Introduction
Neuroendocrine tumors (NETs) were previously
considered extremely rare lesions, which most commonly occur in the
gastroenteropancreatic system (1,2). However,
over the last 30 years, the incidence and prevalence of
gastrointestinal NETs have increased significantly due to increased
awareness and widespread use of gastrointestinal endoscopy
(3,4).
Gastroenteropancreatic NETs, which derive from the neuroendocrine
cell system and have widely divergent clinical presentations, are
relatively infrequent, constituting ~2% of all neoplasms, and are
typically indolent, slow-growing tumors (5,6).
Gastrointestinal NETs are increasing (7); in the USA, the prevalence and the
incidence of gastrointestinal NETs have been calculated to be
35/100,000 and 5/100,000, respectively (4), revealing a 7-fold increase in the last
35 years. This phenomenon may partly reflect the increased number
of diagnoses of benign and incidentally-identified lesions due to
the increased availability of advanced endoscopic and radiological
imaging (8–10). The overall 5-year survival rate for
patients with gastrointestinal NETs has improved by almost 20% in
the past 35 years (1,11,12).
Diagnosis of early-stage NETs remains difficult, and the majority
of patients exhibit locally advanced disease or distant metastasis
at diagnosis (4).
At present, an increasing number of gastrointestinal
NETs are diagnosed at an early stage, and thus may be treated with
local excision (13). According to
the National Comprehensive Cancer Network guidelines and American
Joint Committee on Cancer staging of gastrointestinal NETs
(14), T1 tumors that are limited to
the lamina propria or submucosa and present a diameter of <1 cm
(the diameter of colorectal and appendix ≤2 cm), are considered
appropriate for endoscopic resection (15,16).
However, previous studies have reported that patients with such
tumors have experienced lymphatic-vascular involvement and exhibit
the risk of recurrence and metastasis following endoscopic
resection (13). In the present
study, the clinical and pathological features of 78
gastrointestinal NET patients were analyzed to identify appropriate
methods for early diagnosis and treatment of this type of
tumors.
Materials and methods
Patients
The medical records of 78 patients with
histopathologically diagnosed gastrointestinal NETs that were
treated at The Second Affiliated Hospital of Zhejiang University
School of Medicine (Hangzhou, China) between January 2007 and March
2013 were retrospectively analyzed. The patient cohort included 42
(53.8%) male patients and 36 (46.2%) female patients with a mean
age at diagnosis of 58 years (range, 15–82 years) (Table I).
 | Table I.Characteristics of 78 patients with
gastrointestinal neuroendocrine tumors. |
Table I.
Characteristics of 78 patients with
gastrointestinal neuroendocrine tumors.
| Parameter | Patients, n (%) |
|---|
| Gender |
|
| Male | 42 (53.8) |
|
Female | 36 (46.2) |
| Mean age, years
(range) | 58 (15–82) |
| Primary tumor
site |
|
|
Esophagus | 11 (14.1) |
|
Duodenum | 6 (7.7) |
|
Stomach | 30 (38.5) |
|
Colon | 6 (7.7) |
|
Rectum | 23 (29.5) |
|
Appendix | 2 (2.6) |
| Distant
metastasis |
|
| pM0 | 54 (69.2) |
| pM1 | 24 (30.8) |
Patients were divided into two groups, namely, a
submucosal NETs group and a deeper invasion NETs group, according
to the depth of tumor invasion. The present study defined
submucosal NETs as tumors that were restricted to the lamina
propria or submucosal layers, with or without lymph node (LN) and
distant organ metastasis. Deeper invasion NETs were defined as
tumors that had infiltrated into the muscularis propria or deeper
structures, with or without LN and distant organ metastasis. The
pathological characteristics of the submucosal NETs group were
investigated according to the tumor diameter (≤5.0, 5.1–10.0 or
>10.0 mm). A minute tumor was defined as a tumor of ≤5.0 mm in
diameter.
The submucosal NETs group consisted of 24 patients
(11 males and 13 females) with a mean age of 57 years (range, 29–70
years). The tumors in this group penetrated the mucosa and were
restricted to the submucosa. The deeper invasion NETs group
consisted of 54 patients (31 males and 23 females) with a mean age
of 62 years (range, 15–82 years).
Macroscopic appearance of tumors
According to the Paris classification of superficial
neoplastic lesions (17,18), early-stage gastric cancer is
classified into three types: Slightly elevated type (type 0-I),
flat type (type 0-II) and concaved type (type 0-III). Endoscopy was
performed using the GIF-XQ240, GIF-H260, GIF-H260Z, CF-240I/L,
CF-H260AI and PCF-Q260AZI endoscopes (Olympus Corporation, Tokyo,
Japan) Based on the endoscopic characteristics of the NETs,
submucosal NETs were classified into two types: Elevated type (type
0-Is), which consists of elevating lesions such as submucosal
tumors, and elevated depressed type (type 0-Is+IIc), which consists
of an elevating lesion with a depressed lesion. Deeper invasion
NETs were endoscopically classified into four types: Borrmann's
type I, Borrmann's type II, Borrmann's type III type and Borrmann's
type IV (19).
NET growth pattern
Based on the tumor growth pattern, gastrointestinal
NETs were classified as exhibiting an expansive or infiltrative
pattern. Certain NETs with an infiltrative growth pattern exhibited
various invasion directions, including infiltration towards the
anterior and lateral sides of the tumors.
Histopathological classification
All specimens were dissected at a maximal section.
