Introduction
Glioma originates from nerve epithelium-derived
cells (1), accounting for 44.6% of
the central nervous system tumors. It is the most common malignant
tumor in the central nervous system with a high incidence and high
mortality rate (2). Despite several
achievements in cancer therapy in recent years, no effective
treatment for glioma has been found. Currently, surgery-based
comprehensive treatment is the treatment of choice for glioma, but
the prognosis of comprehensive treatment remains poor.
Nevertheless, this treatment can improve the recovery rate of
glioma to a certain extent with 5-year survival rate of <5%
(3,4).
Therefore, there is a huge interest in developing a new means of
treatment for brain glioma. The application of traditional Chinese
medicine for treating the malignant tumors has a long history in
China, but traditional Chinese medicine has failed to be recognized
in the medical field due to the complexity of Chinese herbal
medicinal ingredients and unclear antitumor mechanism. Thus, there
are several restrictions in conducting clinical trials for
traditional Chinese remedies (5,6). In recent
years, several studies have been conducted on the adverse side
effects associated with semi-synthetic and synthetic chemotherapy
drugs used for treatment of tumors. As a result, there is interest
in research on the natural antitumor compounds extracted from
Chinese medicines. The Garcinia plants are known for their
therapeutic effects and there is great interest in the medical
effects of Garcinia. There are studies on using
Garcinia for its anti-HIV, antibiosis, antioxidation,
anti-inflammation, anti-malarial, insecticide and antitumor
activities (7–10). Morelloflavone substances in the
Garcinia plant have been shown to have broad-spectrum
antitumor effects, although their action mechanism remains to be
determined (11).
We extracted various plant substances from
Garcinia plants and then screened the flavonoid substances
using LC-MS. The specific structure of these compounds was analyzed
using nuclear magnetic resonance and the effects of different
compounds on glioma U87 cells and rat glioma C6 cells were
investigated. We also analyzed the relationship between the
structure of compounds and their anti-glioma effects. This study
provides a theoretical reference for further research on
anti-glioma agents.
Materials and methods
Experimental materials
The materials used for the present study were: Ethyl
alcohol, methyl alcohol (chromatographic grade; Sinopharm Chemical
Ltd., Shanghai, China); ultra-pure water (Milli-Q); gambogic tree
trunk; deuterated methanol, dimethyl sulfoxide (DMSO); and human
glioma U87 and rat glioma C6 cells (ATCC).
Experimental method
Extraction of active substances of gambogic tree
trunk
Gambogic tree trunk extract (50.00 g) was broken
into pieces and 70% of ethyl alcohol was added. It was transferred
to a water bath (80°C) for 8 h, and the leaching liquid was
collected and the supernatant after the leaching liquid was
centrifuged at 2,000 × g for 15 min and filtrated. A rotary
evaporator was used to remove the solvent for freeze-drying, and it
was stored at −20°C.
Chromatographic separation of gambogic leaching
liquid
Based on Waters preparative high performance liquid
chromatography (Pre-HPLC), the XBridge C18 (5 µm, 21.2×250 mm)
chromatographic column (Waters, Milford, MA, USA) was used to
isolate the gambogic leaching liquid. Conditions for chromatography
were as follows: Column temperature was 40°C; flow velocity was 4
ml/min; mobile A phase was methyl alcohol, mobile B phase was 0.1%
formic acid aqueous solution, pH 5.0; time 0 min, A (40%), B (60%);
3 min, A (40%), B (60%); 25 min, A (40%), B (60%); 27 min, A
(100%), B (0%); 37 min, A (40%), B (60%); and 45 min, A (40%), B
(60%). The effluent components were collected according to the
chromatographic peak and C18 solid phase extraction column was used
to desalt each component. Components were freeze-dried by
degreasing solvent and stored at −20°C.
Screening the biflavone substances
High resolution mass spectrometry (HRMS TOF; Waters,
Milford, MA, USA) was employed to identify the collected
components. Each component was dissolved in the methyl alcohol by
connecting the HRMS to the ultra-performance liquid chromatography
(UPLC; Waters). Waters BEH C18 (1.7 µm, 2×150 mm) chromatographic
column was used for injecting samples directly and each component
was identified with HRMS (MassLynx 4.1 analysis method) and by
consulting literature and comparing CAS database.
