Introduction
Oral squamous cell carcinoma (OSCC) is the sixth
most common malignancy worldwide, accounting for ~90% of malignant
oral lesions (1). Despite
improvements in its clinical management, OSCC continues to have a
high local recurrence and a poor 5-year survival rate (2,3). Local
tumor recurrence develops in ~60% of patients, and metastases
affect 15–25%, leading to a poor prognosis and encouraging further
research on factors that might correlate with disease outcome
(4).
At present, the tumor-node-metastasis (TNM) staging
system is described as the most effective prognostic tool for tumor
survival (5,6). Furthermore, the clinical characteristics
of patients, including their age, gender and smoking and drinking
habits, are important for selecting the appropriate therapeutic
strategy, and for determining the risk of complications and the
prognosis of several types of cancer (7,8).
Therefore, the identification of factors associated with a poor
prognosis has emerged as an important issue in the management of
OSCC. Previous studies have evaluated various clinicopathological
parameters as prognostic factors of OSCC, and the age (7) and smoking status (8) of patients, extracapsular spread in the
cervical lymph nodes (9), TNM stage
(5,10), status of the surgical resection
margin, bone involvement and the size of the mandibulectomy
(11), as well as the tumor size and
microvascular invasion (12,13), have been shown to be independent
prognostic factors in patients with OSCC. However, previous studies
have assessed only few or groups of risk factors that might
influence the prognosis, and the association between these factors
and the prognosis of OSCC is inconsistent and complex. Therefore,
it is important to identify characteristics that may be suggestive
of a poor prognosis in patients with OSCC.
The construction of tissue microarrays (TMAs), in
which multiple samples are incorporated into a single paraffin
block, allows for the immunohistochemical analysis of large numbers
of tissue samples simultaneously, thereby standardizing
immunohistochemical staining (14).
Not only does this markedly reduce costs, but it also increases the
number of studies that can be performed on a small piece of tissue,
since smaller cores of tissue are used rather than cut sections
(15). By using TMAs, Lourenço et
al (16) demonstrated that
claudin expression patterns were related to the evolution and
prognosis of OSCC. Furthermore, Liu et al (17) reported that the expression levels of
carbonic anhydrases (CAs) I/II in OSCC samples may be used to
predict local tumor growth in OSCC patients. However, there has yet
to be a comparable analysis of OSCC patients from the mainland
Chinese population.
The present study has taken into consideration
numerous characteristics and prognostic factors, in order to
identify important independent risk factors of a poor prognosis in
a relatively large cohort of Chinese patients with OSCC. In
addition, this cohort of patients was used to construct the first
OSCC TMAs from mainland Chinese patients.
Patients and methods
Study participants
After obtaining approval from the West China
Hospital Ethics Board (Chengdu, China), a cohort of 232
histologically confirmed OSCC patients was recruited from the West
China Hospital of Stomatology, Sichuan University (Chengdu, China)
between February 2002 and October 2009. Patients were eligible for
data collection if they met the following inclusion criteria: i)
They had been histologically diagnosed with oral squamous cell
carcinoma; and ii) they had been treated with primary surgery. The
exclusion criteria were: i) Recurrent or metastatic disease; and
ii) malignant disease of the salivary glands, tonsils and
oropharynx or hypopharynx.
Data collection
Clinicopathological parameters were obtained by a
registry review, and included: i) Demographic data, such as age,
gender and history of smoking and alcohol consumption; ii)
tumor-related information, including location, size, TNM stage [the
patients were staged according to the American Joint Committee on
Cancer TNM classification system (18)], histological differentiation and
lymphatic metastasis; and iii) status at the last follow-up (April
30, 2011), mainly the condition of survival or mortality.
Statistical analysis
Categorical variables are expressed as frequencies
and percentages. To compare the data between two groups, the
Pearson χ2 test was implemented for qualitative
variables. Univariate logistic regression (χ2 test with
Yate's continuity correction) was performed for the demographic and
clinical characteristic variables that were significantly
associated with an increased risk of mortality. Factors with
P<0.25 in the univariate analysis were included for multivariate
logistic regression analysis to identify the independency of these
factors (the α-level of significance was relaxed to 0.25 in order
to avoid missing important variables). Independent variables with
P<0.05 in the multiple logistic regression analysis were
selected for receiver operating characteristic (ROC) curve
analysis. Plots of sensitivity (true-positive fraction) vs.
1-specificity (false-positive fraction) were constructed, and the
diagnostic performance of the significant independent variable was
expressed as the area under the ROC curve (AUC). All statistical
analyses were performed using SPSS 17.0 software (SPSS Inc.,
Chicago, IL, USA). For unadjusted comparisons, P<0.05 was
considered to indicate a statistically significant difference.
