Introduction
Cordyceps militaris is a fungus that
parasitizes Lepidoptera larvae and has been extensively used
as a folk tonic food and crude drug (1). Cordyceps militaris has long been
considered to have natural medicinal properties, such as
anti-angiogenic, anti-tumor, anti-viral, anti-inflammatory and
hypoglycemic effects (1–8).
Cordycepin, also termed 3′-deoxyadenosine, is a
nucleoside analogue from C. militaris (9) and has been reported to demonstrate
numerous notable biological and pharmacological properties,
including immunological stimulation, anti-cancer and anti-viral
effects (10–15), a stimulating effect on interlukin-10
production as an immune modulator (16) and preventing hyperlipidermia (17).
Apoptosis, also termed programmed cell death, is a
key regulator of tissue homeostasis and is characterized by typical
morphological and biochemical hallmarks, including cell shrinkage,
membrane blebbing, chromatin condensation and nuclear fragmentation
(18). The mechanisms for foreign
chemicals disrupting cell functions and causing tissue damage have
been associated with the triggering of apoptosis signal
transduction pathways, consisting of the intrinsic (mitochondrial)
and extrinsic (death receptor) pathways (19).
Although cordycepin has been shown to have a
cytotoxic effect on HepG2 cells, the detailed molecular mechanisms
have not been well elucidated (17,19). In
the present study, the mechanistic understanding of how cordycepin
mediates apoptosis in HepG2 cells was investigated and the
intrinsic and extrinsic apoptosis pathways were the main focus.
Materials and methods
Materials
Dried Cordyceps militaris was purchased from
Cordyceps Garden Biotechnology Company (Fuzhou, China). The
macroprous adsorption resin NKA-II was purchased from the Chemical
Plant of Nankai University (Tianjin, China). Sulforhodamine B
(SRB), 4′,6-diamidino-2-phenylindole (DAPI), propidium iodide (PI)
and tetraethylbenzimidazolylcarbocyanine iodide (JC-1) were
purchased from Sigma-Aldrich (St. Louis, MO, USA). The following
antibodies were purchased from Sangon Biotech Co., Ltd. (Shanghai,
China): Mouse monoclonal anti-Fas (1:3,000; catalog no. D198888);
rabbit polyclonal anti-Fas ligand (1:3,000; catalog no. D262701);
mouse monoclonal anti-Fas associated with death domain protein
(FADD; 1:3,000; catalog no. D199671); rabbit polyclonal
anti-caspase-8 (1:2,000; catalog no. D155240); rabbit polyclonal
anti-caspase-9 (1:2,000; catalog no. D220078); rabbit ployclonal
anti-caspase-10 (1:2,000; catalog no. D260010); rabbit monoclonal
anti-caspase-3 (1:3,000; catalog no. D120074); mouse monoclonal
anti-B-cell lymphoma-2 (Bcl-2; 1:3,000; catalog no. D198628);
rabbit polyclonal anti-Bcl-2 associated X protein (Bax; 1:3,000;
catalog no. D120073); mouse monoclonal anti-BH3 interacting domain
death agonist (Bid; 1:3,000; catalog no. D198911); and rabbit
polyclonal anti-cytochrome c (1:2,000; catalog no. D110006).
Rabbit polyclonal anti-mouse IgG (1:3,000; catalog no. sc-358920)
and goat anti-rabbit IgG (1:3,000; catalog no. sc-2768) antibodies
were purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX,
USA). The Mitochondrial Membrane Potential Assay kit with JC-1,
radioimmunoprecipitation assay (RIPA) lysis buffer and Cell
Mitochondria Isolation kit were purchased from Beyotime Institute
of Biotechnology (Haimen, China).
Cell lines and culture
HepG2 cell lines were purchased from the Culture
Center of the Institute of Basic Medical Sciences of the Chinese
Academy of Medical Sciences (Beijing, China). The cells were
maintained in Dulbecco's modified Eagle's medium (DMEM; Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with
10% fetal bovine serum (Invitrogen; Thermo Fisher Scientific,
Inc.), 100 IU/ml penicillin and 100 µg/ml streptomycin
(Sigma-Aldrich), and cultured at 37°C for 48 h in a humidified
atmosphere consisting of 95% air and 5% CO2.
Isolation and purification of
cordycepin
A total of 150 g dried fruiting bodies of C.
militaris were cleaned with distilled water, dried in a dark
and ventilated place and crushed prior to soaking in 500 ml
distilled water overnight at room temperature, and then boiled in
distilled water for 3 h. Subsequent to filtration to remove debris,
the filtrate (crude aqueous extract) was concentrated in a rotary
evaporator (model no. 0010001820; IKA® Works Guangzhou,
Guangzhou, China). Ethanol was added and the mixture was left at
room temperature overnight. The precipitate obtained subsequent to
filtration was discarded and the supernatant was lyophilized to
yield the crude sample. The crude extract was chromatographed on a
NKA-II column (Sigma-Aldrich) and eluted with 50% (v/v) ethanol to
collect the fractions, which were then further purified by high
performance liquid chromatography (HPLC). The Lab Alliance HPLC
system (model no. 2690/2695; Waters Technologies Ltd., Shanghai,
China), including two Series III pumps (model no. 90–2489 rev M;
Scientific Systems, Inc. State College, PA, USA) and anabsorbance
detector (model no. 500; Waters Technologies Ltd.) set at 254 nm,
connected to a Cs420 Hardware integrator (model no. Cs420; Waters
Technologies Ltd.). The column was a 250×10 mm reversed-phase Merck
Millipore C18 ODS column (Merck Millipore, Darmstadt, Germany) with
an internal diameter of 12 µm. The temperature was set at 30°C and
the elution conditions were as follows: Flow rate, 0.8 ml/min; and
solvent, 15% methanol.
Antiproliferative activity assay of
cordycepin
Various concentrations of cordycepin (0, 125, 250
and 500 µM) were inoculated into 96-well microplates when the cell
concentration was adjusted to 1×105 cells/ml. Cultures
in triplicate were treated with cordycepin for 48 h and the control
wells received only maintenance medium (DMEM). Cellular responses
were colorimetrically evaluated by an SRB assay. Briefly, the cells
were fixed with 25% trichloroacetic acid (Sigma-Aldrich) and washed
and stained with 0.4% SRB. Subsequent to the cell-bound SRB being
solubilized by the addition of 10 mM Tris-HCl, bound SRB was
colorimetrically assessed using an ELISA microplate reader (MK3;
Thermo Fisher Scientific, Inc.) at 490 nm. Cell growth inhibition
was expressed as a percentage of the untreated control absorbance
following the subtraction of the mean background absorbance.
