Introduction
Despite increasingly successful initial therapies
for various cancers, recurrent secondary tumours remain a problem
(1). Malignant tumour cells are
heterogeneous; only a small proportion of cells in solid tumour
cancer stem cells are reportedly able to form colonies in
clonogenic assays in vitro (2). Cancer stem cells have been identified in
numerous types of malignancies (3–5). As a
result, increasing attention has been focused on cancer stem cells
in oncological research. However, there have been few reports on
oral and maxillofacial malignant tumours.
Cluster of differentiation 133 (CD133; formerly
known as AC133) is a highly-conserved antigen that is the human
homologue of mouse Prominin-1, which was initially identified as a
5-transmembrane cell surface glycoprotein, and was classified as a
marker for primitive haematopoietic and neural stem cells. CD133 is
also considered a universal marker of organ-specific stem cells and
tumour-initiating cells. CD133 protein plays an important role in
supporting tumour growth (6,7). In addition, CD133+ cells are
involved in tumourigenesis, invasion, metastasis, drug resistance
and disease relapse (8). CD133 is
detectable in a range of solid tumours, but there has been little
research on its role in oral and maxillofacial tumours. Based on a
previous study on the characterisation of cancer stem cells, CD133
has been identified as a common marker for cancer stem cells
(9). It is therefore reasonable to
suspect that the CD133 antigen is a marker for cancer stem cells
associated with tongue squamous carcinoma. The purpose of the
present study was to determine whether CD133 is a surface marker of
tongue squamous carcinoma stem cells. In this study, immunomagnetic
beads were used to select and purify CD133+ tumour
cells, which were then cultured. The proliferative ability of these
cells was observed in vitro.
Materials and methods
Cell preparation
The Tca-8113 cell line was obtained from the Ninth
Affiliated People's Hospital, Shanghai Jiao Tong University
(Shanghai, China) and stored in liquid nitrogen.
Single cell culture in vitro
The Tca-8113 cells (2×105) were thawed
and cultured in RPMI-1640 medium supplemented with 10% foetal calf
serum (FCS; Hangzhou Sijiqing Biological Engineering Materials Co.,
Ltd., Hanzhou, China) in 100% relative humidity at 37°C in an
atmosphere containing 5% CO2. Cells in the exponential
growth phase were collected after trypsin-ethylenediamine
tetra-acetic acid digestion and washed twice in phosphate-buffered
saline (PBS). The cell pellets were then suspended in PBS, and the
cells were counted. Using the limited dilution method, the cell
concentration was changed to 10–15/ml, and the cell suspension with
uniform distribution was then transferred (1 cell/100 µl) to single
wells using a pipette. The wells in which single cells were
successfully isolated (verified by inverted phase contrast
microscopy) were marked. The cells were transferred in this manner
for a total of 384 wells (4 plates). The culture was repeated three
times at the same time in vitro. Changes in population and
cell morphology were observed using an inverted microscope.
Sphere formation
To test their ability for growth and sphere
formation in suspension, the cells were trypsinized and passed
through a 40-µm filter to obtain a single cell suspension. In
total, 10 ml of media containing 2×104 cells was added
to 96-well ultra-low attachment plates (Corning Inc., Corning, NY,
USA). After 7 days, the plates were visually assayed for the
formation of floating spheres. To assess the ability of primary
spheres to form secondary spheres, the primary spheres were
collected by centrifugation (1,000 × g for 5 min) and again
digested to single-cell suspensions, and passed through a 40-µm
filter. A total of 10 ml of medium containing 2×104
cells was added to 96-well ultra-low attachment plates (Corning
Inc.). To determine the viability and self-renewal abilities of the
constituent cells, after 7 days, secondary spheres were collected
and placed into adherent plates to assess their colony-formation
patterns.
Flow cytometry (FCM)
The Tca-8113 cells were digested with 0.25% trypsin,
and the digestion was terminated with culture media. The cells were
washed twice in 0.01% PBS and suspended by centrifugation
(centrifuge radius, 132 mm; 1,000 rpm; 5 min). Phycoerythrin
(PE)-conjugated CD133 antibody (20 µl; 1:20 dilution; catalog no.
130080801; eBioscience, San Diego, CA, USA) was added to the fluid.
The cells were then incubated for 1 h at room temperature in the
dark. The isotype control was immunoglobulin (Ig)G-PE
(eBioscience). The cells were rewashed in 0.01% PBS and detected
using FCM (FACStarplus; BD Biosciences, Franklin Lakes, NJ, USA).
The PE conjugate was excited with an argon laser at 488 nm and was
collected at 575/26 nm.
Immunocytochemistry
The adherent Tca-8113 cells were digested with 0.25%
trypsin, transferred to cover slips and placed in culture dishes
with small amounts of RPMI 1640 culture medium (Gibco; Thermo
Fisher Scientific, Inc., Rockville, MD, USA). Further medium was
added to the culture dishes after the cells had adhered to the
cover slips. The cells were then cultivated for 48–72 h. The cells
were fixed for 5 min in acetone and washed in 0.01% PBS. Next, 30
µl fluorescein isothiocyanate (FITC)-labelled anti-CD133 antibody
(catalog no. 46133182; R&D Systems Inc., Minneapolis, MN, USA)
was added to cover the cells, which were incubated for 1 h at room
temperature in the dark. The cells were then washed with 0.01% PBS,
mounted in anhydrous glycerol and observed using a fluorescence
microscope.
Magnetic sorting of CD133+
cells and in vitro culture
The Tca-8113 cells were conventionally cultured with
10% FCS. The cells in the exponential growth phase were digested
(with 0.25% trypsin) and counted. The cell density was reduced to
3–5×107 cells/ml. The Tca-8113 cells (1×108
cells/0.3 ml) were completely suspended in incubation fluid (PBE)
consisting of 0.5% NBS, 2 mm EDTA (pH 7.2) and PBS. FC
receptor-blocking pharmacon (100 µl; Miltenyi Biotec Inc., Bergisch
Gladbach, Germany) was added to the cells, which were incubated for
30 min at 4°C. Next, 0.1 ml of CD133 antibody-conjugated magnetic
beads (catalog no. 130-091-895; Miltenyi Biotec, Inc.) was added to
the cells for 30 min at 4°C. The final volume was 0.5 ml. The cells
were washed in 1 ml PBE, centrifuged (940 × g for 10 min) and
suspended completely in 0.5 ml PBE. The CD133+ cells
were labelled with primary CD133/1 antibody (mouse IgG1, 111 per
million cells; Miltenyi Biotec Inc.), magnetically labelled with
rat anti-mouse IgG1 microbeads (Miltenyi Biotec Inc.; 2,011 per 10
million cells), and separated on a magnetic-activated cell sorting
(MACS) LS column (Miltenyi Biotec Inc.). All the procedures were
performed according to the manufacturer's instructions.
In vitro CD133+ cell
reproductive test
CD133+, CD133– and primary
cells were respectively separated using MACS. After these cells
were prepared as single-cell suspensions, they were transferred to
96-well microwell plates with 1–2×103 cells/well.
