Introduction
Sclerosing angiomatoid nodular transformation of the
spleen (SANT) is an extremely rare benign lesion of the spleen
(1,2),
which was first characterized by Martel et al (1) in 2004. To date, a total of 133 cases of
SANT have been reported in the English literature; however, the
exact incidence rate remains unclear. The majority of patients are
asymptomatic at presentation and SANT is identified incidentally on
imaging. For symptomatic patients, common presentations are
incidental splenic mass, abdominal discomfort or pain. There is a
slight female preponderance and middle-aged adults are
predominantly affected (1). The
majority of cases undergo traditional or laparoscopic splenectomy,
and diagnosis is confirmed using histopathological examination. No
recurrence of SANT has been reported following a splenectomy;
therefore, traditional or laparoscopic splenectomy is considered to
be a curative technique for the management of SANT (2). By contrast, due to absent definitive
imaging signs and varying growth patterns, it remains challenging
to form a clearly pre-operative diagnosis of SANT compared with
other splenic tumors or malignant lesions. The present study
reports a novel case of SANT and discusses the characteristics of
imaging, immunohistochemical profile and differential diagnosis,
which may result in improved management of SANT.
Case report
A 29-year-old man presented to the Department of
Surgery, The First Affiliated Hospital, Sun Yat-sen University
(Guangzhou, China) on March 30, 2014 with a 1-year history of left
upper quadrant and back discomfort. The discomfort was
intermittent, but had become progressively worse since it first