Compounds were considered to have potent growth inhibitory activity
when the reduction in SRB absorbance was >25% compared with the
untreated control cells. The half maximal inhibitory concentration
(IC50) values were calculated from the dose-response
curves.
Assessment of apoptosis by DAPI
staining
HepG2 cells were seeded into a 50 ml culture flask
at a density of 1×105 cells/ml for 24 h. Subsequent to
treatment with or without cordycepin (0, 125, 250 and 500 µM) at
37°C for 48 h, the cells were collected and washed with
phosphate-buffered saline (PBS). Cell pellets were fixed with 4%
paraformaldehyde for 10 min and washed three times with PBS. The
cells were then incubated with 5 µg/ml DAPI for 20 min. Subsequent
to washing with PBS, the cells were observed under a fluorescence
microscope (model no. Bx51; Olympus Corporation, Tokyo, Japan) and
images were captured.
Assessment of apoptosis by flow
cytometry
HepG2 cells cultured with or without cordycepin at
37°C for 48 h were harvested, washed with PBS and fixed with 70%
cold ethanol at 4°C for 4 h. The fixed cells were washed and
stained with a PI solution containing 20 µg/ml PI and 10 mg/ml
RNase (Takara Biotech, Inc., Dalian, China) in PBS for 20 min in
the dark. The stained cells were then analyzed by flow cytometry
using fluorescence-activated cell sorting (FACS) and the
FACSCalibur system (BD Biosciences, Franklin Lakes, NJ, USA). In
addition, subsequent to being treated with various concentrations
of cordycepin (0, 125, 250 and 500 µM) for 48 h, the harvested
HepG2 cells were washed in PBS and incubated in a freshly prepared
JC-1 solution at 37°C for 20 min. Cell-associated fluorescence was
also measured by FACS.
Western blot analysis
HepG2 cells were treated for 48 h with various
concentrations of cordycepin (0, 125, 250 and 500 µM) and harvested
and lysed in RIPA lysis buffer that contained 1% NP-40, 0.5% sodium
deoxycholate and 0.1% SDS. The cell lysates were resolved by sodium
dodecyl sulfate-polyacrylamide gel electrophoresis and
electrophoretically transferred to a polyvinylidene fluoride
membrane, and then blocked for 1 h with 5% fat-free milk in PBS
with Tween-20 (PBST) at room temperature. The membrane was
immunoblotted overnight with antibodies in PBST at 4°C. Subsequent
to being washed with PBST, the membrane was detected by enhanced
chemiluminescence (Kodak, Rochester, NY, USA). Equal loading was
confirmed by probing with an antibody against β-actin (1:3000;
catalog no. sc-8432; Santa Cruz Biotechnology, Inc.). Mitochondrial
and cytosolic fractions were prepared in order to detect cytochrome
c. Following treatment with the indicated concentrations of
cordycepin for 48 h, HepG2 cells were harvested and disposed using
the Cell Mitochondria Isolation kit, according to the
manufacturer's protocol. Finally, the mitochondrial pellet and
cytosolic supernatant were separated using a centrifuge (model no.
5415C; Eppendorf, Hamburg, Germany).
Results
Isolation and purification of
cordycepin
Two fractions from crude extract, termed A and B,
were eluted using NKA macroporous resin (data not shown). Fraction
A was then purified on a HPLC preparative column followed by an
analytical column using CH3OH:H2O = 15:85
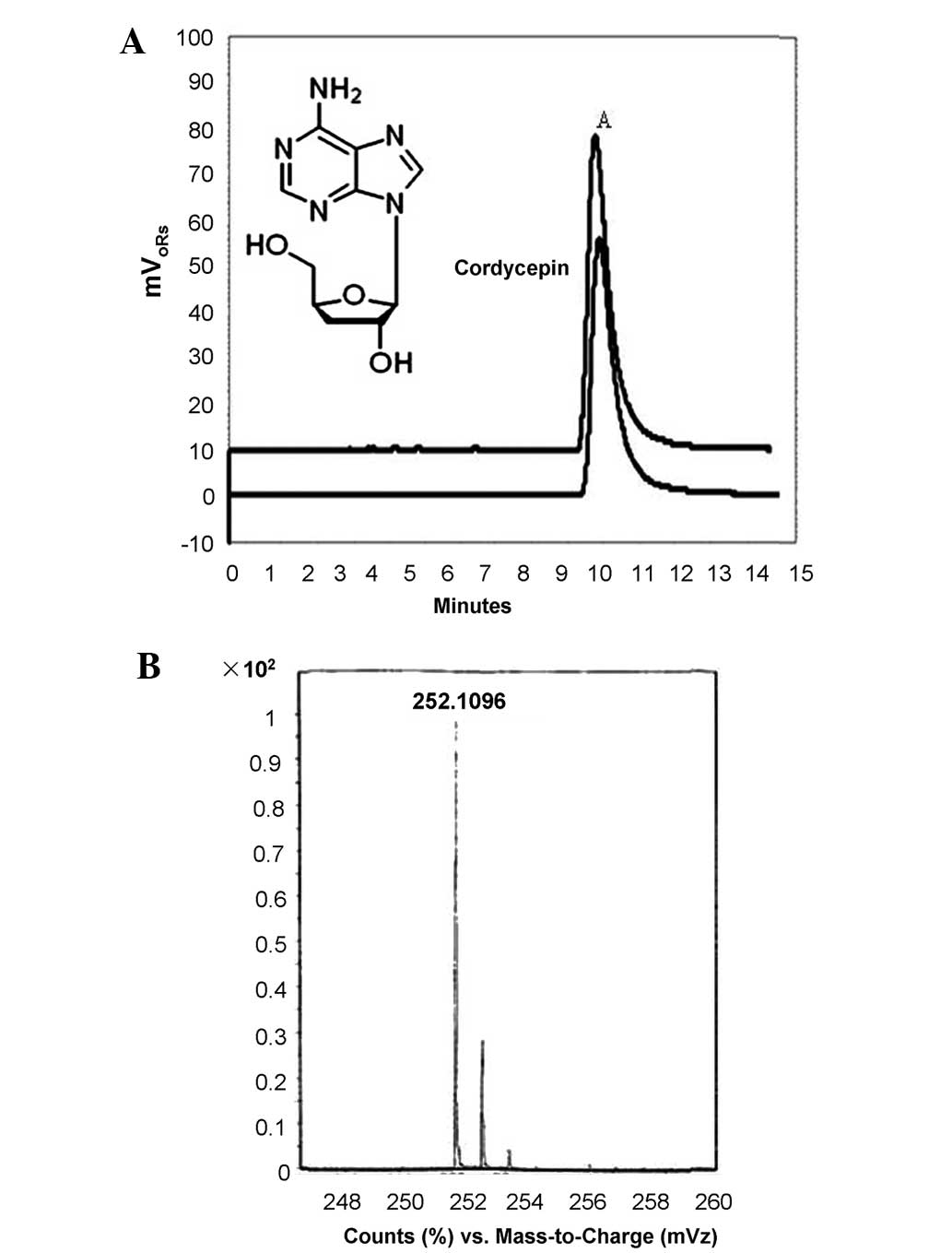
(v/v) as the mobile phase. The retention time of fraction A was
~10.5 min, which is consistent with that of standard cordycepin
(Fig. 1A). In addition, mass
spectrometric analysis revealed that the m/z of fraction A was
252.1096 Da, which corresponds with the m/z of standard cordycepin
(Fig. 1B).
