Introduction
Esophageal cancer is a common malignancy and is the
sixth leading cause of cancer-associated mortality worldwide
(1). Esophageal cancer has a striking
geographical distribution; for example, with an increased
prevalence in the north and central regions of China (2). Histopathologically, the vast majority of
esophageal cancer cases in China are of the esophageal squamous
cell carcinoma (ESCC) type (3).
Currently, there are limited clinical approaches for the early
diagnosis and treatment of ESCC, resulting in a 5-year survival
rate of ~10% for patients (3). A
better understanding of the molecular events involved in the
development of ESCC may offer opportunities to identify diagnostic
markers, therapeutic targets or prognostic indicators for this
disease.
Cellular senescence, a physiological program of
irreversible growth arrest that is triggered by multiple factors
such as oncogenic stress and DNA damage, is considered to be
important for the development of cancer (4). Senescent cells exhibit a characteristic
increase of senescence associated-β-galactosidase (SA-β-Gal)
activity together with profound alterations in protein secretion
that are collectively called the senescence-associated secretory
phenotype (SASP) or senescence-messaging secretome (5). Among these secreted molecules,
plasminogen activator inhibitor-1 (PAI1), matrix metalloproteinases
(MMPs), chemokines such as chemokine (C-X-C motif) ligand 1 (CXCL1)
and interleukin (IL)8, proinflammatory cytokines, including IL1 and
IL6, and other molecules are involved in insulin-like growth factor
(IGF), transforming growth factor-β (TGF-β), tumor necrosis factor
(TNF) and interferon (IFN) signaling (5–8).
Senescence is a defense against potentially dangerous mutations,
locking the afflicted cells into a permanent state of arrest
(9). However, the mechanism by which
cancer cells escape from senescence and progress to malignancy is
poorly studied.
Gender determining region Y-box 4 (SOX4) is a member
of the SOX transcription factor family that is characterized by a
highly conserved sequence in the high-mobility group DNA-binding
domain (10). SOX4 has been shown to
be important for numerous developmental processes, including
embryonic cardiac, thymocyte and nervous system development
(11). SOX4 is highly upregulated in
a number of human cancers, including breast cancer, hepatocellular
carcinoma, colon cancer and leukemia (12–15). The
expression and activity of SOX4 are regulated by various signals,
including the epidermal growth factor receptor, TGF-β and
Wnt/β-catenin pathways (10,16). In addition, the deregulated expression
of SOX4 has been shown to induce an epithelial-to-mesenchymal
transition and metastasis in cancer cells (17). The present study demonstrated that
SOX4 was upregulated and mediated an antisenescence effect in ESCC,
thus elucidating the important role of SOX4 in the progression of
ESCC.
Materials and methods
Clinical samples and cell culture
The present study was approved by the Ethics
Committee of Xinxiang Medical University, Xinxiang, China. Written
consent was obtained from all participants. Human ESCC tumor and
adjacent non-tumor tissues were collected from 14 ESCC patients at
the Anyang Tumor Hospital (Anyang, China) and frozen in liquid
nitrogen. None of the patients received pre-operative chemical or
radiation therapy.
ESCC gene expression microarray data was downloaded
from NCBI Gene Expression Omnibus database (accession number,
GSE23400; Affymetrix Human Genome U133A Array platform) (18). The Cancer Genome Atlas (TCGA) RNA-Seq
data (https://tcga-data.nci.nih.gov/tcga/) was used to
investigate the gene expression profile in stomach
adenocarcinoma.
The human ESCC KYSE410 and KYSE510 cell lines were
cultured in RPMI-1640 (Hyclone; GE Healthcare Life Sciences, Logan,
UT, USA) containing penicillin (100 U/ml), streptomycin (100 mg/ml;
Beyotime Institute of Biotechnology, Haimen, China), and 10% fetal
bovine serum (Hyclone; GE Healthcare Life Sciences) at 37°C in a
humidified incubator supplemented with 5% CO2 in air.
Doxorubicin was purchased from Meilun Pharmaceutical Co., Ltd.
(Dalian, China) and dissolved in double-distilled water (1.0 g/ml)
for storage and diluted with phosphate-buffered saline (PBS) prior
to use.
