Introduction
Colorectal cancer (CRC) is considered to develop
slowly via the progressive accumulation of genetic mutations
(1,2).
Genes that regulate cell growth and differentiation must be altered
in cancerous cells in the process of tumorigenesis (3,4). Markers
of CRC may provide the basis for decision-making regarding
intensive chemotherapy or molecule-targeting drugs in CRC patients
(5–7).
Therefore, the identification of markers may assist in cancer
prevention, detection and prognostic prediction (5,8,9), thereby increasing survival rates
(10). Molecular markers (11) have their own clinical significance in
CRC (12).
In CRC, both sigmoidoscopy and colonoscopy are
considered to be the gold standards regarding detection rates.
However, these clinical examinations have drawbacks in terms of
their risk and inconvenience (13,14).
Molecular markers of CRC present in the peripheral blood of
patients, including carcinoembryonic antigen and carbohydrate
antigen 19-9, have been discussed in numerous reports, despite
exhibiting poor specificity (15). In
addition to the fecal occult blood test, the molecular detection of
CRC using human feces has attracted attention in recent years
(16–18). In fact, feces gather shedding cells
from the colonic tract, including CRC cells, and respond to
localized malignance (7,19,20). Not
only DNA but also messenger (m)RNA molecules that are present in
human feces faithfully represent CRC manifestations (17,21–24). For
this reason, human feces are potentially appropriate material to
gain an understanding of CRC development (25,26).
Gene expression is used for classifying tumors or
predicting prognoses (27). The
active genetic molecules that are differentially expressed in feces
may be non-invasive candidates to indicate the pathogenic processes
that underlie pharmacological responses. Studies of active genes in
human feces have revealed specific molecular signatures of
different CRC patients (28,29). Previously, several genes were reported
as having differential expression in the feces of CRC patients
(21,30). Furthermore, a number of these genes
were correlated with cancer (20,21,24,31–34).
The expression of the most significant of these genes must be
characterized and explored in CRC cells (21,35,36).
To verify the clinical credibility of fecal
molecules, the present study first assessed the stability of mRNAs
from human fecal samples that were stored under different
conditions. Subsequently, the most significant genes in CRC were
verified using quantitative polymerase chain reaction (qPCR) in
different CRC cell lines. The present results may shed light on the
selection of the best treatment option for individual patients
according to their significant fecal molecules.
Materials and methods
Quantitation of the mouse β-actin gene
in human feces
To simulate the sloughed colonic cells present in
human feces, 1×104 mouse embryonic fibroblast cells
[National Institutes of Health (NIH) 3T3 cells, gifted by Dr
Shih-Ming Huang, National Defense Medical Center, Taipei, Taiwan]
were added into 0.5 g of feces from a healthy volunteer (a
37-year-old male). The present study was approved by the
Institutional Review Board of Cathay General Hospital (Taipei,
Taiwan) as a research study. Each NIH 3T3-containing fecal sample
was stored under different conditions (Fig. 1) in our specific buffer (30). The fecal total RNA was extracted and
reverse transcribed into complementary (c)DNA as detailed in our
previous reports (21,30). The mouse β-actin gene (NM_007393) was
specifically quantified by qPCR on a LightCycler 1.5 instrument
(Roche Diagnostics GmbH, Mannheim, Germany), according to the
standard protocol (37). The primers
and the TaqMan® probe used for quantifying the mRNA
levels of mouse β-actin are listed in Table I. In addition, the quantification
cycle (Cq) value was used to indicate the expression level of the
detected gene (38).
 | Table I.Primers and TaqMan® probe
for quantifying the messenger RNA levels of mouse β-actin. |
Table I.
Primers and TaqMan® probe
for quantifying the messenger RNA levels of mouse β-actin.
| Gene (symbol) | Accession no. | Primers
(5′-3′) | UPL no. |
|---|
| Mus musculus actin, beta
(Actb) | NM_007393 | F:
AAGGCCAACCGTGAAAAGAT | #56 |
|
|
| R:
GTGGTACGACCAGAGGCATAC |
|
Colonic cell lines and cell
culture
In the present study, one normal colonic cell line
[CCD-18Co, American Type Culture Collection (ATCC) CRL-1459], three
early-stage CRC cell lines (SW1116, ATCC CCL-233; LS123, ATCC
CCL-255; and SW480, ATCC CCL-228; ATCC, Manassas, VA, USA), and
three late-stage CRC cell lines (SW620, ATCC CCL-227; Caco-2, ATCC
HTB-37; and T84, ATCC CCL-248; ATCC) were used (39,40). In
addition, one metastatic CRC cell line [CC-M3, Bioresource
Colletion and Research Center (BCRC) 60450] was purchased from BCRC
(Hsinchu, Taiwan) (41). With the
exception of SW1116, SW480 and SW620, which were cultured in
Leibovitz's L-15 Medium in a non-CO2 incubator, other cells were
cultured at 37°C in a humidified 5% CO2 incubator with the medium
recommended by ATCC, such as Eagle's minimum essential medium or
Dulbecco's modified Eagle's medium. All culture medium contained
fetal bovine serum to a final concentration of 10%.
Extraction of total cellular RNA and
reverse transcription
Total cellular RNA was extracted from cultured cells
using TRIzol reagent (Thermo Fisher Scientific, Inc., Waltham, MA,
USA) according to the manufacturer's protocol. Subsequently, 1 µg
of total RNA was reverse transcribed into single-stranded cDNA
using 0.5 µg of oligo(dT) primer and a High Capacity cDNA Reverse
Transcription kit (Applied Biosystems; Thermo Fisher Scientific,
Inc.).