occurred. There was no history of fever, weight loss and other
medical issues, and the patient's respiratory, digestive and
urinary systems were normal. Abdominal examination found no
positive physical signs. Hematological parameters, and liver and
kidney function tests were unremarkable. On computed tomography
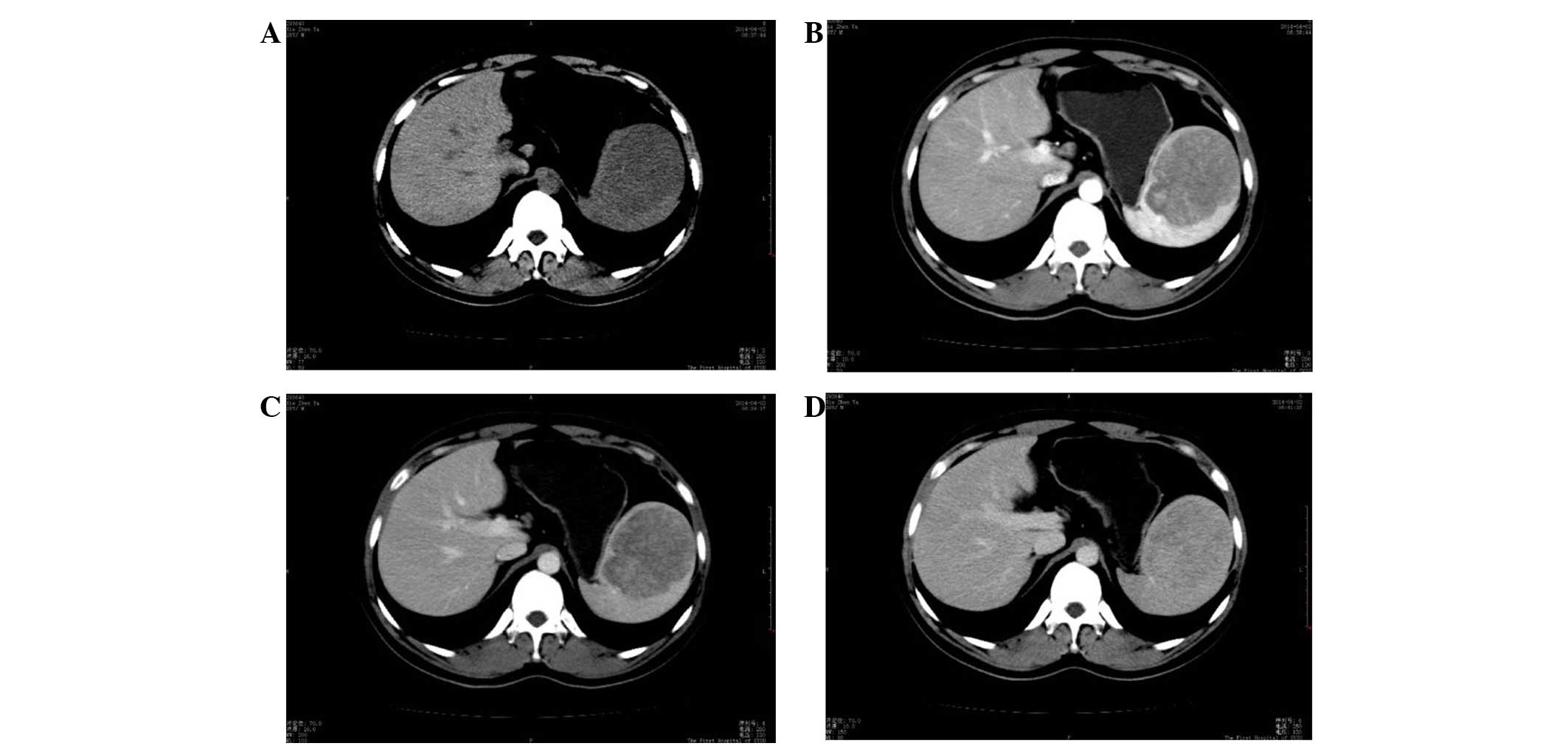
(CT) scan, a round mass of 76×74 mm was observed in the spleen,
which showed slightly low density on a plain scanning CT image
(Fig. 1A). CT values of the mass and
the surrounding normal splenic tissue were ~40 and 42 HU,
respectively, prior to the injection of contrast medium. The mass
showed heterogeneous moderate enhancement with a value of 66 HU,
which was clearly lower than that of the surrounding normal spleen
(136 HU) on arterial phase-enhanced CT scanning. Certain regions
within the lesion were significantly enhanced (Fig. 1B). The CT value of the mass was
slightly increased (80 HU) on portal venous phase-enhanced CT
scanning, but remained lower than that of the surrounding spleen
(116 HU) (Fig. 1C). The densities of
the mass and the normal surrounding spleen were close on delayed
enhanced CT scanning after a delay of 3 min (Fig. 1D). The patient underwent a
splenectomy. A well-circumscribed mass measuring 7 cm in diameter
was noted in the cross-sections of the resected specimen. On gross
examination, the mass exhibited a gray-white or gray-red cut
surface with fibrous septa traversing throughout and was divided
into discrete complete or incomplete nodules.
Resected tissues underwent routine preparation for
hematoxylin and eosin staining, and light microscopy confirmed the
nodular appearance observed during the gross examination.