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was isolated with TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
according to the manufacturer's specifications, and quantified
using optical density measurements from a spectrophotometer at 260
nm. RNA (2 µg) was reverse transcribed into complementary DNA
(cDNA) using Moloney Murine Leukemia Virus Reverse Transcriptase
(Fermentas; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. RT-qPCR analysis was performed using SYBR
Green-based detection on an ABI Step One Plus instrument (Thermo
Fisher Scientific, Inc.). Following denaturation at 95°C for 2 min,
qPCR was performed for 40 cycles consisting of 95°C for 15 sec,
60°C for 15 sec and 72°C for 30 sec. Each experiment was performed
three times independently. The following primers were used for
qPCR: SOX4, 5′-GACCTGCTCGACCTGAACC-3′ (sense) and
5′-CCGGGCTCGAAGTTAAAATCC-3′ (antisense); and glyceraldehyde
3-phosphate dehydrogenase (GAPDH), 5′-CTGGGCTACACTGAGCACC-3′
(sense) and 5′-AAGTGGTCGTTGAGGGCAATG-3′ (antisense) as the control.
The relative expression of the gene of interest was normalized to
GAPDH and calculated with 2−ΔΔCq method (19).
Western blotting
Cells (5×106) in 6-cm dishes were washed
once with cold PBS and harvested by scraping into
radioimmunoprecipitation assay lysis buffer on ice. Protein
concentration was determined by the Bradford assay (Beyotime
Institute of Biotechnology). The extracted proteins (40 µg/lane)
were separated by 10% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis and transferred to polyvinylidene fluoride
membranes. The membranes were first blocked with 5% (w/v) nonfat
milk in Tris-buffered saline and Tween 20, and then probed with
either rabbit polyclonal SOX4 (1:2,000 dilution; catalog no.
17919-1-AP; ProteinTech Group, Inc., Chicago, IL, USA) or rabbit
monoclonal GAPDH (1:5,000 dilution; product no. 2118L; Cell
Signaling Technology, Inc., Danvers, MA, USA) primary antibodies at
4°C overnight. Subsequent to washing 4 times with Tris-buffered
saline and Tween 20, the membranes were incubated with the
horseradish peroxidase-conjugated secondary antibodies (product no.
7074; Cell Signaling Technology, Inc.) for 1 h at room temperature.
The signals were detected using an enhanced chemiluminescence
detection kit (Thermo Fisher Scientific, Inc.).
Plasmid construction and
transfection
SOX4-targeting DNA sequences (sense,
5′-GATCCGCGACAAGATCCCTTTCATTTCAAGAGAATGAAAGGGATCTTGTCGCTGA-3′ and
antisense,
5′-AGCTTCAGCGACAAGATCCCTTTCATTCTCTTGAAATGAAAGGGATCTTGTCGCG-3′) were
synthesized, annealed and inserted into pSilencer 4.1-CMV
expression vectors (Ambion; Thermo Fisher Scientific, Inc.).
KYSE410 and KYSE510 cells were seeded into 6-well plates and
transfected with 1 µg/well short hairpin RNA (shRNA) or pSilencer
4.1 CMV empty vector plasmids in Lipofectamine 2000 reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) to produce shSOK4 and
vector cell groups, respectively. Culture medium containing 100
µg/ml geneticin (G418; Beyotime Institute of Biotechnology) was
used to grow the cells for 2 weeks at 37°C to select for stably
transfected cells.
Colony forming assay and SA-β-Gal
staining
Cells were seeded into 6-well plates at 200
cells/well and cultured for 10 days at 37°C. The cells were then
washed with PBS, fixed in 4% paraformaldehyde and stained with
Coomassie brilliant blue.
Doxorubicin-induced cancer cell senescence was
analyzed using a β-galactosidase staining kit (Beyotime Institute
of Biotechnology) following the manufacturer's instructions.