Effect of cordycepin on HepG2 cell
viability
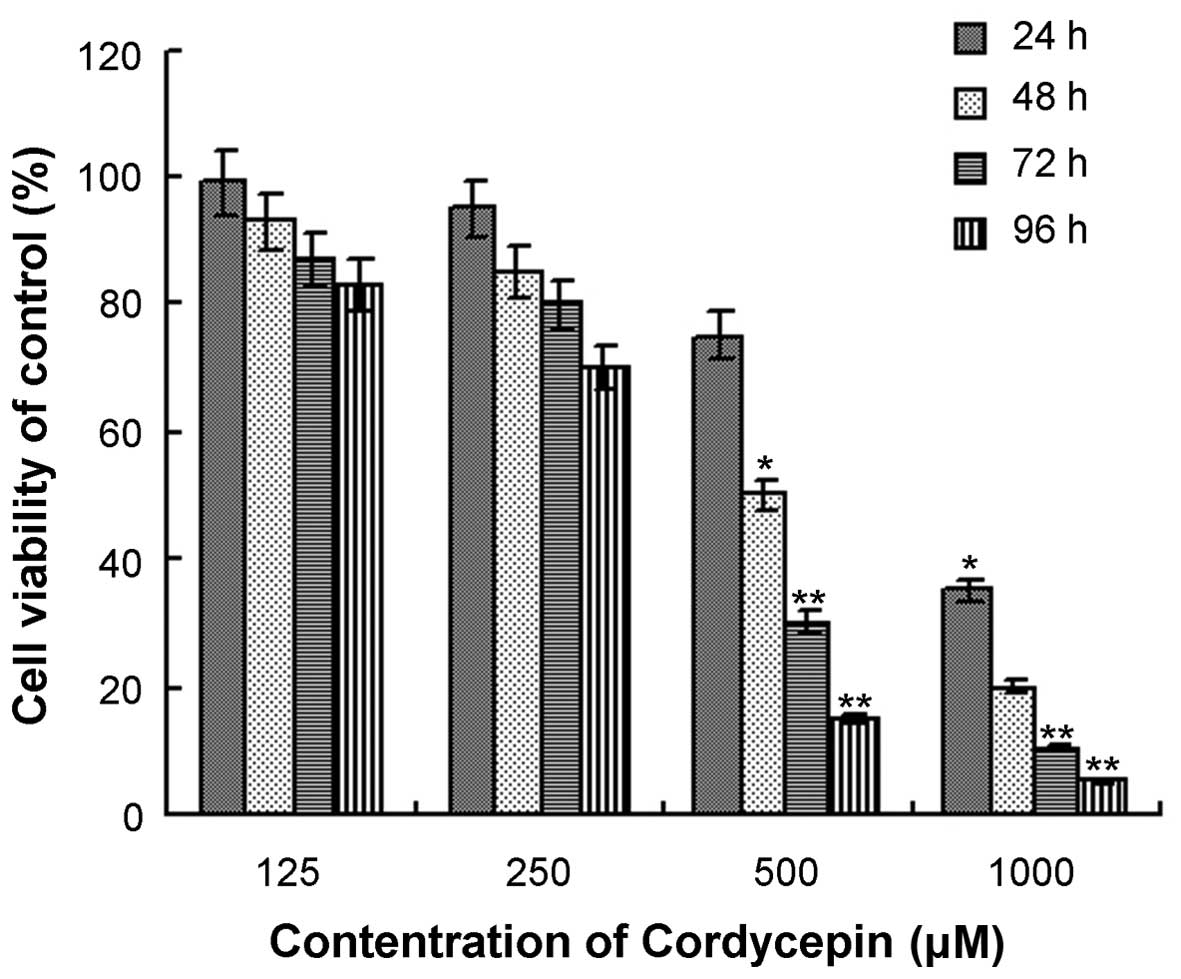
Cordycepin exhibited the ability to inhibit the
proliferation of HepG2 cells in a time- and dose-dependent manner
(Fig. 2). The IC50 values
for HepG2 cells treated with cordycepin for 24, 48, 72 and 96 h
were 735±3.67, 497.5±2.49, 385±1.93 and 307.5±1.54 µM,
respectively.
