Introduction
Epithelial ovarian cancer (EOC) is the most lethal
type of gynecological malignancy (1).
Each year, almost 22,000 new diagnoses of EOC are made in the
United States, and ~14,000 mortalities occur due to disease
progression (1). The majority of
patients in the early stages of the disease are free of any
symptoms; thus, the disease is often of an advanced stage when
diagnosed (2). Despite efforts made
towards the improvement of primary treatment, over two thirds of
treated patients relapse and require a sufficient second-line
treatment strategy, which is typically palliative (3).
In cases of platinum-sensitive relapse, the standard
treatment approach for tumor recurrence occurring at ≥12 months
after the primary platinum-based chemotherapy is a re-challenge of
platinum-containing regimens; several large trials have proven
superiority of platinum-based chemotherapy as a second line therapy
compared to non-platinum base chemotherapy (4–6). By
contrast, patients relapsing within the first 6 months after
primary treatment are considered to have platinum-resistant
disease, and the treatment options, as well as the prognosis of the
patients, are limited (7). Patients
who experience tumor recurrence between 6 and 12 months after
primary treatment are considered to have partially
platinum-sensitive disease (8). Thus,
the necessity for active and tolerable drugs for patients with
recurrent EOC is urgent, particularly for cases of
non-platinum-sensitive relapse.
In patients with platinum-refractory EOC, a number
of cytostatic agents, including paclitaxel, topotecan and
gemcitabine, have been trialled and have demonstrated activity
(7–9).
Furthermore, pegylated liposomal doxorubicin has been reported to
be active and well-tolerable as second-line treatment option
(10–16). However, pegylated liposomal
doxorubicin presents a specific toxicity profile, including palmar
plantar erythrodysesthesia (PPE) most prominently, which limits
feasibility of this regimen (17,18).
Non-pegylated liposomal doxorubicin (Myocet®) has been
studied in clinical trials in the treatment of several types of
solid tumor, including metastatic breast cancer (16). Due to its different pharmacological
formulation, which lacks a pegylated membrane around the
doxorubicin-carrying liposomes, non-pegylated liposomal doxorubicin
should cause less PPE. This hypothesis is driven from preclinical
experiences (17) and has been
confirmed by clinical evidence (12–16).
However, few data are currently available on the potential role of
this drug in the treatment of recurrent ovarian cancer.
The present prospective multicentric non-randomized
single-arm phase II trial was conducted to gain further data
regarding the activity and tolerability of non-pegylated liposomal
doxorubicin in patients with recurrent ovarian cancer, relapsing
within 12 months after primary platinum-containing therapy.
Patients and methods
Trial design
This prospective non-randomized single-arm phase II
trial was a multicenter study in nine German sites. The majority of
the patients were treated in the Department of Gynecology and
Obstetrics of the University Hospital of Heidelberg (Heidelberg,
Germany). The study was designed to assess the toxicity and
efficacy of non-pegylated liposomal doxorubicin
(Myocet®; Cephalon, Inc.; Teva Pharmaceutical
Industries, Ltd., Frazer, PA, USA) in patients with recurrent
non-platinum-sensitive ovarian cancer, which was defined as relapse
within 12 months after the end of platinum-containing first-line
chemotherapy. Other studies have shown that patients with recurrent
ovarian cancer within 12 months after platinum-based first line
chemotherapy do not benefit by a second surgery; these patients
should be treated with a second-line chemotherapy (19). The current study was approved by the
institutional ethics committee of the University of Heidelberg. All
patients signed an informed consent prior to the beginning of
treatment.
Treatment plan
All patients received non-pegylated liposomal
doxorubicin in a 1-h intravenous (i.v.) infusion every 21 days for
a maximum of 6 cycles, except in cases of disease progression
during treatment. A prolongation of treatment for a further 2
cycles was allowed following the clinical decision of local
investigators. The trial commenced with a dosage of 75
mg/m2. Due to the experience of myelosuppression grade
III in 3 of the first 5 treated patients, a dose reduction to 60
mg/m2 d1q22 was implemented and reviewed as an amendment
to the trial. Premedication consisted of dexamethasone (8 mg, i.v.)
and granisetron (1 mg i.v.). In addition, patients received
dexamethasone (4 mg twice daily) on days 2 and 3. Treatment was
discontinued in cases of progressive disease or unacceptable
toxicity, or as per the patient's preference.
