predictive
variables for the presence of prostate cancer on repeat biopsy. The
value of these factors as predictors of PCa detection on repeat
biopsy was subsequently analyzed using a logistic regression
analysis. The variables entered into the model were PSA at repeat
biopsy, fPSA/tPSA ratio, prostate volume, PSAD and presence of
histological inflammation. Among these factors, histological
inflammation was the only independent predictor for the presence of
a malignant lesion on repeat biopsy (odds ratio, 0.027;
P=0.01).
 | Table III.Results of the multivariate analysis
of predictive variables for the presence of prostate cancer on
repeat biopsy. |
Table III.
Results of the multivariate analysis
of predictive variables for the presence of prostate cancer on
repeat biopsy.
| Factor | P-value | Relative hazard
ratio | 95% confidence
interval |
|---|
| Serum PSA value at
repeat biopsy | 0.64 | 0.87 | 0.50–1.51 |
| fPSA/tPSA
ratio | 0.34 | 0.93 | 0.82–1.07 |
| Prostate
volume | 0.80 | 0.98 | 0.86–1.12 |
| PSA density | 0.47 | 571 |
1.5×10−5-2.1×1010 |
| Histological
inflammation | 0.01 | 0.027 | 0.001–0.54 |
Characteristics of prostate cancer
patients diagnosed by repeat biopsies
Table IV lists the
characteristics of all 16 patients diagnosed with PCa. Of these 16
patients, 14 (87.5%) had localized disease and 2 (12.5%) had
metastasis. The clinical stages were T1c, T2a and T2b in 4 (25%),
10 (62.5%) and 2 (12.5%) patients, respectively. The Gleason scores
were 3+3, 3+4 and ≥4+4 in 6 (37.5%), 3 (18.8%) and 7 (43.8%)
patients, respectively.
 | Table IV.Characteristics of 16 patients
diagnosed with prostate cancer by repeat biopsy. |
Table IV.
Characteristics of 16 patients
diagnosed with prostate cancer by repeat biopsy.
| Parameters | n |
|---|
| Serum PSA value at
repeat biopsy, ng/ml |
|
|
>3–10 | 6 |
|
>10–20 | 5 |
|
>20–50 | 4 |
|
>50–100 | 1 |
| Clinical T
stage |
|
|
T1c | 4 |
|
T2a | 10 |
|
T2b | 2 |
| Clinical N/M
stage |
|
|
N0M0 | 14 |
|
N1M0 | 1 |
|
N0M1 | 1 |
| Gleason score |
|
|
3+3 | 6 |
|
3+4 | 3 |
|
>4+4 | 7 |
| Number of positive
cores |
|
|
1–2 | 6 |
|
3–4 | 6 |
|
5–10 | 2 |
|
10–15 | 2 |
Of the 16 cases, 2 were compatible with the widely
used criteria for predicting clinically insignificant PCa,
consisting of T1c, PSA ≤10 ng/ml, Gleason score ≤6 and <50%
cancer involvement in any core (18).
Of these, 1 patient was subsequently determined to have significant
cancer. The PSA level, the clinical stage, and the Gleason score of
this patient were 5.73 ng/ml, T1cN0M0, 3+3, respectively, and 3/24
cores were positive for cancer. When the patient was subsequently
treated with radical retropubic prostatectomy (RRP), pT2c
significant disease and a Gleason score of 4+5 in the prostatectomy
specimen were determined. By contrast, the other patient was
confirmed to have clinically insignificant cancer. The detailed
clinical findings of this patient were as follows: PSA at repeat
biopsy, 4.48 ng/ml; clinical stage, T1cN0M0; Gleason score, 3+3;
and 1/24 cores positive for cancer. This patient subsequently
underwent RRP; however, the pathological stage was pT0.
Adverse effects
Major complications associated with biopsy that
required subsequent inpatient intervention occurred in 3 patients
(3.8%): 2 patients experienced urinary retention requiring
temporary catheterization; the other patient experienced severe
septicaemia with Escherichia coli that required intravenous
antibiotic administration, and this patient made a full recovery
two weeks after the procedure.
Discussion
To date, PSA measurement has been the most valuable
criterion upon which to base a diagnosis of PCa (19). The benefits of increasing the number
of biopsy cores and/or expanding the number of regions sampled have
been demonstrated in several studies. Pepe et al (5) compared 12-core with 18-core biopsies,
and identified significant differences in the detection rates,
which were determined to be 35 and 51%, respectively. Ficarra et
al (6) demonstrated a correlation
between a detection rate and a core count. Initial biopsy protocols
have been gradually extended to improve the detection rate through
more representative sampling of all areas in the prostate (5,6).