Statistical analysis
Data were presented as the mean ± standard deviation
and analyzed using the Wilcoxon paired t-test or Spearman's rank
correlation coefficient for analysis of the association between
SOX4 and senescence marker expression. Statistical calculations
were performed with Graphpad Prism 5.0 (GraphPad Software Inc., San
Diego, CA, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
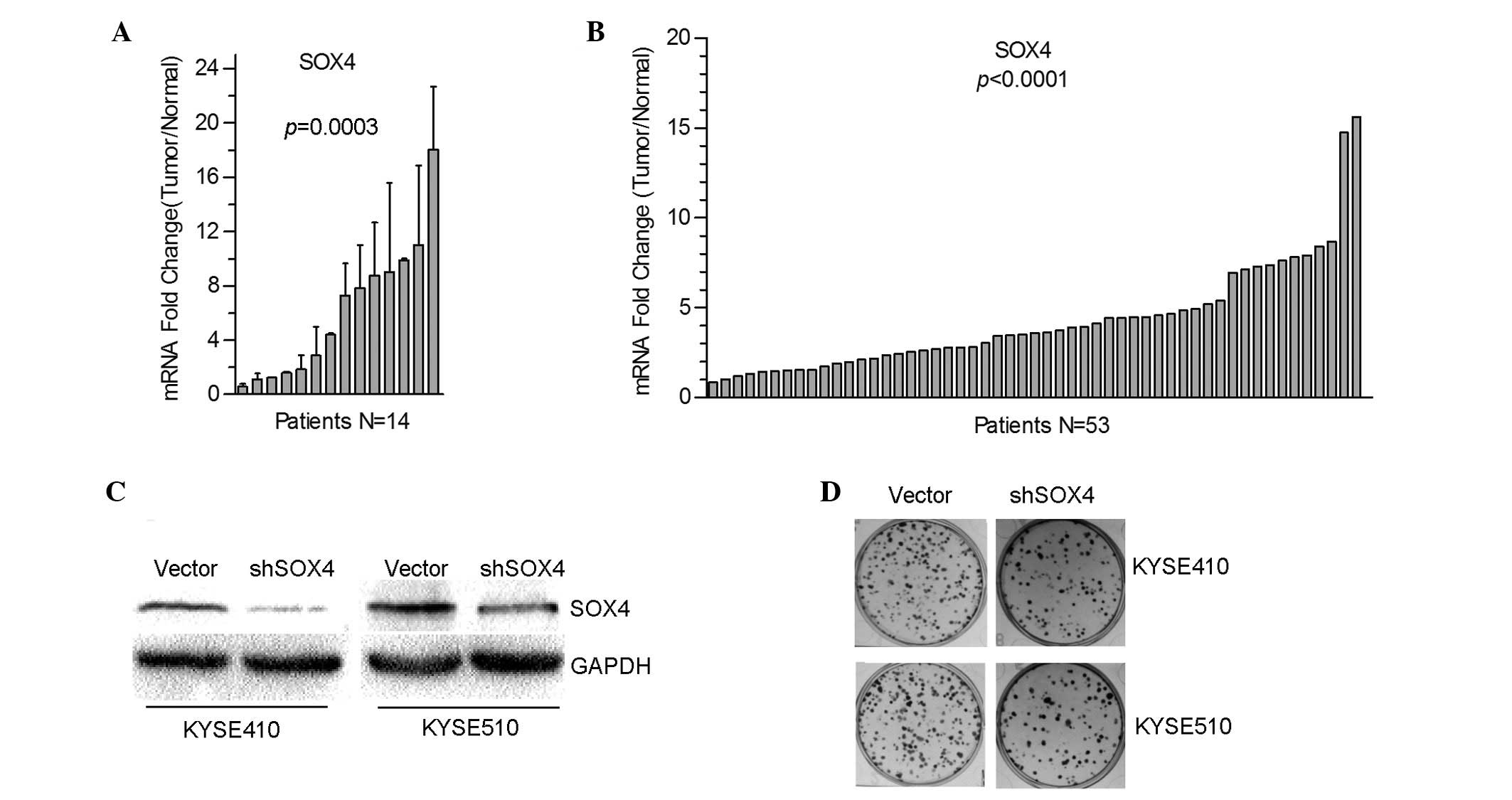
SOX4 is upregulated in ESCC
Tumor and adjacent non-tumor tissues were collected
from 14 ESCC patients from Anyang Tumor Hospital between May 2011
and October 2013, and the expression of SOX4 in tumor and paired
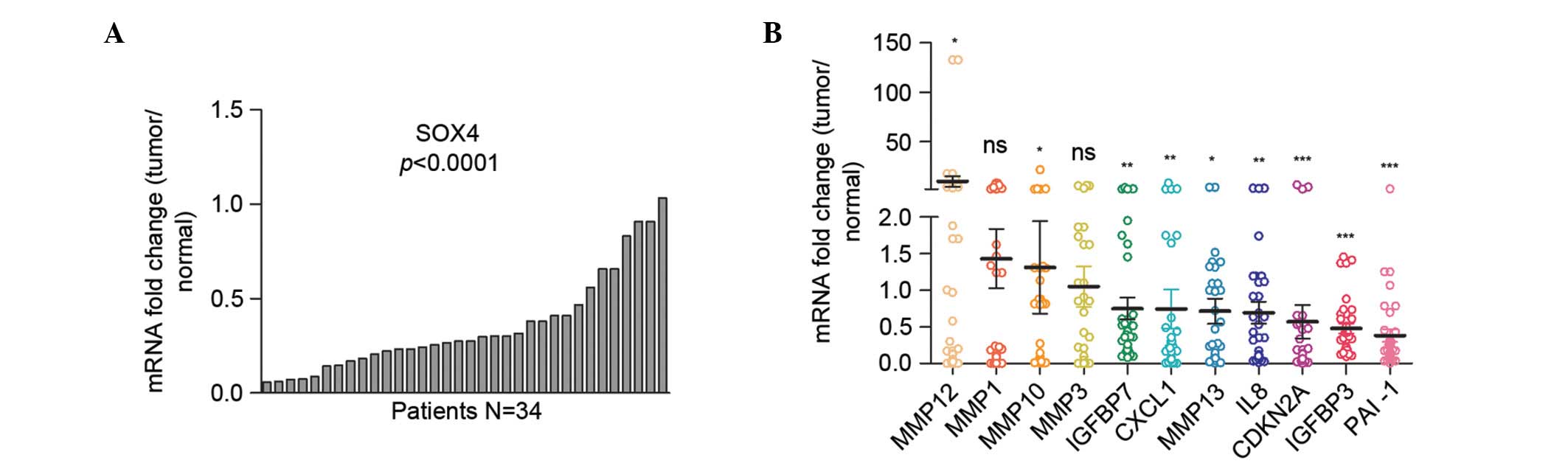
non-tumor tissues was compared using RT-qPCR. As shown in Fig. 1A, the messenger RNA (mRNA) levels of
SOX4 were upregulated in 92% patients (13/14 cases), with 64% (9/14
cases) exceeding normal levels by 2-fold. The cDNA microarray data
deposited at NCBI Gene Expression Omnibus database (accession
number, GSE23400) (18), which
included 53 ESCC samples and 53 matched normal samples that were
collected from the Thoracic Surgery Department of Shanxi Cancer
Hospital (Taiyuan, China) between 1998 and 2001, were then
examined. The SOX4 levels in tumors were found to be upregulated in
52 cases compared with the normal tissues, with 77% (41/53 cases)