Eligibility criteria
Women aged between 18 and 75 years with
histologically confirmed EOC, cancer of the fallopian tube or
peritoneal cancer, with a prior therapy consisting of
platinum-containing chemotherapy and a second-line chemotherapy
situation, were included in this study if the platinum-free
interval was <12 months. Patients were required to have an
evaluable or measurable tumor mass on computed tomography (CT) or
magnetic resonance imaging (MRI) scans, or progression of the
disease in terms of significant increase of cancer antigen 125
(CA125; >25% compared to the lowest value prior to the
commencement of non-pegylated liposomal doxorubicin therapy, or
nadir), a life expectancy of >3 months, satisfactory bone marrow
function [white blood cell count >2,0×109/l; absolute
neutrophil count >1,5×109/l; platelet count
>100×109/l; hemoglobin >10 g/dl (if necessary
after transfusion)], satisfactory renal function (serum creatinine
<1.25 × upper norm of calculated creatinine clearance >60
ml/min) and satisfactory hepatic function (bilirubin <1.25 ×
upper norm or <5 × upper norm with hepatic metastasis;
transaminases <3 × upper norm or <5 × upper norm with hepatic
metastasis), and an Eastern Cooperative Oncology Group performance
status ≤2 as well as a left ventricular ejection fraction ≥50%.
Patients were excluded from the trial, if one of the
following criteria was met: Previous anthracycline treatment;
secondary malignancy, with the exception of ductal carcinoma in
situ or cervical intraepithelial neoplasia; patients lacking an
evaluable or measurable tumor mass and not showing a significant
increase of CA125; cardiac arrhythmias, cardiac insufficiency or
history of myocardial infarction within the previous 6 months;
acute infection not allowing cytotoxic treatment; severe
comorbidities, such as uncontrolled infections; synchronous or
scheduled radiotherapy; or major psychiatric diseases not allowing
treatment within trial conditions.
Outcome assessment
The primary trial objective was observing the
activity of non-pegylated liposomal doxorubicin in terms of
response. The secondary objective was to evaluate toxicity,
duration of response, overall survival (OS) time and
progression-free survival (PFS) time.
Response was evaluated on every 3 therapy cycles by
CT/MRI and CA125 response, as well as every 3 months during 18
months of follow-up. Evaluation of response was assessed according
to the World Health Organization criteria (20), as follows: Complete remission, total
disappearance of measurable tumor mass; partial remission, tumor
mass reduction >50%; stable disease, reduction of tumor mass
<50% or progression <25%; or progressive disease, new tumor
manifestations or progression of the tumor mass >25% or increase
of CA125 >25%).
The clinical benefit rate (CBR) was defined as the
rate of patients with complete remission or partial remission, or
with stable disease for >6 months. PFS was defined as the time
from diagnosis to disease progression or recurrence, or to the date
of mortality or last known contact (whichever occurred first.) OS
was determined as time interval between randomization and mortality
or last contact. Toxicity and tolerability analyses were performed
in all patients who completed ≥1 cycle of therapy. Hematological
and non-hematological toxicities were evaluated and graded
according to the National Cancer Institute (NCI) Common Toxicity
Criteria (21).
Data management and statistical
analysis
All data were collected and saved using the
electronic documentation system SAS version 9.2 (SAS Institute,
Inc., Cary, NC, USA) according to good clinical practice in a
pseudonymized form (22). Data are
expressed as the absolute and relative frequencies, or the median
and range. Data from survival analyses are presented as the mean or
median survival rates and 95% confidence intervals. Fisher's exact
test was used to compare statistical differences between patient
subgroups. Statistical significance was considered to be indicated
by P<0.05. Data from toxicity analyses, PFS, OS and CBR were
evaluated with methods of descriptive statistics. All analyses,
except the primary endpoint, have an explorative character.