Repeat saturation biopsies have also been developed
in an effort to reduce the high false-negative rate of initial
biopsies. Several studies introduced modified techniques for repeat
biopsy involving an increased number of cores in order to sample
the entire gland. Merrick et al (10) demonstrated a positive correlation
between the detection rate and core count in a 50-core repeat
biopsy series. In a study by Borboroglu et al (11), an average of 22.5 cores was obtained
(depending on prostate size) in patients with previous negative
sextant biopsies, and cancer was identified in 30% of patients.
Similarly, Stewart et al (12)
obtained an average of 23 cores throughout the whole prostate of
patients who had previously had negative sextant biopsies, and
cancer was detected in 34% of cases.
To date, there are no established procedures for
repeat saturation biopsy. Abdollah et al (9) evaluated 332 patients who underwent a
transrectal 24-core repeat biopsy compared with 140 who underwent a
24-core transperineal biopsy, and concluded that transperineal and
transrectal saturation biopsies have a similar PCa detection rate
in patients undergoing a repeated saturation biopsy. On the other
hand, a number of studies have demonstrated that tumors
subsequently identified upon repeat biopsy are most frequently
found in the anterior prostate and/or transition zone (20,21). In
the present study, a transperineal route was used for repeat biopsy
as the majority of the patients had undergone initial biopsies via
the transrectal approach. Geometric considerations dictate that the
apex and the anterior region of the prostate are better sampled via
the transperineal route, while the base is better sampled via the
transrectal approach. However, in the current study, the sites in
which the cancer was detected were distributed uniformly, and there
did not seem to be any trends in the sampling site (data not
shown).
The detection of clinically insignificant PCa is an
inevitable risk of repeat biopsy (14). The majority of patients in the current
study were considered to have significant cancer. In particular, 2
patients (12.5%), whose cancer was not detected in the initial
biopsy, had metastatic disease. Thus, the present saturation
protocol has demonstrated generally favorable results. However, 1
patient (6.25%) was considered to have insignificant cancer in the
current study. It is supposed that clinically insignificant disease
is more likely to be detected with more extensive sampling, which
is why extensive sampling remains controversial. A study by Zaytoun
et al (22) suggested that
almost a third of PCa detected in the repeat saturation biopsy was
clinically insignificant. Conversely, a number of reports have
demonstrated that saturation repeat biopsy may not increase the
detection of clinically insignificant tumors (23,24).
Discriminating between PCa and BPD is difficult,
particularly in patients with initial negative biopsies (25,26). The
serum PSA level at repeat biopsy is one of the strongest predictors
of a positive repeat biopsy (25,26).
However, it is generally recognized that PSA lacks specificity for
PCa detection (14). Several
clinically available PSA-associated factors, including the
free-to-total PSA ratio, PSAD and PSAV, have been examined in an
attempt to improve the efficiency of repeat biopsies; however, the
value of these parameters remains the subject of considerable
debate.
The significance of the prostate volume for the
detection of PCa is thought to differ between the initial and
repeat biopsies (27–29). For initial biopsies, previous studies
have reported a decreasing probability of detecting cancer when the
same number of biopsy cores is taken from glands of increasing
size, indicating a potential benefit of increasing the number of
core samples in larger glands (27).
In addition, several studies have demonstrated an inverse
correlation between the PCa detection rate in repeat saturation
biopsies and the prostate volume; that is, there was a lower PCa
detection rate in large prostates compared with smaller glands
(28,29).
Consistent with previous reports (9,11,22,26,28,30),
the current study demonstrated that a higher PSA level at repeat
biopsy, lower prostate volume and elevated PSAD, were associated
with a higher rate of PCa detection. In the context of repeat
biopsy, intensive saturation biopsies should be recommended in
patients with a smaller prostate volume or increased PSAD.
DRE is indispensable for PCa screening, and is a
meaningful indication for an initial biopsy (14). Abnormal findings on MRI and TRUS are
also significant predictors of PCa, particularly in the initial
biopsy setting (31). By contrast, in
the repeat biopsy setting, the present study demonstrated that the
incidence of abnormal findings on DRE, TRUS and/or MRI in patients
with PCa was not significantly higher than that in patients with no
evidence of cancer. We hypothesize that this was due to a selection
bias, i.e. patients with well-visualized cancers had already been
diagnosed in the initial biopsies, and a biased population
therefore remained in the repeat biopsy setting.