Hematoxylin (catalog no., 130608; Shanghai Boao Biotechnology Co.,
Ltd., Shanghai, China) and eosin (catalog no., 20130729; Shanghai
SSS Reagent Co., Ltd., Shanghai, China) staining and
immunohistochemical staining for synuclein-α (rabbit anti-human
polyclonal antibody; cat. no. ZM-0506; dilution, 1:300),
chromogranin A (EP38; rabbit anti-human polyclonal antibody; cat.
no. ZA-0507; dilution, 1:200), neuron-specific enolase (E27; mouse
anti-human polyclonal antibody; cat. no. ZM-0203; dilution, 1:200)
and Ki-67 (MIB1; mouse anti-human polyclonal antibody; cat. no.
ZM-0167; dilution, 1:300) (Beijing Zhongshan Golden Bridge
Biotechnology Co., Ltd., Beijing, China) were subsequently
performed using the One-Step Universal kit (containing endogenous
peroxidase blocking solution, HRP-Polymer and DAB Diluent; catalog
no., PV-8000; Beijing Zhongshan Golden Bridge Biotechnology Co.,
Ltd.). Antibodies were incubated for 32 min at 37°C. Stained
sections were examined using a Nikon ECLIPSE 50I microscope (Nikon
Corporation, Tokyo, Japan). According to the 2010 World Health
Organization International Classifications of Tumors (20), NETs were classified into four
subtypes: NET G1, NET G2, neuroendocrine carcinoma (large or small
cell carcinoma) and mixed adenoneuroendocrine carcinoma.
Statistical analysis
All statistical analyses were performed with SPSS
version 20.0 statistical software (IBM SPSS, Armonk, NY, USA). The
χ2 test was used to evaluate the associations between the depth of
invasion and clinicopathological variables of the patients and the
association between LN metastasis and clinicopathological
variables. Survival was estimated according to the Kaplan-Meier
product limit estimator, and differences observed among patient
subgroups were assessed by the log-rank test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Characteristics of gastrointestinal
NET patients
A total of 78 patients with gastrointestinal NETs
were included in the present study. The baseline characteristics of
the patients are presented in detail in Table I. The median age of the patients was
58 years old. Out of 78 patients, 42 (53.8%) were men and 36
(46.2%) were women. The primary tumor sites were the esophagus
(11/78; 14.1%), the duodenum (6/78; 7.7%), the stomach (30/78;
38.5%), the colon (6/78; 7.7%), the rectum (23/78; 29.5%) and the
appendix (2/78; 2.6%). In total, 24 patients (30.8%) possessed
distant metastasis, while the remaining 54 (69.2%) had no distant
metastasis.
Association between patients'
clinicopathological characteristics and depth of tumor
invasion
The association between the clinicopathological
characteristics of the patients and the depth of tumor invasion is
summarized in Table II.
 | Table II.Association between
clinicopathological characteristics and depth of tumor invasion in
78 gastrointestinal NET patients. |
Table II.
Association between
clinicopathological characteristics and depth of tumor invasion in
78 gastrointestinal NET patients.
| Parameter | Patients, n | Submucosal NETs, n
(%) | Deeper invasion NETs,
n (%) | P-value |
|---|
| Gender |
|
|
|
0.350 |
| Male | 42 | 11 (45.8) | 31 (57.4) |
|
|
Female | 36 | 13 (54.2) | 23 (42.6) |
|
| Age, years |
|
|
|
0.409 |
|
>30 | 6 | 1 (4.2) | 5 (9.3) |
|
|
31–60 | 33 | 14 (58.3) | 19 (35.2) |
|
|
>60 | 39 | 9 (37.5) | 30 (55.6) |
|
| Tumor location |
|
|
|
0.026 |
|
Esophagus | 11 | 2 (8.3) | 9 (16.7) |
|
|
Duodenum | 6 | 2 (8.3) | 4 (7.4) |
|
|
Stomach | 30 | 6 (25.0) | 24 (44.4) |
|
|
Colon | 6 | 0 (0.0) | 6 (11.1) |
|
|
Rectum | 23 | 14 (58.3) | 9 (16.7) |
|
|
Appendix | 2 | 0 (0.0) | 2 (3.7) |
|
| Histopathological
classification |
|
|
| <0.001 |
| NET
G1 | 20 | 20 (83.3) | 0 (0.0) |
|
| NET
G2 | 29 | 3 (12.5) | 26 (48.1) |
|
|
NEC | 9 | 0 (0.0) | 9 (16.7) |
|
|
MANEC | 20 | 1 (4.2) | 19 (35.2) |
|
| Tumor diameter,
mm |
|
|
| <0.001 |
|
≤10.0 | 20 | 20 (83.3) | 0 (0.0) |
|
|
>10.0 | 58 | 4 (16.7) | 54 (100.0) |
|
| Macroscopic
appearance |
|
|
| <0.001 |
| 0-Is
type | 18 | 18 (75.0) | 0 (0.0) |
|
|
0-Is+IIc type | 6 | 6 (25.0) | 0 (0.0) |
|
|
Bormann's I type | 18 | 0 (0.0) | 18 (33.3) |
|
|
Bormann's II type | 13 | 0 (0.0) | 13 (24.1) |
|
|
Bormann's I+II type | 11 | 0 (0.0) | 11 (20.4) |
|
|
Bormann's III type | 9 | 0 (0.0) | 9 (16.7) |
|
|
Bormann's IV type | 3 | 0 (0.0) | 3 (5.6) |
|
| Growth pattern |
|
|
| <0.001 |
|
Expansive | 9 | 9 (37.5) | 0 (0.0) |
|
|
Infiltrative | 69 | 15 (62.5) | 54 (100.0) |
|
| Lymphatic-vascular
involvement |
|
|
| <0.001 |
|
Absent | 20 | 15 (62.5) | 5 (9.3) |
|
|
Present | 58 | 9 (37.5) | 49 (90.7) |
|
| lymph node
metastasis |
|
|
| <0.001 |
|
Negative | 28 | 19 (79.2) | 9 (16.7) |
|
|
Positive | 50 | 5 (20.8) | 45 (83.3) |
|
| Distant
metastasis |
|
|
|
0.001 |
|
pM0 | 54 | 23 (95.8) | 31 (57.4) |
|
|
pM1 | 24 | 1 (4.2) | 23 (42.6) |
|
No significant differences were identified between
age (P=0.409) and gender groups (P=0.350) in terms of depth of
tumor invasion. However, a significant correlation was identified
between depth of tumor invasion at diagnosis and histopathological
classification (P<0.001), diameter of the tumor (P<0.001),
macroscopic appearance (P<0.001), growth pattern (P<0.001),
lymphatic-vascular involvement (P<0.001), LN metastasis
(P<0.001) and distant metastasis (P=0.001).