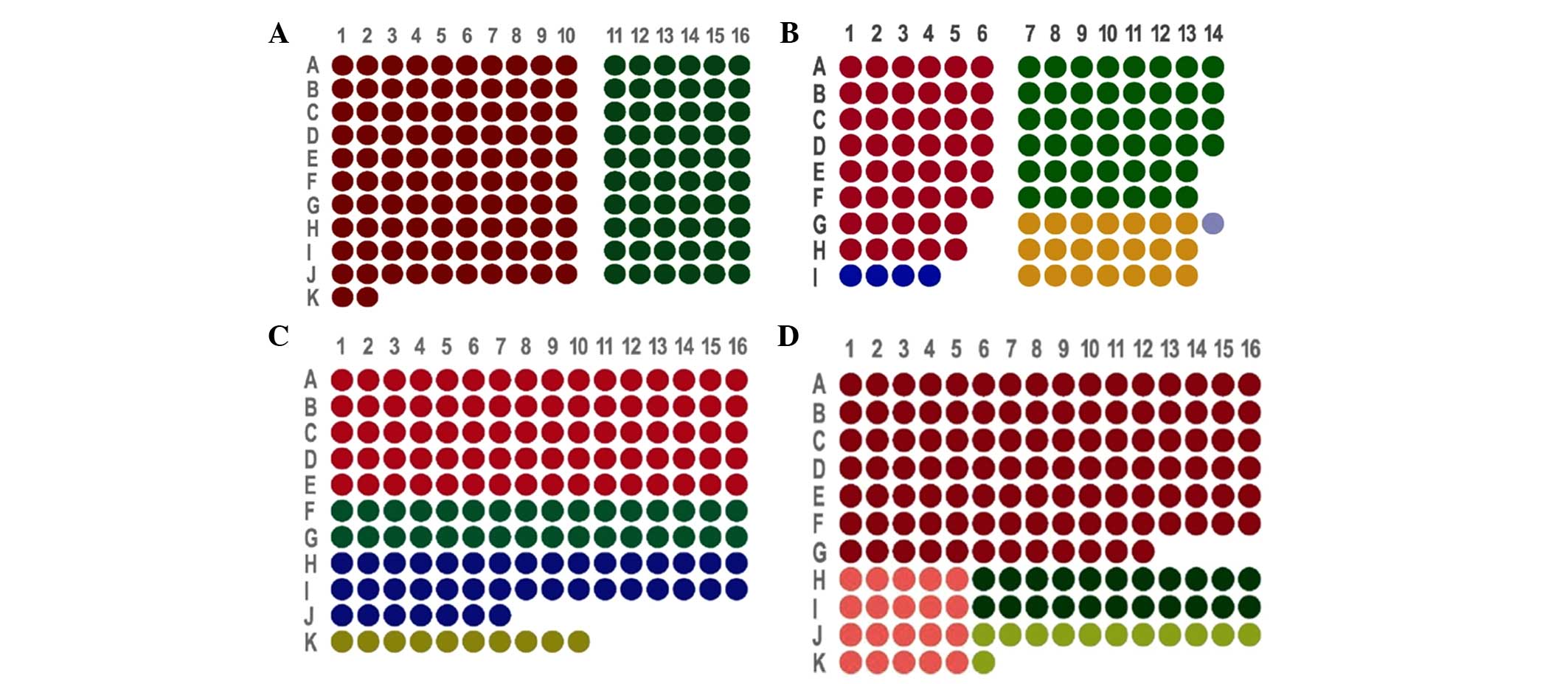
TMAs design and construction
According to the characteristics of OSCC donor
paraffin wax (OSCC tissues with or without lymph node metastasis,
the pericarcinomatous tissues or the metastatic lymph node tissues
that met the inclusion criteria), we designed four sets of arrays,
the arrangements of which were as follows: i) TMA1, which included
162 tissue cores from 81 patients (Fig.
1A); ii) TMA2, which included 118 tissue cores from 58 patients
(Fig. 1B); iii) TMA3, which included
161 tissue cores from 161 patients (Fig.
1C); and iv) TMA4, which included 162 tissue cores from 162
patients (Fig. 1D).
The representative pathology regions were observed
by hematoxylin and eosin (HE) staining, after which the HE-stained
sections were overlaid onto the surface of the corresponding donor
blocks to guide the transfer of tissue cores from morphologically
representative sites into defined loci in the recipient block using
a steel stylet. Tissue cores with a diameter of 1.5 mm, and the
recipient blocks, were heated in a 52°C oven until they had fused
to become the TMA blocks. Consecutive sections (4-µm thick) were
cut, and every 10 slides were collected to assess by HE staining.
The specific processes were performed as described previously
(14).
Results
Clinical characteristics
Clinicopathological parameters of 232 patients with
OSCC, including 175 fatal cases and 57 non-fatal cases, were
analyzed. The 232 patients with OSCC included 169 males and 63
females, with an age range of 24–83 years. The 57 non-fatal cases
included 37 males and 20 females, of which 26 patients were aged
>60 years. Of the 175 fatal cases, 132 were male and 43 were
female, and 82 patients were aged >60 years. There was no
significant difference in age and gender between the non-fatal and
fatal groups (P=0.918 and 0.121, respectively; Table I). Similarly, no significant
differences in smoking and alcohol consumption were observed
between the fatal and non-fatal groups (smoking habit, 53.1 vs.