Quantification of the mRNA levels of
target genes in cells
The genes of interest were quantified in CCD-18Co
cells or in the different CRC cell lines using the
TaqMan® qPCR approach, as aforementioned. The
amplification primers and TaqMan® probes from the
Universal ProbeLibrary Set, Human (Roche Diagnostics GmbH) used are
listed in Table II. To avoid errors
caused by sample-to-sample differences in RNA quantity, the
normalization of each gene was performed using the level of
glyceraldehyde 3-phosphate dehydrogenase (GAPDH, NM_002046).
LightCycler 4.05 Software (Roche Diagnostics GmbH) was used to
analyze the PCR kinetics.
 | Table II.Primers and TaqMan® probes
for quantifying the messenger RNA levels of target genes. |
Table II.
Primers and TaqMan® probes
for quantifying the messenger RNA levels of target genes.
| Gene (symbol) | Accession no. | Primers
(5′-3′) | UPL no. |
|---|
| Solute carrier
family 15, member 4 (SLC15A4) | NM_145648 | F:
GAGCAGTCACACAGACTTTGGT | #71 |
|
|
| R:
CAGGAGGGTAGCTCCTTGAA |
|
| Cluster of
differentiation 44 | NM_001202555 | F:
CAAGCAGGAAGAAGGATGGAT | #41 |
| (CD44) |
| R:
AACCTGTGTTTGGATTTGCAG |
|
| 3-oxoacid
CoA-transferase 1 | NM_000436 | F:
ACTGGGTGTGATTTTGCAGTT | #84 |
| (OXCT1) |
| R:
GCAGCCTGGTACAAATATCCA |
|
| Placenta-specific
8 | NM_016619 | F:
CGTCGCAATGAGGACTCTCT | #56 |
| (PLAC8) |
| R:
CTCTTGATTTGGCAAAGAGTACAA |
|
| Growth
arrest-specific 2 | NM_005256 | F:
TGGGAGAAAAGATCCTCTTCATT | #75 |
| (GAS2) |
| R:
TCAACAAATACCCTGCAAAAGTT |
|
| Glyceraldehyde
3-phosphate dehydrogenase (GAPDH) | NM_002046 | F:
CTCTGCTCCTCCTGTTCGAC | #60 |
|
|
| R:
ACGACCAAATCCGTTGACTC |
|
Statistical analysis
Gene expressions of two groups were analyzed for
significance using the Student's t-test. The calculations were made
with SPSS software (v.16.0; SPSS, Inc. Chicago, IL, USA). P<0.05
was considered to indicate a statistically significant
difference.
Results
Feasibility of total fecal RNA as a
marker of CRC and custom-made microarrays for CRC patients
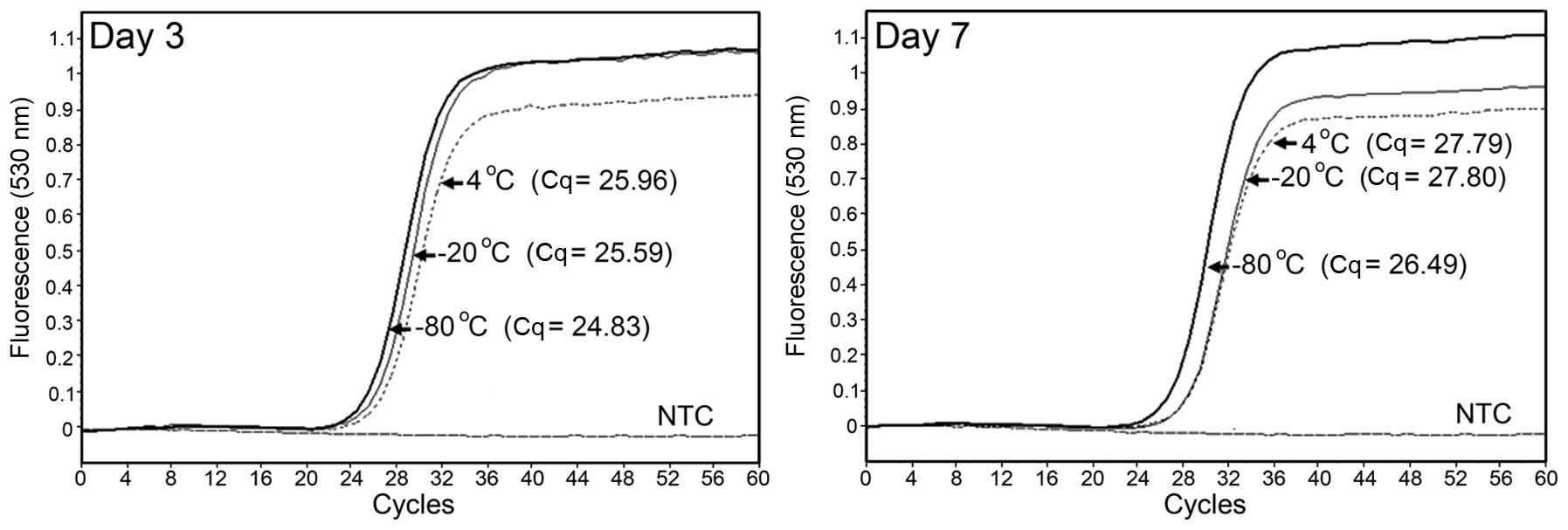
As indicated in Fig.
1, three Cq values (25.96 at 4°C, 25.59 at −20°C and 24.83 at
−80°C) after a 3-day storage were not remarkably different to those
detected at day 0 (25.61). Similar results were obtained after a
7-day storage; however, the difference was slightly larger. By
applying this technique of fecal RNA purification, numerous genes
that were expressed differentially in the feces of CRC patients
were identified, as assessed by analysis of whole-genome
oligonucleotide microarrays (30). As
summarized in Table III, genes with
a significantly differential expression (>2-fold, P<0.05)
were selected from the feces of a group of 16 subjects that
consisted of 5 noncancerous individuals and 11 CRC patients at
different American Joint Committee on Cancer (AJCC) stages (2 at
AJCC stage I, 3 at stage II, 3 at stage III and 3 at stage IV).
 | Table III.Genes with potentially clinical
significance in feces of CRC patients. |
Table III.