Previously, the presence of high-grade prostatic
intraepithelial neoplasia (HGPIN) and/or ASAP was demonstrated to
be a useful predictor of the repeat biopsy outcome (28,32). The
role of HGPIN as a precursor of PCa remains controversial.
According to recent guidelines, a HGPIN diagnosis no longer
represents an indication for immediate repeat biopsy (33). By contrast, ASAP indicates the
presence of suspicious glands with insufficient cytological or
architectural atypia for a definitive diagnosis of prostatic
adenocarcinoma (30). In the present
study, the incidence of PCa was not significantly higher in
patients with ASAP. This may have been due to the limited number of
patients with ASAP in this cohort, and so these findings are not
conclusive. Further studies are required to clarify the prognostic
value of the presence of ASAP.
Serum PSA levels may be increased not only in
patients with PCa, but also in those with non-malignant conditions,
including benign prostatic hyperplasia, prostatic manipulation and
even asymptomatic and chronic prostatitis (19,34).
Prostatic inflammation is detected in some biopsy specimens, and
subclinical prostatitis is known to cause increases in the serum
PSA level (35–37). Morote et al (13) retrospectively studied 284 patients
with no evidence of cancer on sextant ultrasound-guided biopsies.
Benign tissue without inflammation was detected in 23.2% of the
patients, whilst 68.3% had chronic prostatitis and 8.4% had acute
prostatitis, suggesting that prostatic inflammation is common in
specimens of BPD. Our group previously reported that histological
prostatic inflammation was significantly correlated with the serum
PSA level and a negative biopsy result (14). Similarly, Terakawa et al
(38) reported that histological
inflammation in initial biopsy specimens was associated with a
negative initial biopsy outcome in men with a serum PSA level of
10–50 ng/ml.
In the current study, histological inflammation was
found to be independently associated with a lower risk of PCa, even
on repeat biopsy. Asymptomatic inflammation may cause increased PSA
in some men, leading to repeat prostate biopsy (14). A more selective repeat biopsy strategy
would help avoid unnecessary repeat biopsies in men with an
increased PSA level.
The limitations of this study include its
retrospective design, its being performed at a single center using
a single arm, and the relatively small number of patients. The
results may have been biased by the patient selection for repeat
biopsy. The probability of detecting clinically insignificant PCa
is still uncertain based on this study due to the limited number of
patients; further prospective studies with large populations are
necessary in order to clarify this issue. Nevertheless, even with
these limitations, the current results suggest that saturation
repeat biopsy may be effective in patients for whom PCa is
persistently suspected despite a negative initial biopsy.
Although numerous trials have been published, the
number and most appropriate location of the cores, in addition to
the timing and the criteria to perform repeat biopsy, remains
matters of debate. However, saturation biopsy appears to be
effective in patients with a persistent suspicion of PCa following
a negative initial biopsy. A relatively small prostate volume in a
patient with an elevated PSA level (i.e. a high PSAD) may be a
useful indicator of the results of a repeat saturation biopsy.
Furthermore, patients with asymptomatic inflammation appear to have
a lower risk of PCa. It should be considered that inflammation may
cause persistent elevated PSA in patients with a negative initial
biopsy, leading to unnecessary repeat biopsy.
Acknowledgements
The authors would like to thank Professor Joji Imura
of the Department of Diagnostic Pathology, Graduate School of
Medicine and Pharmaceutical Sciences for Research, University of
Toyama, for his valuable advice and suggestions with regard to
pathological findings.