Results
Patients and treatments
Between February 2005 and January 2011, 29 patients
with recurrent ovarian cancer within 12 months after
platinum-containing primary treatment were randomized to this trial
in nine German sites. The characteristics of the patients are
presented in Table I. The median age
of the patients was 60 years (range, 25–75 years). The majority of
the patients had primarily been diagnosed with low-grade EOC
(55.2%) of International Federation of Gynecology and Obstetrics
(FIGO) stage IIIC (48.3%) (23). All
but 3 patients initially presented with FIGO stage III/IV
disease.
 | Table I.Patient characteristics (n=29). |
Table I.
Patient characteristics (n=29).
| Parameter | Value |
|---|
| Age, years; median
(range) | 60 (25–75) |
| Histology of primary
tumor, n |
|
|
Adenocarcinoma | 3 |
| Serous
carcinoma | 8 |
| Papillary
serous carcinoma | 12 |
| Clear
cell carcinoma | 1 |
|
Other | 5 |
| Grade, n |
|
| 1 | 0 |
| 2 | 10 |
| 3 | 16 |
| X | 3 |
| Primary tumor FIGO
stage, n |
|
| I | 2 |
| IIA | 0 |
| IIB | 1 |
| IIIA | 2 |
| IIIB | 4 |
| IIIC | 14 |
| IV | 5 |
| Missing
data | 1 |
| WHO performance
status prior to start of Myocet® chemotherapy, n |
| 0 | 16 |
| 1 | 11 |
| 2 | 2 |
| Missing
data | 0 |
| Duration of
first-line pretreatment with platinum, days; median (range) | 112 (72–234) |
Second-line therapy with non-pegylated liposomal
doxorubicin consisted of 75 mg/m2 given every 3 weeks as
1-h i.v. infusions for a total of 6–8 cycles. As grade III
hematological toxicities occurred in 3 of the first 5 treated
patients, the treatment schedule was modified to 60
mg/m2 d1q22. Within the subsequent 24 patients, only 1
patient required a dose reduction to 50 mg/m2 according
to protocol after the third cycle of chemotherapy, due to a
non-hematological toxicity.
A total of 124 cycles of non-pegylated liposomal
doxorubicin were administered. There were 11 patients who completed
≥6 cycles of therapy, and 4 patients who completed 8 cycles of
chemotherapy, respectively. Early discontinuation of therapy was
necessary in 15 cases (due to progressive disease in 46.7% of the
cases and due to non-hematological toxicities in 20%,
respectively).
Activity data
Of the included patients, 20 were available for
response evaluation (10 with initial relapse at <6 months after
platinum-containing therapy, and 10 patients with initial relapse
6–12 months after platinum-containing therapy). There were 9
patients who were not available for response evaluation, mostly due
to premature discontinuation of therapy because of progression,
toxicity or personal decision following the first cycle of therapy.
The overall response rate of the patients, all of whom initially
had measurable disease, was 20%; 1 patient (initial relapse within
6–12 months after platinum-containing therapy) experienced a
complete remission, whereas 3 patients (15%) experienced partial
remission (all 3 patients with initial relapse within 6–12 months
after platinum-containing therapy). A further 6 patients (30%)
exhibited stable disease (3 patients with initial relapse <6
months after platinum-containing therapy; 3 patients with initial
relapse within 6–12 months after platinum-containing therapy),
consistent with a clinical benefit rate of 50%. In 8 patients
(40%), a progression of the disease was observed (5 patients with
initial relapse <6 months after platinum-containing therapy; 3
patients with initial relapse within 6–12 months after
platinum-containing therapy). Table
II shows all response data in detail. Serum levels of CA125
during the study period were available for 25 patients; in summary,
the median serum levels of CA125 were 246 U/ml [standard deviation
(SD), ±2,260.76 U/ml] prior to treatment, 231.60 U/ml (SD, ±530.38
U/ml) at the end of treatment, and 116.3 U/ml (SD, ±617.3) at the
end of the 18-month follow-ups.
 | Table II.Clinical response rates and survival
data (entire study population, n=29). |
Table II.