Samples from each group were placed into 3 wells, and 0.2 ml RPMI
1640 complete culture solution was placed in each well. The cells
were cultured in 100% relative humidity at 37°C in an atmosphere
containing 5% CO2. Cell proliferation was examined on
days 1, 3, 5 and 7. The viable count was quantified by an optical
density (OD) at 450 nm absorbance and with Cell Counting kit-8
(CCK-8; Beyotime Institute of Biotechnology, Haimen, China).
Finally, to compare the growth rates of the 3 cell groups, a cell
growth curve was drawn according to the association between the
date and absorbance.
CD133+ cell differentiation
potency test in vitro
Single CD133+ cells were cultured in RPMI
1640 complete culture solution with 10% foetal bovine serum, in
100% relative humidity at 37°C, in an atmosphere containing 5%
CO2. The culture fluid was changed once every 2 days.
The percentage of cells expressing CD133+ was evaluated
on days 0, 4, 8, 12 and 16 using FCM.
Tumourigenic capacity of
CD133+ cells in NOD/SCID mice
A total of 90 NOD/SCID male mice, classified into 3
groups and weighing 40–50g, were purchased form the Animal Breeding
Center of The Second Military Medical University of Chinese
People's Liberation Army (Shanghai, China). Mice were maintained at
the animal room (specific pathogen-free) of Tongji Stomatology
School (Shanghai, China), at 20°C with 50% humidity. All
experiments were approved by the Ethics Committee of Tongji
Stomatology School.
CD133+ and CD133– cells
obtained by MACS from the Tca-8113 cell line and unsorted cells
were subcutaneously inoculated into the left and right axillas, and
the backs of NOD/SCID mice, respectively, with 1×105
cells/ml in 3 injection points per mouse. The tumourigenic capacity
and different phenotypes of tumour cells from the Tca-8113 cell
line in the NOD/SCID mice were observed every 2 days after
inoculation for a total of 8 weeks. The mice were sacrificed by an
overdose of anesthesia drugs injected into the vein.
Statistical analysis
All data are presented as the mean ± standard error.
Results were analysed using analysis of variance or t-test with
SPSS version 14.0 software (SPSS, Inc., Chicago, IL, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
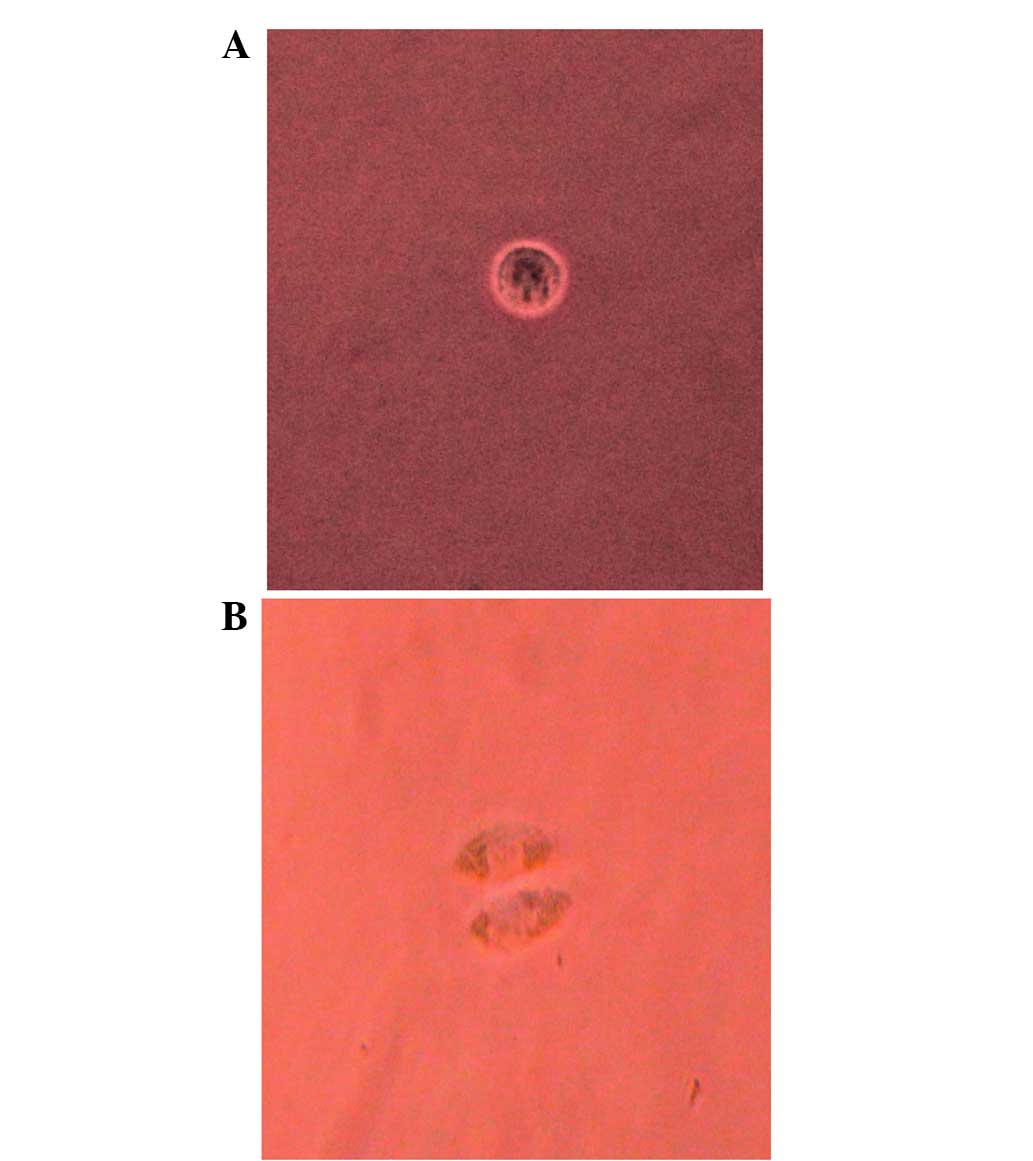
Single-cell culture in vitro
At first, the Tca-8113 cells seeded into the 96-well
microwell plates were round and exhibited halation and translucent
circumferentia. After 12 h, certain cells adhered to the substrates
and other cells demonstrated vacuoles in their centres or were
fragmented and dead. After 24 h, almost all the cells were adhered
to the substrates and had begun to differentiate; these cells were
polygonal with abundant cytoplasm and displayed polynuclear
anachromasis. After 8 days, only 10.85% of the cells demonstrated a
stronger proliferative capability; this was true of 5.23 and 5.09%
of the cells by days 12 and 16, respectively (Table I; Figs.
1 and 2).
 | Table I.Survival and proliferation of
Tca-8113 single-cell culture in vitro. |
Table I.