Assessment of apoptosis by DAPI
staining
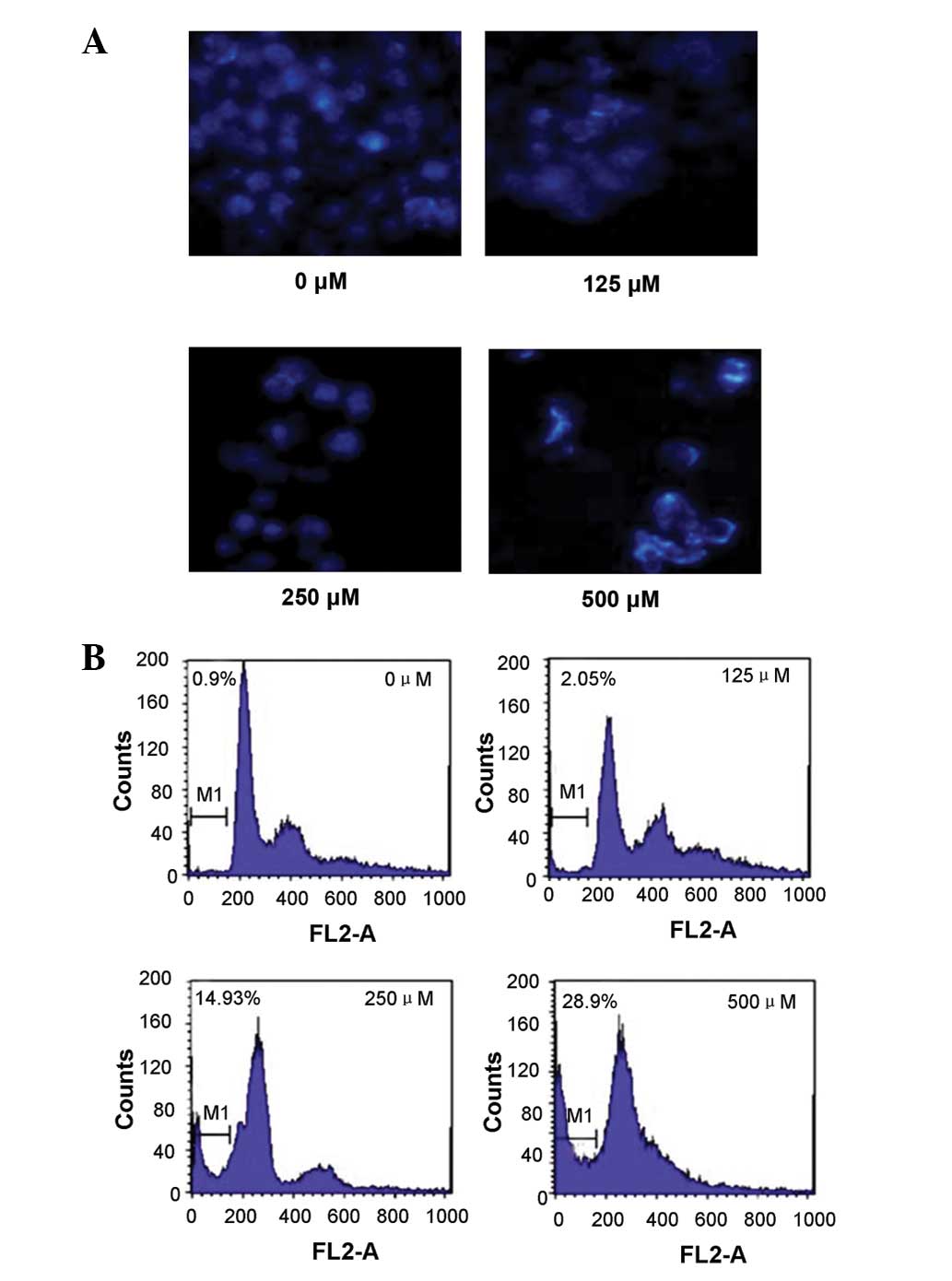
The fluorescence microscopic examination with DAPI
staining revealed that morphological changes, such as chromatin
condensation, nuclear shrinkage or fragmentation and apoptotic body
formation, occurred in HepG2 cells subsequent to being treated with
cordycepin for 48 h (Fig. 3A).
Assessment of apoptosis by flow
cytometry
The flow cytometric analysis with PI staining
revealed that the cells treated with various concentrations of
cordycepin for 48 h exhibited an apoptotic hypodiploid sub-G1 peak
in a concentration-dependent manner (Fig.
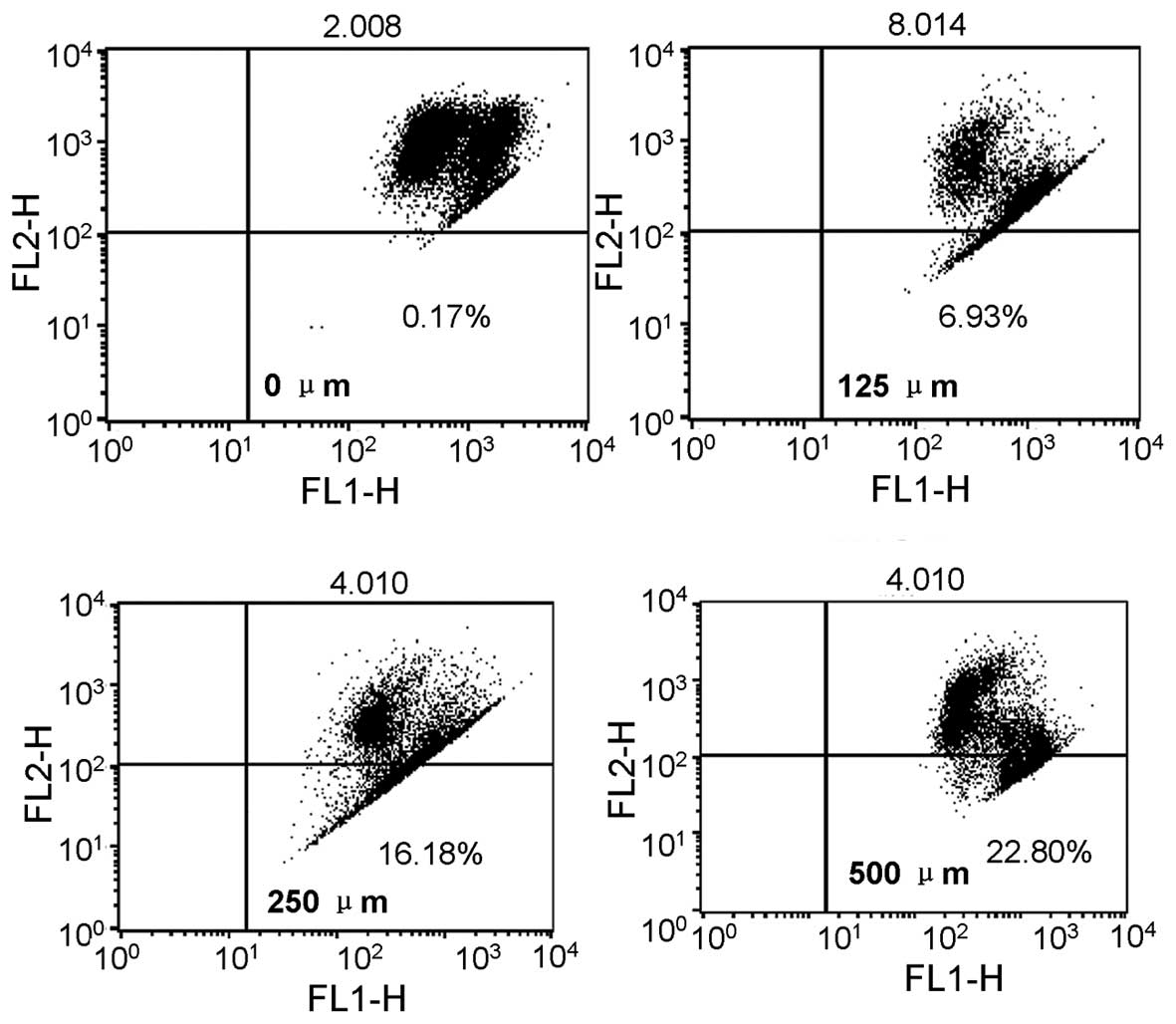
3B). The flow cytometric analysis with JC-1 staining showed the
respective green fluorescence intensity ratios, which indicated the
apoptotic HepG2 cells, were 5.72, 18.58, 32.12 and 36.65%,
respectively (Fig. 4).