43.9%, respectively; alcohol consumption, 50.9 vs. 50.9%,
respectively; P=0.223 and 0.998, respectively). The baseline
clinical presentations were similar between the fatal and non-fatal
groups in terms of the tumor stage, size and location (P=0.117,
0.799 and 0.972, respectively). However, lymphatic metastasis was
significantly more frequent in fatal patients than in non-fatal
patients (78.3 vs. 17.5%, respectively; P=0.002; Table I). In addition, well-differentiated
OSCC was significantly associated with fatal cases (P=0.049). The
basic characteristics of the 232 study participants are shown in
Table I.
 | Table I.Clinical characteristics of fatal and
non-fatal oral squamous cell carcinoma cases. |
Table I.
Clinical characteristics of fatal and
non-fatal oral squamous cell carcinoma cases.
| Variable | Fatal (n=175) | Non-fatal
(n=57) | P-value |
|---|
| Gender (%
male) | 132 (75.4) | 37 (64.9) | 0.121 |
| Age (years) |
|
| 0.918 |
|
<40 | 14 (8.1) | 4 (7.0) |
|
|
40–60 | 77 (44.5) | 27 (47.4) |
|
|
>60 | 82 (47.4) | 26 (45.6) |
|
| Smoking | 93 (53.1) | 25 (43.9) | 0.223 |
| Alcohol
consumption | 89 (50.9) | 29 (50.9) | 0.998 |
| Location |
|
| 0.972 |
| Buccal
mucosa | 25 (14.3) | 7 (12.3) |
|
|
Tongue | 48 (27.4) | 17 (29.8) |
|
|
Gingiva | 26 (14.9) | 8 (14.0) |
|
| Other
sites | 76 (43.4) | 25 (43.9) |
|
| Tumor size
(cm) |
|
| 0.799 |
|
<2 | 54 (33.5) | 16 (29.6) |
|
|
2–4 | 84 (52.2) | 31 (57.4) |
|
| ≥4 | 23 (14.3) | 7 (13.0) |
|
| Differentiated
type |
|
| 0.049a |
|
Well | 158 (91.3) | 55 (98.2) |
|
|
Moderately | 10 (5.8) | 0 (0.0) |
|
|
Poorly | 5 (2.9) | 1 (1.8) |
|
| TNM stage |
|
| 0.117 |
| I | 27 (15.9) | 10 (18.2) |
|
| II | 34 (20.0) | 18 (32.7) |
|
|
III | 42 (24.7) | 7 (12.7) |
|
| IV | 67 (39.4) | 20 (36.4) |
|
| Lymphatic
metastasis | 137 (78.3) | 10 (17.5) | 0.002a |
Determinants of a poor OSCC
prognosis
The associations between the clinical
characteristics of patients with OSCC and a poor prognosis were
evaluated. Univariate logistic regression analysis revealed that
the male gender (P=0.123), a history of smoking (P=0.225), tumor
stages I and III (P=0.129 and 0.147, respectively), and lymphatic
metastasis (P=0.002) were significantly associated with patient
survival (Table II).
 | Table II.Univariate logistic regression
analysis. |
Table II.
Univariate logistic regression
analysis.
| Variable | B | SE | P-value | OR (95% CI) |
|---|
| Gender (male) | −0.506 | 0.328 | 0.123a | 0.603
(0.317–1.147) |
| Age (years) |
|
|
|
|
|
<40 | 0.104 | 0.610 | 0.864 | 1.110
(0.336–3.668) |
|
40–60 | −0.101 | 0.317 | 0.751 | 0.904
(0.486–1.684) |
|
>60b |
|
| 0.919 |
|
| Smoking | 0.373 | 0.307 | 0.225a | 1.452
(0.795–2.649) |
| Alcohol
consumption | 0.000 | 0.305 | 0.998 | 0.999
(0.550–1.817) |
| Location |
|
|
|
|
| Buccal
mucosa | 0.161 | 0.486 | 0.740 | 1.175
(0.453–3.044) |
|
Tongue | −0.074 | 0.364 | 0.839 | 0.929
(0.455–1.897) |
|
Gingiva | 0.067 | 0.465 | 0.886 | 1.069
(0.429–2.662) |
| Other
sitesb |
|
| 0.972 |
|
| Tumor size
(cm) |
|
|
|
|
|
<2b |
|
| 0.800 |
|
|
2–4 | −0.220 | 0.354 | 0.535 | 0.803
(0.401–1.606) |