In the submucosal NETs group, 9 (37.5%) patients
exhibited an expansive growth pattern and 15 (62.5%) exhibited an
infiltrative growth pattern, of which 10 cases infiltrated towards
the anterior side of the tumor, 2 cases infiltrated towards the
lateral side of the tumor and 3 cases infiltrated towards the
anterior and lateral sides of the tumor. In the deeper invasion
NETs group, 54 (100.0%) cases exhibited an infiltrative growth
pattern.
Association between patients'
clinicopathological factors and LN metastasis in submucosal
gastrointestinal NETs
The association between different clinical and
pathological factors of submucosal NETs and the risk of LN
metastasis is presented in Table
III.
 | Table III.Association between
clinicopathological factors and LN metastasis in 24 patients with
submucosal NETs. |
Table III.
Association between
clinicopathological factors and LN metastasis in 24 patients with
submucosal NETs.
|
| LN metastasis |
|
|---|
|
|
|
|
|---|
| Parameter | Patients, n | Negative, n
(%) | Positive, n
(%) | P-value |
|---|
| Gender |
|
|
| 0.209 |
|
Male | 11 | 10 (52.6) | 1 (20.0) |
|
|
Female | 13 | 9 (47.4) | 4 (80.0) |
|
| Age, years |
|
|
| 0.774 |
|
<30 | 1 | 0 (0.0) | 1 (20.0) |
|
|
31–60 | 14 | 13 (68.4) | 1 (20.0) |
|
|
>60 | 9 | 6 (31.6) | 3 (60.0) |
|
| Tumor location |
|
|
| 0.231 |
|
Esophagus | 2 | 2 (10.5) | 0 (0.0) |
|
|
Duodenum | 2 | 2 (10.5) | 0 (0.0) |
|
|
Stomach | 6 | 5 (26.3) | 1 (20.0) |
|
|
Rectum | 14 | 10 (52.6) | 4 (80.0) |
|
| Histopathological
classification |
|
|
| <0.001 |
| NET
G1 | 20 | 19 (100.0) | 1 (20.0) |
|
| NET
G2 | 3 | 0 (0.0) | 3 (60.0) |
|
|
MANEC | 1 | 0 (0.0) | 1 (20.0) |
|
| Tumor diameter,
mm |
|
|
| 0.238 |
|
≤5.0 | 7 | 7 (36.8) | 0 (0.0) |
|
|
5.1–10.0 | 13 | 9 (47.4) | 4 (80.0) |
|
|
>10.0 | 4 | 3 (15.8) | 1 (20.0) |
|
| Macroscopic
appearance |
|
|
| 0.799 |
| 0-Is
type | 18 | 15 (78.9) | 3 (60.0) |
|
|
0-Is+IIc type | 6 | 4 (21.0) | 2 (40.0) |
|
| Growth pattern |
|
|
| 0.081 |
|
Expansive | 9 | 8 (42.1) | 1 (20.0) |
|
|
Infiltrative | 15 | 11 (57.9) | 4 (80.0) |
|
| Envelope |
|
|
| 0.607 |
|
Complete | 6 | 6 (31.6) | 0 (0.0) |
|
|
Incomplete | 11 | 7 (36.8) | 4 (80.0) |
|
|
N/A | 7 | 6 (31.6) | 1 (20.0) |
|
| Lymphatic-vascular
involvement |
|
|
| 0.027 |
|
Absent | 15 | 14 (73.7) | 1 (20.0) |
|
|
Present | 9 | 5 (26.3) | 4 (80.0) |
|
| Distant
metastasis |
|
|
| 0.049 |
|
pM0 | 23 | 19 (100.0) | 4 (80.0) |
|
|
pM1 | 1 | 0 (0.0) | 1 (20.0) |
|
A significant correlation was identified between
high-grade tumors with lymphatic or venule invasion or distant
metastasis and an increased risk of nodal metastases (P<0.001,
P=0.027 and P=0.049, respectively).