Glossary
Abbreviations
Abbreviations:
|
PCa
|
prostate cancer
|
|
BPD
|
benign prostatic disease
|
|
PSA
|
prostate-specific antigen
|
|
PSAD
|
PSA density
|
|
PSAV
|
PSA velocity
|
|
DRE
|
digital rectal examination
|
|
MRI
|
magnetic resonance imaging
|
|
TRUS
|
transrectal ultrasonography
|
|
ASAP
|
atypical small acinar
proliferation
|
|
HGPIN
|
high-grade prostatic intraepithelial
neoplasia
|
|
RRP
|
radical retropubic prostatectomy
|
|
SD
|
standard deviation
|
References
|
1
|
Ouyang RC, Kenwright DN, Nacey JN and
Delahunt B: The presence of atypical small acinar proliferation in
prostate needle biopsy is predictive of carcinoma on subsequent
biopsy. BJU Int. 87:70–74. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hodge KK, McNeal JE, Terris MK and Stamey
TA: Random systematic versus directed ultrasound guided transrectal
core biopsies of the prostate. J Urol. 142:71–75. 1989.PubMed/NCBI
|
|
3
|
Rabbani F, Stroumbakis N, Kava BR, Cookson
MS and Fair WR: Incidence and clinical significance of
false-negative sextant prostate biopsies. J Urol. 159:1247–1250.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Norberg M, Egevad L, Holmberg L, Sparèn P,
Norlèn BJ and Busch C: The sextant protocol for ultrasound-guided
core biopsies of the prostate underestimates the presence of
cancer. Urology. 50:562–566. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pepe P and Aragona F: Prostate needle
biopsy: 12 vs. 18 cores - is it necessary? Urol Int. 74:19–22.
2005.PubMed/NCBI
|
|
6
|
Ficarra V, Novella G, Novara G, Galfano A,
Pea M, Martignoni G and Artibani W: The potential impact of
prostate volume in the planning of optimal number of cores in the
systematic transperineal prostate biopsy. Eur Urol. 48:932–937.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Schröder FH, Hugosson J, Roobol MJ,
Tammela TL, Ciatto S, Nelen V, Kwiatkowski M, Lujan M, Lilja H,
Zappa M, et al: Screening and prostate-cancer mortality in a
randomized European study. N Engl J Med. 360:1320–1328. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Andriole GL, Crawford ED, Grubb RL III,
Buys SS, Chia D, Church TR, Fouad MN, Gelmann EP, Kvale PA, Reding
DJ, et al: Mortality results from a randomized prostate-cancer
screening trial. N Engl J Med. 360:1310–1319. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Abdollah F, Novara G, Briganti A, Scattoni
V, Raber M, Roscigno M, Suardi N, Gallina A, Artibani W, Ficarra V,
et al: Transrectal versus transperineal saturation rebiopsy of the
prostate: Is there a difference in cancer detection rate? Urology.
77:921–925. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Merrick GS, Gutman S, Andreini H,
Taubenslag W, Lindert DL, Curtis R, Adamovich E, Anderson R, Allen
Z, Butler W and Wallner K: Prostate cancer distribution in patients
diagnosed by transperineal template-guided saturation biopsy. Eur
Urol. 52:715–723. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Borboroglu PG, Corner SW, Riffenburgh RH
and Amling CL: Extensive repeat transrectal ultrasound-guided
prostate biopsy in patient with previous benign sextant biopsies. J
Urol. 163:158–162. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Stewart CS, Leibovich BC, Weaver AL and
Lieber MM: Prostate cancer diagnosis using a saturation needle
biopsy technique after previous negative sextant biopsies. J Urol.
166:86–92. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Morote J, Lopez M, Encabo G and de Torres
IM: Effect of inflammation and benign prostatic enlargement on
total and percent free serum prostatic specific antigen. Eur Urol.
37:537–540. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kato T, Suzuki H, Komiya A, Imamoto T,
Naya Y, Tobe T and Ichikawa T: Clinical significance of urinary
white blood cell count and serum C-reactive protein level for
detection of non-palpable prostate cancer. Int J Urol. 13:915–919.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hellmann F, Verdi M, Schlemper BR Jr and
Caponi S: 50th anniversary of the Declaration of Helsinki: The
double standard was introduced. Arch Med Res. 45:600–601. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Epstein JI, Allsbrook WC Jr, Amin MB and
Egevad LL: ISUP Grading Committee: The 2005 International Society
of Urological Pathology (ISUP) Consensus Conference on Gleason
Grading of prostatic carcinoma. Am J Surg Pathol. 29:1228–1242.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Krieger JN, Nyberg L Jr and Nickel JC: NIH
consensus definition and classification of prostatitis. JAMA.
282:236–237. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tosoian JJ, Trock BJ, Landis P, Feng Z,
Epstein JI, Partin AW, Walsh PC and Carter HB: Active surveillance
program for prostate cancer: An update of the Johns Hopkins
experience. J Clin Oncol. 29:2185–2190. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Oesterling JE: Prostate specific antigen:
A critical assessment of the most useful tumor marker for
adenocarcinoma of the prostate. J Urol. 145:907–923.