Structure identification
The biflavone substances were prepared again to make
the quantity of biflavone substances to reach the concentration
necessary for nuclear magnetic resonance. Magnetic resonance
spectroscopy (NMR; Bruker ARX 400; Karlsruhe, Germany) was used to
confirm and analyze their structure. The sample solvent of nuclear
magnetic resonance was CD3OD and the internal sample
standard was TMS. Data acquired were analyzed by MestreNova 4.1
(San Diego, CA, USA).
Inhibition rate of U87 cells of biflavone
substances by MTT detection
The biflavone substances with different structures
were dissolved in DMSO, and the compound concentration was 0, 10,
20, 40 and 80 µmol/l. The sample of 0 µmol/l was taken as the blank
control. The specific experimental operation methods were similar
to those reported by Vandamme et al (12).
The calculation method for obtaining the inhibition
rate was: IR inhibition rate (%) = (1 - OD value of experimental
port/OD value of blank control port) × 100%. The mean of the
inhibition rate was the end value and the inhibition rate of blank
control port was 0 according to the calculation.
Preparation of glioma animal model
To prepare rat glioma cell C6 xenograft tumor model
we followed the method reported by Li et al (13) with a slight modification. Approval for
the animal experiments was received from The First Affiliated
Hospital of Liaoning Medical University (Liaoning, China).
Statistical analysis
SPSS 18.0 statistical software (Chicago, IL, USA)
was used to conduct statistical analysis. The mean ± standard
deviation (SD) was used to record the independent experimental data
and the comparison among various groups was analyzed using one-way
ANOVA. Inter-group comparisons were tested by t-test. P<0.05 was
considered to indicate a statistically significant difference.
Results
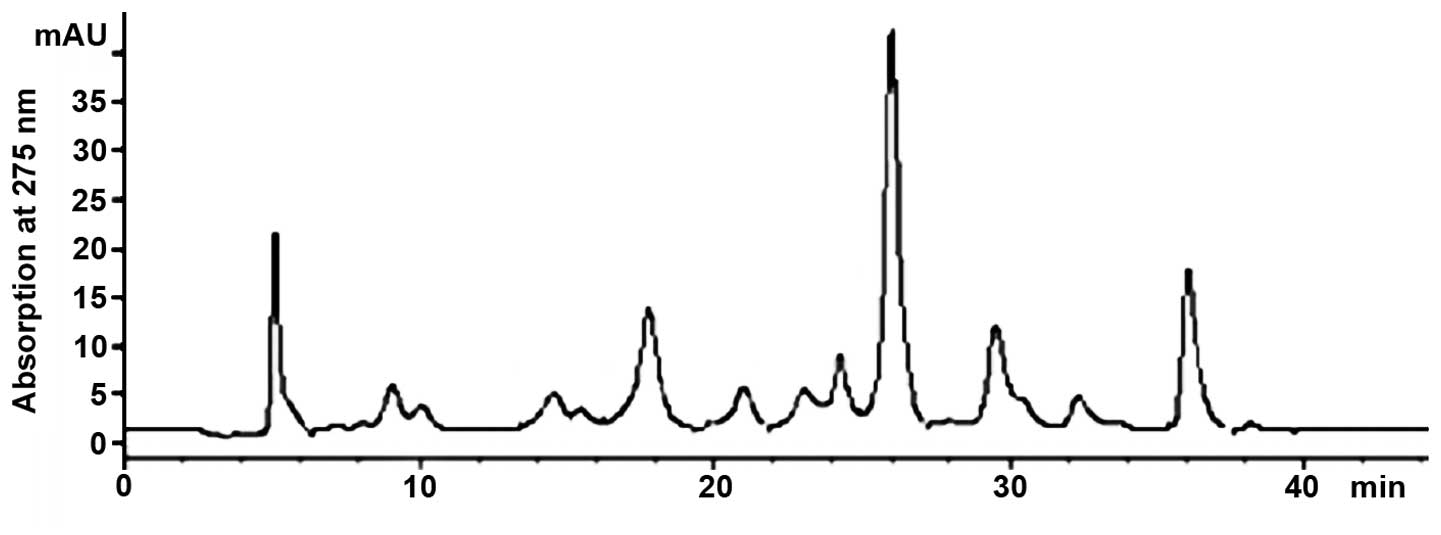
Pre-HPLC isolation of gambogic
extraction solution
The ethanol water solution was used to extract the
active ingredients from the gambogic tree trunk. Due to the fact
that flavonoids contain benzene and pyranoid rings in their
structure we used 275 nm wavelength to detect the ultraviolet
absorption band. To ensure rapid isolation of flavonoids, the
gradient elution method was used. The gradient of methanol started
from 40% and gradually increased to 100% in order to elute all
components. Results for final chromatographic separation are
presented in Fig. 1. The chromatogram
showed that the separation degree between the chromatographic peaks
was improved. Components were eluted from chromatographic column
and collected according to the chromatographic peaks (18 components
in total). Due to the presence of formic acid in the mobile phase,
collected components were desalinated and purified, and C18 solid
phase extraction column was used to absorb active components.