Genes with potentially clinical
significance in feces of CRC patients.
| Comparison | Number of
genesa | Representative
gene | Used cell
lines |
|---|
| Normalb vs. CRC | 180 | Solute carrier
family 15, member 4 | CCD-18Co, SW1116,
LS123, Caco-2 and T84 |
| Normal vs. AJCC
stage I | 167 | Cluster of
differentiation 44 | CCD-18Co and
SW1116 |
| Non-metastasis vs.
metastasis | 9 | 3-oxoacid
CoA-transferase 1 | SW480 and
CC-M3 |
| Non-recurrence vs.
recurrence | 22 | Placenta-specific 8
and growth arrest-specific 2 | SW480 and
SW620 |
Validation of genes that were
differentially expressed in CRC cell lines
Partial genes that were highly expressed in the
feces of CRC patients were verified using CRC cell lines (Figs. 2–5 and
Table III). For example, solute
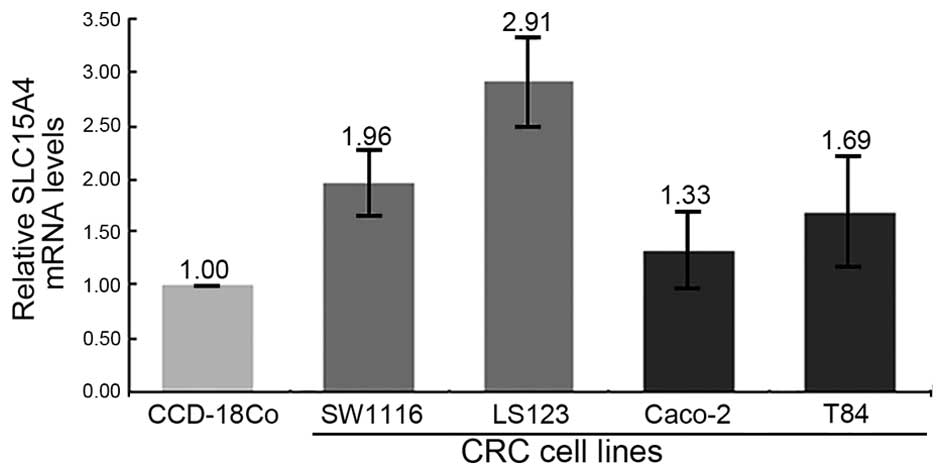
carrier family 15, member 4 (SLC15A4, NM_145648) was upregulated in
the majority of feces of CRC patients. The expression of SLC15A4
was also increased in four CRC cell lines (SW1116, LS123, Caco-2
and T84) at different AJCC stages compared with the normal colonic
cell line CCD-18Co (Fig. 2).
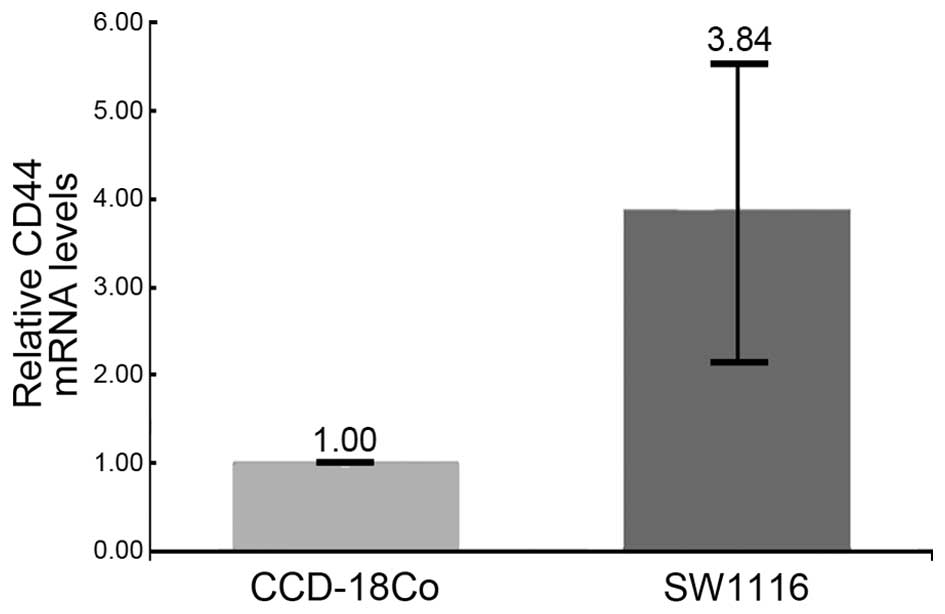
To identify the initial stages of CRC, 167 genes
that exhibited differential expression in early-stage CRC or in CRC
patients without symptoms were selected. Among these 167 genes, a
cell-surface molecule, cluster of differentiation (CD)44