Clinical response rates and survival
data (entire study population, n=29).
|
| Relapse (n=20),
n |
|
|---|
|
|
|
|
|---|
| Clinical
response | <6 months | 6–12 months | % |
|---|
| Complete
remission | 0 | 1 | 5 |
| Partial
remission | 0 | 3 | 15 |
| Stable disease | 3 | 3 | 30 |
| Progressive
disease | 5 | 3 | 40 |
| Not evaluable | 2 | 0 | 10 |
A total of 25 patients were available for progress
evaluation in follow-up: 22 of these patients (88%) showed
progression of disease (11 patients with initial relapse <6
months after platinum-containing therapy; 11 patients with initial
relapse within 6–12 months after platinum-containing therapy). The
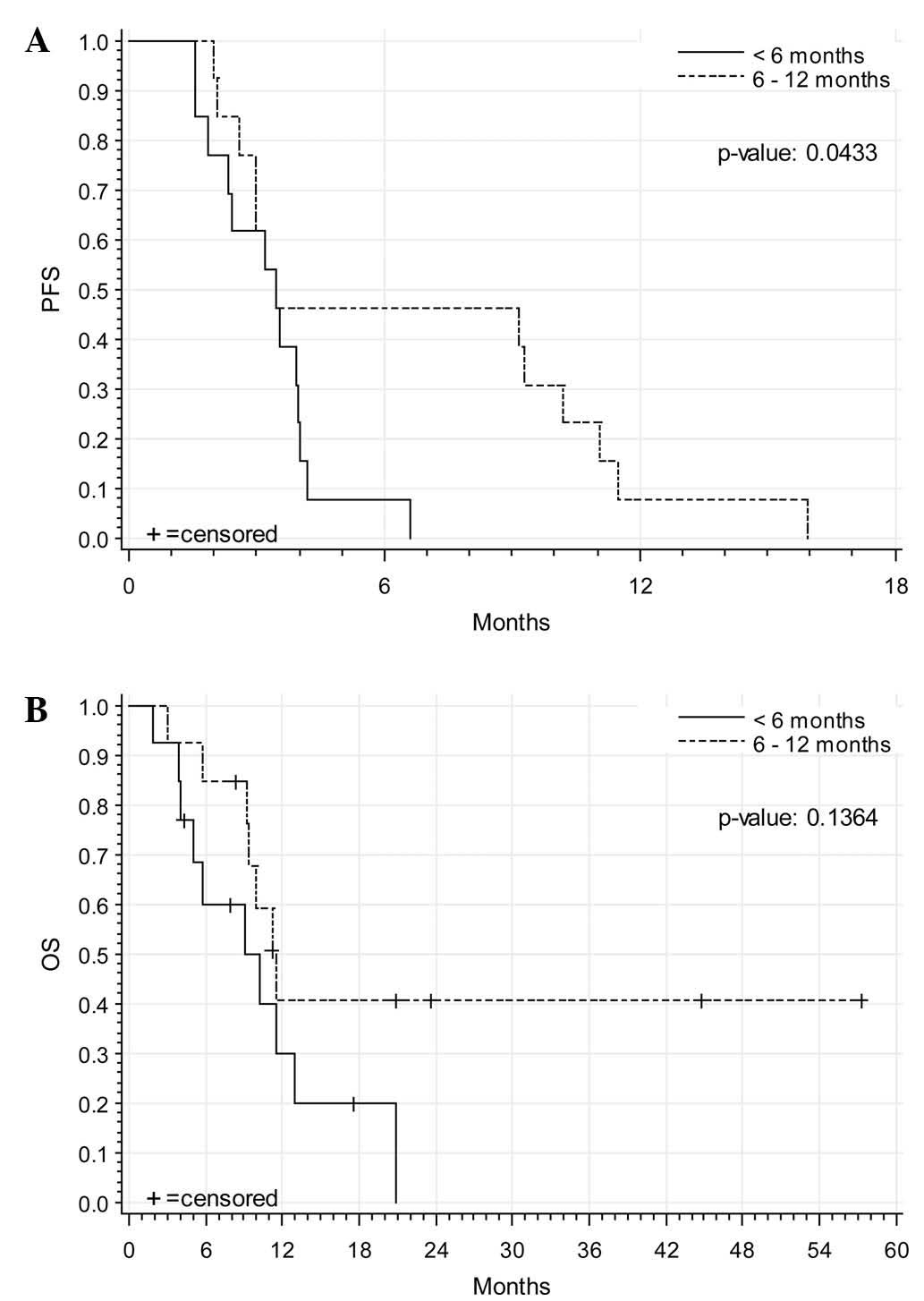
median PFS was 3.45 months (SD, ±0.74) (3.45 months in patients
with initial relapse <6 months after platinum-containing
therapy; 3.45 months in patients with initial relapse within 6–12
months after platinum-containing therapy). The median OS was 10.2
months (SD, ±1.39) (9.04 months in patients with initial relapse
<6 months after platinum-containing therapy; 11.47 patients with
initial relapse within 6–12 months after platinum-containing
therapy). A total of 10 patients were still alive at last
follow-up. Further chemotherapy was administered in 61.9% of the
patients during follow-up; topotecan was the most common
subsequently administered chemotherapy, with a median of 3 cycles.