Ultra-pure water was then used to elute salts and impurities and
finally the methanol was used to elute active substances from the
solid phase extraction column. Active substances were freeze-dried
after the solvent was removed and then stored at −20°C.
Screening of biflavone substances
We used HRMS to analyze the structure of biflavones.
Due to the fact that all 18 components mentioned in the present
study were isolated and purified by liquid chromatography, the
direct injection technique was used for HRMS. Components structures
were determined according to the accurate molecular mass and ion
fragments information, and finally the biflavone substances were
determined by comparing our data with those available in the
literature (14,15) and CAS database. Five biflavone
substances were finally selected (Table
I).
 | Table I.HRMS information of biflavone
substances. |
Table I.
HRMS information of biflavone
substances.
|
Substancesa | Retention time | Measured molecular
mass | Actual molecular
mass | Molecular
formula | Error m/z (ppm) | Molecular
fragment |
|---|
| 1 | 17.8 | 751.1505 | 751.1510 |
C36H31O18 | −0.7 | 151.0031, 285.0392,
435.0708, 437.0866, 445.0556 |
| 2 | 18.6 | 735.1564 | 735.1561 |
C36H31O17 | +0.4 | 125.0237, 269.0448,
403.0818, 429.0609, 447.0717 |
| 3 | 24.6 | 589.0972 | 589.0982 |
C30H22O13 | −1.7 | 125.0237, 151.0028,
285.0393, 435.0713, 463.0660 |
| 4 | 26.6 | 573.1043 | 573.1033 |
C30H22O12 | +1.7 | 125.0237, 296.0313,
419.0761, 447.0711 |
| 5 | 32.7 | 557.1084 | 557.1083 |
C30H22O11 | +0.1 | 125.2038, 269.0443,
296.0317, 403.0813, 431.0764 |
Although HRMS is a fast and effective method to
determine the molecular structure of different compounds, structure
of biflavones cannot be completely determined just by mass
spectrometry. In order to determine the specific structure and
position of each functional group they should be analyzed by
nuclear magnetic resonance. Therefore, we used a relatively HRMS
and prepared large quantities of samples to for nuclear magnetic
resonance.
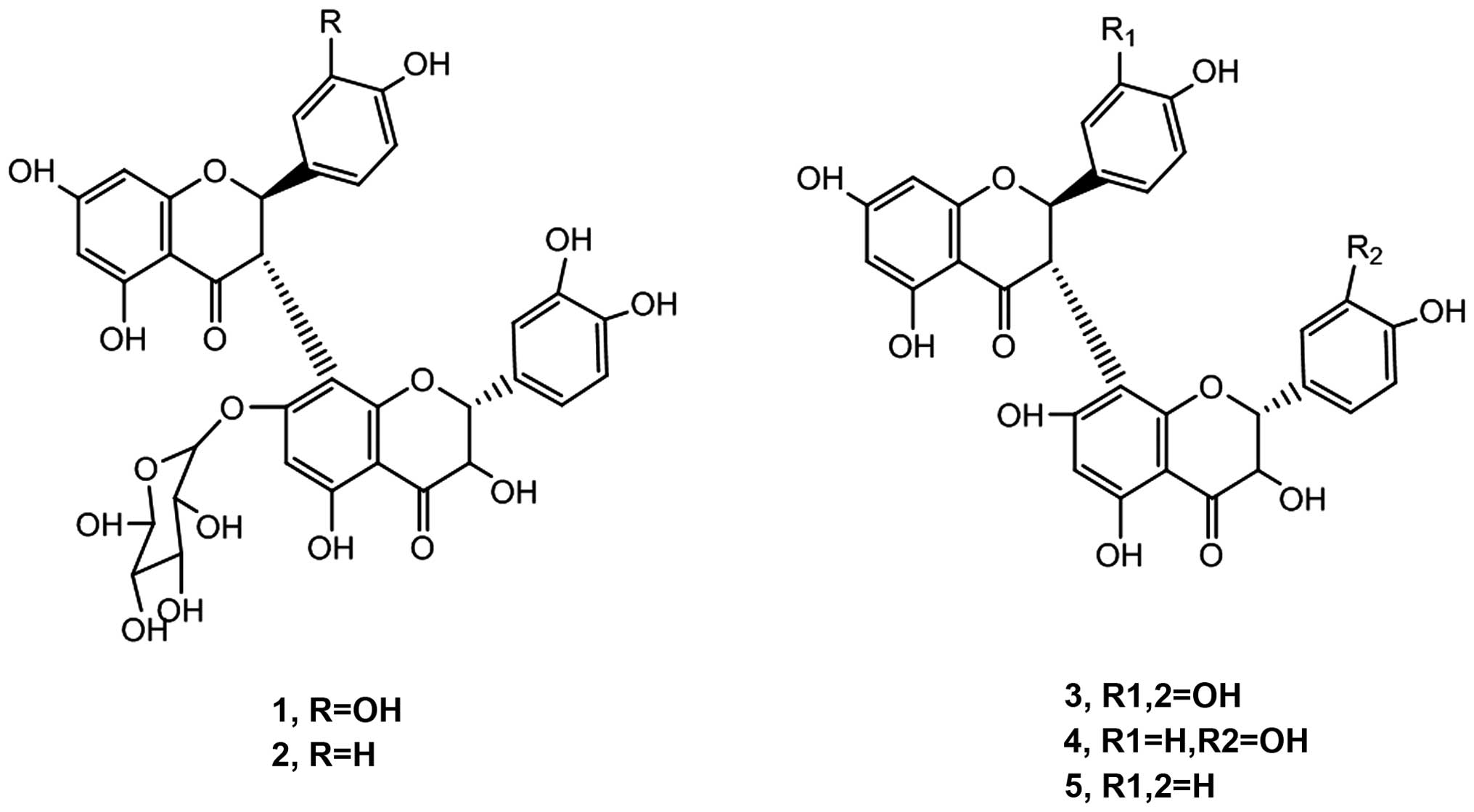
Structure determination of biflavone
substances by nuclear magnetic resonance