In the submucosal NETs group, 7 minute tumors (≤5.0
mm) exhibited an expansive growth pattern (0-Is type), with no
evidence of lymphatic-vascular involvement, LN or distant
metastasis. Tumors measuring 5.1–10.0 mm in diameter exhibited a
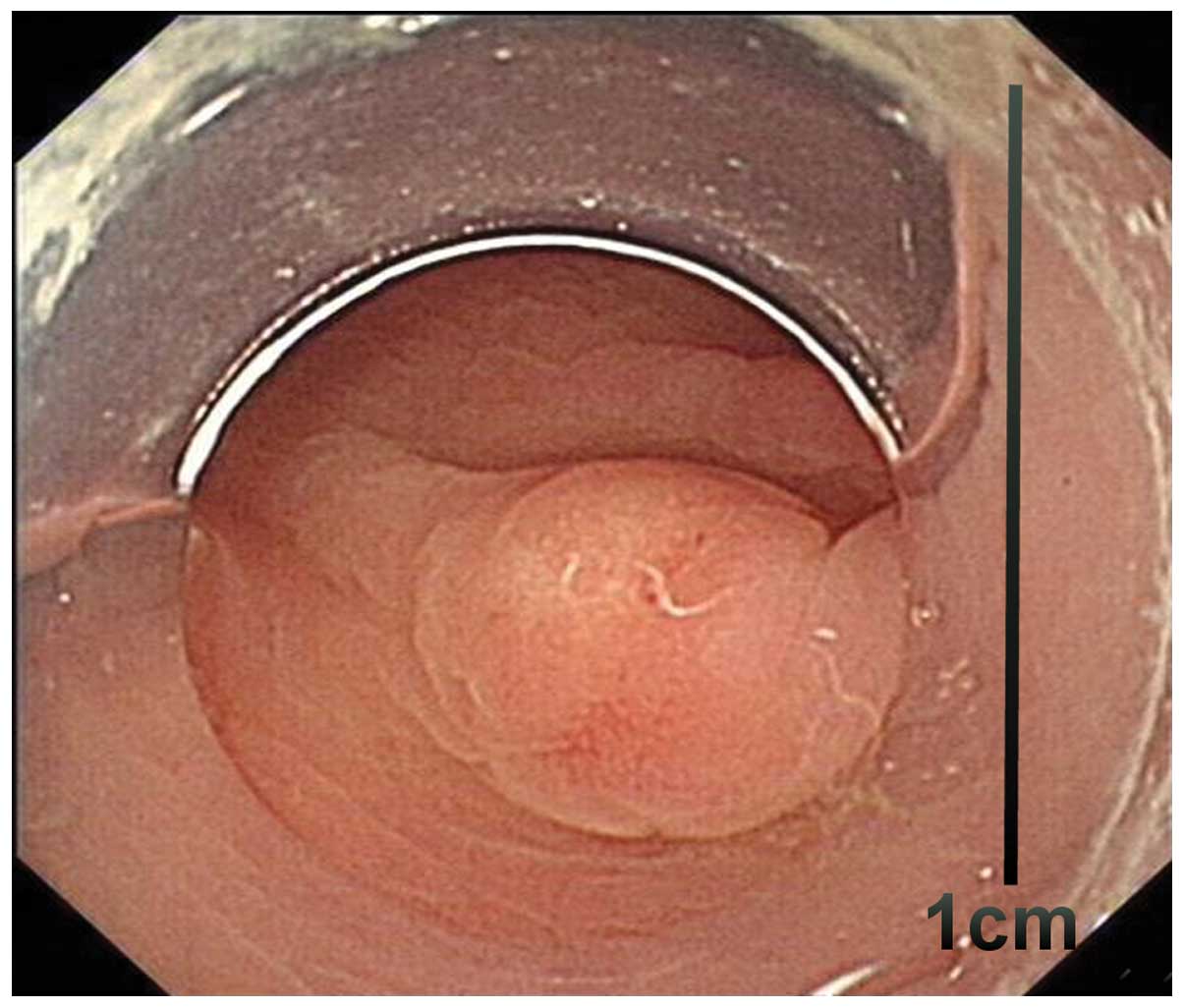
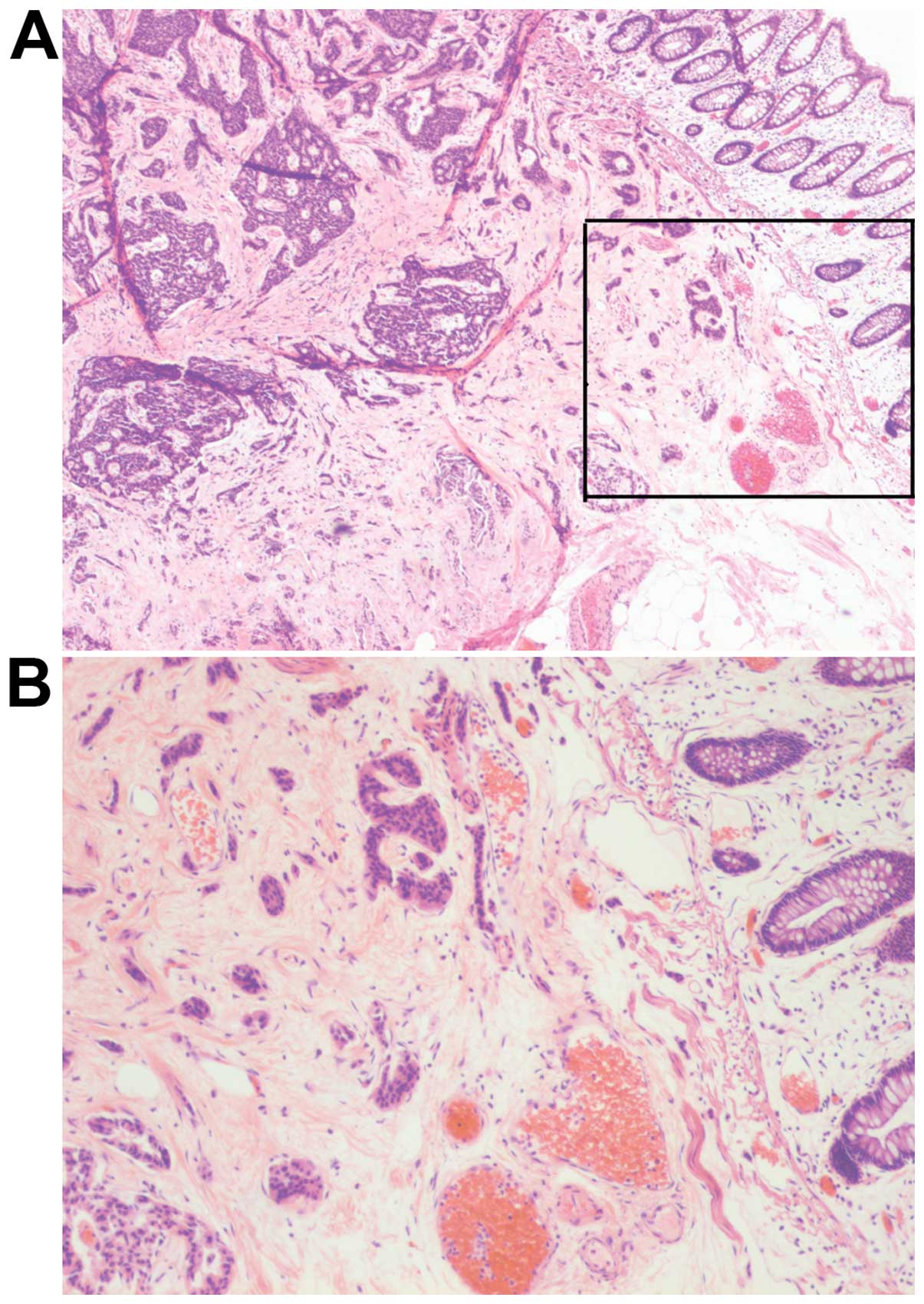
high rate of infiltrative growth (84.6%; 11/13) (Figs. 1 and 2),
lymphatic-vascular involvement (46.2%; 6/13), LN metastasis (30.8%;
4/13) and distant metastasis (7.7%; 1/13). Tumors measuring
>10.0 mm in diameter also exhibited a high rate of
lymphatic-vascular involvement (75.0%; 3/4) and LN metastasis
(25.0%; 1/4). No significant difference in LN metastasis was
identified between the three tumor diameter categories
(P=0.238).
Therapy
Of the 24 submucosal NETs, 1 case (4.2%) of liver
metastasis was identified in a patient who had undergone resection
of a primary tumor. In the other 23 cases, 14 cases (60.9%)
underwent endoscopic resection of the primary tumor and 5 cases
(21.7%) received surgery following endoscopic resection for
suspected positive margin or lymphatic-vascular involvement. The
other 9 cases (39.1%) underwent surgery.
In the deeper invasion NETs group, 23 (42.6%)
patients with distant metastasis and 31 (57.4%) patients without
distant metastasis underwent surgical resection of the primary
tumor. Among the 23 patients exhibiting distant metastasis, 14
underwent surgical resection of the primary tumor: 4 cases (17.4%)
underwent resection of the metastatic disease and 10 cases (43.5%)
received systemic therapy, which included somatostatin analogues