Cordycepin-induced apoptosis signaling
pathway
Cordycepin increased the expression levels of Fas
and FADD, but had no effect on FasL expression. Additionally, the
level of procaspase-8 decreased, while the level of cleaved
caspase-8 was elevated (Fig. 5A). The
expression of the Bcl-2 family proteins was also detected and the
results showed that cordycepin enhanced the expression level of
truncated Bid (tBid) and decreased the level of Bid, but had little
effect on the levels of Bax and Bcl-2 (Fig. 5B). A decreased level of cytochrome
c was detected in the mitochondrial fraction and an
increased level was detected in the cytosolic fraction (Fig. 5C). Furthermore, the results also
showed that HepG2 cells treated with cordycepin for 48 h underwent
significant cleavage of precaspase-9 and precaspase-3 (Fig. 5C).
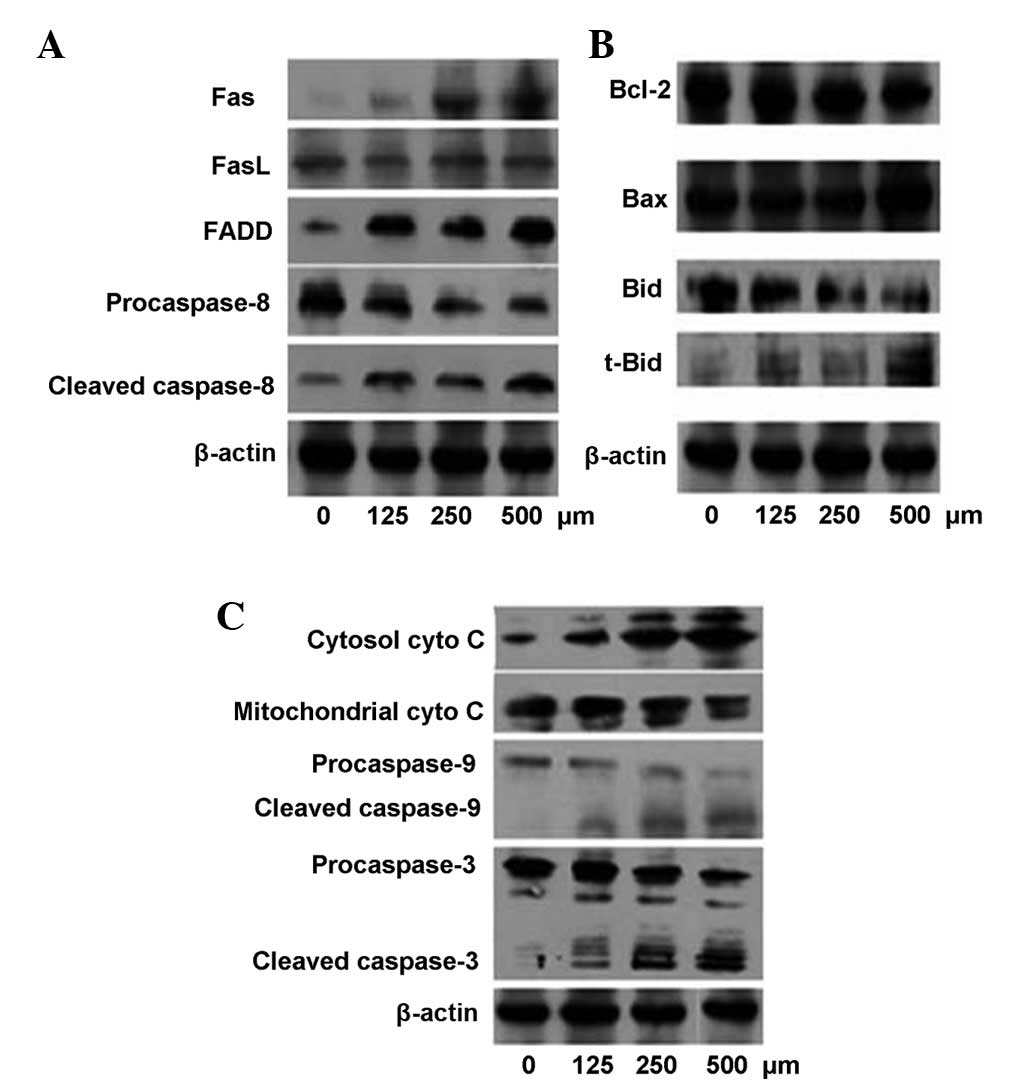 | Figure 5.Western blot analysis of the signaling
pathway involved in cordycepin-induced apoptosis. (A) Effects of
cordycepin on the expression levels of Fas, FasL, FADD and
caspase-8. (B) Effects of cordycepin on the expression level of the
Bcl-2 family proteins. (C) Effects of cordycepin on the expression
level of cyto C, caspase-9 and caspase-3. FasL, Fas ligand; FADD,
Fas-associated death domain protein; Bcl-2, B-cell lymphoma-2; Bax,
Bcl-2-associated X protein; Bid, BH3 interacting domain death
agonist; t-Bid, truncated Bid; cyto C, cytochrome c. |
Discussion
As a natural compound, cordycepin plays a key role
in cancer therapy and induces apoptosis in numerous cells by
targeting molecules and pathways (20–22).
However, studies investigating the induction of apoptosis by
cordycepin through the extrinsic and intrinsic signaling pathways
in HepG2 cell are currently available (21,22). The
present study investigated the effect of cordycepin on HepG2 cells
and the molecular pathway by which cordycepin induces apoptosis.
Subsequently, it was demonstrated that cordycepin induced apoptosis
by the activation of caspase, interaction between Fas and FADD, and
modulation of the protein levels of Bid and tBid.