Five biflavone substances were dissolved in
CD3OD and 1H and 13C spectrum was
then analyzed by nuclear magnetic resonance and the specific
structures of these compounds were determined by comparing them
with literature (16–18) and spectrum database (Fig. 2). After determination of the biflavone
molecular structures, we investigated the inhibition effects of
these substances on glioma and then analyzed the possible
relationship between the structure and the function.
Inhibition effect on U87 cells
Different concentrations of each biflavone substance
were added to cells and after 24 h the inhibition rate on glioma
U87 cells was determined (Table II).
Our results showed that the five biflavone substances demonstrated
some inhibitory activities on U87 glioma cells. The inhibition rate
of compound no. 1 was the highest followed by compound no. 2. The
inhibition rate of compound no. 3 had no significant difference
from that of compound no. 4, while the inhibition rate of compound
no. 5 was the lowest.
 | Table II.Proliferation inhibition effect of
biflavone substances on glioma U87 cell (%, mean ± SD). |
Table II.
Proliferation inhibition effect of
biflavone substances on glioma U87 cell (%, mean ± SD).
| Concentration
(µmol/l) | Compound 1 | Compound 2 | Compound 3 | Compound 4 | Compound 5 |
|---|
| 0 |
0.13±0.11 |
0.14±0.09 |
0.08±0.12 |
0.11±0.10 |
0.09±0.15 |
| 10 |
12.24±0.24 |
14.10±0.55 |
6.75±0.41 |
6.12±0.33 |
2.23±0.54 |
| 20 |
19.78±0.28 |
17.64±0.86 |
8.17±0.96 |
8.43±0.35 |
1.85±0.21 |
| 40 |
25.66±0.68 |
32.73±0.94 |
12.31±0.68 |
11.92±0.68 |
4.31±0.35 |
| 80 |
55.64±0.65a |
49.82±0.91a,b |
21.17±0.84a–c |
22.78±0.75a–c |
9.87±0.66a–d |
Inhibition of five biflavone
substances on cell C6
To verify the inhibition activity of biflavone
substances on xenograft tumor cells, the axillary xenograft tumor
model of rat glioma C6 cells was used. Rats were injected with 1
mg/kg of cisplatin and 800 µg/kg of biflavone on the seventh day
after the inoculation of glioma C6 cells. The results are presented
in Table III.
 | Table III.Inhibition effect of biflavone
substances on the growth of rat glioma cell C6 xenograft
tumors. |
Table III.