| ≥4 | −0.027 | 0.517 | 0.959 | 0.974
(0.353–2.682) |
| Differentiated
type |
|
|
|
|
|
Wellb |
|
| 0.882 |
|
|
Moderately | 20.148 |
1.271×104 | 0.999 | 0.562
(0.000–5.347) |
|
Poorly | 0.554 | 1.107 | 0.617 | 1.741
(0.199–15.226) |
| TNM |
|
|
|
|
|
Ib |
|
| 0.129a |
|
| II | −0.357 | 0.471 | 0.448 | 0.700
(0.278–1.762) |
|
III | 0.799 | 0.551 | 0.147a | 2.222
(0.755–6.545) |
| IV | 0.216 | 0.449 | 0.631 | 1.241
(0.514–2.994) |
| Lymphatic
metastasis | 1.120 | 0.369 | 0.002a | 3.064
(1.487–6.313) |
Table III shows the
final model for the predictors in the multivariate logistic
regression analysis to identify factors that were independently
associated with a poor prognosis. The risk of mortality in patients
with lymphatic metastasis was 3.421-times higher than those with
normal lymph nodes, after adjusting for gender, smoking history and
TNM stage (95% confidence interval, 1.609–7.273; P=0.001), which
was demonstrated as independent prognostic factor in all OSCCs. The
male gender (P=0.077), a history of smoking (P=0.438) and the TNM
stage (P>0.05) were not shown to be independent prognostic
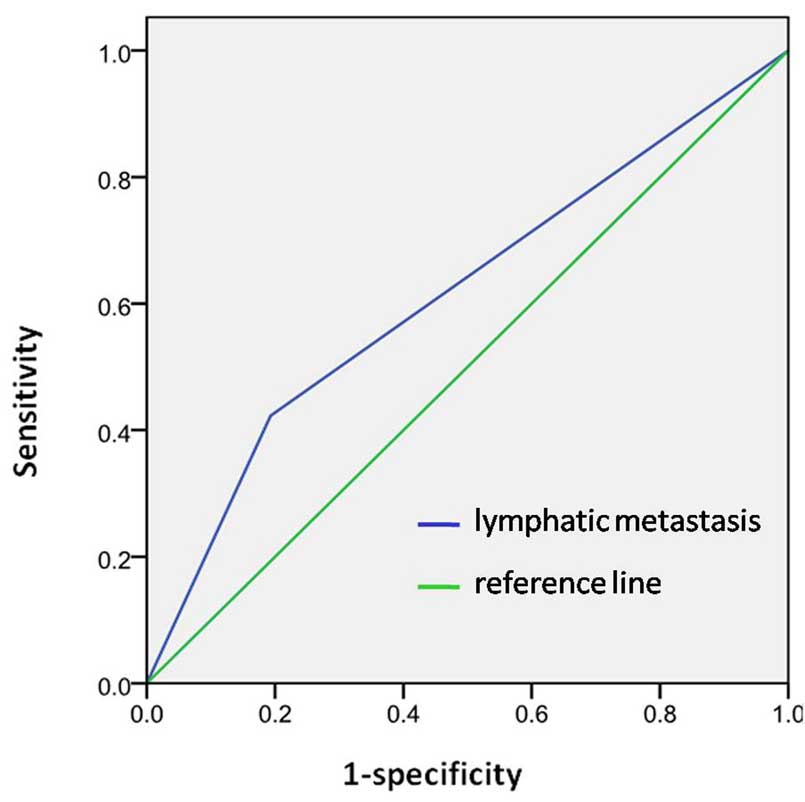
factors of OSCC. The AUC for the prediction model was 0.62
(Fig. 2).
 | Table III.Multivariate logistic regression
analysis. |
Table III.
Multivariate logistic regression
analysis.
| Variable | B | SE | P-value | OR (95% CI) |
|---|
| Gender (male) | −0.607 | 0.343 | 0.077 | 0.545
(0.278–1.067) |
| Smoking | 0.304 | 0.392 | 0.438 | 1.356
(0.629–2.923) |
| TNM |
|
|
Ia |
|
| 0.516 |
|
| II | −0.357 | 0.483 | 0.460 | 0.700
(0.271–1.804) |
|
III | 0.392 | 0.573 | 0.494 | 1.480
(0.481–4.550) |
| IV | −0.206 | 0.477 | 0.666 | 0.814
(0.319–2.074) |
| Lymphatic
metastasis | 1.230 | 0.385 | 0.001b | 3.421
(1.609–7.273) |
HE staining of TMAs
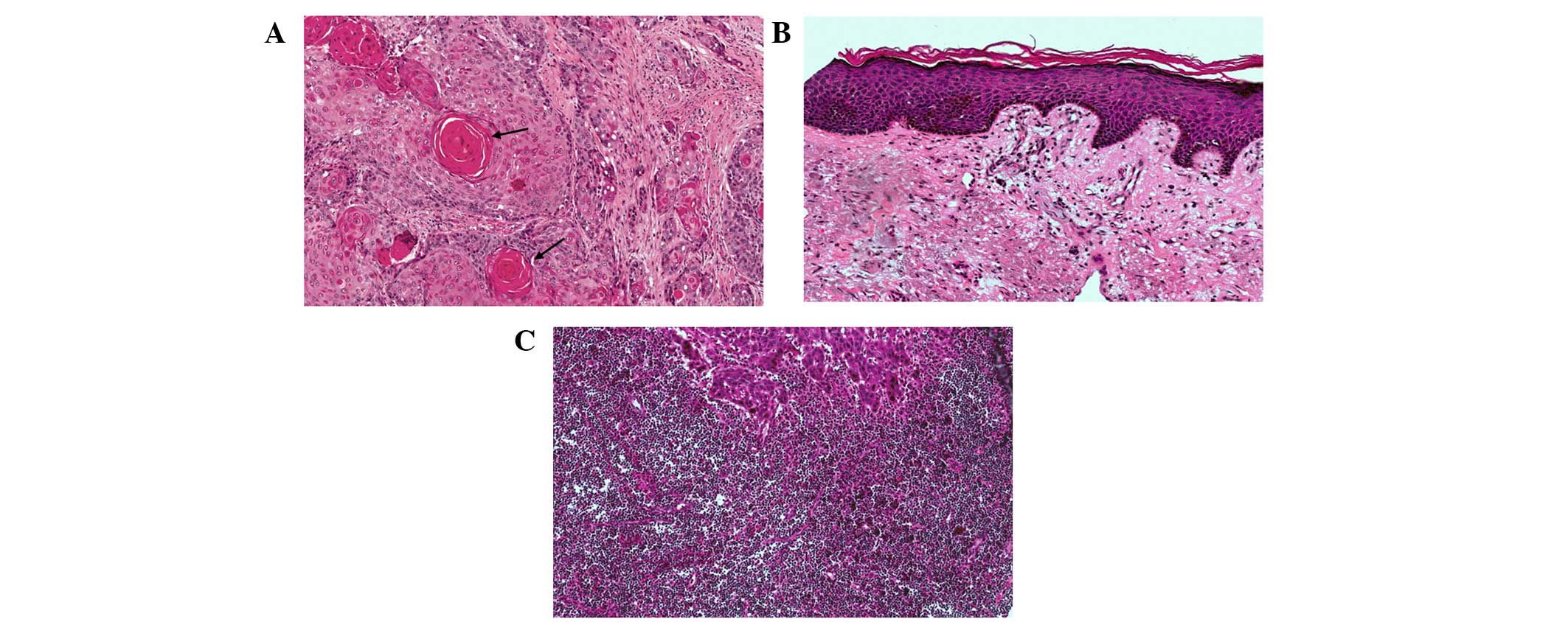
HE staining of the four TMAs is shown in Fig. 3. HE staining identified cancerous
tissues (Fig. 4A), pericarcinomatous
tissues (Fig. 4B) and metastatic
lymph node tissues (Fig. 4C).
Discussion
A previous study suggested that cohort studies could
be employed to support the existence of associations between
suspected risk factors and disease outcome (19). OSCC accounts for ~90% of malignant
oral lesions, and is widely recognized as the most frequently
occurring malignant tumor of oral structures (2). The mortality rate of OSCC is relatively
high, with a 5-year survival rate of 50% (20). Therefore, the present study analyzed
clinical symptoms and pathological parameters in 232 cases of OSCC,
including 175 that were fatal and 57 that were non-fatal, in order
to identify factors associated with a poor prognosis. The
parameters of gender, age, smoking and drinking habits, tumor
location, size and stage, as well as lymphatic metastasis, were
analyzed. Notably, a multiple regression analysis identified
lymphatic metastasis as an important independent factor associated
with a fatal outcome of OSCC. To the best of our knowledge, this is
one of the largest studies to assess numerous factors as survival
predictors in patients with OSCC.
Previous reports on risk factors and a poor