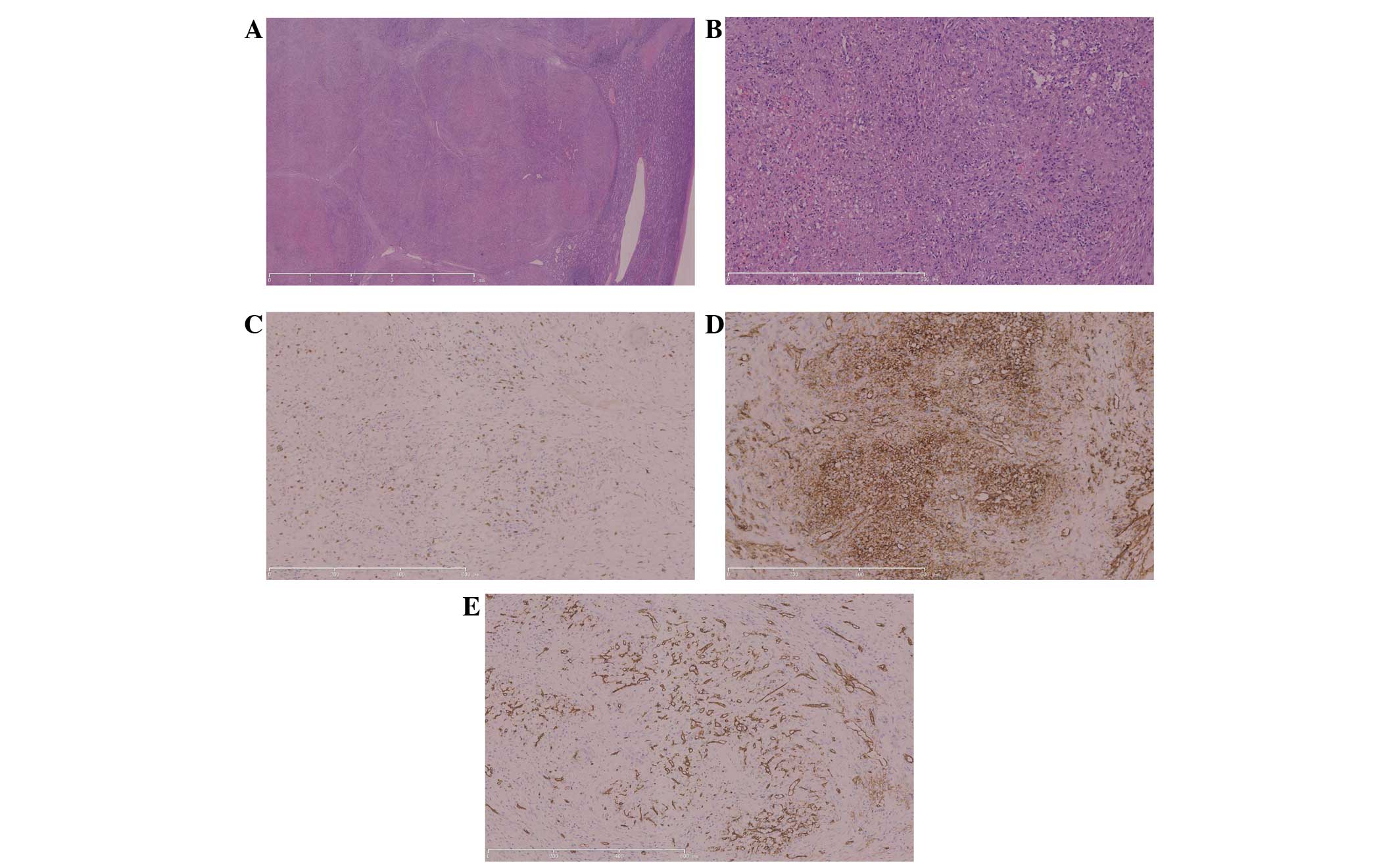
Observation under a light-contrast microscope showed that the
nodules were separated by fibrous or fibrosclerotic stroma with
hemosiderophages, fibroblasts and lympho-mononuclear cells
(Fig. 2A). Within the nodules,
extravasated erythrocytes and hemosiderin pigment were plentiful,
and oval stromal cells, red blood cells and mild chronic
inflammatory cell infiltration were also present (Fig. 2B). In addition, three distinct types
of blood vessels were also observed: Splenic cord-type capillaries,
sinusoid-type spaces and small veins. The results of
immunohistochemistry tests revealed that the endothelial cells in
these blood vessels showed heterogenous immunohistological profiles
of cluster of differentiation (CD)8-, CD31- and CD34-positive
cells, which indicated their derivation from sinusoidal,
capillary-like and vein-like elements (Fig. 2C-E). Immunohistochemistry was
performed using the following rabbit polyclonal antibodies, which
were purchased from Abcam (Cambridge, MA, USA): Anti-CD8 (catalog
no., ab4055; dilution, 1:200), anti-CD31 (catalog no., ab28364;
dilution, 1:50) and anti-CD34 (catalog no., ab110643; dilution,
1:200) Horseradish peroxidase and intelliPATH FLXDAB DAB Chromogen
kit (Biocare Medical, Inc., Concord, CA, USA) were used for
visualization. These findings and the characteristic macroscopic
features led to the diagnosis of SANT.