exceeding the normal upregulation by 2-fold (Fig. 1B). Together, these results
demonstrated that SOX4 was significantly upregulated in ESCC.
SOX4 knockdown impairs ESCC cell
proliferation
To investigate the function of SOX4 in ESCC, a shRNA
to target SOX4 was constructed and transfected it into the two ESCC
KYSE410 and KYSE510 cell lines. Following selection with 100 µg/ml
of G418 for 2 weeks, two cell lines were obtained in which SOX4
were stably knocked down (Fig. 1C). A
colony formation assay was performed, which indicated that
decreased SOX4 expression was significantly associated with
decreased colony numbers in KYSE410 and KYSE510 cells compared with
the vector group (Fig. 1D).
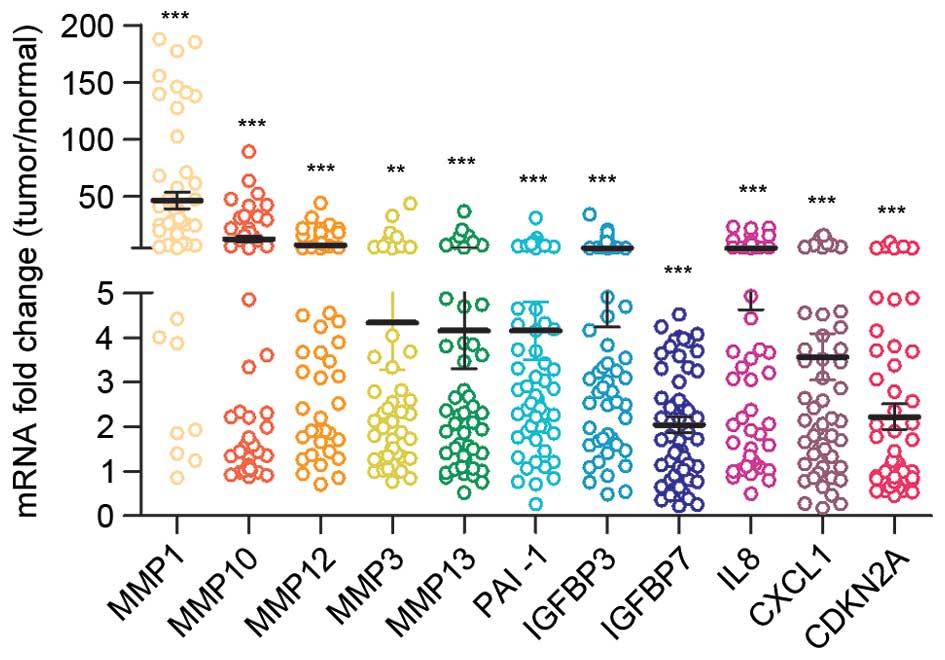
ESCC has an associated senescent
microenvironment
The gene expression profile in ESCC was then
examined using the GSE23400 dataset (18). As shown in Fig. 2, among the most upregulated genes in
ESCC tumor tissues were numerous matrix-remolding proteinases,
including MMP1, MMP10, MMP12, MMP3 and MMP13, chemokines such as
CXCL1 and inflammatory factors such as IL8. This gene expression
signature had the characteristics of SASP (5,20,21). Other important senescence mediators
such as PAI-1, IGF-binding proteins (IGFBPs) and cyclin-dependent
kinase inhibitor 2A (CDKN2A) were also upregulated in ESCC tumor
tissues (21). These data suggested
the senescent microenvironment in ESCC.