It is well known that mitochondria play a major role
in several stress-induced cell death pathways, and damage to
mitochondria with subsequent loss of mitochondrial membrane
potential has been known to be the point of no return in apoptotic
cascades (20,23). In the present study, it was found that
cordycepin had an inhibitory effect on the viability of HepG2 cells
by DAPI staining and could increase the fraction of sub-G1 cells by
PI staining. The JC-1 assay also revealed that cordycepin-induced
collapse of the mitochondrial membrane occurred in a
concentration-dependent manner. All these findings demonstrated
that cordycepin may induce apoptosis in HepG2 cells.
In mammalian cells, apoptosis occurs via either the
extrinsic (receptor-dependent) or the intrinsic
(mitochondria-dependent) pathway, with each involving caspase
activation. Fas is a TNF-receptor that transduces the apoptotic
signal into cells (23,24). FADD is an apoptotic adaptor molecule
that recruits caspase-8 or caspase-10 to activated Fas (25). FasL is a cytokine that binds to Fas,
and it has been proposed that Fas-mediated apoptosis involves the
interaction between Fas and FasL (26). In the present study, cordycepin
increased the expression levels of Fas and FADD, without changing
the level of FasL, and the expression of cleaved caspase-8 was
elevated. Therefore, the present study hypothesizes that cordycepin
may stimulate Fas/FADD signaling independently of FasL and
subsequently activate caspase-8 in HepG2 cells.
Fas may induce caspase-mediated cleavage of p22 Bid
into a major p15, and minor p13 and p11 product; the major
proteolytic product p15 tBid allows the release of cytochrome
c (27–29). The mitochondrial membrane potential
was shown to be decreased by the JC-1 staining analysis and
confirmed the increased cytosolic cytochrome c by western
blotting. The activation cascade of caspase-9 and caspase-3, which
are responsible for apoptosis execution, was detected. In
particular, caspase-3 plays a central role in the execution of
apoptosis and its activation requires the activation of initiator
caspases, such as caspase-8 or caspase-9, in response to
pro-apoptotic stimuli (30).
Caspase-8 is crucial for triggering apoptosis via the death
receptor pathway and its activation requires the binding of death
ligands to death receptors (31). In
addition, in the prior time course assay, the active forms of
caspase-8 was detected notably earlier than that of caspase-3 and
−9, which clearly demonstrated that caspase-8 may act as an
upstream initiator and the extrinsic pathway was involved in
cordycepin-induced apoptosis.
The Bcl-2 family proteins play an essential role in
apoptotic progress, such as Bcl-2 protein (anti-apoptosis) and Bax
protein (pro-apoptosis). These proteins are regulators of
mitochondrial membrane permeability and intermembrane space protein
efflux, according to the opposing fractions of the anti-apoptosis
members and pro-apoptosis members (32,33). Bax
accelerates programmed cell death and undergoes a conformational
change that causes translocation to the mitochondrial membrane,
leading to the release of cytochrome c, which then triggers
apoptosis (34). Cordycepin enhanced
the expression level of Bax and decreased the expression level of
Bid in the present study.
Cleaved Bid causes cytochrome c efflux from
mitochondria, which in turn leads to the activation of caspase-9
and caspase-3 (35). In addition,
other Bcl-2 family molecules, such as the proapoptotic protein Bax
and anti-apoptotic protein Bcl-2, are also key regulators of
apoptosis, which control the release of mitochondrial cytochrome
c by modulating the permeability of the outer mitochondrial
membrane (36). The upregulation of
the ratio of Bax/Bcl-2 caused by cordycepin was confirmed by
immunoblotting. The release of cytochrome c from
mitochondria was also relevant to the dissipation of the
mitochondrial membrane potential (ΔΨm) (37). In the present study, cordycepin
treatment caused a significant decrease of ΔΨm in HepG2 cells and
increased cytosolic cytochrome c. These findings indicated
that activation of caspase-3 in cordycepin-induced apoptosis may
result from direct caspase-8 cleavage and caspase-8-mediated
caspase-3 activation through the intrinsic pathway, which is
regulated by the Bcl-2 family proteins.
It has been shown that cordycepin is an analogue of
adenosine (22). The possibility that
the adenosine receptor is involved in the apoptosis of certain
types of cancer cells, including ovarian (38), breast (39) and renal (40) cancer cells, has also been proposed.
Thus, additional studies investigating whether cordycepin induces
apoptosis through adenosine receptors in HepG2 cells may be
valuable.
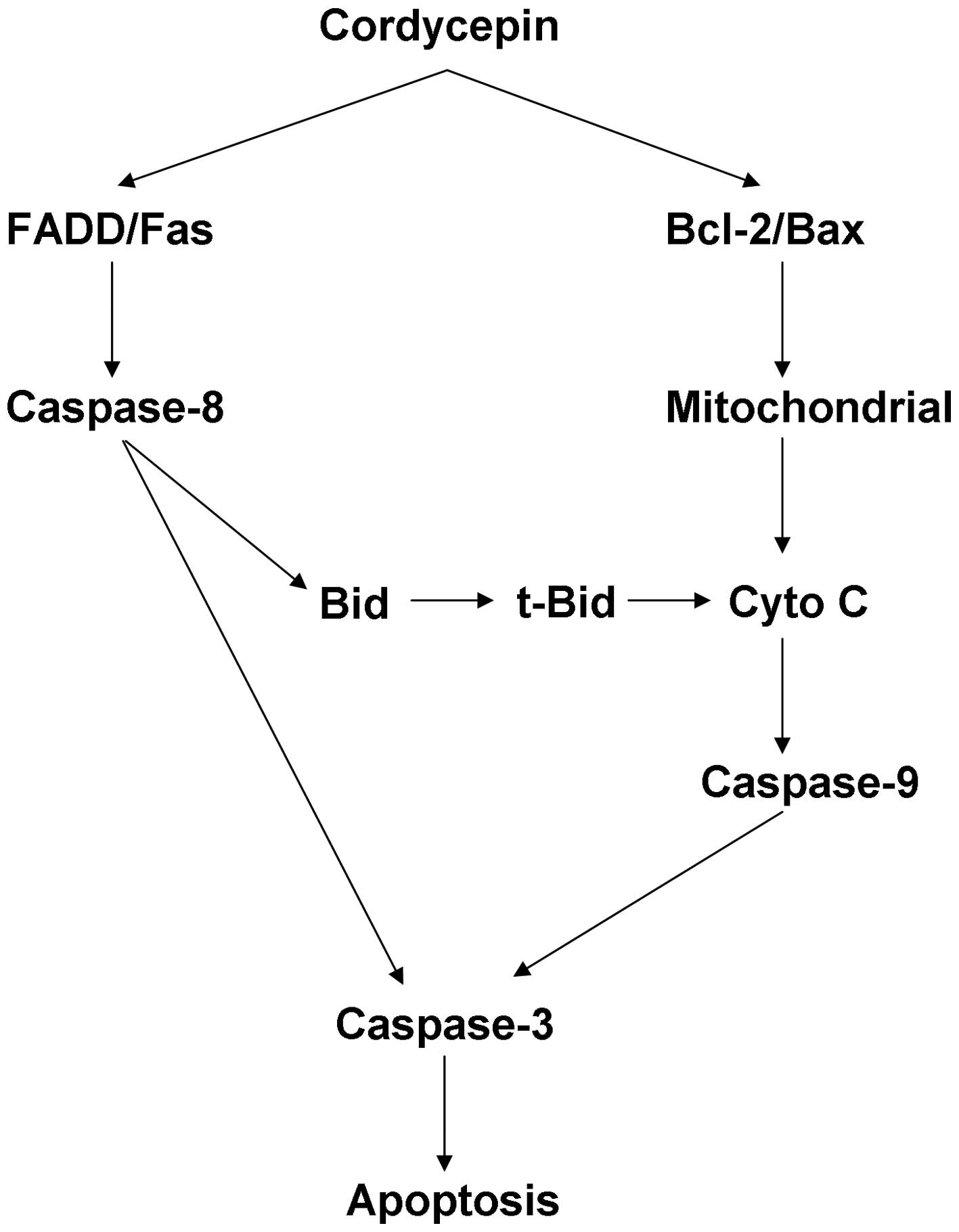
In conclusion, cordycepin-induced apoptosis in HepG2
cells may be initiated by the FADD mediated signal pathway and
regulated by the Bcl-2 family proteins that can change the