Survival and proliferation of
Tca-8113 single-cell culture in vitro.
|
| Surviving
cells |
|
|
|---|
|
|
|
|
|
|---|
|
| Dividing | One cell | Dead cells |
|---|
|
|
|
|
|
|---|
| Time | n | % | n | % | n | % |
|---|
| 12 h |
0.00 |
0.00 | 323.40 | 95.57 |
15.00 |
4.43 |
| 24 h | 287.86 | 85.07 |
35.20 | 10.40 |
15.34 |
4.53 |
| 8 days |
36.72 | 10.85 |
62.35 | 18.42 | 239.33 | 70.72 |
| 12 days |
17.70 |
5.23 |
46.14 | 13.63 | 274.56 | 81.13 |
| 16 days |
17.23 |
5.09 |
29.51 |
8.72 | 291.66 | 86.19 |
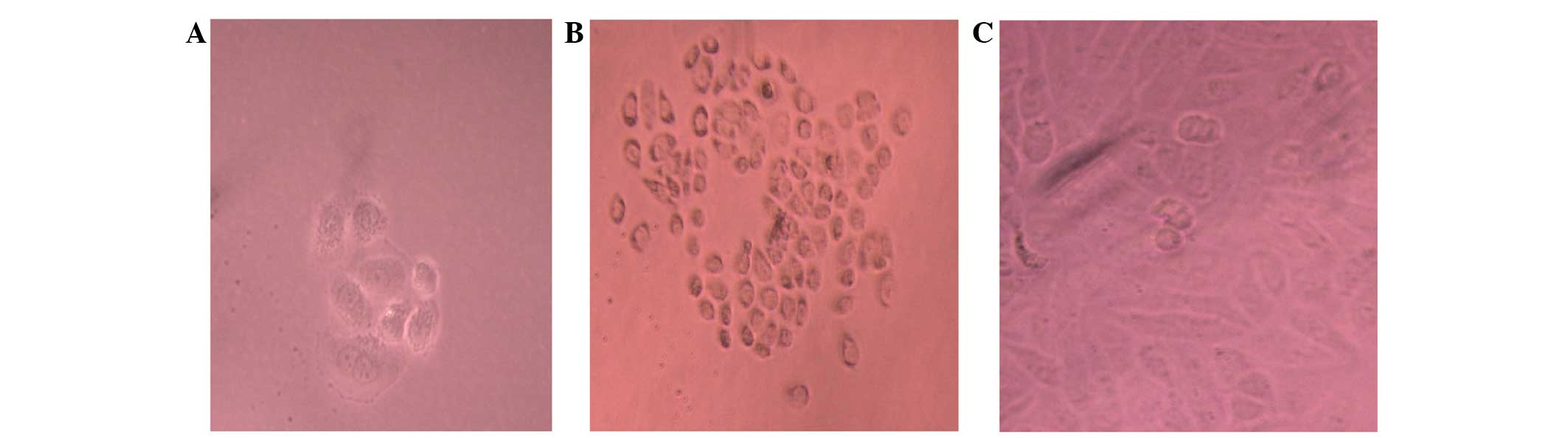
Sphere formation in suspension
The Tca-8113 cells were observed forming floating
clusters of cells within 7 days of plating into non-adherent
plates. Primary sphere formation by these cells is illustrated in
Fig. 3. When the primary spheres were
dissociated and re-plated, spheres from all three groups
demonstrated an ability to form secondary spheres and an increase
in the total number of spheres. When cells forming secondary
spheres were dissociated and placed into standard tissue culture
plates, they adhered and formed colonies with primarily holoclone
morphologies (Fig. 3).
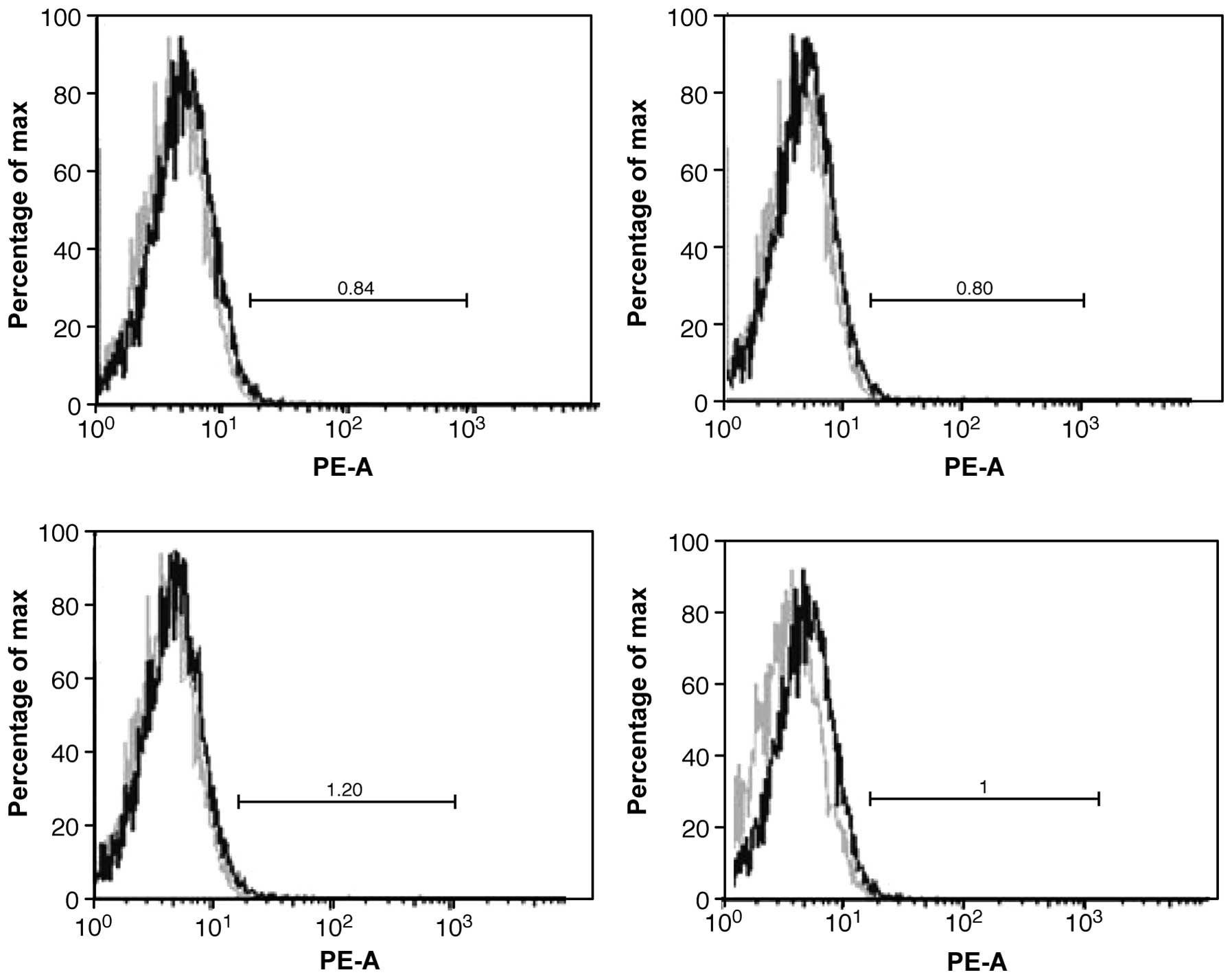
CD133 expression by FCM
Relatively few tumour cells expressed CD133.
Approximately 0.95% of the cells were highly expressed on the cell
membrane (Fig. 4).