Inhibition effect of biflavone
substances on the growth of rat glioma cell C6 xenograft
tumors.
| Groups | Dosage (µg/kg) | Weight (g) | Tumor change (g) | Inhibition rate
(%) |
|---|
| Control | 0 | 7.13 | 2.33±1.11 | 0 |
| Compound 1 | 800 | 5.62 | 0.95±0.55 |
66.73±0.12a |
| Compound 2 | 800 | 6.77 | 1.23±0.62 |
65.21±0.35a |
| Compound 3 | 800 | 4.89 | 2.28±0.47 |
52.12±0.51a,b |
| Compound 4 | 800 | 5.12 | 2.59±0.41 |
53.31±0.28a,b |
| Compound 5 | 800 | 6.08 | 4.41±0.53 |
31.84±0.33a–c |
Our results showed that the inhibition activity of
biflavone substances on glioma C6 cells was similar to the
inhibition effect in the case of U87 cells. Compound no. 1 was the
most potent compound among the five. Compound no. 2 was the second
and compound no. 3 had no significant difference compared to
compound no. 4. The inhibition rate of compound no. 5 was the
lowest.
Discussion
Glioma is one of the most common malignant tumors
occurring in the central nervous system, and the incidence of
glioma accounts for 50–60% of brain tumors (19,20).
Although surgery remains the method of choice for treating
malignant glioma, the efficacy of surgery has not greatly improved
in recent years. Thus, the trial of drug therapy or assisted
surgery therapy has a certain practical significance and broad
applications. There are studies on adverse effects associated with
synthetic and semi-synthetic drugs used in chemotherapy, therefore
people are gradually paying more attention to the research on
natural antitumor compounds extracted from traditional Chinese
medicine. As type of traditional Chinese medicine, the
Garcinia has various types of medical effects and the
biflavone substances that the carcinia contains have broad-spectrum
antitumor effects, but the action mechanism of the these biflavone
substances are still unclear.
Our results showed that the inhibition effects of
biflavone substances on glioma U87 and C6 cells were alike. The
inhibition effect of compound no. 1 was the strongest followed by
compound no. 2 whose inhibition effect was significantly greater
than that of compounds nos. 3–5. From studying the structure of all
five compounds we detected the presence of a glucose chain in the
structure of compound nos. 1 and 2. The preliminary
structure-function relationship showed that the circumscribed
glucose chain in the biflavone compounds could enhance the
inhibition effect. It is possible that the glucose chain was
responsible for these adverse effect on the cell DNA (21).
Compound no. 5, had R1=R2=H, while compounds nos.
1–4 R1=R2=hydroxyl group. It was hypothesized that the reduction of
hydroxyl group in biflavone substances may reduce their inhibition
effects. Additionally, the inhibition activity of compound no. 2
was significantly lower than that of compound no. 1. The existence
of hydroxyl group can stimulate the formation of external as well
as internal hydrogen bonds within and between molecules and
indirectly enhance the interaction between molecules and improved
the inhibition rate on glioma.
In future, more biflavone substances will be
isolated and screened from different parts of Garcinia
plants and their anti-tumoral activity will be verified. This may
lay a more solid foundation for the further verification and
exploration of the possible link between biflavone structure and
its function.
References
|
1
|
Johnson BE, Mazor T, Hong C, Barnes M,
Aihara K, McLean CY, Fouse SD, Yamamoto S, Ueda H, Tatsuno K, et
al: Mutational analysis reveals the origin and therapy-driven
evolution of recurrent glioma. Science. 343:189–193. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lee Titsworth W, Murad GJ, Hoh BL and
Rahman M: Fighting fire with fire: the revival of thermotherapy for
gliomas. Anticancer Res. 34:565–574. 2014.PubMed/NCBI
|
|
3
|
Mirimanoff RO: High-grade gliomas: reality
and hopes. Chin J Cancer. 33:1–3. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Giovagnoli AR, Meneses RF, Silvani A,
Milanesi I, Fariselli L, Salmaggi A and Boiardi A: Quality of life
and brain tumors: what beyond the clinical burden? J Neurol.