Additionally, 7.1% of patients underwent a second surgery during
follow-up. Fig. 1 demonstrates the
corresponding Kaplan-Meier analyses for PFS and OS.
Toxicity
All 29 patients were evaluable for toxicity. A total
of 105 adverse events occurred. The majority of the adverse events
were documented in the NCI groups of ‘blood/bone marrow’,
‘gastrointestinal’ and ‘constitutional symptoms’. Grade III/IV
toxicity was predominantly documented for hematological toxicities:
Anemia, neutropenia and leukopenia grade III were documented for 1
patient each, whereas leukopenia and low granulocytes grade I/II
were common, occurring in 17 (58.6%) and 16 (55.2%) patients,
respectively. Anemia grade I/II was documented in 28 patients.
Thrombocytopenia grade I/II was documented for 14 patients. Blood
transfusions were given in 9 cases (7.4%) and growth factors in 26
cases (21.5%).
The most common non-hematological toxicity was
nausea (grade I/II, 14 patients; grade III, 1 patient), and fatigue
as a constitutional symptom was documented in 9 patients for grade
I/II and in 2 patients for grade III. No PPE was observed in any
patient. There were 3 patients with grade III allergic reactions
(flush) on infusion that could easily be managed by steroid
infusions. There was 1 grade III increase of aspartate
aminotransferase/alanine aminotransferase. All of the other adverse
events documented were of a lower grade. Table III summarizes the observed
hematological and non-hematological toxicities observed.
 | Table III.Toxicities in the patient population
(n=29). |
Table III.
Toxicities in the patient population
(n=29).
|
| Grade, n |
|
|---|
|
|
|
|
|---|
| Parameter | I/II | III/IV | Total |
|---|
| Hematological |
|
|
|
|
Neutropenia | 16 | 1 | 17 |
|
Leukopenia | 17 | 1 | 18 |
|
Anemia | 28 | 1 | 29 |
|
Thrombocytopenia | 14 | 0 | 14 |
|
Non-hematological |
|
|
|
|
Allergic reaction | 2 | 3 | 4 |
|
Nausea | 14 | 1 | 15 |
|
Vomiting | 4 | 0 | 4 |
|
Alopecia | 0 | 0 | 0 |
|
Fever | 2 | 0 | 2 |
|
Fatigue | 9 | 2 | 11 |
|
Mucositis/stomatitis | 2 | 0 | 2 |
A total of 12 severe adverse events occurred. In 9
cases, hospitalization was the reason for the evaluation as
‘severe’. Mortality due to thrombosis during therapy occurred in 1
patient; however, this fatality was doubtfully related to the
treatment. The median duration of the severe adverse events was 3
days.
Discussion
Despite significant improvements in primary
treatment efforts for EOC, over two-thirds of these patients
relapse and require a second-line therapy (3). In particular, for patients with
non-platinum-sensitive disease, there is an urgent necessity for
developing drugs that offer satisfactory antitumoral activity as
well as mild toxicity, in order to maintain an acceptable quality
of life.