CD133 expression illustrated by
immunocytochemical staining
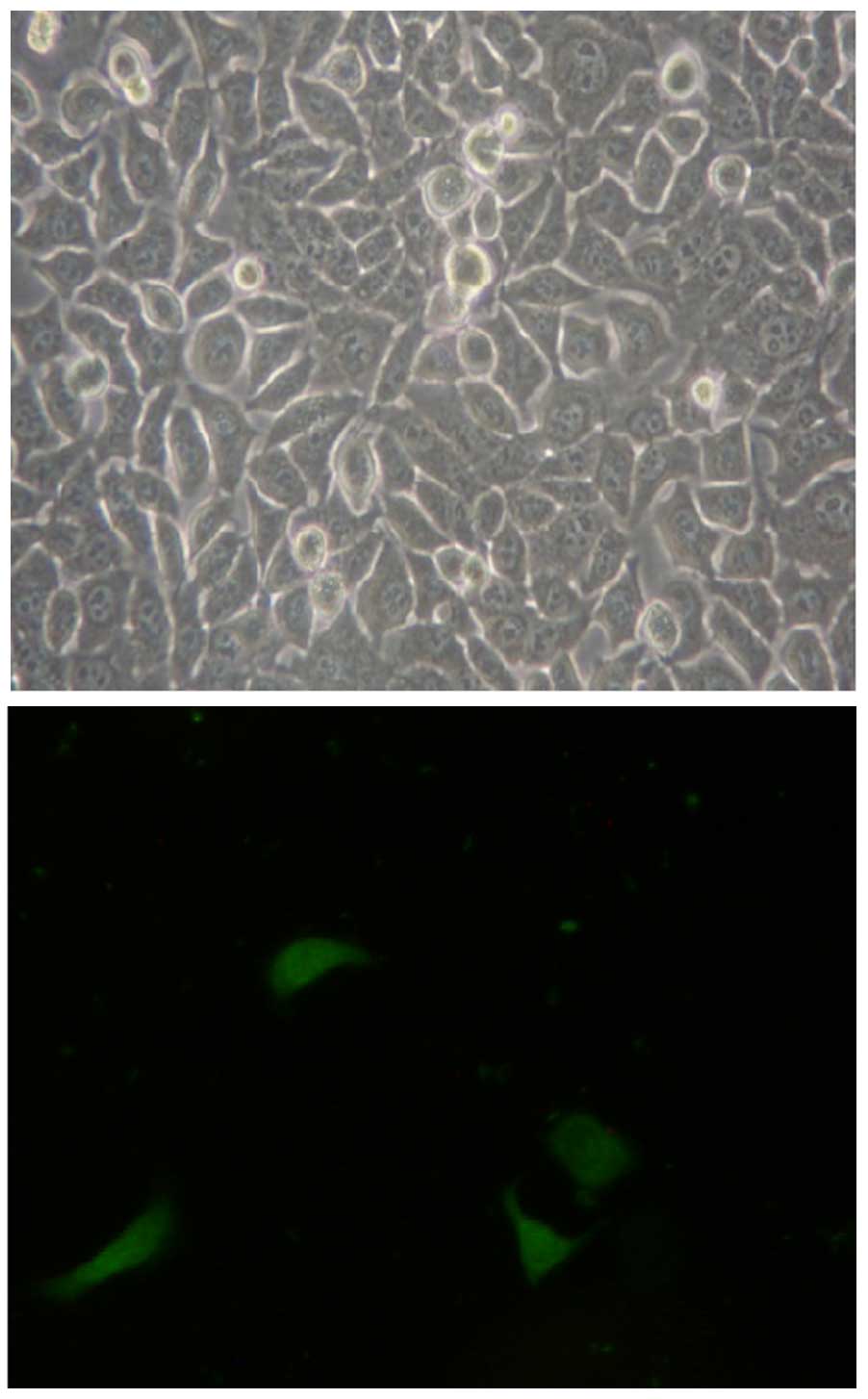
As observed under fluorescence microscopy, CD133
cells were labelled with an FITC-CD133 antibody; its green
fluorescence appeared to have a scattered distribution, which
indicated that CD133 was distributed on the cell membranes. Only a
low percentage of whole-tongue squamous Tca8113 carcinoma cells
expressed CD133 (Fig. 5).
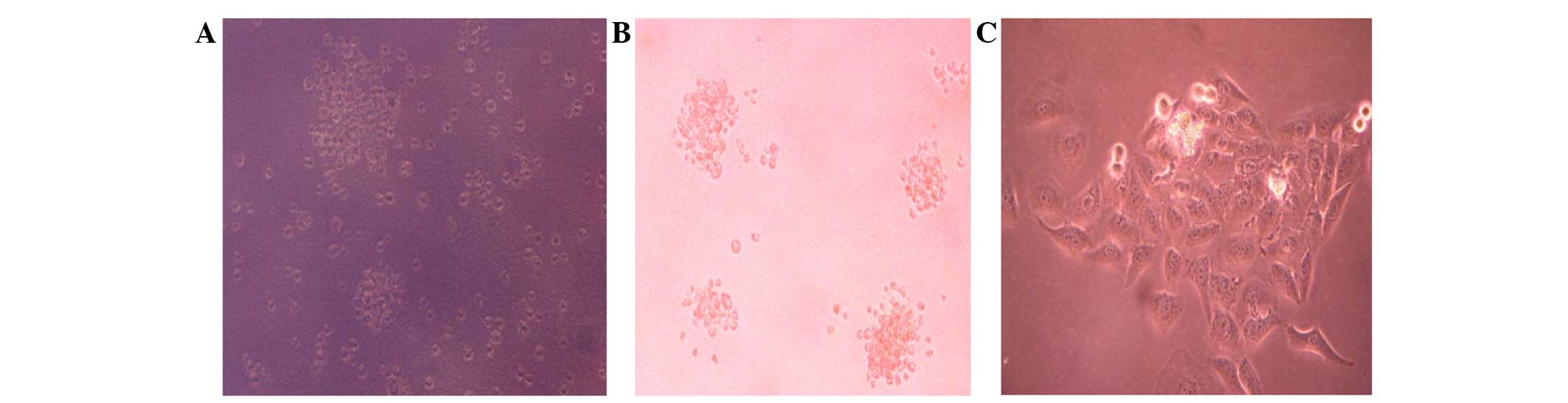
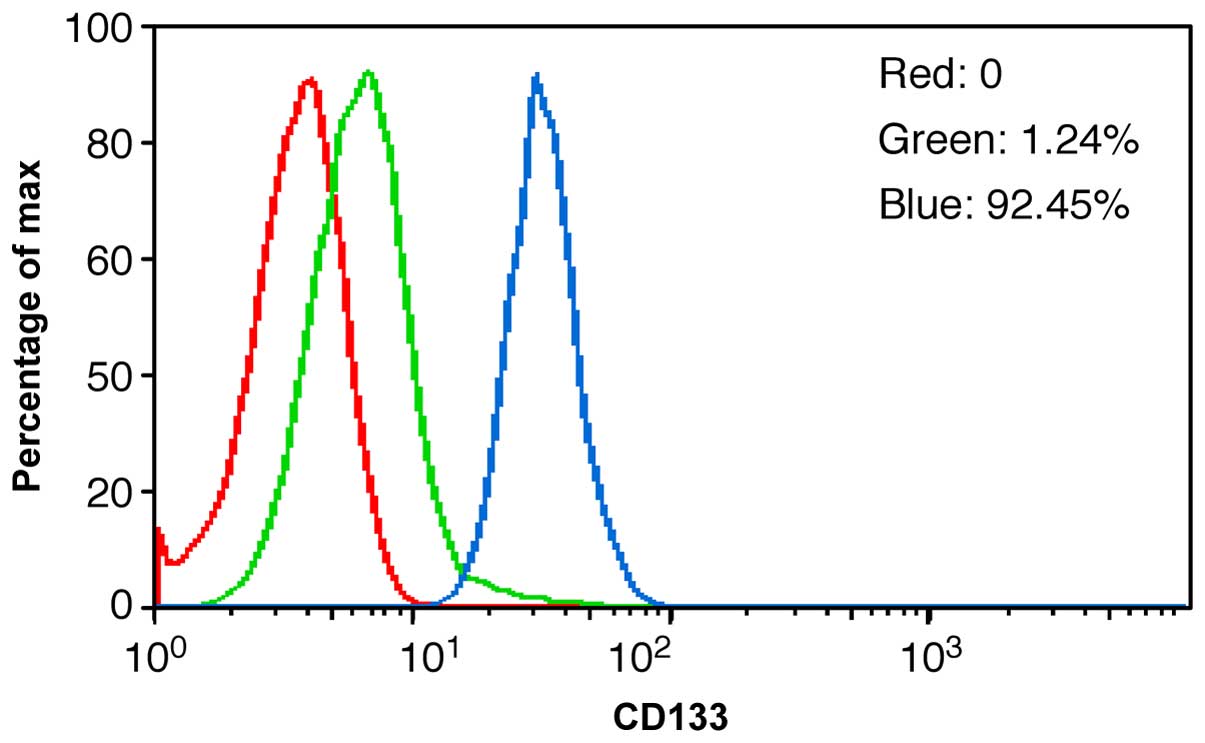
CD133+ cells purified by
MACS
Sustainable separate tumour cells were highly
purified; 92.45% of the separated cells expressed CD133. MACS
appeared to be a better method than FCM for identifying