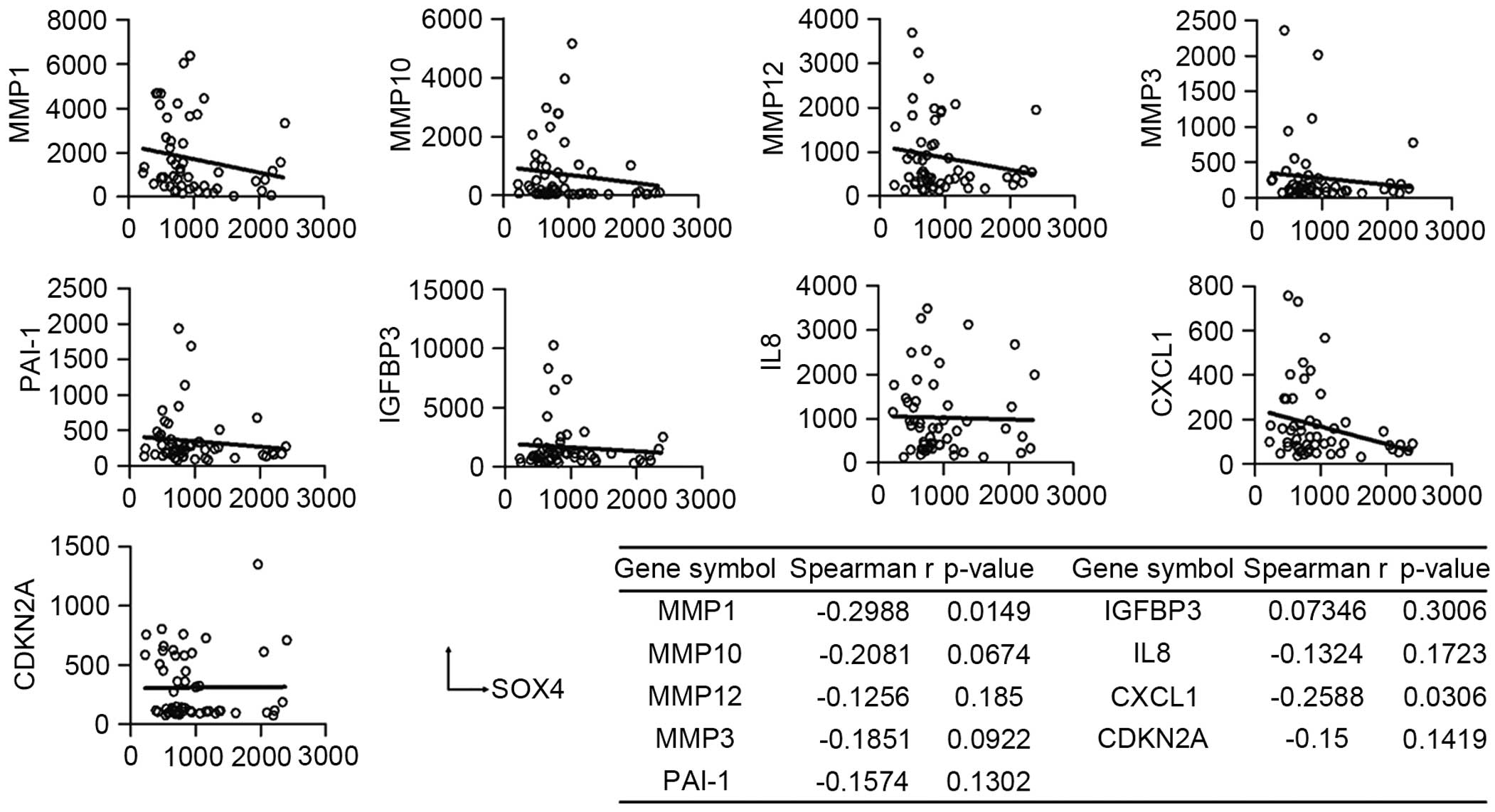
SOX4 is inversely correlated with
senescence markers
To investigate the role of SOX4 in the senescent
phenotype of ESCC, Spearman's rank correlation coefficient analysis
was performed to assess the association between SOX4 and senescence
markers. Fig. 3 shows that in ESCC
tumor tissues, the mRNA levels of SOX4 were inversely correlated
with the levels of MMP1 (P=0.0149), MMP10 (P=0.0674), MMP12, MMP3,
PAI-1, IL8, CXCL1 (P=0.0306) and CDKN2A (P=0.1419). These results
suggested that SOX4 may have an antisenescence function in
ESCC.