(Sandostatin LAR; 30 mg on day 1, every 28 days; Novartis
International AG, Basel, Switzerland) and chemotherapy (100 mg/m2
etoposide on days 1–3 and 25 mg/m2 cisplatin on days 1–3; every 21
days, for 4–6 cycles).
Follow-up
The mean follow-up period, post-surgery or
treatment, was 40 months. In total, 12 (15.4%) of the 78 patients
succumbed to the disease during the follow-up period. All patients
in the submucosal NETs group survived (follow-up time, 33 months).
No evidence of recurrence or metastasis was identified in 23
(95.8%) cases, whereas rectal NETs with liver metastasis was
diagnosed in 1 (4.2%) patient. This patient was a 29-year-old male,
with an 8-mm primary tumor (type 0-Is; NET G2), which exhibited
infiltrative growth, lymphatic-vascular involvement and was
LN-positive. The patient had undergone resection of the primary
tumor and no evidence of recurrence or disease progression was
identified during the follow-up period. In the submucosal NETs
group, the 3-year survival and disease-free survival rates were
100.0 and 95.8%, respectively. In the deeper invasion NETs group,
12 (22.2%) patients succumbed to the disease, and the 3-year
survival rate was 83.3%.
A significant correlation was identified between
submucosal NETs or lower grade tumors and prolonged overall
survival (P<0.001 and P=0.009, respectively; Table IV). No significant differences were
identified between age or gender and overall survival (P=0.276 and
P=0.089, respectively).
 | Table IV.Kaplan-Meier survival analysis of
gastrointestinal NETs patients with regard to clinicopathological
factors. |
Table IV.