CD133+ expression, as it was less damaging to the cells
and better retained their proliferative ability (Fig. 6).
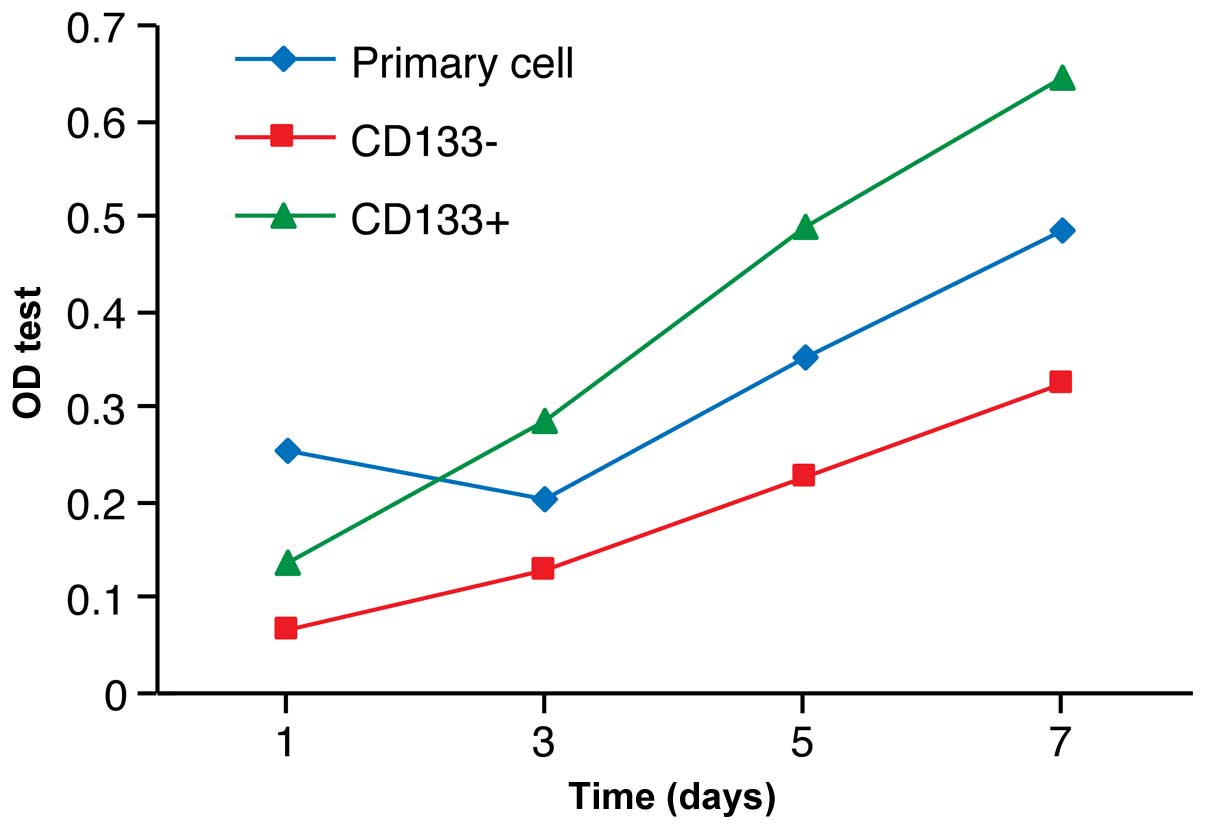
Proliferation activity of
CD133+ cells in vitro
The CD133+ cells grew quickly between
days 3 and 5, while the CD133– cells grew much more
slowly, indicating less proliferative activity (Fig. 7).
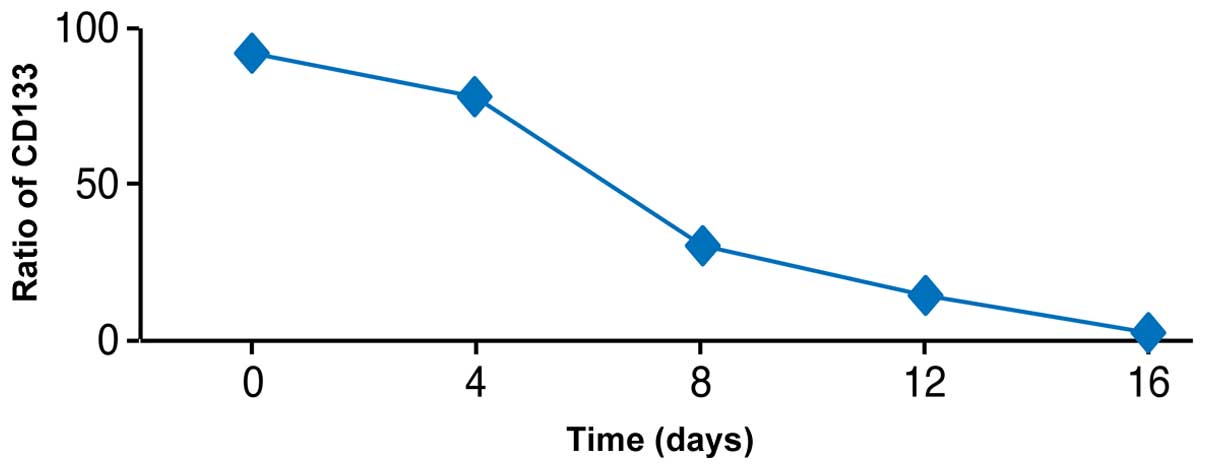
Differentiation potency of
CD133+ cells in vitro
The proportion of CD133+ cells decreased
in culture over time. During 16 days of culture, the percentage of
CD133+ cells decreased from 92.45 to 1.62% (similar to
CD133 expression ratios in sustainable separate cells) (Fig. 8).