(NM_001202555) was upregulated (42)
in the SW1116 cell line (3.84-fold), which was previously diagnosed
as AJCC stage I (Fig. 3). To date,
the majority of the genetic research conducted on metastatic or
recurrent CRC is confined to a single molecule (43–45). It is
known that distant metastasis is the major cause of mortality in
CRC patients (46). However, few
studies have investigated the profiles of genetic variation in
these CRC patients. Thus, fecal total RNA was used to distinguish
metastatic or recurrent CRC patients from other CRC patients in the
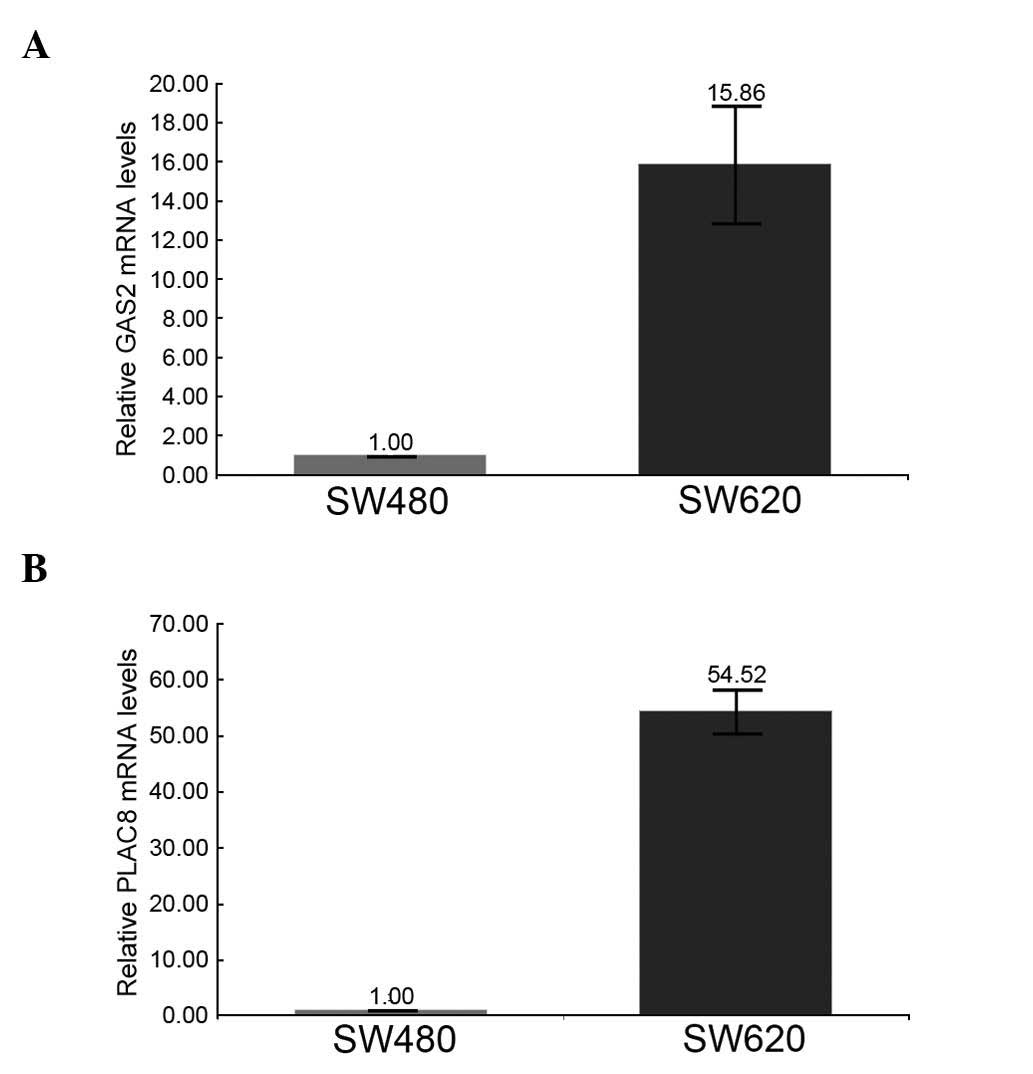
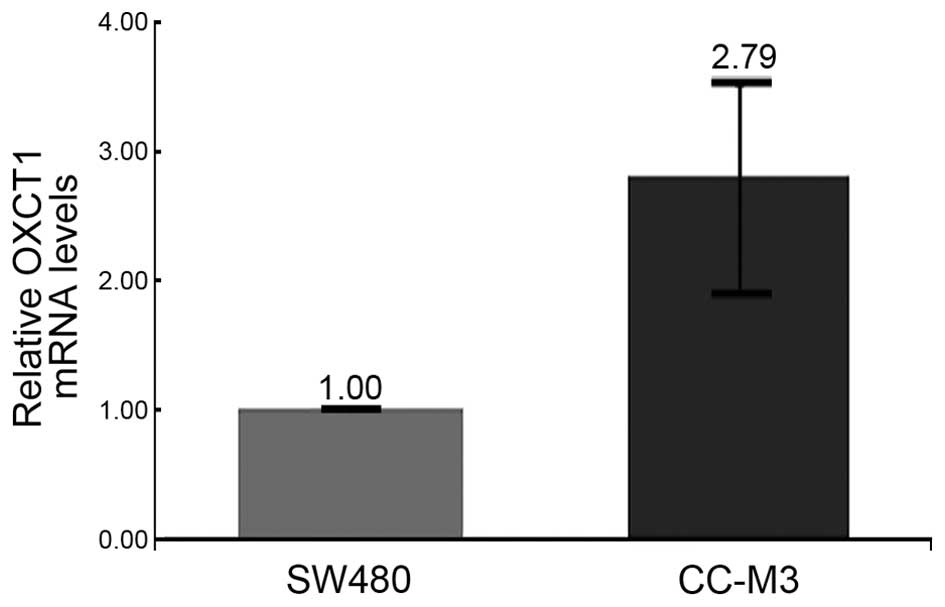
present study. The results revealed that one gene involved in
extrahepatic ketone-body catabolism, 3-oxoacid CoA-transferase 1
(OXCT1, NM_000436), was expressed at higher levels (2.79-fold) in
the metastatic CRC cell line CC-M3 than in the non-metastatic cell
line SW480 (Fig. 4). In addition, two
genes, growth arrest-specific 2 (GAS2, NM_005256) and
placenta-specific 8 (PLAC8, NM_016619), which are involved in
recurrent CRC, were detected in the SW620 cell line, which is the
lymph-node metastatic derivative of the SW480 cell line (47,48). As
indicated in Fig. 5, both GAS2
(15.86-fold) and PLAC8 (54.52-fold) were upregulated in the SW620
cell line compared with the SW480 cell line.