Kaplan-Meier survival analysis of
gastrointestinal NETs patients with regard to clinicopathological
factors.
| Parameter | Patients, n | Median overall
survival, monthsa | 3-year survival
rate, %a | 95% CI | P-value |
|---|
| Gender |
|
|
|
|
0.276 |
|
Male | 42 | 80.4 | – | 70.2–90.6 |
|
|
Female | 36 | 73.5 | – | 60.1–87.0 |
|
| Age, years |
|
|
|
|
0.089 |
|
<30 | 6 | 61.8 | – | 36.6–87.1 |
|
|
31–60 | 33 | 88.9 | – | 79.4–98.4 |
|
|
>60 | 39 | 66.2 | – | 54.1–78.4 |
|
| Histopathological
classification |
|
|
|
| <0.001 |
| NET
G1 | 20 | – | 100.0 | – |
|
| NET
G2 | 29 | – | 100.0 | – |
|
|
NEC | 9 | – |
44.4 | – |
|
|
MANEC | 20 | – |
85.0 | – |
|
| Depth of
invasion |
|
|
|
|
0.009 |
|
Submucosal | 24 | – | 100.0 | – |
|
| Deeper
invasion | 54 | – |
83.3 | – |
|
Discussion
As the incidence of gastrointestinal NETs has
increased, endoscopic therapy has become more common for the
treatment of this type of tumors (3,4).
Therefore, studies investigating the safety, potential risks and
indications of endoscopic therapy have become particularly
important.
Several studies have demonstrated that the
histological classification, grading, LN metastasis,
lymphatic-vascular involvement and perineural invasion of
gastrointestinal NETs are associated with the infiltrative growth
of the tumor, staging and prognosis (4,21–23). For early-stage gastric cancer, tumors
exhibiting an expansive growth pattern and elevated macroscopic
type are less invasive, whereas poorly differentiated tumors are
associated with a higher risk of deep infiltration, LN and distant
metastasis (24,25). In the present study, histologically
submucosal NETs exhibited a higher level of differentiation,
expansive growth and small diameter compared with deeper invasion
NETs. In the deeper invasion NETs group, an infiltrative growth
pattern and tumors of mixed and poorly differentiated type were
more common than in the submucosal NETs group. These findings
indicated that poorly differentiated NETs usually exhibit an
infiltrative growth pattern and are more likely to invade the
muscularis propria and deeper layer and exhibit LN and distal
metastasis.
Thomas et al (26) analyzed 104 gastric carcinoids, the
majority of which exhibited a diameter of <2.0 cm, and observed
that the infiltration depth was restricted to the mucosa and
submucosa. Soga (27) investigated
1,914 gastrointestinal carcinoids and hypothesized that the
diameter of the tumor was closely associated with metastasis.
Gastrointestinal submucosa carcinoids exhibited a metastasic rate
of 16.4% as a whole and minute carcinoids (≤5 mm) revealed a
metastasic rate of 6.0% on average. In the present study, the
majority of lesions measuring <5.0 mm in diameter exhibited a
slightly elevated macroscopic type and an expansive growth pattern
without lymphatic-vascular involvement or distant metastasis.
However, a high number of tumors measuring 5.1–10.0 mm in diameter
exhibited an infiltrative growth pattern, lymphatic-vascular
involvement and nodal metastasis. In the deeper invasion NETs
group, all lesions measured >10.0 mm and exhibited an
infiltrative growth pattern. This indicated that at the initial
stage of growth (≤5.0 mm in diameter), NETs exhibit an expanding
growth pattern, and gradually develop an infiltrating growth
pattern as the tumor increases in diameter (>5.0 mm).
Subsequently, the tumor may invade through the submucosal layer,
infiltrating the muscular and deeper layers. In addition, tumors
may grow towards the mucosa in an elevated way, leading to mucosal
erosions and ulcers (Borrmann's type II or III tumors). Thus, the
present authors hypothesize that minute tumors with an expansive
growth pattern present a relatively inert stage of tumor
development and therefore, such tumors are suitable for endoscopic
therapy.
At present, surgery is considered the curative
treatment for NETs with muscular layer or deeper infiltration, with
or without LN metastasis (13,28).
Treatment for metastatic NETs includes, surgery, radiotherapy,
chemotherapy and palliative care, depending on the individual case
(28). If a tumor is limited to the
mucosa or submucosa, it may be resected by surgery or by endoscopy
using endoscopic mucosal resection or endoscopic submucosal
dissection (15,16,29–31).
Endoscopic resection is not only a curative method, but it also
exhibits minimal trauma and a lower cost than other surgical
procedures. For gastrointestinal epithelial tumors, if the tumor is
diagnosed when limited to the mucosa or submucosa, >50% tumors
are curable, with a 5-year survival rate of 80–90% (32). Generally, the indicators for
endoscopic resection are as follows: The gastrointestinal NET is
limited to the mucosa or submucosa; the diameter of the tumor is
<1.0 cm; and there is no evidence of LN or distant metastasis
(15,16). Certain studies have reported
endoscopic resection for tumors measuring >1.0 cm in diameter
(33–35). In the present study, 6 cases with
lesions measuring ≤5.0 mm and 8 cases with 5.1–10.0-mm lesions in
the submucosa were treated with tumor resection by endoscopy. A
total of 5 patients underwent surgery following endoscopic
resection for suspected positive margin or lymphatic-vascular
involvement. No patients exhibited recurrence or metastasis during
the follow-up period. These results indicated that surgery
subsequent to endoscopic resection may achieve curable effects for
patients with high risk factors. In the present study, submucosa
NETs measuring 5.1–10.0 mm in diameter exhibited a high rate of
lymphatic-vascular involvement (46.2%) and LN metastasis (30.8%),
which suggests that the indicators for endoscopic resection of
gastrointestinal NETs should be more stringent than that for
early-stage gastrointestinal adenocarcinoma. For ≤5.0-mm lesions of
the slightly elevated type, endoscopic resection is a safe
treatment method, whereas for gastrointestinal NETs of 5.1–10.0 mm
in diameter, endoscopic resection must be performed with
caution.