prognosis of OSCC have indicated the existence of various and
inconsistent determinants (7,12). Grimm (12) retrospectively reviewed pathological
parameters of patients with OSCC, including age, gender, site
distribution, tumor size, lymph node involvement and grading, and
demonstrated that an increased tumor size and microvascular
invasion were independent prognostic factors for predicting
survival in patients with OSCC. However, Rossi et al
(7) reported that patients diagnosed
before reaching 65 years-of-age showed an improved prognosis, as
compared with patients diagnosed when aged >65 years, while the
gender distribution was almost equal.
The TNM staging system has been widely accepted as
the basis of cancer staging, since it provides a reasonably precise
description of the extent of disease (21). The main failing of the system,
however, is that it does not include information about the clinical
features of the patient. Ribeiro et al (5) demonstrated that survival estimates may
be improved by the addition of patient clinical features to the TNM
staging system, generating a more powerful and precise system in
the determination of prognosis.
Kang et al (22) reported that the presence of
pathological node metastases was an independent predictor of the
5-year outcomes of 467 patients, although, only for those patients
with well-differentiated OSCC. Kawakita et al (8) conducted a retrospective cohort analysis
of 222 patients with OSCC, in which they assessed the association
between smoking status of patients and clinical outcome.
Furthermore, Munoz Guerra et al (11) analyzed a 20-year cohort of 106
patients to assess the association between a worse prognosis and
factors related to the surgical treatment of oral cancer.
No definitive assumptions regarding the prognostic
factors for OSCC outcome can be drawn, due to the incomplete design
or small sample size of previous studies (15–17).
Therefore, the present study aimed to screen as many factors as
possible, including age, gender, history of smoking and alcohol
consumption, tumor location and size, TNM stage, histological
differentiation and lymphatic metastasis, in a relatively large
cohort, in order to obtain a more precise estimation of the
independent predictors for a worse prognosis. Univariate logistic
regression analysis revealed that the smoking status, male gender,
tumor stages I and III, and lymphatic metastasis were significantly
associated with clinical outcomes. However, following a
multivariate logistic regression analysis, only lymph node
metastasis was identified as an independent risk factor for a poor
prognosis.
An association between smoking and the risk of
developing OSCC has previously been demonstrated (23,24);
however, the relationship between the smoking status and clinical
outcome of a patient remains inconclusive. Previous studies have
reported that tobacco smoke promotes tumor hypoxia associated with
resistance to radiotherapy (RT) and upregulation of the epidermal
growth factor receptor, and demonstrated that a mutation in the p53
gene was associated with resistance to apoptosis (25–27).