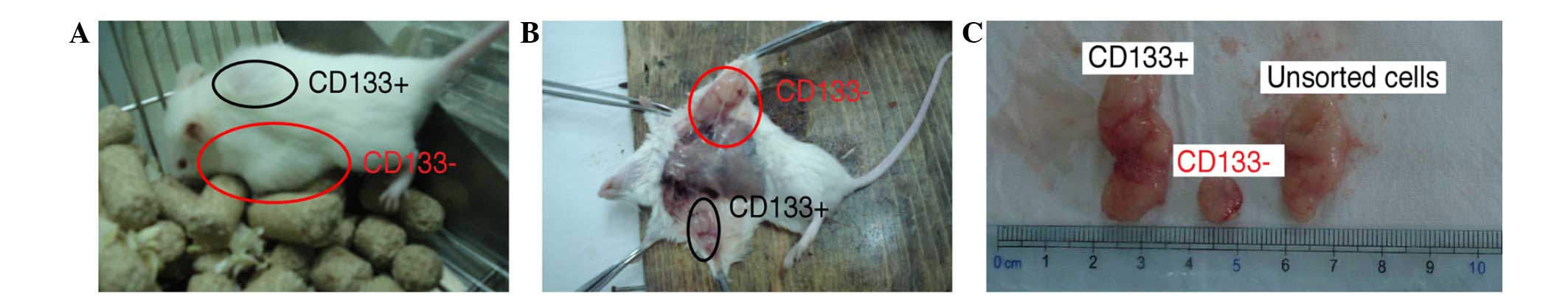
Tumourigenic capacity of
CD133+ cells in NOD/SCID mice
The NOD/SCID mice were sacrificed according to the
national animal care laws. The subcutaneous tumours were exposed
after the NOD/SCID mice were sacrificed. The tumours generated from
the CD133+ cells were markedly larger compared with the
tumours generated from the CD133– cells. In addition, in
terms of the tumourigenic time, the CD133+ cells could
generate tumours in ~1 week, whereas CD133– cells
required 10 to 14 days or longer (Fig.
9).
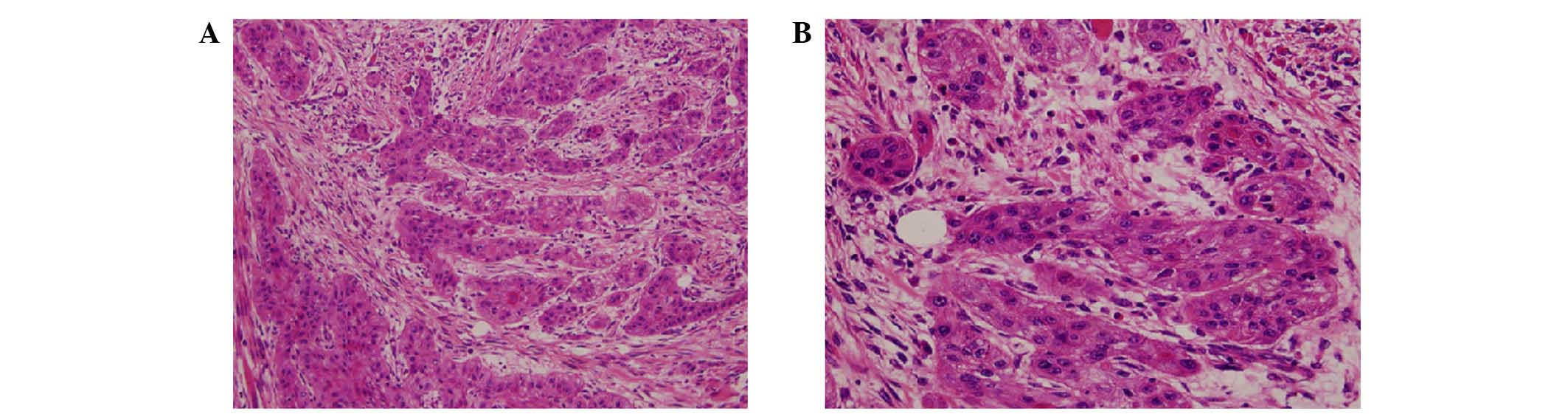
Haematoxylin and eosin (HE) staining
in tongue squamous carcinoma tissues
The histopathological HE staining demonstrated that
a large number of oblate and pleomorphic cells were present in the
tissues, with an abundant and eosinophilic cytoplasm. The nuclei
(blue) were large, with substantial atypia. The nucleocytoplasmic
ratio was high, with clear nucleoli and multiple mitotic phases,
and the presence of cancer nests was observed, indicating that the
tissues generated from the CD133 cells and unsorted cells
transplanted into the NOD/SCID mice were cancerous (Fig. 10).
Number of CD133 cells in NOD/SCID
mice
The tumourigenic rate of CD133+ cells was
markedly higher than that of the CD133– cells, which
reached almost 50%. Thus, CD133– cells also exhibit a
strong tumourigenic ability with a certain degree of stemness
(Table II).
 | Table II.Comparison of the number of cancers
generated from different cell types. |
Table II.
Comparison of the number of cancers
generated from different cell types.
| Classification | Generated, n | None, n | Total, n |
|---|
|
CD133+ | 24 | 6 | 30 |
| Primary | 22 | 8 | 30 |
|
CD133– | 15 | 15 | 30 |
| Total | 61 | 29 | 90 |
Discussion
Although research regarding cancer stem cells could
open a wide range of clinical possibilities, identifying these
cells has been difficult. Certain data indicate that tumour stem
cells in a number of cancers are derived from adult stem cells
(e.g., haematological neoplasms, breast cancer and human brain
glioma) (10,11). These cells are the foundation of
tumourigenesis, but only account for a small portion of tumour
cells. Bonnet and Dick (12) found
and confirmed the existence of tumour stem cells initially by
isolating CD34+/CD38– stem cells in acute
myeloblastic leukaemia cells. Al-Hajj et al (3) first verified the existence of tumour
stem cells in solid tumours by identifying tumour stem cells with
the characteristic marker
Lin–ESA+/CD44+/CD24–/low
in a breast carcinoma NOD/SCID mouse model. Recently, Singh et
al (4) identified
CD133+ as a cell-surface marker in brain tumours. A
study by Jordan (13) also
demonstrated the oncogenicity of CD133+ cells, which
further supports the hypothesis of tumour stem cells.