In the present study, overall prognosis was
favorable. Survival time was significantly higher in early-stage
and lower grade tumors compared with later-stage and higher grade
tumors. For gastrointestinal carcinomas, once the tumor has
progressed out of the range of being resectable, the patient is
expected to possess a relatively poor prognosis. Thus, earlier
diagnosis and improved systemic treatment for advanced disease is
urgently required.
In conclusion, the present retrospective study
revealed that gastrointestinal submucosal NETs regularly exhibit a
slightly elevated macroscopic type, and are usually small low-grade
tumors. These features may aid oncologists to diagnose
gastrointestinal NETs at an early stage. Gastrointestinal NETs of
<5.0 mm in diameter with a slightly elevated macroscopic type
may require endoscopic resection, while NETs measuring 5.1–10.0 mm
in diameter must be considered carefully prior to attempting
surgery, due to the potential risk of lymphatic-vascular
involvement and LN metastasis.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (Beijing, China; grant nos.
81201640 and 81372302), Zhejiang Provincial Natural Science
Foundation of China (Hangzhou, China; grant no. LY16H160026), the
National Health Key Special Fund (Beijing, China; grant no.
200802112) and the Key Project of Zhejiang Province (Hangzhou,
China; grant no. 2013C03044-5).
References
|
1
|
Modlin IM, Lye KD and Kidd M: A 5-decade
analysis of 13,715 carcinoid tumors. Cancer. 97:934–959. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pape UF, Berndt U, Müller-Nordhorn J,
Böhmig M, Roll S, Koch M, Willich SN and Wiedenmann B: Prognostic
factors of long-term outcome in gastroenteropancreatic
neuroendocrine tumours. Endocr Relat Cancer. 15:1083–1097. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hauso O, Gustafsson BI, Kidd M, Waldum HL,
Drozdov I, Chan AK and Modlin IM: Neuroendocrine tumor
epidemiology: Contrasting Norway and North America. Cancer.
113:2655–2664. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yao JC, Hassan M, Phan A, Dagohoy C, Leary
C, Mares JE, Abdalla EK, Fleming JB, Vauthey JN, Rashid A and Evans
DB: One hundred years after ‘carcinoid’: Epidemiology of and
prognostic factors for neuroendocrine tumors in 35,825 cases in the
United States. J Clin Oncol. 26:3063–3072. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Berge T and Linell F: Carcinoid tumours.
Frequency in a defined population during a 12-year period. Acta
Pathol Microbiol Scand A. 84:322–330. 1976.PubMed/NCBI
|
|
6
|
Oberg K and Eriksson B: Endocrine tumours
of the pancreas. Best Pract Res Clin Gastroenterol. 19:753–781.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Modlin IM, Oberg K, Chung DC, Jensen RT,
de Herder WW, Thakker RV, Caplin M, Fave G Delle, Kaltsas GA,
Krenning EP, et al: Gastroenteropancreatic neuroendocrine tumours.
Lancet Oncol. 9:61–72. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Scherübl H, Cadiot G, Jensen RT, Rösch T,
Stölzel U and Klöppel G: Neuroendocrine tumors of the stomach
(gastric carcinoids) are on the rise: Small tumors, small problems?
Endoscopy. 42:664–671. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Scherübl H: Rectal carcinoids are on the
rise: Early detection by screening endoscopy. Endoscopy.
41:162–165. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Scherübl H, Jensen RT, Cadiot G, Stölzel U
and Klöppel G: Neuroendocrine tumors of the small bowels are on the
rise: Early aspects and management. World J Gastrointest Endosc.
2:325–334. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Strosberg J, Gardner N and Kvols L:
Survival and prognostic factor analysis of 146 metastatic
neuroendocrine tumors of the mid-gut. Neuroendocrinology.
89:471–476. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zar N, Garmo H, Holmberg L, Rastad J and
Hellman P: Long-term survival of patients with small intestinal
carcinoid tumors. World J Surg. 28:1163–1168. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Scherübl H, Jensen RT, Cadiot G, Stölzel U
and Klöppel G: Management of early gastrointestinal neuroendocrine
neoplasms. World J Gastrointest Endosc. 3:133–139. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kulke MH, Benson AB III, Bergsland E,
Berlin JD, Blaszkowsky LS, Choti MA, Clark OH, Doherty GM, Eason J,
Emerson L, et al: Neuroendocrine tumors. J Natl Compr Canc Netw.
10:724–764. 2012.PubMed/NCBI
|
|
15
|
Zhou FR, Huang LY and Wu CR: Endoscopic
mucosal resection for rectal carcinoids under micro-probe
ultrasound guidance. World J Gastroenterol. 19:2555–2559. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yamaguchi N, Isomoto H, Nishiyama H,
Fukuda E, Ishii H, Nakamura T, Ohnita K, Hayashi T, Kohno S, Nakao
K and Shikuwa S: Endoscopic submucosal dissection for rectal
carcinoid tumors. Surg Endosc. 24:504–508. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Participants in the Paris Workshop, . The
Paris endoscopic classification of superficial neoplastic lesions:
Esophagus, stomach, and colon: November 30 to December 1, 2002.