Kawakita et al (8)
hypothesized that the pre-treatment smoking status of patients with
OSCC was associated with survival, but only in patients receiving
chemoradiotherapy (CRT)/RT, thus suggesting that the impact of
smoking on patient survival may differ according to the treatment
method. In addition, several studies have reported that smoking
cessation during RT was beneficial to clinical outcome (28–30). In
the present study, smoking was not considered an independent
factor.
In the present study, the age and gender
distributions of patients were not significantly different between
the fatal and non-fatal cases, which was mainly consistent with
previous reports (1,7). Even when the incidence of OSCC has been
shown to be age- or gender-related (31), it is difficult to consider them as
independent prognostic factors.
OSCC often metastasizes to the lymph nodes, which
was shown in the present study to be the most important predictor
of patient survival. Approximately 50% of patients with OSCC have
detectable lymph node involvement, and <40% of patients with
lymph node metastasis survive for 5 years, as compared with 90% of
patients without metastasis (32).
Therefore, lymph node metastasis may be considered important for
predicting the prognosis of patients with OSCC. Generally, the TNM
staging system, which is used to describe the anatomical extension
of the disease, is considered the most important prognostic factor
(5,6),
which is inconsistent with the results of the present study. The
TNM staging system consists of three parts (tumor, lymph node and
metastasis), and it may be that the tumor size is not as important
as lymphatic metastasis in determining the prognosis of a patient;
thus, the tumor size may be a confounding factor when analyzing the
TNM stage as a whole. For example, Kang et al (22) hypothesized that, in OSCC patients with
pathologically negative node disease, a tumor depth of ≥8 mm
(rather than tumor size) was the only independent prognostic factor
for 5-year outcomes. However, the error caused by the small sample
size for each stage of TNM system may be the reason for this
result.
Increasingly, studies have focused on identifying
the molecular markers associated with the diagnosis, treatment and
prognosis of OSCC (33). The TMA is a
high-throughput method that has been used due to its high
efficiency, large-scale, ability to save time, small sample
requirements, and standardization (14). To date, few previous studies have
employed OSCC TMAs. Chen et al (34) used an OSCC TMA to demonstrate that the
expression of the fascin protein may have an important role in the
progression of OSCC, while Chien et al (35) determined that the expression of CA XII
in OSCC samples could predict the progression of OSCC and survival
of OSCC patients. In order to form an experimental platform for the
further detection of markers associated with OSCC, the present
study used the tumor tissues of 232 patients with OSCC to construct
TMAs. Each chip contained the target tissue cores, including
carcinoma tissues, pericancerous tissues and lymph nodes. The
epidemiological and clinical data of the patients used for
construction of the TMAs was completely recorded. To the best of
our knowledge, these are the first OSCC TMAs of the mainland
Chinese population to be constructed, and include a large sample
size and detailed records. They will form the experimental base for
the further exploration of the molecular targets associated with
OSCC.
The present study had some limitations. First, tumor
differentiation has been shown to affect the treatment outcome in
patients with OSCC, and poor differentiation typically indicates
that the tumor cells have lost the characteristics of epithelial
cells and have a more primitive nature (36,37). Chen
et al (38) reported that a
poorly-differentiated tumor was an independent risk factor for
subsequent distant metastasis in patients with locoregionally
advanced OSCC. However, the results of the present study suggested
that tumor differentiation was not significantly associated with a
poor outcome in a univariate logistic regression analysis. The
reason for this could be that the number of patients with poorly-
and moderately-differentiated tumors in the cohort was too small
(10 moderately and 6 poorly), especially compared with the large
number of patients with well-differentiated tumors (213
participants); this may have generated large standard errors and
inaccurate regression coefficients. Second, this study overlooked
some other factors, including comorbidities and treatment method,
which may have affected the prognosis. Third, since the specific
survival time of each patient was not obtained, a survival analysis
could not be performed to estimate the time until death. Fourth, no
definitive assumptions could be drawn due to the relatively small
number of subjects in this retrospective cohort study. Therefore, a
future OSCC cohort should include more participants, especially the
current groups of small population.
In conclusion, the present study demonstrated that
lymphatic metastasis was an independent risk factor for the
prediction of a poor prognosis in a large OSCC patient cohort, in
which a large number of clinicopathological variables were
screened. However, more balanced cohort data are required to
identify more precise prognostic factors. Furthermore, using this
cohort, the first OSCC TMAs to be reported for a mainland Chinese
population were successfully established.
Acknowledgements
The present work was supported by grants from the
National Natural Science Foundation of China (grant nos. 81321002,
81472533 and 81500855), the International Science and Technology
Cooperation Program of China (grant no. 2012DFA31370), the
Chongqing Research Program of Basic Research and Frontier
Technology (grant no. cstc2015jcyjA10029) and the Program for
Innovation Team Building at the Institutions of Higher Education in
Chongqing in 2016.