Discussion
The differentiation of CRC patients from non-cancer
individuals or CRC patients with different clinical characteristics
is crucial in CRC treatment. However, the current staging system
used for CRC, which is based on the tumor-node-metastasis
classification, does not yield a reliable personalized prediction
of prognosis (49). This can be
improved by employing molecular parameters in addition to the
staging system (50).
In recent years, human feces have been used as
research material in CRC (51). Both
fecal DNA and RNA are known to represent CRC-related molecular
targets (51–53). Our previous studies also reported
various molecules that are differentially expressed in human feces
(30). Significant gene profiles were
acquired computationally by comparing different group settings
according to clinical characteristics. In the present study, the
feasibility of total fecal RNA as a marker of CRC was first
verified using exogenous mouse cells. Subsequently, different CRC
cell lines were used to validate the differentially expressed genes
in feces. The significant molecules detected in CRC cell lines may
provide novel insights into colorectal carcinogenesis and
personalized prediction in a non-invasive manner using human
feces.
For example, upregulation of SLC15A4 was detected in
the feces of CRC patients and in CRC cell lines at different stages
(AJCC stages I–IV). Expression of SLC15A4, a histidine transporter,
was previously observed in the gastrointestinal tract (54). This histidine transporter coordinates
mechanistic target of rapamycin-dependent inflammatory responses
and may promote colitis (55,56). In the present study, two early-stage
CRC cell lines, SW1116 and LS123, exhibited a higher expression
level of SLC15A4 than normal colonic cells and late-stage CRC cell
lines. Furthermore, an anti-inflammatory function may contribute to
antitumor activity (57). Thus,
SLC15A4 may participate in the initial inflammation-induced
colorectal dysplasia. This result may be associated with another
marker, CD44, which was detected in the feces of CRC patients at
AJCC stage I. CD44 is of functional importance for tumor initiation
and progression in CRC (58). In
another animal study, downregulated CD44 was able to reduce tumor
growth significantly (59). Recently,
CD44 was further proposed to contribute to targeted therapeutic
strategies due to its role in sensing the extracellular environment
(60). These findings are in
agreement with the results obtained in the present study for the
fecal samples of early-stage cancer groups and for SW1116 cells.
Taken together, our findings revealed that the detection of SLC15A4
and CD44 in feces may aid to identify the initial CRC cells.
Another gene that was identified in the feces of CRC patients at
AJCC stage IV was OXCT1 (61–63). Increased expression of OXCT1 has been
observed in numerous human cancers. As detected in the present
study in metastatic CRC, a substantially elevated level of
expression of OXCT1 has been reported in association with
metastatic cancers (62,63), which suggests that OXCT1 may be a
potential marker of late-stage CRC and can be detected in
feces.
In fact, the two genes involved in CRC recurrence
described in the current study were also reported in other human
cancers. For example, GAS2 was expressed at a high level in chronic
myeloid leukemia cells, and the inhibition of GAS2 impaired tumor
growth. PLAC8 was also upregulated in other human leukemic cells
and induced apoptosis resistance (64). In addition, PLAC8 overexpression was
further linked to intestinal stem cells in CRC (65). Our current results appear to agree
with these reports due to the high expression levels of GAS2 and
PLAC8 detected in the feces of relapsed CRC patients and in the
recurrent CRC cell line SW620.
Genes involved in CRC tumorigenesis or with
uncharacterized functions may be potential markers that could aid
in CRC detection, diagnosis, treatment or prognostic prediction
(66). However, upregulated genes
were frequently observed during the process of CRC tumorigenesis
(67). In the present study, CRC cell
lines were used to validate the genes that were significantly
upregulated in the feces of CRC patients. Our results suggest that
CRC cell lines can respond to differential gene expression in
feces. Thus, the present study focused on detecting fecal RNA in
association with tumor initiation, recurrence and liver metastasis
in CRC. Clinically, pathological factors, alone or in combination,
cannot perfectly identify CRC patients or make a personalized
prediction of recurrence (49,68). The
molecules involved in CRC pathogenesis may act as markers of early
CRC diagnosis or may be used to stratify susceptible patients into
appropriate screening or surveillance programs (69). In other words, the genetic
understanding of CRC has led to the introduction of molecular
proposals that exemplify the knowledge translated from basic
science to clinical care (10). The
possible clinical application of non-invasive molecules provides a
useful platform in molecular medicine and translational research.
Genes expressed in the feces of CRC patients varied in the present
study according to the clinical characteristics of the individuals,
and these differential expression levels also arose in the
corresponding CRC cell lines. In conclusion, feces represent a good
marker of CRC and can be interpreted using the appropriate CRC cell
lines.
Acknowledgements
The current study was supported by grants (grant
numbers CGH-MR-10119 and CGH MR-A10218) from Cathay General
Hospital (Taipei, Taiwan).