The patient remained asymptomatic and there was no
evidence of recurrence subsequent to a follow-up period of 13
months. Written informed consent was obtained from the patient for
the publication of the present study.
Discussion
The prevalence of splenic tumors is rare, and the
majority are vascular or inflammatory in origin (3,4). As a
novel entity that differed from other splenic vascular tumors, SANT
was first defined by Martel et al (1) in 2004. However, prior to its
identification, this lesion was possibly reported in other studies
under the names of different splenic vascular lesions, such as
hamartoma, hemangioma or hemangioendothelioma (4). The present study reviews the current
knowledge on SANT, beginning with the present case, and discusses
other cases reported in the medical English literature.
In a review of the existing English literature, a
total of 133 cases of SANT have been found to be reported in ~50
studies after the condition was first defined in 2004 (2). The cases consisted of 59 males and 74
females, with a female-to-male ratio of 1.25:1, which is similar to
the results recently reported by Falk et al in 2012
(2), but lower than the ratio of 2:1
reported by Diebold et al in 16 splenectomy specimens
(5). Therefore, the gender ratio may
change as more cases are reported. The median age of the patients
in the present literature review was 44 years, and the majority
(94/133 cases) were aged between 30 and 60 years. Thus, SANT
predominantly affects the middle-aged population. In these cases,
>80 lesions were asymptomatic and were found incidentally during
imaging. For symptomatic patients, abdominal pain or discomfort was
predominant, but certain presentations included a palpable mass,
flank pain, pelvic pain, anemia, thrombocytopenia, long-standing
fevers and night sweats.
Pathological features were the key for the diagnosis
of SANT, as originally described by Martel et al (1) in a series of 25 patients and confirmed
in multiple subsequent case series by other researchers. The
present literature review summarizes these features as follows:
Firstly, the size of the lesion varied widely from 0.1–17 cm in
diameter among the 133 cases of SANT. Secondly, gross examination
showed a well-circumscribed but non-encapsulated bosselated mass,
with multiple individual or confluent red-brown or tan-white
nodules separated by whitish fibrotic stroma. In addition, the
lesion was slightly firmer than the rest of the spleen. This
classic appearance is diagnostic for SANT (6). Moreover, as shown in Fig. 1, the microscopic examination of SANT
usually reveals a multinodular growth pattern composed of a
variable mixture and sieve-like arrangement of vascular spaces that
are often surrounded by dense collagen fibrosis or fibroid rims.
The circumscription of nodules by collagen and the nature of the
intervening stroma can vary among individual tumors. Centrally, the
nodules contain vascular channels of varying sizes lined with thick
endothelium interspersed with ovoid or spindle cells. Numerous
erythrocytes, myofibroblasts, plasma cells, siderophages, and
inflammatory cells can appear in these nodules. Notably, no
markedly cytological atypia, mitotic figures, histiocytic giant
cells or necrosis were observed in the lesions.
The classic appearance of SANT with regard to the
immunohistochemical profiles includes three distinct types of blood
vessels and endothelial cells stained with CD34, CD8 or CD31,
respectively (7). The first type of
vessels consists of well-formed cord capillaries in an organized
lobular arrangement in endothelial cells positive for CD34 and
CD31, but negative for CD8. The second type of vessels is
consistent with splenic sinusoids and includes cells negative for
CD34, but positive for CD31 and CD8. The third type consists of
small veins that are arranged in intricate mesh-like patterns and
include cells negative for CD34 and CD8, but positive for CD31. In
the studies reviewed, these classic appearances were observed in
almost all specimens (7). Moreover,
none of the cells in the lesions stained positively for CD23,
anaplastic lymphoma kinase, CD30, CD68 or Epstein-Barr virus latent
membrane protein 1.