261:894–904. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ji B, Shi J, Cheng X, Zhou J, Zhou Q, Cao
C and Pang J: Association analysis of two candidate polymorphisms
in the tumour necrosis factor-α gene with osteoarthritis in a
Chinese population. Int Orthop. 37:2061–2063. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lin SZ: Era of stem cell therapy for
regenerative medicine and cancers: an introduction for the special
issue of Pan Pacific Symposium on Stem Cells and Cancer Research.
Cell Transplant. 24:311–312. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Boakye PA, Brierley SM, Pasilis SP and
Balemba OB: Garcinia buchananii bark extract is an effective
anti-diarrheal remedy for lactose-induced diarrhea. J
Ethnopharmacol. 142:539–547. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Castro J, Balemba OB, Blackshaw LA and
Brierley SM: Garcinia buchananii bark extract inhibits nociceptors,
with greater efficacy during inflammation. Gastroenterology.
140(Suppl 1): S8662011.
|
|
9
|
Kisangau DP, Lyaruu HV, Hosea KM and
Joseph CC: Use of traditional medicines in the management of
HIV/AIDS opportunistic infections in Tanzania: a case in the Bukoba
rural district. J Ethnobiol Ethnomed. 3:29–37. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ye ZN, Yu MY, Kong LM, Wang WH, Yang YF,
Liu JQ, Qiu MH and Li Y: Biflavone Ginkgetin, a novel Wnt
inhibitor, suppresses the growth of medulloblastoma. Nat Prod
Bioprospect. 5:91–97. 2015. View Article : Google Scholar
|
|
11
|
Pang X, Yi T, Yi Z, Cho SG, Qu W, Pinkaew
D, Fujise K and Liu M: Morelloflavone, a biflavonoid, inhibits
tumor angiogenesis by targeting rho GTPases and extracellular
signal-regulated kinase signaling pathways. Cancer Res. 69:518–525.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Vandamme M, Robert E, Pesnel S, Barbosa E,
Dozias S, Sobilo J, Lerondel S, Le Pape A and Pouvesle JM:
Antitumor effect of plasma treatment on U87 glioma xenografts:
preliminary results. Plasma Process Polym. 7:264–273. 2010.
View Article : Google Scholar
|
|
13
|
Li XQ, Ouyang ZG, Zhang SH, Liu H, Shang
Y, Li Y and Zhen YS: Synergistic inhibition of angiogenesis and
glioma cell-induced angiogenesis by the combination of temozolomide
and enediyne antibiotic lidamycin. Cancer Biol Ther. 15:398–408.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Stark TD, Losch S, Frank O, Balemba OB and
Hofmann T: Purification procedure for
(2R,3S,2′R,3′R)-manniflavanone and its minor (2R,3S,2′S,3′S)-isomer
from Garcinia buchananii stem bark extract. Eur Food Res Technol.
240:1075–1080. 2015. View Article : Google Scholar
|
|
15
|
Xu G, Kan WL, Zhou Y, Song JZ, Han QB,
Qiao CF, Cho CH, Rudd JA, Lin G and Xu HX: Cytotoxic
acylphloroglucinol derivatives from the twigs of Garcinia cowa. J
Nat Prod. 73:104–108. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bing L, Gao-Sheng H, Ling-Ling H, He-Yu L
and Jing-Ming J: Extraction and determination of biflavone
components in ginkgo leaves. Chinese Herbal Med. 17:20142014.
|
|
17
|
Xueping J and Keli C: Research on
biflavone compounds in Selaginella moellendorfii Hieron. Chin J
Pharmaceuticals. 1:20092009.
|
|
18
|
Lianzhu Z: Treatment and possible effect
of total flavonoids of Selaginella tamariscina and amentoflavone in
cognitive disorder model (unpublished PhD dissertation). Jilin
University. 2013.
|
|
19
|
Lipeng L: Experimental research on the
treatment of C6brain glioma by nimustine combined with cisplatin
(unpublished Master dissertation). Tianjin Medical University.
2008.
|
|
20
|
Flavahan WA, Drier Y, Liau BB, Gillespie
SM, Venteicher AS, Stemmer-Rachamimov AO, Suvà ML and Bernstein BE:
Insulator dysfunction and oncogene activation in IDH mutant
gliomas. Nature. 529:110–114. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bao S, Wu Q, McLendon RE, Hao Y, Shi Q,
Hjelmeland AB, Dewhirst MW, Bigner DD and Rich JN: Glioma stem
cells promote radioresistance by preferential activation of the DNA
damage response. Nature. 444:756–760. 2006. View Article : Google Scholar : PubMed/NCBI
|