References
|
1
|
Warnakulasuriya S: Global epidemiology of
oral and oropharyngeal cancer. Oral Oncol. 45:309–316. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kamarajan P, Garcia-Pardo A, D'Silva NJ
and Kapila YL: The CS1 segment of fibronectin is involved in human
OSCC pathogenesis by mediating OSCC cell spreading, migration, and
invasion. BMC Cancer. 10:3302010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wu CH, Wu TY, Li CC, Lui MT, Chang KW and
Kao SY: Impact of diabetes mellitus on the prognosis of patients
with oral squamous cell carcinoma: A retrospective cohort study.
Ann Surg Oncol. 17:2175–2183. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Genden EM, Ferlito A, Bradley PJ, Rinaldo
A and Scully C: Neck disease and distant metastases. Oral Oncol.
39:207–212. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ribeiro KC, Kowalski LP and Latorre MR:
Impact of comorbidity, symptoms, and patients' characteristics on
the prognosis of oral carcinomas. Arch Otolaryngol Head Neck Surg.
126:1079–1085. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fan S, Tang QL, Lin YJ, Chen WL, Li JS,
Huang ZQ, Yang ZH, Wang YY, Zhang DM, Wang HJ, et al: A review of
clinical and histological parameters associated with contralateral
neck metastases in oral squamous cell carcinoma. Int J Oral Sci.
3:180–191. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rossi V, Tarozzi M, Lodi G, Sardella A,
Demarosi F and Carrassi A: Clinical aspect and survival rates in
subject with oral cancer: A retrospective cohort study. Minerva
Stomatol. 56:591–601. 2007.(In English, Italian). PubMed/NCBI
|
|
8
|
Kawakita D, Hosono S, Ito H, Oze I,
Watanabe M, Hanai N, Hasegawa Y, Tajima K, Murakami S, Tanaka H and
Matsuo K: Impact of smoking status on clinical outcome in oral
cavity cancer patients. Oral Oncol. 48:186–191. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shaw RJ, Lowe D, Woolgar JA, Brown JS,
Vaughan ED, Evans C, Lewis-Jones H, Hanlon R, Hall GL and Rogers
SN: Extracapsular spread in oral squamous cell carcinoma. Head
Neck. 32:714–722. 2010.PubMed/NCBI
|
|
10
|
Kreppel M, Dreiseidler T, Rothamel D, Eich
HT, Drebber U, Zöller JE and Scheer M: The role of clinical versus
histopathological staging in patients with advanced oral squamous
cell carcinoma treated with neoadjuvant radiochemotherapy followed
by radical surgery. J Craniomaxillofac Surg. 41:22–27. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Muñoz Guerra MF, Gías L Naval, Campo FR
and Pérez JS: Marginal and segmental mandibulectomy in patients
with oral cancer: A statistical analysis of 106 cases. J Oral
Maxillofac Surg. 61:1289–1296. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Grimm M: Prognostic value of
clinicopathological parameters and outcome in 484 patients with
oral squamous cell carcinoma: Microvascular invasion
(V+) is an independent prognostic factor for OSCC. Clin
Transl Oncol. 14:870–880. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ratajczak-Wrona W, Jablonska E, Antonowicz
B, Dziemianczyk D and Grabowska SZ: Levels of biological markers of
nitric oxide in serum of patients with squamous cell carcinoma of
the oral cavity. Int J Oral Sci. 5:141–145. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu CL, Prapong W, Natkunam Y, Alizadeh A,
Montgomery K, Gilks CB and van de Rijn M: Software tools for
high-throughput analysis and archiving of immunohistochemistry
staining data obtained with tissue microarrays. Am J Pathol.
161:1557–1565. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kononen J, Bubendorf L, Kallioniemi A,
Bärlund M, Schraml P, Leighton S, Torhorst J, Mihatsch MJ, Sauter G
and Kallioniemi OP: Tissue microarrays for high-throughput
molecular profiling of tumor specimens. Nat Med. 4:844–847. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lourenço SV, Coutinho-Camillo CM, Buim ME,
Pereira CM, Carvalho AL, Kowalski LP and Soares FA: Oral squamous
cell carcinoma: Status of tight junction claudins in the different
histopathological patterns and relationship with clinical
parameters. A tissue-microarray-based study of 136 cases. J Clin
Pathol. 63:609–614. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu CM, Lin YM, Yeh KT, Chen MK, Chang JH,
Chen CJ, Chou MY, Yang SF and Chien MH: Expression of carbonic
anhydrases I/II and the correlation to clinical aspects of oral
squamous cell carcinoma analyzed using tissue microarray. J Oral
Pathol Med. 41:533–539. 2012.PubMed/NCBI
|
|
18
|
Edge SB and Compton CC: The American Joint
Committee on Cancer: The 7th edition of the AJCC cancer staging
manual and the future of TNM. Ann Surg Oncol. 17:1471–1474. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Doll R: Cohort studies: History of the
method. II. Retrospective cohort studies. Soz Praventivmed.
46:152–160. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang Z, Jiang L, Huang C, Li Z, Chen L,
Gou L, Chen P, Tong A, Tang M, Gao F, et al: Comparative proteomics
approach to screening of potential diagnostic and therapeutic
targets for oral squamous cell carcinoma. Mol Cell Proteomics.