CT imaging cannot specifically distinguish SANT from
various other types of vascular lesions in the spleen, but it is
the major method to find SANT lesions (8). In the 133 cases of SANT identified in
the present review, CT imaging was performed in almost all of the
patients, and the CT features of SANT were relatively unique. As
described in a previous study (9),
SANT generally presents as a well-circumscribed, solitary, isodense
or hypodense mass in the spleen on plain CT scanning images in the
portal and late portal venous phase, with or without a small
central calcification. In multiphase imaging, a number of SANT
lesions showed a ‘spoke-and-wheel’ pattern (10). Detailed study of radiological and
pathological findings revealed that this spoke-and-wheel morphology
corresponded to a central, stellate fibrous stroma with fibrous
septa that separated angiomatoid nodules (9). Moreover, positron-emission tomography-CT
and single-photon emission CT/CT (SPECT/CT) were also used for the
diagnosis of SANT. As a benign lesion, SANT should not be avid for
18F-fluorodeoxyglucose (FDG) (11). However, at least 2 cases of SANT
showed mild to moderate FDG avidity, and the lesion appeared as a
hypermetabolic-activity mass in the FDG-positron emission
tomography image, simulating a neoplasm (11,12). This
feature suggests that SANT lesions may have a higher glycolytic
activity than the surrounding splenic parenchyma. Moreover, only 1
study (11) described a finding of
SANT based on 99mTc-sulfur colloid SPECT/CT imaging, and
normal tracer activity was observed within the liver and spleen,
but not within the splenic lesion. This appearance confirmed the
absence of reticuloendothelial elements within splenic SANT.
Only a small number of cases of SANT included
magnetic resonance imaging (MRI) findings, which limits any broad
statements regarding the MRI imaging of SANT. Karaosmanoglu et
al (10) first described the MRI
findings of SANT, and reported that the lesion presented as a
central hyperintense area that was consistent with hemorrhage on
fat-saturated precontrast T1-weighted images. However, on
T2-weighted images, the lesion appeared as a spoke-and-wheel
pattern, which was similar to the pattern obtained by multiphase
imaging with CT. Subsequent studies (1,12–14) have described the MRI features of SANT,
and numerous investigators have suggested that the spoke-and-wheel
pattern from MRI and multiphase CT imaging may be useful for the
diagnosis of SANT.
Only a few studies on ultrasonography for the
diagnosis of SANT have appeared in the English literature, and
therefore, the sonographic features of SANT are poorly described
(15–19). On gray-scale ultrasonography, SANT
usually appears as a heterogeneously hypoechoic mass with bright
linear echoes accompanied by acoustic shadowing in the center
(16,17). These linear echoes usually show blood
signals on color Doppler images and an arterial waveform on
spectral Doppler analysis. Contrast-enhanced ultrasound examination
has better temporal resolution than contrast-enhanced CT and can
dynamically visualize blood-flow distribution in the vascular phase
of the tumor. With the aid of ultrasound contrast agents such as
Sonazoid (Daiichi-Sankyo, Tokyo, Japan) or SonoVue (Bracco, Milan,
Italy), SANT appears as a spoke-and-wheel enhancement that extends
radially from the center of the mass during the early vascular
phase (16).
CT, MRI and ultrasonography can provide various
types of information on SANT, but the images alone are not usually
sufficient to radiologically distinguish SANT from other benign
splenic lesions. To address this shortcoming, Gutzeit et al
(16) first reported the use of
ultrasound-guided core needle biopsy (CNB) for the diagnosis of
SANT. Although the study suggested that ultrasound-guided CNB is
safe, another study (1) reported
opposing views due to the danger of bleeding and tumor
dissemination. Further imaging studies must be performed to provide
more reliable information for the diagnosis of SANT.
The differential diagnosis of SANT involves
consideration of several other benign splenic lesions and malignant
vascular lesions. These include hemangioma, lymphangioma, littoral
cell angioma, hamartoma, angiosarcoma and inflammatory pseudotumor
(IPT).