Glossary
Abbreviations
Abbreviations:
|
CRC
|
colorectal cancer
|
|
SLC15A4
|
solute carrier family 15, member 4
|
|
CD44
|
cluster of differentiation 44
|
|
OXCT1
|
3-oxoacid CoA-transferase 1
|
|
PLAC8
|
placenta-specific 8
|
|
GAS2
|
growth arrest-specific 2
|
|
Cq
|
quantification cycle
|
|
GAPDH
|
glyceraldehyde 3-phosphate
dehydrogenase
|
References
|
1
|
Vogelstein B, Fearon ER, Hamilton SR, Kern
SE, Preisinger AC, Leppert M, Nakamura Y, White R, Smits AM and Bos
JL: Genetic alterations during colorectal-tumor development. J Engl
Med. 319:525–532. 1988. View Article : Google Scholar
|
|
2
|
Jass JR: Colorectal cancer: A multipathway
disease. Crit Rev Oncog. 12:273–287. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kong YW, Ferland-McCollough D, Jackson TJ
and Bushell M: microRNAs in cancer management. Lancet Oncol.
13:e249–e258. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
You JS and Jones PA: Cancer genetics and
epigenetics: Two sides of the same coin? Cancer Cell. 22:9–20.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Center MM, Jemal A, Smith RA and Ward E:
Worldwide variations in colorectal cancer. CA Cancer J Clin.
59:366–378. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chun P and Wainberg ZA: Adjuvant
Chemotherapy for Stage II Colon Cancer: The role of molecular
markers in choosing therapy. Gastrointest Cancer Res. 3:191–196.
2009.PubMed/NCBI
|
|
7
|
Matsuyama T, Ishikawa T, Mogushi K,
Yoshida T, Iida S, Uetake H, Mizushima H, Tanaka H and Sugihara K:
MUC12 mRNA expression is an independent marker of prognosis in
stage II and stage III colorectal cancer. Int J Cancer.
127:2292–2299. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kimura Y, Sumiyoshi M and Baba K:
Antitumor activities of synthetic and natural stilbenes through
antiangiogenic action. Cancer Sci. 99:2083–2096. 2008.PubMed/NCBI
|
|
9
|
Watine JC and Bunting PS: Mass colorectal
cancer screening: Methodological quality of practice guidelines is
not related to their content validity. Clinical Biochem.
41:459–466. 2008. View Article : Google Scholar
|
|
10
|
Soreide K, Berg M, Skudal BS and Nedreboe
BS: Advances in the understanding and treatment of colorectal
cancer. Discov Med. 12:393–404. 2011.PubMed/NCBI
|
|
11
|
Sinicrope FA, Shi Q, Smyrk TC, et al:
Molecular markers identify subtypes of stage III colon cancer
associated with patient outcomes. Gastroenterology. 148:88–99.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Matsuda T, Chiu HM, Sano Y, Fujii T, Ono A
and Saito Y: Surveillance colonoscopy after endoscopic treatment
for colorectal neoplasia: From the standpoint of the Asia-Pacific
region. Digestive Endoscopy. 28:342–347. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Blázquez C, Geelen MJ, Velasco G and
Guzmán M: The AMP-activated protein kinase prevents ceramide
synthesis de novo and apoptosis in astrocytes. FEBS Lett.
489:149–153. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Belletti B, Nicoloso MS, Schiappacassi M,
Chimienti E, Berton S, Lovat F, Colombatti A and Baldassarre G:
p27(kip1) functional regulation in human cancer: A potential target
for therapeutic designs. Curr Med Chem. 12:1589–1605. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang SH, Lin JK, Lai CR, Chen CC, Li AF,
Liang WY and Jiang JK: Risk factors for peritoneal dissemination of
colorectal cancer. J Surg Oncol. 87:167–173. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Huang CJ, Yang SH, Lee CL, Cheng YC, Tai
SY and Chien CC: Ribosomal protein S27-like in colorectal cancer: A
candidate for predicting prognoses. PLoS One. 8:e670432013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Imperiale TF, Ransohoff DF, Itzkowitz SH,
Levin TR, Lavin P, Lidgard GP, Ahlquist DA and Berger BM:
Multitarget stool DNA testing for colorectal-cancer screening. N
Engl J Med. 370:1287–1297. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ng JM and Yu J: Promoter hypermethylation
of tumour suppressor genes as potential biomarkers in colorectal
cancer. Int J Mol Sci. 16:2472–2496. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Giusti L, Iacconi P, Da Valle Y, Ciregia
F, Ventroni T, Donadio E, Giannaccini G, Chiarugi M, Torregrossa L,
Proietti A, et al: A proteomic profile of washing fluid from the
colorectal tract to search for potential biomarkers of colon
cancer. Mol Biosyst. 8:1088–1099. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Young GP and Bosch LJ: Fecal tests: From
blood to molecular markers. Curr Colorectal Cancer Rep. 7:62–70.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Huang CJ, Chien CC, Yang SH, Chang CC, Sun
HL, Cheng YC, Liu CC, Lin SC and Lin CM: Faecal ribosomal protein
L19 is a genetic prognostic factor for survival in colorectal
cancer. J Cell Mol Med. 12:1936–1943. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Takai T, Kanaoka S, Yoshida K, Hamaya Y,
Ikuma M, Miura N, Sugimura H, Kajimura M and Hishida A: Fecal
cyclooxygenase 2 plus matrix metalloproteinase 7 mRNA assays as a
marker for colorectal cancer screening. Cancer Epidemiol Biomarkers
Prev. 18:1888–1893. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hamaya Y, Yoshida K, Takai T, Ikuma M,
Hishida A and Kanaoka S: Factors that contribute to faecal
cyclooxygenase-2 mRNA expression in subjects with colorectal
cancer. Br J Cancer. 102:916–921. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yang SH, Huang CJ, Lee CL, Liu CC, Chien
CC and Chen SH: Fecal RNA detection of cytokeratin 19 and ribosomal
protein L19 for colorectal cancer. Hepatogastroenterology.