1991.PubMed/NCBI
|
|
20
|
Kawakami S, Kihara K, Fujii Y, Masuda H,
Kobayashi T and Kageyama Y: Transrectal ultrasound-guided
transperineal 14-core systematic biopsy detects apico-anterior
cancer foci of T1c prostate cancer. Int J Urol. 11:613–618. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shannon BA, McNeal JE and Cohen RJ:
Transition zone carcinoma of the prostate gland: A common indolent
tumor type that occasionally manifests aggressive behaviour.
Pathology. 35:467–471. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zaytoun OM, Moussa AS, Gao T, Fareed K and
Jones JS: Office-based transrectal saturation biopsy improves
prostate cancer detection compared to extended biopsy in the repeat
biopsy population. J Urol. 186:850–854. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bastian PJ, Mangold LA, Epstein JI and
Partin AW: Characteristics of insignificant clinical T1c prostate
tumors. A contemporary analysis. Cancer. 101:2001–2005. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Epstein JI, Sanderson H, Carter HB and
Scharfstein DO: Utility of saturation biopsy to predict
insignificant cancer at radical prostatectomy. Urology. 66:356–360.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gann PH, Fought A, Deaton R, Catalona WJ
and Vonesh E: Risk factors for prostate cancer detection after a
negative biopsy: A novel multivariable longitudinal approach. J
Clin Oncol. 28:1714–1720. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Djavan B, Fong YK, Ravery V, Remzi M,
Horninger W, Susani M, Kreuzer S, Boccon-Gibod L, Bartsch G and
Marberger M: Are repeat biopsies required in men with PSA levels
< or =4 ng/ml? A multi-institutional prospective European study.
Eur Urol. 47:38–44. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Brawer MK: The influence of prostate
volume on prostate cancer detection. Eur Urol Suppl. 1:35–39. 2002.
View Article : Google Scholar
|
|
28
|
Campos-Fernandes JL, Bastien L, Nicolaiew
N, Robert G, Terry S, Vacherot F, Salomon L, Allory Y, Vordos D,
Hoznek A, et al: Prostate cancer detection rate in patients with
repeated extended 21-sample needle biopsy. Eur Urol. 55:600–606.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sajadi KP, Kim T, Terris MK, Brown JA and
Lewis RW: High yield of saturation prostate biopsy for patients
with previous negative biopsies and small prostates. Urology.
70:691–695. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Oderda M and Gontero P: High-grade
prostatic intraepithelial neoplasia and atypical small acinar
proliferation: Is repeat biopsy still necessary? BJU Int.
104:1554–1556. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Inahara M, Suzuki H, Nakamachi H, Kamiya
N, Shimbo M, Komiya A, Ueda T, Ichikawa T, Akakura K and Ito H:
Clinical evaluation of transrectal power doppler imaging in the
detection of prostate cancer. Int Urol Nephrol. 36:175–180. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chan TY and Epstein JI: Follow-up of
atypical prostate needle biopsies suspicious for cancer. Urology.
53:351–355. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Heidenreich A, Aus G, Bolla M, Joniau S,
Matveev VB, Schmid HP and Zattoni F: European Association of
Urology: EAU guidelines on prostate cancer. Eur Urol. 53:68–80.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Stamey TA, Yang N, Hay AR, McNeal JE,
Freiha FS and Redwine E: Prostate-specific antigen as a serum
marker for adenocarcinoma of the prostate. N Engl J Med.
317:909–916. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yaman O, Göğüş C, Tulunay O, Tokatli Z and
Ozden E: Increased prostate-specific antigen in subclinical
prostatitis: The role of aggressiveness and extension of
inflammation. Urol Int. 71:160–164. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Schatteman PH, Hoekx L, Wyndaele JJ,
Jeuris W and Van Marck E: Inflammation in prostate biopsies of men
without prostatic malignancy or clinical prostatitis: Correlation
with total serum PSA and PSA density. Eur Urol. 37:404–412. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kandirali E, Boran C, Serin E, Semercioz A
and Metin A: Association of extent and aggressiveness of
inflammation with serum PSA levels and PSA density in asymptomatic
patients. Urology. 70:743–747. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Terakawa T, Miyake H, Kanomata N, Kumano
M, Takenaka A and Fujisawao M: Inverse association between
histologic inflammation in needle biopsy specimens and prostate
cancer in men with serum PSA of 10–50 ng/ml. Urology. 72:1194–1197.
2008. View Article : Google Scholar : PubMed/NCBI
|