Hemangiomas of the spleen are the most common benign
tumors that arise from sinusoidal epithelial cells (20). Usually smaller than 2 cm, the tumors
show cavernous configurations on imaging (20). Histologically, the endothelial cells
are positive for CD31 and CD34, and are negative for CD8, CD21 and
CD68 (4). A characteristic difference
is that hemangiomas lack the typical trivascular pattern of
proliferation observed in SANT (4).
Lymphangioma is a benign splenic tumor that arises
from the lymphatic endothelium (21).
The tumor usually presents as either a solitary nodule or a diffuse
process, and capillary and cavernous forms also exist (6). Microscopic examination reveals cystic
spaces lined with single- or multi-layered endothelium, with
occasional papillary excrescences (2,5,21). Immunostaining shows the lymphatic
endothelia are strongly positive for D2-40 immunostain (Ventana,
Tucson, AZ), and negative for CD21 and CD8 (2,21).
Littoral cell angioma is a vascular tumor that is
derived from the littoral cells (22). Histological examination shows that
these cells line the splenic sinuses and exhibit both endothelial
and histocytic phenotypes (2).
Significantly, littoral cell angioma has a characteristic
CD34−/CD68+/CD21+/CD8−
immunophenotype, which is extensively different from that of SANT
(2).
Splenic hamartoma is another rare vasoformative
lesion of the spleen (23).
Microscopically, it displays predominantly red pulpy elements in a
disorganized fashion, with scanty fibrous trabeculae (6). On immunohistochemistry, it is positive
for factor VIII, CD31, CD8 and type IV collagen, but is negative
for CD34, CD21 and CD68 (6). The
typical difference between the tumor and SANT is the absence of a
nodular growth pattern in splenic hamartoma and the presence of the
classical immunophenotype in SANT (2).
Angiosarcoma is the most common malignant primary
tumor of the spleen (24). The
histological features of angiosarcoma include irregular,
anastomosing vascular channels, with marked cytological atypia,
brisk mitoses and invasion. The atypical cells have a vascular
endothelial phenotype and are positive for CD68 and CD8 (24). The characteristics that differentiate
SANT from angiosarcoma include the absence of a nodular growth
pattern, and the presence of invasion, cytological atypia and
mitosis (2).
IPT is a splenic lesion that is particularly similar
to SANT (25). Microscopic
examination reveals a mass comprising spindle cells, with features
of fibroblasts and myofibroblasts in association with a mixed
inflammatory infiltrate and a variable hypocellular
fibrocollagenous stroma (3,25). These features are also present in
SANT, but IPT lacks angiomatoid nodules and shows a
CD34−/CD8−/CD21− phenotype
(3).
There is currently no sensitive and specific way to
diagnose SANT without a tissue sample, and on the basis of
conventional imaging studies, it is pre-operatively difficult to
rule out the possibility of a malignant neoplasm. As
aforementioned, an ultrasound-guided CNB of the spleen has been
used for tissue diagnosis (16), but
the problem of bleeding or tumor dissemination may be an unwanted
effect of this procedure. Thus, in the majority of cases,
splenectomy has become the recommended technique. With the
development of laparoscopic instruments and techniques during the
past decades, laparoscopic surgery has been deployed extensively in
numerous surgical fields. Since the introduction of laparoscopic
splenectomy by Delaitre and Maignien in 1991 (26), this procedure has been recognized as a
safe and effective treatment for certain splenic diseases. To date,
>30 patients with SANT have undergone laparoscopic splenectomy,
and no serious problems have been observed during or after this
procedure (27–30). Hence, laparoscopic splenectomy may be
a safe and effective procedure for the diagnosis and treatment of
SANT, particularly when malignancy cannot be ruled out.
Overall, the present study reports a novel case of
SANT that underwent splenectomy successfully. By comparison to
other cases, the lesion exhibited no outstanding difference on
presentation and characters during imaging. Therefore, when
solitary lesions are identified in the spleen during imaging
examination, clinicians and radiologists should be conscious of the
possibility of SANT. To overcome these challenges, a thorough
histopathological examination and immunohistochemical analysis are
necessary.