In the present study, the isolation and
characterisation of a highly tumourigenic subpopulation of cells
were described in human tongue squamous carcinoma. We believe that
this is the first description of the isolation of malignant
progenitors from human tongue squamous carcinoma. Over the past
several years, CD133 has been identified as a cancer stem cell
marker, including stem cells for cancer of adult brain, prostatic
carcinoma, colon carcinoma and liver cancer (5,14–17). Five separate criteria have been
established for cancer stem cells: i) The cells can self-renew; ii)
they are part of a small minority of the total tumour cell
population; iii) they present a reproducible tumour phenotype; iv)
they are capable of multipotent differentiation into
non-tumourigenic cells; and v) they carry distinct cell surface
antigenic phenotypes, permitting consistent isolation (18,19).
CD133+ cancer stem cells are capable of unlimited
self-renewal (20). Therefore, we
hypothesized that cancer stem cells may exist in tongue squamous
carcinoma. Further investigations will be performed to determine
whether the CD133 antigen is a surface marker of tumour stem cells,
and to assess the biological activity and proliferation state of
CD133+ cells in the human tongue squamous carcinoma
Tca8113 cell line.
Malignant tumours are capable of unlimited
self-renewal and heterogeneity (21).
In the present study, monoclonal tongue squamous carcinoma cells
were cultured in vitro after being monoclonally cultured
in vitro; the proliferation ratio was similar to that of
primary tumour cells, which indicated that only a small percentage
of tumour cells possessed clone capacity, heterogeneity and the
characteristics of tumour stem cells in vitro. Fluorescence
immunocytology demonstrated the distribution of CD133+
cells in the Tca8113 cell line. Green fluorescence was distributed
discretely among the cells; only 0.95% of the Tca8113 cells
expressed the CD133 antigen, indicating that a low proportion of
tongue squamous carcinoma cells were CD133+.
Studies have demonstrated that normal stem cells and
malignant tumour stem cells can be derived from nervous system
tissue (the growth of which has been suspended) (22). Subsequently, this phenomenon was also
observed in normal breast tissue and malignant breast cancer,
allowing cancer stem cells to be isolated from malignant breast
cancer. Further studies indicated that suspended growth was a
characteristic of cancer stem cells (23). Current research regarding head and
neck malignant cell lines has found that suspended cells (and the
secondary cultures of these cells) in the tongue Tca-8113 cell
lines are also able to form ‘tumour spheres’ (24). After re-plating the ‘tumour spheres’
to adhesive culture plates, the present study found that these
cells retained a strong capacity for self-renewal and
proliferation, indicating that the tongue squamous cell carcinoma
Tca-8113 cell line has its own cancer stem cells.
Cell Counting kit-8 is a novel method of measuring
cell viability. The principle is coincidental with the MTT method.
CCK-8 may form a hydrosoluble yellow formazan dye. This method is
convenient, and the variation in the results is low. Moreover,
compared with MTT, the OD and linear correlation are better and
offer a more sensitive and reproducible assay. The present study
found that the CD133+ tumour cells had a significantly
higher rate of proliferation than the CD133– tumour
cells and the primary cells. Among these three cell types, the
proliferation rate of CD133– cells was the slowest and
that of CD133+ cells was the strongest.
MACS involves combining magnetic beads with
mono-antibodies to a cell-surface antigen. The cells displaying the
antigen are adsorbed to the magnetic beads and held in a magnetic
field. In the present study, MACS was utilised to isolate positive
and negative cells from the primary tumour cells. MACS offered a
stable and repeatable methodology, separated the majority of the
cells of interest (purity of separation >90%) and only slightly
affected the energy state of the cells (25). Moreover, post-separation contamination
and cell damage were reduced.
The cell growth of the CD133+ cells was
faster than that of the CD133– or unsorted tumour cells.
The proportion of CD133+ cells decreased in culture over
time (decreasing from 92.45% initially to 1.62% at day 12),
indicating that the CD133+ cells differentiated over
time. This phenomenon is consistent with stem cell bionomics. The
percentage of tumour stem cells was extremely low (0.01–5%), but
the percentage of persistent tumour cells was >5%. The Tca-8113
cell lines in certain studies (26)
have been found to establish a tumour phenotype. Researcher bias
may also be an issue.
The establishment of animal models provides an ideal
testing tool for basic theory studies on the biological behaviour
and regulation of metastasis of malignant tumours and tumourigenic
mechanisms. By analysing the CD133 cells transplanted into NOD/SCID
mice, Singh et al (4) found
that 100 CD133+ cells could lead to tumourigenesis in
NOD/SCID mice. However, transplantation of 50,000–100,000
CD133– cells did not lead to tumourigenesis. A study by
Jordan (13) further confirmed the
tumourigenesis of CD133+ cells. O'Brien et al
(5) used tumours from patients with
colon cancer to inject CD133 antibodies into NOD/SCD mice, and the
result demonstrated that CD133+ had a strong
tumourigenic ability. The CD133+ tumour cells in the
NOD/SCID mice were again transplanted into new NOD/SCID mice, and
the result demonstrated that the newly formed tumours had a similar
phenotypic heterogeneity as the original tumours.
The present study also used NOD/SCID mice.
Suspensions of CD133+, CD133– and unsorted
cells (5×105/-2.5×108/ml) from the tongue
squamous cell line were subcutaneously injected into the mice using
a syringe (after disinfection). After 2 months, the subcutaneous
tumours were exposed following sacrifice of the NOD/SCID mice, and
the tumours generated from the CD133+ cells were
significantly larger than those generated from the
CD133– cells. Regarding tumourigenesis time, the
CD133+ cells required ~1 week and the CD133–
cells required 10 to 14 days, or even longer. However, the
CD133– cells also demonstrated a tumourigenic
capacity.
The present study was based on the observation that
the Tca-8113 cell line is heterogeneous and could include cancer
stem cells. Such cells could exist in suspended growth, but be able
to form ‘tumour spheres’. The proportion of CD133+ cells
to other tumour cells was only 0.95%; moreover, the
CD133+ cells were capable of unlimited self-renewal and
proliferation. In brief, we consider CD133 to be a phenotype of
tumour stem cells (possibly in combination with other markers).
This conclusion will require further research. However,
identification of the function of CD133 may increase our
understanding of its role in tumourigenesis.
According to the literature, there are some stem
cells in CD133– populations (27). The present study also found that
CD133– cells possess reproductive activity to a certain
extent, suggesting that tumour stem cells may be present in this
subpopulation. Nevertheless, it is worthwhile to investigate
whether CD133 is a specific marker in tongue squamous carcinoma.
Research in this area may elucidate the mechanism of tumourigenesis
and provide an original premise for developing antitumour drugs in
the future (28).
In conclusion, it is possible that CD133 may be a
phenotypic marker of tumour stem cells in the Tca-8113 cell line of
tongue squamous cancer. The surface markers of tumour stem cells of
tongue squamous cancer may be a combination of various cell
phenotypes. If this is the case, CD133 can be used to purify stem
cells and identify surface markers of the Tca-8113 cell line in
order to study protein expression and signal transduction.