Gastrointest Endosc. 58(Suppl 6): S3–S43. 2003.PubMed/NCBI
|
|
18
|
Endoscopic Classification Review Group, .
Update on the Paris classification of superficial neoplastic
lesions in the digestive tract. Endoscopy. 37:570–578. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Borrmann R: Tumors of the stomach and
duodenumHandbook of Special Pathological Anatomy and Histology:
Digestive Tract. Borchardt H, Borrmann R, Christeller E, Dietrich
A, Fischer W, Von Gierke E, Hauser G, Kaiserling C, Koch M, Koch W,
et al: 4. 1st. Springer Vienna; Berlin: pp. 8651926, (In
German).
|
|
20
|
Bosman FT, Carneiro F, Hruban RH and
Theise ND: WHO Classification of Tumours of the Digestive System.
3. 4th. IARC Press; Lyon: 2010
|
|
21
|
Pape UF, Jann H, Müller-Nordhorn J,
Bockelbrink A, Berndt U, Willich SN, Koch M, Röcken C, Rindi G and
Wiedenmann B: Prognostic relevance of a novel TNM classification
system for upper gastroenteropancreatic neuroendocrine tumors.
Cancer. 113:256–265. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Garcia-Carbonero R, Capdevila J,
Crespo-Herrero G, Díaz-Pérez JA, Martínez Del Prado MP, Orduña V
Alonso, Sevilla-García I, Villabona-Artero C, Beguiristain-Gómez A,
Llanos-Muñoz M, et al: Incidence, patterns of care and prognostic
factors for outcome of gastroenteropancreatic neuroendocrine tumors
(GEP-NETs): Results from the National Cancer Registry of Spain
(RGETNE). Ann Oncol. 21:1794–1803. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cho MY, Sohn JH, Jin SY, Kim H, Jung ES,
Kim MJ, Kim KM, Kim WH, Kim JM, Kang YK, et al: Gastrointestinal
Pathology Study Group of Korean Society of Pathologists: Proposal
for a standardized pathology report of gastroenteropancreatic
neuroendocrine tumors: Prognostic significance of pathological
parameters. Korean J Pathol. 47:227–237. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yoshino T, Shimoda T, Saito A, Nakanishi
Y, Yusuke T, Shirasu T and Miura S: Macroscopic features and its
clinical treatment of stomach type differentiated adenocarcinoma in
early gastric cancer. Stomach and Intestines. 34:513–525. 1999.(In
Japanese).
|
|
25
|
Japanese Gastric Cancer Association, .
Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric
cancer. 14:113–123. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Thomas RM, Baybick JH, Elsayed AM and
Sobin LH: Gastric carcinoids. An immunohistochemical and
clinicopathologic study of 104 patients. Cancer. 73:2053–2058.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Soga J: Early-stage carcinoids of the
gastrointestinal tract: An analysis of 1,914 reported cases.
Cancer. 103:1587–1595. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ramage JK, Ahmed A, Ardill J, Bax N, Breen
DJ, Caplin ME, Corrie P, Davar J, Davies AH, Lewington V, et al:
Guidelines for the management of gastroenteropancreatic
neuroendocrine (including carcinoid) tumours (NETs). Gut. 61:6–32.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Basuroy R, Srirajaskanthan R, Prachalias
A, Quaglia A and Ramage JK: Review article: The investigation and
management of gastric neuroendocrine tumours. Aliment Pharmacol
Ther. 39:1071–1084. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Heo J, Jeon SW, Jung MK, Kim SK, Shin GY,
Park SM, Ahn SY, Yoon WK, Kim M and Kwon YH: A tailored approach
for endoscopic treatment of small rectal neuroendocrine tumor. Surg
Endosc. 28:2931–2938. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kwon YH, Jeon SW, Kim GH, Kim JI, Chung
IK, Jee SR, Kim HU, Seo GS, Baik GH, Choi KD and Moon JS: Long-term
follow up of endoscopic resection for type 3 gastric NET. World J
Gastroenterol. 19:8703–8708. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sano T, Sasako M, Kinoshita T and Maruyama
K: Recurrence of early gastric cancer. Follow-up of 1475 patients
and review of the Japanese literature. Cancer. 72:3174–3178. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sung HY, Kim SW, Kang WK, Kim SY, Jung CK,
Cho YK, Park JM, Lee IS, Choi MG and Chung IS: Long-term prognosis
of an endoscopically treated rectal neuroendocrine tumor: 10-year
experience in a single institution. Eur J Gastroenterol Hepatol.
24:978–983. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yokoyama S, Takifuji K, Tani M, Kawai M,
Naka T, Uchiyama K and Yamaue H: Endoscopic resection of duodenal
bulb neuroendocrine tumor larger than 10 mm in diameter. BMC
Gastroenterol. 11:672011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Baek IH: Endoscopic submucosal dissection
or conventional endoscopic mucosal resection is an effective and
safe treatment for rectal carcinoid tumors: A retrospective study.
J Laparoendosc Adv Surg Tech A. 20:329–331. 2010. View Article : Google Scholar : PubMed/NCBI
|