References
|
1
|
Martel M, Cheuk W, Lombardi L,
Lifschitz-Mercer B, Chan JK and Rosai J: Sclerosing angiomatoid
nodular transformation (SANT): Report of 25 cases of a distinctive
benign splenic lesion. Am J Surg Pathol. 28:1268–1279. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Falk GA, Nooli NP, Morris-Stiff G, Plesec
TP and Rosenblatt S: Sclerosing angiomatoid nodular transformation
(SANT) of the spleen: Case report and review of the literature. Int
J Surg Case Rep. 3:492–500. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Thipphavong S, Duigenan S, Schindera ST,
Gee MS and Philips S: Nonneoplastic, benign, and malignant splenic
diseases: Cross-sectional imaging findings and rare disease
entities. AJR Am J Roentgenol. 203:315–322. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Önder S, Kosemehmetoglu K, Himmetoglu Ç,
Firat P and Uner A: Sclerosing angiomatoid nodular transformation
(SANT) of spleen: A case report describing cytology, histology,
immunoprofile and differential diagnosis. Cytopathology.
23:129–132. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Diebold J, Le Tourneau A, Marmey B, Prevot
S, Müller-Hermelink HK, Sevestre H, Molina T, Billotet C, Gaulard
P, Knopf JF, et al: Is sclerosing angiomatoid nodular
transformation (SANT) of the splenic red pulp identical to
inflammatory pseudotumour? Report of 16 cases. Histopathology.
53:299–310. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Agrawal M, Uppin SG, Bh S, Suppin M NB and
Challa S: Sclerosing angiomatoid nodular transformation of the
spleen: A new entity or a new name? Turk Patoloji Derg. Apr
9–2014.(Epub ahead of print). PubMed/NCBI
|
|
7
|
Pradhan D and Mohanty SK: Sclerosing
angiomatoid nodular transformation of the spleen. Arch Pathol Lab
Med. 137:1309–1312. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Raman SP, Singhi A, Horton KM, Hruban RH
and Fishman EK: Sclerosing angiomatoid nodular transformation of
the spleen (SANT): Multimodality imaging appearance of five cases
with radiology-pathology correlation. Abdom Imaging. 38:827–834.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lewis RB, Lattin GE Jr, Nandedkar M and
Aguilera NS: Sclerosing angiomatoid nodular transformation of the
spleen: CT and MRI features with pathologic correlation. AJR Am J
Roentgenol. 200:W353–W360. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Karaosmanoglu DA, Karcaaltincaba M and
Akata D: CT and MRI findings of sclerosing angiomatoid nodular
transformation of the spleen: Spoke wheel pattern. Korean J Radiol.
9(Suppl): S52–S55. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Thacker C, Korn R, Millstine J, Harvin H,
Van Lier Ribbink JA and Gotway MB: Sclerosing angiomatoid nodular
transformation of the spleen: CT, MR, PET, and
99(m)Tc-sulfur colloid SPECT CT findings with gross and
histopathological correlation. Abdom Imaging. 35:683–689. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lee D, Wood B, Formby M and Cho T: F-18
FDG-avid sclerosing angiomatoid nodular transformation (SANT) of
the spleen: Case study and literature review. Pathology.
39:181–183. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bamboat ZM and Masiakos PT: Sclerosing
angiomatoid nodular transformation of the spleen in an adolescent
with chronic abdominal pain. J Pediatr Surg. 45:E13–E16. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chikkappa MG, Morrison C, Lowe A, Antrim
R, Swirsky DM and Gokhale J: Case report and magnetic resonance
images of sclerosing angiomatoid nodular transformation (SANT) of
the spleen. BMJ Case Rep. 2009:bcr07.2009.21312009.PubMed/NCBI
|
|
15
|
Cao JY, Zhang H and Wang WP:
Ultrasonography of sclerosing angiomatoid nodular transformation in
the spleen. World J Gastroenterol. 16:3727–3730. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gutzeit A, Stuckmann G and
Dommann-Scherrer C: Sclerosing angiomatoid nodular transformation
(SANT) of the spleen: Sonographic finding. J Clin Ultrasound.