57:710–715. 2010.PubMed/NCBI
|
|
25
|
Steele RJ, Kostourou I, McClements P,
Watling C, Libby G, Weller D, Brewster DH, Black R, Carey FA and
Fraser C: Effect of repeated invitations on uptake of colorectal
cancer screening using faecal occult blood testing: Analysis of
prevalence and incidence screening. BMJ. 341:c55312010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tonus C, Sellinger M, Koss K and Neupert
G: Faecal pyruvate kinase isoenzyme type M2 for colorectal cancer
screening: A meta-analysis. World J Gastroenterol. 18:4004–4011.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Raspe E, Decraene C and Berx G: Gene
expression profiling to dissect the complexity of cancer biology:
Pitfalls and promise. Semin Cancer Biol. 22:250–260. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bernal G: Use of RNA isolated from feces
as a promising tool for the early detection of colorectal cancer.
Int J Biol Markers. 27:e82–e89. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shi M, Beauchamp RD and Zhang B: A
network-based gene expression signature informs prognosis and
treatment for colorectal cancer patients. PLoS One. 7:e412922012.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chien CC, Chang CC, Yang SH, Chen SH, et
al: A homologue of the drosophila headcase protein is a novel tumor
marker for early-stage colorectal cancer. Oncol Rep. 15:919–926.
2006.PubMed/NCBI
|
|
31
|
Yang SH, Chien CC, Chen CW, Li SY and
Huang CJ: Potential of faecal RNA in diagnosing colorectal cancer.
Cancer Lett. 226:55–63. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chang CC, Yang SH, Chien CC, Chen SH, Pan
S, Lee CL, Lin CM, Sun HL, Huang CC, Wu YY, et al: Clinical meaning
of age-related expression of fecal cytokeratin 19 in colorectal
malignancy. BMC cancer. 9:3762009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kato I, Badsha KZ, Land S, Nechvatal JM,
Matherly LH, Tarca AL, Majumdar AP, Basson MD and Ram JL: DNA/RNA
markers for colorectal cancer risk in preserved stool specimens: A
pilot study. Tumori. 95:753–761. 2009.PubMed/NCBI
|
|
34
|
Yang RN, Yang SH, Chang CC, Chien CC, Pan
S and Huang CJ: Upregulation of fecal cytokeratin 19 is associated
with prognosis in older colorectal cancer patients. Genet Test Mol
Biomarkers. 14:703–708. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liebig C, Agarwal N, Ayala GE, Verstovsek
G, Tuszynski GP and Albo D: Angiocidin inhibitory peptides decrease
tumor burden in a murine colon cancer model. J Surg Res.
142:320–326. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wu CY, Lee WH, Wang JY, Chiang H, Chang
JL, Tsai WC, Sheu LF and Jin JS: Tissue microarray-determined
expression profiles of cyclooxygenase-2 in colorectal
adenocarcinoma: Association with clinicopathological parameters.
Chin J Physiol. 49:298–304. 2006.PubMed/NCBI
|
|
37
|
Altangerel O, Cao S, Meng J, et al:
Chronic neutrophilic leukemia with overexpression of EVI-1, and
concurrent CSF3R and SETBP1 mutations: A case report. Oncology
letters. 10:1694–1700. 2015.PubMed/NCBI
|
|
38
|
Hellemans J, Mortier G, De Paepe A,
Speleman F and Vandesompele J: qBase relative quantification
framework and software for management and automated analysis of
real-time quantitative PCR data. Genome biology. 8:R192007.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Tien LT, Chien CC, Yang SH, Lin CM, Wu YY
and Huang CJ: p53-Dependent Expression of Ribosomal Protein
S27-Like in Colorectal Cancer. Fu Jen J Med. 8:11–17. 2010.
|
|
40
|
Christensen J, El-Gebali S, Natoli M,
Sengstag T, Delorenzi M, Bentz S, Bouzourene H, Rumbo M, Felsani A,
Siissalo S, et al: Defining new criteria for selection of
cell-based intestinal models using publicly available databases.
BMC genomics. 13:2742012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wu CC, Tsai FM, Shyu RY, Tsai YM, Wang CH
and Jiang SY: G protein-coupled receptor kinase 5 mediates
Tazarotene-induced gene 1-induced growth suppression of human colon
cancer cells. BMC cancer. 11:1752011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Godar S, Ince TA, Bell GW, Feldser D,
Donaher JL, Bergh J, Liu A, Miu K, Watnick RS, Reinhardt F, et al:
Growth-inhibitory and tumor-suppressive functions of p53 depend on
its repression of CD44 expression. Cell. 134:62–73. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Elzagheid A, Emaetig F, Buhmeida A, Laato
M, El-Faitori O, Syrjänen K, Collan Y and Pyrhönen S: Loss of MUC2
expression predicts disease recurrence and poor outcome in
colorectal carcinoma. Tumour Biol. 34:621–628. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Herszenyi L, Hritz I, Lakatos G, Varga MZ
and Tulassay Z: The behavior of matrix metalloproteinases and their
inhibitors in colorectal cancer. Int J Mol Sci. 13:13240–13263.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Lu Y, Jingyan G, Baorong S, Peng J, Xu Y
and Cai S: Expression of EGFR, Her2 predict lymph node metastasis
(LNM)-associated metastasis in colorectal cancer. Cancer Biomark.