The current trial demonstrated for the first time
prospectively that non-pegylated liposomal doxorubicin is active in
the treatment of recurrent ovarian cancer. With a response rate of
20%, the activity of this drug is similar and comparable to other
single-agent treatment options in this setting (7–9). In
addition, PFS and OS times of 3.5 and 10.2 months, respectively,
are also consistent with previously reported survival data from
other second-line trials (11).
An advantage of non-pegylated liposomal doxorubicin
is the specific, moderate toxicity profile of the drug. Following
dose modification during the trial to a 60 mg/m2 d1q22
schedule, few grade III/IV hematological toxicities were
subsequently observed in the patients. Furthermore, no alopecia or
PPE were noted, indicating a remarkable clinical difference against
pegylated liposomal doxorubicin treatment (16,17), in
which PPE can be expected in ~50% of cases at standard dosages of
50 mg/m2 q4w, and grade III PPE may develop in 20% of
the patients (17).
A clinical particularity of non-pegylated liposomal
doxorubicin that was indicated from the current trial data was that
there appears to be a relevant allergenic potential of the drug (3
patients experienced a grade III allergic reaction). This confirms
previously reported experiences, and should lead the treating
physician to specific caution in this respect (24). Nevertheless, this adverse reaction
occurred only in 10%, and was clinically uncomplicatedly
manageable, resolving completely without any residuals. A
pathophysiological explanation of this phenomenon is currently
lacking; it can only be speculated that the presence of pegylating
proteins on the surface of the liposomes may cover their allergenic
potential, as this adverse event is not commonly reported from
pegylated liposomal doxorubicin.
In summary, non-pegylated liposomal doxorubicin
appears to be a useful additional treatment option for patients
with recurrent non-platinum-sensitive disease.
Acknowledgements
This trial was realized with a scientific grant of
Cephalon, Inc. (Teva Pharmaceutical Industries, Ltd., Frazer, PA,
USA).
Glossary
Abbreviations
Abbreviations:
|
CA125
|
cancer antigen 125
|
|
CBR
|
clinical benefit rate
|
|
CT
|
computed tomography
|
|
EOC
|
epithelial ovarian cancer
|
|
FIGO
|
International Federation of Gynecology
and Obstetrics
|
|
MRI
|
magnetic resonance imaging
|
|
OS
|
overall survival
|
|
PFS
|
progression-free survival
|
|
PPE
|
palmar plantar erythrodysesthesia
|
|
SD
|
standard deviation
|
References
|
1
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hennessy BT, Colemann RL and Markmann M:
Ovarian Cancer. Lancet. 374:1371–1382. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Armstrong DK: Relapsed ovarian cancer:
Challenges and management strategies for a chronic disease.
Oncologist. 7(Suppl 5): 20–28. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Piccart MJ, Du Bois A, Gore ME, Neijt JP,
Pecorelli S and Pujade-Lauraine E: A new standard of care for
treatment of ovarian cancer. Eur J Cancer. 36:10–12. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bookman MA: Standard treatment in advanced
ovarian cancer in 2015 The state of the art. Int J Gynecol Cancer.
15:(Suppl 3). S212–S220. 2005. View Article : Google Scholar
|
|
6
|
du Bois A, Herrstedt J, Hardy-Bessard AC,
Müller HH, Harter P, Kristensen G, Joly F, Huober J,
Avall-Lundqvist E, Weber B, et al: Phase III trial of carboplatin
plus paclitaxel with or without gemcitabine in first-line treatment
of epithelial ovarian cancer. J Clin Oncol. 28:4162–4169. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Naumann RW and Coleman RL: Management
strategies for recurrent platinum-resistant ovarian cancer. Drugs.