alternation of mitochondrial membrane permeability and cause the
mitochondria mediated apoptosis signal pathway (Fig. 6). To the best of our knowledge, the
present study is the first to report apoptosis induced by
cordycepin in human liver cancer HepG2 cells.
References
|
1
|
Won SY and Park EH: Anti-inflammatory and
related pharmacological activities of cultured mycelia and fruiting
bodies of Cordyceps militaris. J Ethnopharmacol. 96:555–561. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yoo HS, Shin JW, Cho JH, Son CG, Lee YW,
Park SY and Cho CK: Effects of Cordyceps militaris extract on
angiogenesis and tumor growth. Acta Pharmacol Sin. 25:657–665.
2004.PubMed/NCBI
|
|
3
|
Hattori M, Isomura S, Yokoyama E, Ujita M
and Hara A: Extracellular trypsin-like proteases produced by
Cordyceps militaris. J Biosci Bioeng. 100:631–636. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Park C, Hong SH, Lee JY, Kim GY, Choi BT,
Lee YT, Park DI, Park YM, Jeong YK and Choi YH: Growth inhibition
of U937 leukemia cells by aqueous extract of Cordyceps militaris
through induction of apoptosis. Oncol Rep. 13:1211–1216.
2005.PubMed/NCBI
|
|
5
|
Zhang G, Huang Y, Bian Y, Wong JH, Ng TB
and Wagn H: Hypoglycemic activity of the fungi Cordyceps militaris,
Cordyceps sinensis, Tricholoma mongolicum and Omphalia lapidescens
in streptozotocin-induced diabetic rats. Appl Microbiol Biotechnol.
72:1152–1156. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ohta Y, Lee JB, Hayashi K, Fujita A, Park
DK and Hayashi T: In vivo anti-influenza virus activity of an
immunomodulatory acidic polysaccharide isolated from Cordyceps
militaris grown on germinated soybeans. J Agric Food Chem.
55:10194–10199. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lee H, Kim YJ, Kim HW, Lee DH, Sung MK and
Park T: Induction of apoptosis by Cordyceps militaris through
activation of caspase-3 in leukemia HL-60 cells. Biol Pharm Bull.
29:670–674. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xu FL, Lee YL, Tsai WY, Lin SJ, Yang ZQ,
Yang CC, Liu HY, Cheng L, Xiao H and Wen L: Effect of cordycepin on
Hantaan virus 76–118 infection of primary human embryonic pulmonary
fibroblasts-characterization of apoptotic effects. Acta Virol.
49:183–193. 2005.PubMed/NCBI
|
|
9
|
Nakamura K, Konoha K, Yoshikawa N,
Yamaguchi Y, Kagota S, Shinozuka K and Kunitomo M: Effect of
cordycepin (3′-deoxyadenosine) on hematogenic lung metastatic model
mice. In Vivo. 19:137–141. 2005.PubMed/NCBI
|
|
10
|
Nakamura K, Yoshikawa N, Yamaguchi Y,
Kagota S, Shinozuka K and Kunitomo M: Antitumor effect of
cordycepin (3′-deoxyadenosine) on mouse melanoma and lung carcinoma
cells involves adenosine A3 receptor stimulation. Anticancer Res.
26:43–47. 2006.PubMed/NCBI
|
|
11
|
Yoshikawa N, Nakamura K, Yamaguchi Y,
Kagota S, Shinozuka K and Kunitomo M: Antitumour activity of
cordycepin in mice. Clin Exp Pharmacol Physiol. 31(Suppl 2):
S51–S53. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lee SJ, Kim SK, Choi WS, Kim WJ and Moon
SK: Cordycepin causes p21WAF1-mediated G2/M cell-cycle arrest by
regulating c-Jun N-terminal kinase activation in human bladder
cancer cells. Arch Biochem Biophys. 490:103–109. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhou X, Luo L, Dressel W, Shadier G,
Krumbiegel D, Schmidtke P, Zepp F and Meyer CU: Cordycepin is an
immunoregulatory active ingredient of Cordyceps sinensis. Am J Chin
Med. 36:967–980. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shin S, Lee S, Kwon J, Moon S, Lee S, Lee
CK, Cho K, Ha NJ and Kim K: Cordycepin suppresses expression of
diabetes regulating genes by inhibition of
lipopolysaccharide-induced inflammation in macrophages. Immune
Netw. 9:98–105. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Thomadaki H, Scorilas A, Tsiapalis CM and
Havredaki M: The role of cordycepin in cancer treatment via
induction or inhibition of apoptosis: Implication of
polyadenylation in a cell type specific manner. Cancer Chemother
Pharmacol. 61:251–265. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhou X, Meyer CU, Schmidtke P and Zepp F:
Effect of cordycepin on interleukin-10 production of human
peripheral blood mononuclear cells. Eur J Pharmacol. 453:309–317.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Guo P, Kai Q, Gao J, Lian ZQ, Wu CM, Wu CA
and Zhu HB: Cordycepin prevents hyperlipidemia in hamsters fed a
high-fat diet via activation of AMP-activated protein kinase. J
Pharmacol Sci. 113:395–403. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lomonosova E and Chinnadurai G: BH3-only
proteins in apoptosis and beyond: An overview. Oncogene. 27(Suppl
1): S2–S19. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Seitz SJ, Schleithoff ES, Koch A, Schuster
A, Teufel A, Staib F, Stremmel W, Melino G, Krammer PH, Schilling T
and Müller M: Chemotherapy-induced apoptosis in hepatocellular
carcinoma involves the p53 family and is mediated via the extrinsic
and the intrinsic pathway. Int J Cancer. 126:2049–2066.