11:219–226. 2012.PubMed/NCBI
|
|
46
|
Hur K, Toiyama Y, Schetter AJ, et al:
Identification of a metastasis-specific MicroRNA signature in human
colorectal cancer. J Nat Can Ins. 107:2015.
|
|
47
|
Bordonaro M and Lazarova DL: Determination
of the Role of CBP- and p300-Mediated Wnt Signaling on Colonic
Cells. JMIR Res Prot. 5:e662016. View Article : Google Scholar
|
|
48
|
Huang CJ, Lee CL, Yang SH, et al:
Upregulation of the growth arrest-specific-2 in recurrent
colorectal cancers, and its susceptibility to chemotherapy in a
model cell system. Biochim Biophys Acta. 1862:1345–1353. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Miyake M, Takemasa I, Matoba R, Tanino M,
Niijima S, Ikeda M, Yamamoto H, Sekimoto M, Kuhara S, Okayama T, et
al: Heterogeneity of colorectal cancers and extraction of
discriminator gene signatures for personalized prediction of
prognosis. Int J Oncol. 39:781–789. 2011.PubMed/NCBI
|
|
50
|
Sudoyo AW: Biomolecular markers as
determinants of patients selection for adjuvant chemotherapy of
sporadic colorectal cancers. Acta Med Indones. 42:45–50.
2010.PubMed/NCBI
|
|
51
|
Carroll MR, Seaman HE and Halloran SP:
Tests and investigations for colorectal cancer screening. Clinical
Biochem. 47:921–939. 2014. View Article : Google Scholar
|
|
52
|
Pox C: Colon cancer screening: Which
non-invasive filter tests? Dig Dis. 1:(Suppl 1). S56–S59. 2011.
View Article : Google Scholar
|
|
53
|
Miller S and Steele S: Novel molecular
screening approaches in colorectal cancer. J Surg Oncol.
105:459–467. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Bhardwaj RK, Herrera-Ruiz D, Eltoukhy N,
Saad M and Knipp GT: The functional evaluation of human
peptide/histidine transporter 1 (hPHT1) in transiently transfected
COS-7 cells. Eur J Pharm Sci. 27:533–542. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Kobayashi T, Shimabukuro-Demoto S,
Yoshida-Sugitani R, Furuyama-Tanaka K, Karyu H, et al: The
histidine transporter SLC15A4 coordinates mTOR-dependent
inflammatory responses and pathogenic antibody production.
Immunity. 41:375–388. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Sasawatari S, Okamura T, Kasumi E,
Tanaka-Furuyama K, Yanobu-Takanashi R, Shirasawa S, Kato N and
Toyama-Sorimachi N: The solute carrier family 15A4 regulates TLR9
and NOD1 functions in the innate immune system and promotes colitis
in mice. Gastroenterology. 140:1513–1525. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Ye Q, Zheng Y, Fan S, Qin Z, Li N, Tang A,
Ai F, Zhang X, Bian Y, Dang W, et al: Lactoferrin deficiency
promotes colitis-associated colorectal dysplasia in mice. PLoS One.
9:e1032982014. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Rao G, Wang H, Li B, Huang L, Xue D, et
al: Reciprocal interactions between tumor-associated macrophages
and CD44 positive cancer cells via osteopontin/CD44 promote
tumorigenicity in colorectal cancer. Clin Cancer Res. 19:785–797.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Perez A, Neskey DM, Wen J, Pereira L,
Reategui EP, Goodwin WJ, Carraway KL and Franzmann EJ: CD44
interacts with EGFR and promotes head and neck squamous cell
carcinoma initiation and progression. Oral Oncol. 49:306–313. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Yu S, Cai X, Wu C, Wu L, Wang Y, Liu Y, et
al: Adhesion glycoprotein CD44 functions as an upstream regulator
of a network connecting ERK, AKT and Hippo-YAP pathways in cancer
progression. Oncotarget. 6:2951–2965. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Sawai M, Yashiro M, Nishiguchi Y, Ohira M
and Hirakawa K: Growth-inhibitory effects of the ketone body,
monoacetoacetin, on human gastric cancer cells with succinyl-CoA:
3-oxoacid CoA-transferase (SCOT) deficiency. Anticancer Res.
24:2213–2217. 2004.PubMed/NCBI
|
|
62
|
Martinez-Outschoorn UE, Lin Z,
Whitaker-Menezes D, Howell A, Sotgia F and Lisanti MP: Ketone body
utilization drives tumor growth and metastasis. Cell cycle.
11:3964–3971. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Saraon P, Cretu D, Musrap N, Karagiannis
GS, Batruch I, Drabovich AP, van der Kwast T, Mizokami A, Morrissey
C, Jarvi K and Diamandis EP: Quantitative proteomics reveals that
enzymes of the ketogenic pathway are associated with prostate
cancer progression. Mol Cell Proteomics. 12:1589–1601. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Mourtada-Maarabouni M, Watson D, Munir M,
Farzaneh F and Williams GT: Apoptosis suppression by candidate
oncogene PLAC8 is reversed in other cell types. Curr Cancer Drug
Targets. 13:80–91. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Li C, Ma H, Wang Y, Cao Z, Graves-Deal R,
Powell AE, Starchenko A, Ayers GD, Washington MK, Kamath V, et al:
Excess PLAC8 promotes an unconventional ERK2-dependent EMT in colon
cancer. J Clin Invest. 124:2172–2187. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Coghlin C and Murray GI: Biomarkers of
colorectal cancer: Recent advances and future challenges.
Proteomics Clin Appl. 9:64–71. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Okuno K, Yasutomi M, Nishimura N, Arakawa
T, Shiomi M, Hida J, Ueda K and Minami K: Gene expression analysis
in colorectal cancer using practical DNA array filter. Dis Colon
Rectum. 44:295–299. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Kim HJ, Yu MH, Kim H, Byun J and Lee C:
Noninvasive molecular biomarkers for the detection of colorectal
cancer. BMB Rep. 41:685–692. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Zoratto F, Rossi L, Verrico M, Papa A,
Basso E, Zullo A, Tomao L, Romiti A, Lo Russo G and Tomao S: Focus
on genetic and epigenetic events of colorectal cancer pathogenesis:
Implications for molecular diagnosis. Tumour Biol. 35:6195–6206.
2014. View Article : Google Scholar : PubMed/NCBI
|