37:308–311. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim HJ, Kim KW, Yu ES, Byun JH, Lee SS,
Kim JH and Lee JS: Sclerosing angiomatoid nodular transformation of
the spleen: Clinical and radiologic characteristics. Acta Radiol.
53:701–706. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lee M, Caserta M and Tchelepi H:
Sclerosing angiomatoid nodular transformation of the spleen.
Ultrasound Q. 30:241–243. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Watanabe M, Shiozawa K, Ikehara T,
Kanayama M, Kikuchi Y, Ishii K, Okubo Y, Shibuya K and Sumino Y: A
case of sclerosing angiomatoid nodular transformation of the
spleen: Correlations between contrast-enhanced ultrasonography and
histopathologic findings. J Clin Ultrasound. 42:103–107. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Giovagnoni A, Giorgi C and Goteri G:
Tumours of the spleen. Cancer Imaging. 5:73–77. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Singh P, Mathur SK, Singh S, Marwah N,
Kalra R and Arora B: Cystic lymphangioma of the spleen-a case
report. Indian J Pathol Microbiol. 48:202–203. 2005.PubMed/NCBI
|
|
22
|
Emir S, Sozen S, Yazar MF, Altinsoy HB,
Arslan Solmaz O and Ozkan Z: Littoral-cell angioma of the spleen.
Arch Iran Med. 16:189–191. 2013.PubMed/NCBI
|
|
23
|
Di Blasi A, Boscaino A, De Dominicis G and
Marino-Marsilia G: Splenic hamartoma. Pathologica. 97:124–129.
2005.(In Italian). PubMed/NCBI
|
|
24
|
Delacruz V, Jorda M, Gomez-Fernandez C,
Benedetto P and Ganjei P: Fine-needle aspiration diagnosis of
angiosarcoma of the spleen: A case report and review of the
literature. Arch Pathol Lab Med. 129:1054–1056. 2005.PubMed/NCBI
|
|
25
|
Kim HH, Hur YH, Koh YS, Kim JC, Kim HJ,
Kim JW, Kim Y, Lee JH and Cho CK: Sclerosing angiomatoid nodular
transformation of the spleen related to IgG4-associated disease:
Report of a case. Surg Today. 43:930–936. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Delaitre B and Maignien B: Splenectomy by
the laparoscopic approach. Report of a case. Presse Med.
20:22631991.(In French). PubMed/NCBI
|
|
27
|
Budzyński A, Demczuk S, Kumiega B,
Migaczewski M, Matłok M and Zub-Pokrowiecka A: Sclerosing
angiomatoid nodular transformation of the spleen treated by
laparoscopic partial splenectomy. Wideochir Iinne Tech
Maloinwazyjne. 6:249–255. 2011.
|
|
28
|
Kakisaka T, Kamiyama T, Yokoo H, Orimo T,
Wakayama K, Tsuruga Y, Kamachi H, Harada T, Kato F, Yamada Y, et
al: Hand-assisted laparoscopic splenectomy for sclerosing
angiomatoid nodular transformation of the spleen complicated by
chronic disseminated intravascular coagulation: A case report.
Asian J Endosc Surg. 7:275–278. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kim KH, Lee S, Youn SH, Lee MR, Kim MC,
Rha SH and Jung GJ: Laparoscopic splenectomy for sclerosing
angiomatoid nodular transformation of the spleen. Journal of the
Korean Surgical Society. 80(Suppl 1): S59–S62. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mohr Z, Klippel S, Spiethoff A, Trick D
and Willis S: Laparoscopic splenectomy for sclerosing angiomatoid
nodular transformation. Chirurg. 82:714–718. 2011.(In German).
View Article : Google Scholar : PubMed/NCBI
|