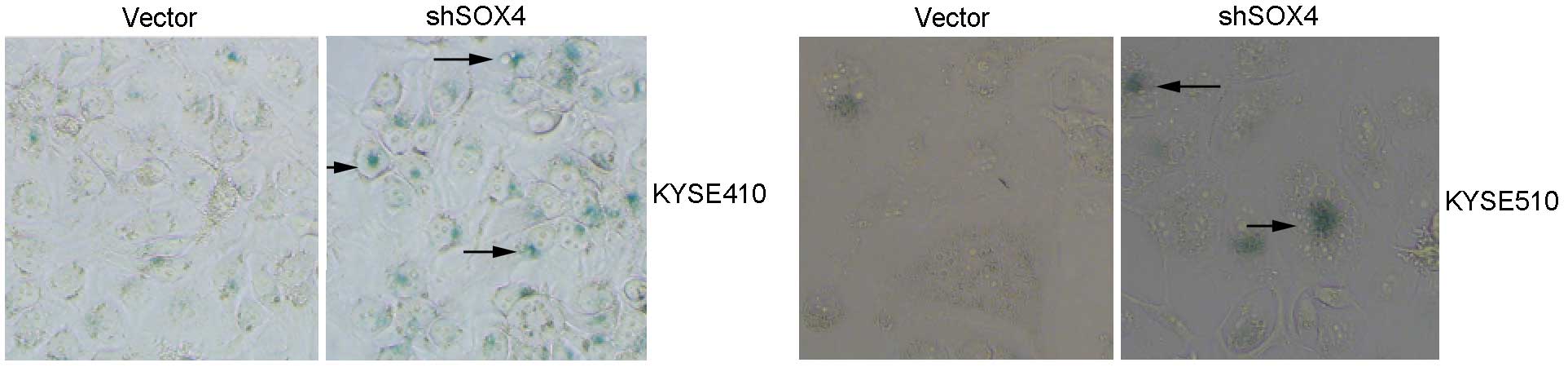
SOX4 knockdown promotes
doxorubicin-induced senescence in ESCC cells
In vitro-cultured cancer cells do not show senescent
phenotypes under normal conditions (20). However, under stresses such as
anticancer drug treatment and ionizing radiation, various cancer
cells undergo senescence. To further elucidate the antisenescence
function of SOX4, KYSE410 and KYSE510 cells that were transfected
with SOX4-targeting shRNA or empty vector were treated with 50
ng/ml doxorubicin for 48 h. The cells were then maintained in
drug-free medium for another 72 h to enter growth-arrest states,
and subsequently stained with SA-β-Gal. In Fig. 4, the SOX4 knockdown groups showed
stronger staining with SA-β-Gal compared with the control groups in
the KYSE410 and KYSE510 cell lines. These results demonstrated the
important role of SOX4 in doxorubicin-induced senescence in ESCC
cells.
Senescent microenvironment is absent
in stomach adenocarcinoma
The TCGA database included the RNA-Seq data of 34
paired tumor and normal tissues of stomach adenocarcinoma and 8
paired tumor and normal tissues of esophageal adenocarcinoma. The
present study then investigated whether the senescence-associated
gene expression signature exist in stomach adenocarcinoma. The mRNA
level of SOX4 was found to be downregulated in stomach
adenocarcinomas (Fig. 5A). Although
the MMP12 and MMP10 were significantly upregulated in stomach tumor
tissues, the mRNA levels of other senescence markers were
downregulated (IGFBP7, CXCL1, MMP13, IL8, CDKN2A, IGFBP3 and PAI-1)
or not significantly changed (MMP1 and MMP3) in tumor tissues
compared with normal tissues (Fig.