71:1397–1412. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pfisterer J and Ledermann JA: Management
of platinum-sensitive recurrent ovarian cancer. Semin Oncol. 33(2
Suppl 6): S12–S16. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cormio G, Loizzi V, Gissi F, Camporeale A,
De Mitri P, Leone L, Putignano G and Selvaggi L: Long-term
topotecan therapy in recurrent or persistent ovarian cancer. Eur J
Gynaecol Oncol. 32:153–155. 2011.PubMed/NCBI
|
|
10
|
Markman M: Pegylated liposomal
doxorubicin: Appraisal of its current role in the management of
epithelial ovarian cancer. Cancer Manag Res. 3:219–225. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gordon AN, Granai CO, Rose PG, Hainsworth
J, Lopez A, Weissman C, Rosales R and Sharpington T: Phase II study
of liposomal doxorubicin in platinum-and paclitaxel-refractory
epithelial ovarian cancer. J Clin Oncol. 18:3093–3100.
2000.PubMed/NCBI
|
|
12
|
Muggia FM, Hainsworth JD, Jeffers S,
Miller P, Groshen S, Tan M, Roman L, Uziely B, Muderspach L, Garcia
A, et al: Phase II study of liposomal doxorubicin in refractory
ovarian cancer: Antitumor activity ad toxicity modification by
liposomal encapsulation. J Clin Oncol. 15:987–993. 1997.PubMed/NCBI
|
|
13
|
Israel VP, Garcia AA, Roman L, Muderspach
L, Burnett A, Jeffers S and Muggia FM: Phase II study of liposomal
doxorubicin in advanced gynecological cancers. Gynecol Oncol.
78:143–147. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Campos SM, Penson RT, Mays AR, Berkowitz
RS, Fuller AF, Goodman A, Matulonis UA, Muzikansky A and Seiden MV:
The clinical utility of liposomal doxorubicin in recurrent ovarian
cancer. Gynecol Oncol. 81:206–212. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Markman M, Kennedy A, Webster K, Peterson
G, Kulp B and Belinson J: Phase 2 trial of liposomal doxorubicin
(40 mg/m(2)) in platinum/paclitaxel-refractory ovarian and
fallopian tube cancers and primary carcinoma of the peritoneum.
Gynecol Oncol. 78:369–372. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bernardi D, Errante D, Stefani M and
Salvagno L: Non-pegylated liposomal doxorubicin in metastatic
breast cancer patients: A valuable therapeutic option requiring
caution. Breast. 19:549–550. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lorusso D, Di Stefano A, Carone V, Fagotti
A, Pisconti S and Scambia G: Pegylated liposomal
doxorubicin-related palmar-plantar erythrodysesthesia (‘hand-foot’
syndrome). Ann Oncol. 18:1159–1164. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Miolo G, Baldo P, Bidoli E, Lombardi D,
Scalone S, Sorio R and Veronesi A: Incidence of palmar-plantar
erythrodysesthesia in pretreated and unpretreated patients
receiving pegylated liposomal doxorubicin. Tumori. 95:687–690.
2009.PubMed/NCBI
|
|
19
|
Meier W, Römisch M and Hepp H: The value
of reoperation in the treatment of ovarian cancer. Geburtshilfe
Frauenheilkd. 53:30–34. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
World Health Organization: WHO handbook
for reporting results of cancer treatment. WHO offset publication.
No. 48. World Health Organization; Geneva, Switzerland: 1979
|
|
21
|
Cancer Therapy Evaluation Program. Common
toxicity criteria for adverse events. Version 3.0. National Cancer
Institute, National Institutes of Health; Bethseda, MD: 2006
|
|
22
|
Food and Drug Administration:
International Conference on Harmonisation. Good Clinical Practice
Consolidated Guideline. 1997.https://www.gpo.gov/fdsys/pkg/FR-1997-05-09/pdf/97-12138.pdfAccessed.
May 25–2016
|
|
23
|
Heintz AP, Odicino F, Maisonneuve P,
Beller U, Benedet JL, Creasman WT, Ngan HY, Sideri M and Pecorelli
S: Carcinoma of the ovary. J Epidemiol Biostat. 6:107–138.
2001.PubMed/NCBI
|
|
24
|
Bokemeyer C and Lipp HP: Allergic
reaction. Compendium of Oncology Standards in Diagnosis and
Therapy. Schmoll HJ, Höffken K and Possinger K: 4th.
Springer-Verlag GmbH; Heidelberg: pp. pp1947–1952. 2006, (In
German).
|