Furthermore, CD133 can be used as a therapy target for the radical
treatment of tumours.
Acknowledgements
The authors would like to thank the Ninth Affiliated
People's Hospital of Shanghai Jiao Tong University for the Tca-8113
cell line, and the animal room of Tongji University School of
Stomatology for assistance with the animal experiments. This study
was supported by the National Natural Science Foundation of China
(grant no. 30700956) and the Shanghai City Natural Science
Foundation (grant no. 06ZR14084).
References
|
1
|
Choi IK and Yun CO: Recent developments in
oncolytic adenovirus-based immunotherapeutic agents for use against
metastatic cancers. Cancer Gene Ther. 20:70–76. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Heppner GH and Miller BE: Tumor
heterogeneity: biological implications and therapeutic
consequences. Cancer metastasis Rev. 2:5–23. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Al-Hajj M, Wicha MS, Benito-Hernandez A,
Morrison SJ and Clarke MF: Prospective identification of
tumorigenic breast cancer cells. Proc Natl Acad Sci USA.
100:3983–3988. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Singh SK, Clarke ID, Terasaki M, Bonn VE,
Hawkins C, Squire J and Dirks PB: Identification of a cancer stem
cell in human brain tumors. Cancer Res. 63:582l–5828. 2003.
|
|
5
|
O'Brien CA, Pollett A, Gallinger S and
Dick JE: A human colon cancer cell capable of initiating tumor
growth in immunodeficient mice. Nature. 445:106–110. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hide T and Kuratsu J: Progress in the
study of brain tumor stem cells as treatment target. Brain Nerve.
61:781–789. 2009.(In Japanese). PubMed/NCBI
|
|
7
|
Binello E and Germano IM: Targeting glioma
stem cells: a novel framework for brain tumors. Cancer Sci.
102:1958–1966. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu G, Yuan X, Zeng Z, Tunici P, Ng H,
Abdulkadir IR, Lu L, Irvin D, Black KL and Yu JS: Analysis of gene
expression and chemoresistance of CDl33+ cancer stem
cells in glioblastoma. Mol Cancer. 5:672006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wei XD, Zhou L, Cheng L and Tian J:
Experimental investigation of CD133 as a putative marker of
tumor-initiating cell in laryngeal carcinoma. Zhonghua Er Bi Yan
Hou Tou Jing Wai Ke Za Zhi. 41:940–944. 2006.(In Chinese).
PubMed/NCBI
|
|
10
|
Yuan X, Curtin J, Xiong Y, Liu G,
Waschsmann-Hogiu S, Farkas DL, Black KL and Yu JS: Isolation of
cancer stem cells from adult glioblastoma multiforme. Oncogene.
23:9392–9400. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Behbod F and Rosen JM: Will cancer stem
cells provide new therapeutic targets? Carcinogenesis. 26:703–711.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bonnet D and Dick JE: Human acute myeloid
leukemia is organized as a hierarchy that originates from a
primitive hematopoietic cell. Nat Med. 3:730–737. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jordan CT: Cancer stem cell biology: From
leukemia to solid tumors. Curr Opin Cell Biol. 16:708–712. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ricci-Vitiani L, Lombardi DG, Pilozzi E,
Biffoni M, Todaro M, Peschle C and De Maria R: Identification and
expansion of human colon-cancer initiating cells. Nature.
445:111–115. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Collins AT, Berry PA, Hyde C, Stower MJ
and Maitland NJ: Prospective identification of tumorigenic prostate
cancer stem cells. Cancer Res. 65:10946–10951. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Suetsugu A, Nagaki M, Aoki H, Motohashi T,
Kunisada T and Moriwaki H: Characterization of CD133+
hepatocellular carcinoma cells as cancer stem/progenitor cells.
Biochem Biophys Res Commun. 351:820–824. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ma S, Chan KW, Hu L, Lee TK, Wo JY, Ng IO,
Zheng BJ and Guan XY: Identification and characterization of
tumorigenic liver cancer stem/progenitor cells. Gastroenterology.
132:2542–2556. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dalerba P, Cho RW and Clarke MF: Cancer
stem cells: Models and concepts. Annu Rev Med. 58:267–284. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Clarke MF, Dick JE, Dirks PB, Eaves CJ,
Jamieson CH, Jones DL, Visvader J, Weissman IL and Wahl GM: Cancer
stem cells-perspectives on current status and future directions:
AACR workshop on cancer stem cells. Cancer Res. 66:9339–9344. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bar EE, Chaudhry A, Lin A, Fan X, Schreck
K, Matsui W, Piccirillo S, Vescovi AL, DiMeco F, Olivi A and
Eberhart CG: Cyclopamine-mediated hedgehog pathway inhibition
depletes stem-like cancer cells in glioblastoma. Stem Cells.
25:2524–2533. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rowehl RA, Burke S, Bialkowska AB, Pettet
DW 3rd, Rowehl L, Li E, Antoniou E, Zhang Y, Bergamaschi R, Shroyer
KR, et al: Establishment of highly tumorigenic human colorectal
cancer cell line (CR4) with properties of putative cancer stem
cells. PLoS One. 9:e990912014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dontu G, Al-Hajj M, Abdallah WM, Clarke MF
and Wicha MS: Stem cells in normal breast development and breast
cancer. Cell Prolif. 36(Suppl 1): S59–S72. 2003. View Article : Google Scholar
|
|
23
|
Patrawala L, Calhoun T,
Schneider-Broussard R, Zhou J, Claypool K and Tang DG: Side
population is enriched in tumorigenic, stem-like cancer cells,
whereas ABCG2+ and ABCG2– cancer cells are
similarly tumorigenic. Cancer Res. 65:6207–6219. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Harper LJ, Piper K, Common J, Fortune F
and Mackenzie IC: Stem cell patterns in cell lines derived from
head and neck squamous cell carcinoma. J Oral Pathol Med.
36:594–603. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nishi H, Nishimura S, Higashiura M, Ikeya
N, Ohta H, Tsuji T, Nishimura M, Ohnishi S and Higashi H: A new
method for histamine release from purified peripheral blood
basophils using monoclonal antibody-coated magnetic beads. J
Immunol Methods. 240:39–46. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zou B, Ji P, Sun S and Qi X: Screen and
analysis of differentially expressed genes related to stem-like
cells in tongue squamous cell carcinoma Tca8113 cell line. Huaxi
Kouqiang Yixue Zazhi. 30:458–462. 2012.(In Chinese). PubMed/NCBI
|
|
27
|
Beier D, Hau P, Proescholdt M, Lohmeier A,
Wischhusen J, Oefner PJ, Aigner L, Brawanski A, Bogdahn U and Beier
CP: CD133+ and CD133– glioblastoma-derived
cancer stem cells show differential growth characteristics and
molecular profiles. Cancer Res. 67:4010–4015. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Taipale J and Beachy PA: The hedgehog and
Wnt signalling pathways in cancer. Nature. 411:349–354. 2001.
View Article : Google Scholar : PubMed/NCBI
|