2010.PubMed/NCBI
|
|
20
|
Lee SY, Debnath T, Kim SK and Lim BO:
Anti-cancer effect and apoptosis induction of cordycepin through
DR3 pathway in the human colonic cancer cell HT-29. Food Chem
Toxicol. 60:439–447. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lee HH, Park C, Jeong JW, Kim MJ, Seo MJ,
Kang BW, Park JU, Kim GY, Choi BT, Choi YH and Jeong YK: Apoptosis
induction of human prostate carcinoma cells by cordycepin through
reactive oxygen species-mediated mitochondrial death pathway. Int J
Oncol. 42:1036–1044. 2013.PubMed/NCBI
|
|
22
|
Chen YH, Wang JY, Pan BS, Mu YF, Lai MS,
So EC, Wong TS and Huang BM: Cordycepin enhances cisplatin
apoptotic effect through caspase/MAPK pathways in human head and
neck tumor cells. Onco Targets Ther. 6:983–998. 2013.PubMed/NCBI
|
|
23
|
Sytwu HK, Liblau RS and McDevitt HO: The
roles of Fas/APO-1 (CD95) and TNF in antigen-induced programmed
cell death in T cell receptor transgenic mice. Immunity. 5:17–30.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lien YC, Daosukho C and St Clair DK: TNF
receptor deficiency reveals a translational control mechanism for
adriamycin-induced Fas expression in cardiac tissues. Cytokine.
33:226–230. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Grunert M, Gottschalk K, Kapahnke J,
Gündisch S, Kieser A and Jeremias I: The adaptor protein FADD and
the initiator caspase-8 mediate activation of NF-κB by TRAIL. Cell
Death Dis. 3:e4142012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Saggioro FP, Neder L, Stavale JN,
Paixão-Becker AN, Malheiros SM, Soares FA, Pittella JE, Matias CC,
Colli BO, Carlotti CG and Franco M: Fas, FasL and cleaved caspases
8 and 3 in glioblastomas: A tissue microarray-based study. Pathol
Res Pract. 210:267–273. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kaufmann T, Strasser A and Jost PJ: Fas
death receptor signalling: Roles of Bid and XIAP. Cell Death
Differ. 19:42–50. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Vogel A, Aslan JE, Willenbring H, Klein C,
Finegold M, Mount H, Thomas G and Grompe M: Sustained
phosphorylation of Bid is a marker for resistance to Fas-induced
apoptosis during chronic liver diseases. Gastroenterology.
130:104–119. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Schmich K, Schlatter R, Corazza N,
Ferreira Sá K, Ederer M, Brunner T, Borner C and Mergort I: Tumor
necrosis factor α sensitizes primary murine hepatocytes to
Fas/CD95-induced apoptosis in a Bim- and Bid-dependent manner.
Hepatology. 53:282–292. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu G, Wang T, Wang T, Song J and Zhou Z:
Effects of apoptosis-related proteins caspase-3, Bax and Bcl-2 on
cerebral ischemia rats. Biomed Rep. 1:861–867. 2013.PubMed/NCBI
|
|
31
|
Hu Q, Cui X, Tao L, Xiu L, Wang T and Wang
X: Staphylococcus aureus induces apoptosis in primary bovine
mammary epithelial cells through Fas-FADD death receptor-linked
caspase-8 signaling. DNA Cell Biol. 33:388–397. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jung EB and Lee CS: Baicalein attenuates
proteasome inhibition-induced apoptosis by suppressing the
activation of the mitochondrial pathway and the caspase-8- and
Bid-dependent pathways. Eur J Pharmacol. 730:116–124. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang J and Li W: Discovery of novel second
mitochondria-derived activator of caspase mimetics as selective
inhibitor of apoptosis protein inhibitors. J Pharmacol Exp Ther.
349:319–329. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gómez-Crisóstomo NP, López-Marure R,
Zapata E, Zazueta C and Martínez-Abundis E: Bax induces cytochrome
c release by multiple mechanisms in mitochondria from MCF7
cells. J Bioenerg Biomembr. 45:441–448. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
De Martino L, Marfé G, Longo M, Fiorito F,
Montagnaro S, Iovane V, Decaro N and Pagnini U: Bid cleavage,
cytochrome c release and caspase activation in canine
coronavirus-induced apoptosis. Vet Microbiol. 141:36–45. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kumar P, Coltas IK, Kumar B, Chepeha DB,
Bradford CR and Polverini P: Bcl-2 protects endothelial cells
against gamma-radiation via a Raf-MEK-ERK-survivin signaling
pathway that is independent of cytochrome c release. Cancer
Res. 67:1193–1202. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lei X, Chen Y, Du G, Yu W, Wang X, Qu H,
Xia B, He H, Mao J, Zong W, et al: Gossypol induces
Bax/Bak-independent activation of apoptosis and cytochrome c
release via a conformational change in Bcl-2. Faseb J.
20:2147–2149. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hajiahmadi S, Panjehpour M, Aghaei M and
Shabani M: Activation of A2b adenosine receptor regulates ovarian
cancer cell growth: involvement of Bax/Bcl-2 and caspase-3. Biochem
Cell Biol. 93:321–329. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Dastjerdi MN, Valiani A, Mardani M and Ra
MZ: Adenosine A1 receptor modifies P53 expression and apoptosis in
breast cancer cell Mcf-7. Bratisl Lek Listy. 117:242–246.
2016.PubMed/NCBI
|
|
40
|
Nagaya H, Gotoh A, Kanno T and Nishizaki
T: A3 adenosine receptor mediates apoptosis in invitro RCC4-VHL
human renal cancer cells by up-regulation AMID expression. J Urol.
189:321–328. 2013. View Article : Google Scholar : PubMed/NCBI
|