5B). Hence, the senescent microenvironment was not evidently
present in stomach adenocarcinoma.
Discussion
Senescent cells are growth-arrested, but remain
metabolically active and can develop a secretory profile named SASP
(4). Senescence is considered to
limit the expansion of early neoplastic cells (5) and is, therefore, a potent
cancer-protective response to oncogenic events. However, the direct
evidence of senescence in cancer tissues is limited (22). Through open-data mining, a plethora of
senescence-associated molecules, which included proteinases,
chemokines and inflammatory factors, were found to be upregulated
in ESCC tumor tissues in the present study. Although the origin of
these senescence makers could not be determined by microarray
assays, this gene expression signature showed the characteristic of
senescence in the ESCC microenvironment. The senescence-associated
gene expression profile was not shown in stomach adenocarcinoma,
suggesting that the senescent phenotype may be organ specific.
Previous studies identified various oncogenes that mutated in ESCC,
including tumor protein 53 (p53), RB transcriptional corepressor 1,
CDKN2A, phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic
subunit α and NOTCH1 (23).
Deregulated oncogenes are well-known inducers of cellular
senescence (9), and may contribute to
the senescent phenotype in ESCC.
The present study demonstrated that SOX4 is
upregulated in ESCC and that SOX4 expression is inversely
correlated with a cohort of senescence markers. SOX4 knockdown
decreased cell proliferation and enhanced doxorubicin-induced
cellular senescence in vitro. These results suggested the
important role of SOX4 in senescence evading in ESCC. Indeed,
Foronda et al recently reported that mice with reduced
whole-body SOX4 expression displayed accelerated aging and reduced
cancer incidence, highlighting the crucial roles of SOX4 in cancer
progression (24). Pan et al
(25) revealed that SOX4 was
upregulated in response to DNA damage, and stabilized p53 protein
by blocking mouse double minute 2 homolog-mediated p53
ubiquitination and degradation. Whether the p53 pathway is required
for the antisenescence function of SOX4 requires additional
investigation.
In conclusion, the current results revealed the
protective role of SOX4 in cancer cell senescence. As senescence
evading is mechanistically implicated in cancer development and
refractory disease, we propose SOX4 targeting as a potential method
of cancer prevention and treatment in the future. However, whether
SOX4 has a similar role in cell senescence accompanied by
chronological aging is a topic that requires further
investigation.
Acknowledgements
The present study was, in part, supported by the
US-Chinese Anti-Cancer Association (Natural Science Foundation of
China, Beijing, China; grant nos. 81071655, 81372149, 91229115 and
81272251), the China Postdoctoral Science Foundation (Beijing,
China; grant no. 2015M572366), the Innovation of Science and
Technology Commission of Shenzhen Municipality (Shenzhen, China;
grant no. JCYJ20130329102515481) and the Key Laboratory Project of
Shenzhen (Shenzhen, China; grant no. ZDSY20130329101130496).
References
|
1
|
Kamangar F, Dores GM and Anderson WF:
Patterns of cancer incidence, mortality, and prevalence across five
continents: Defining priorities to reduce cancer disparities in
different geographic regions of the world. J Clin Oncol.
24:2137–2150. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang SK, Guo LW, Chen Q, Zhang M, Liu SZ,
Quan PL, Lu JB and Sun XB: Prevalence of human papillomavirus 16 in
esophageal cancer among the Chinese population: A systematic review
and meta-analysis. Asian Pac J Cancer Prev. 15:10143–10149. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Xu Y, Yu X, Chen Q and Mao W: Neoadjuvant
versus adjuvant treatment: Which one is better for resectable
esophageal squamous cell carcinoma? World J Surg Oncol. 10:1732012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pérez-Mancera PA, Young AR and Narita M:
Inside and out: The activities of senescence in cancer. Nat Rev
Cancer. 14:547–558. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kuilman T and Peeper DS:
Senescence-messaging secretome: SMS-ing cellular stress. Nat Rev
Cancer. 9:81–94. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Eren M, Boe AE, Murphy SB, Place AT,
Nagpal V, Morales-Nebreda L, Urich D, Quaggin SE, Budinger GR,
Mutlu GM, et al: PAI-1-regulated extracellular proteolysis governs
senescence and survival in Klotho mice. Proc Natl Acad Sci USA.