7:1639–1650. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Schwab W: The present state of the
guideline of the UICC on the TNM system. Arch Klin Exp Ohren Nasen
Kehlkopfheilkd. 191:634–636. 1968.(In German). View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kang CJ, Liao CT, Hsueh C, Lee LY, Lin CY,
Fan KH, Wang HM, Huang SF, Chen IH, Ng SH, et al: Outcome analysis
of patients with well-differentiated oral cavity squamous cell
carcinoma. Oral Oncol. 47:1085–1091. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hashibe M, Brennan P, Benhamou S,
Castellsague X, Chen C, Curado MP, Dal Maso L, Daudt AW, Fabianova
E, Fernandez L, et al: Alcohol drinking in never users of tobacco,
cigarette smoking in never drinkers, and the risk of head and neck
cancer: Pooled analysis in the international head and neck cancer
epidemiology consortium. J Natl Cancer Inst. 99:777–789. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
La Vecchia C, Tavani A, Franceschi S, Levi
F, Corrao G and Negri E: Epidemiology and prevention of oral
cancer. Oral Oncol. 33:302–312. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liloglou T, Scholes AG, Spandidos DA,
Vaughan ED, Jones AS and Field JK: p53 mutations in squamous cell
carcinoma of the head and neck predominate in a subgroup of former
and present smokers with a low frequency of genetic instability.
Cancer Res. 57:4070–4074. 1997.PubMed/NCBI
|
|
26
|
Jensen JA, Goodson WH, Hopf HW and Hunt
TK: Cigarette smoking decreases tissue oxygen. Arch Surg.
126:1131–1134. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Toyooka S, Matsuo K, Shigematsu H, Kosaka
T, Tokumo M, Yatabe Y, Ichihara S, Inukai M, Suehisa H, Soh J, et
al: The impact of sex and smoking status on the mutational spectrum
of epidermal growth factor receptor gene in non small cell lung
cancer. Clin Cancer Res. 13:5763–5768. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Browman GP, Wong G, Hodson I, Sathya J,
Russell R, McAlpine L, Skingley P and Levine MN: Influence of
cigarette smoking on the efficacy of radiation therapy in head and
neck cancer. N Engl J Med. 328:159–163. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen AM, Chen LM, Vaughan A, Sreeraman R,
Farwell DG, Luu Q, Lau DH, Stuart K, Purdy JA and Vijayakumar S:
Tobacco smoking during radiation therapy for head-and-neck cancer
is associated with unfavorable outcome. Int J Radiat Oncol Biol
Phys. 79:414–419. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Meyer F, Bairati I, Fortin A, Gélinas M,
Nabid A, Brochet F and Têtu B: Interaction between antioxidant
vitamin supplementation and cigarette smoking during radiation
therapy in relation to long-term effects on recurrence and
mortality: A randomized trial among head and neck cancer patients.
Int J Cancer. 122:1679–1683. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jainkittivong A, Swasdison S,
Thangpisityotin M and Langlais RP: Oral squamous cell carcinoma: A
clinicopathological study of 342 Thai cases. J Contemp Dent Pract.
10:E033–E040. 2009.PubMed/NCBI
|
|
32
|
Sano D and Myers JN: Metastasis of
squamous cell carcinoma of the oral tongue. Cancer Metastasis Rev.
26:645–662. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Choi S and Myers JN: Molecular
pathogenesis of oral squamous cell carcinoma: Implications for
therapy. J Dent Res. 87:14–32. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chen SF, Yang SF, Li JW, Nieh PC, Lin SY,
Fu E, Bai CY, Jin JS, Lin CY and Nieh S: Expression of fascin in
oral and oropharyngeal squamous cell carcinomas has prognostic
significance-a tissue microarray study of 129 cases.
Histopathology. 51:173–183. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chien MH, Ying TH, Hsieh YH, Lin CH, Shih
CH, Wei LH and Yang SF: Tumor-associated carbonic anhydrase XII is
linked to the growth of primary oral squamous cell carcinoma and
its poor prognosis. Oral Oncol. 48:417–423. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kang CJ, Lin CY, Wang HM, Fan KH, Ng SH,
Lee LY, Chen IH, Huang SF, Liao CT and Yen TC: The number of
pathologically positive lymph nodes and pathological tumor depth
predicts prognosis in patients with poorly differentiated squamous
cell carcinoma of the oral cavity. Int J Radiat Oncol Biol Phys.
81:e223–e230. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Mandal M, Myers JN, Lippman SM, Johnson
FM, Williams MD, Rayala S, Ohshiro K, Rosenthal DI, Weber RS,
Gallick GE and El-Naggar AK: Epithelial to mesenchymal transition
in head and neck squamous carcinoma: Association of Src activation
with E-cadherin down-regulation, vimentin expression, and
aggressive tumor features. Cancer. 112:2088–2100. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chen TC, Hsu CW, Lou PJ, Ko JY, Yang TL,
Chen CN, Chang YL and Wang CP: The clinical predictive factors for
subsequent distant metastasis in patients with locoregionally
advanced oral squamous cell carcinoma. Oral Oncol. 49:367–373.
2013. View Article : Google Scholar : PubMed/NCBI
|