111:7090–7095. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Coppé JP, Patil CK, Rodier F, Krtolica A,
Beauséjour CM, Parrinello S, Hodgson JG, Chin K, Desprez PY and
Campisi J: A human-like senescence-associated secretory phenotype
is conserved in mouse cells dependent on physiological oxygen. PLoS
One. 5:e91882010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kuilman T, Michaloglou C, Vredeveld LC,
Douma S, van Doorn R, Desmet CJ, Aarden LA, Mooi WJ and Peeper DS:
Oncogene-induced senescence relayed by an interleukin-dependent
inflammatory network. Cell. 133:1019–1031. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mooi WJ and Peeper DS: Oncogene-induced
cell senescence-halting on the road to cancer. N Engl J Med.
355:1037–1046. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Vervoort SJ, van Boxtel R and Coffer PJ:
The role of SRY-related HMG box transcription factor 4 (SOX4) in
tumorigenesis and metastasis: Friend or foe? Oncogene.
32:3397–3409. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Penzo-Méndez AI: Critical roles for SoxC
transcription factors in development and cancer. Int J Biochem Cell
Biol. 42:425–428. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang J, Liang Q, Lei Y, Yao M, Li L, Gao
X, Feng J, Zhang Y, Gao H, Liu DX, et al: SOX4 induces
epithelial-mesenchymal transition and contributes to breast cancer
progression. Cancer Res. 72:4597–4608. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hur W, Rhim H, Jung CK, Kim JD, Bae SH,
Jang JW, Yang JM, Oh ST, Kim DG, Wang HJ, et al: SOX4
overexpression regulates the p53-mediated apoptosis in
hepatocellular carcinoma: Clinical implication and functional
analysis in vitro. Carcinogenesis. 31:1298–1307. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lin CM, Fang CL, Hseu YC, Chen CL, Wang
JW, Hsu SL, Tu MD, Hung ST, Tai C, Uen YH and Lin KY: Clinical and
prognostic implications of transcription factor SOX4 in patients
with colon cancer. PLoS One. 8:e671282013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ramezani-Rad P, Geng H, Hurtz C, Chan LN,
Chen Z, Jumaa H, Melnick A, Paietta E, Carroll WL, Willman CL, et
al: SOX4 enables oncogenic survival signals in acute lymphoblastic
leukemia. Blood. 121:148–155. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Scharer CD, McCabe CD, Ali-Seyed M, Berger
MF, Bulyk ML and Moreno CS: Genome-wide promoter analysis of the
SOX4 transcriptional network in prostate cancer cells. Cancer Res.
69:709–717. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tiwari N, Tiwari VK, Waldmeier L, Balwierz
PJ, Arnold P, Pachkov M, Meyer-Schaller N, Schübeler D, van
Nimwegen E and Christofori G: Sox4 is a master regulator of
epithelial-mesenchymal transition by controlling Ezh2 expression
and epigenetic reprogramming. Cancer Cell. 23:768–783. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Su H, Hu N, Yang HH, Wang C, Takikita M,
Wang QH, Giffen C, Clifford R, Hewitt SM, Shou JZ, et al: Global
gene expression profiling and validation in esophageal squamous
cell carcinoma and its association with clinical phenotypes. Clin
Cancer Res. 17:2955–2966. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Elzi DJ, Lai Y, Song M, Hakala K,
Weintraub ST and Shiio Y: Plasminogen activator inhibitor
1-insulin-like growth factor binding protein 3 cascade regulates
stress-induced senescence. Proc Natl Acad Sci USA. 109:12052–12057.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Coppé JP, Desprez PY, Krtolica A and
Campisi J: The senescence-associated secretory phenotype: The dark
side of tumor suppression. Annu Rev Pathol. 5:99–118. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Collado M and Serrano M: Senescence in
tumours: Evidence from mice and humans. Nat Rev Cancer. 10:51–57.
2010. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Song Y, Li L, Ou Y, Gao Z, Li E, Li X,
Zhang W, Wang J, Xu L, Zhou Y, et al: Identification of genomic
alterations in oesophageal squamous cell cancer. Nature. 509:91–95.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Foronda M, Martínez P, Schoeftner S,
Gómez-López G, Schneider R, Flores JM, Pisano DG and Blasco MA:
Sox4 links tumor suppression to accelerated aging in mice by
modulating stem cell activation. Cell Rep. 8:487–500. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pan X, Zhao J, Zhang WN, Li HY, Mu R, Zhou
T, Zhang HY, Gong WL, Yu M, Man JH, et al: Induction of SOX4 by DNA
damage is critical for p53 stabilization and function. Proc Natl
Acad Sci USA. 106:3788–3793. 2009. View Article : Google Scholar : PubMed/NCBI
|