Introduction
Gastric cancer (GC) is one of the most frequently
occurring aggressive malignancies, particularly in East Asian
countries (1–4), with a global incidence of 15.6 and 6.7
cases per 100,000 males and females, respectively in developed
regions, and an incidence of 18.1 and 7.8 cases per 100,000 males
and females, respectively, in less developed areas (4). Furthermore, GC is the second leading
cause of cancer-associated mortality worldwide (1–4),
accounting for 9.2 and 4.2 mortalities per 100,000 males and
females, respectively, in developed areas and 14.4 and 6.5
mortalities per 100,000 males and females, respectively, in less
developed regions (4). A low early GC
diagnosis rate and tumor metastasis are major obstacles in GC
therapy. Novel treatments are urgently required to treat this
malignant cancer. Pre-leukemia transcription factor 3 (PBX3) is a
member of the PBX family of three amino acid loop extension class
homeodomain transcription factors, which are known to regulate gene
expression with a highly conserved homologous domain (5–8). PBX
proteins are also well known for their interaction with other
homologous proteins, such as homeobox (HOX) proteins. The
interaction between PBX and HOX increases the DNA-binding affinity
and promotes the transcription of the downstream target genes
(9–11).
Previously, PBX proteins have been reported to be
involved in a variety of human cancer types and to play important
roles in the progression of human tumors. In prostate cancer, PBX3
is upregulated and can be downregulated by lethal-7d (let-7d)
through post-transcriptional regulation by androgens, and this
effect may be independent of androgen receptors (12). In colorectal cancer, let-7c serves as
a tumor metastasis suppressor by inhibiting PBX3 expression
(13), and PBX3 expression is
significantly associated with lymph node invasion, distant
metastasis and poor overall survival. PBX3 can stimulate the
mitogen-activated protein kinase (MAPK)/extracellular
signal-regulated kinase signaling pathway in colorectal cancer
(6). Additionally, the forced
expression of PBX3 may reverse the effects caused by microRNA-181b
that promote apoptosis, inhibit proliferation and delay
leukemogenesis in cytogenetically abnormal acute myeloid leukemia
(14). In addition, PBX3 is well
known as a critical cofactor of HOXA9 in leukemogenesis, inhibiting
leukemic cell apoptosis (15). PBX3
is also overexpressed in GC and is closely correlated with invasion
depth, clinical stage and differentiation. Furthermore, PBX3
accelerates cell proliferation and colony formation in GC (16). Additionally, blocking the HOX/PBX
dimer has been reported to be a treatment against tumor growth in
ovarian, renal and pancreatic cancer, and in non-small cell lung
cancer (17–19). These findings suggest that PBX3 acts
as an oncogenic gene in the progression of numerous cancer
types.
However, the mechanisms through which PBX3 affects
intracellular signal transduction in GC remain to be determined,
and the study of further biological behaviors of PBX3 in GC are
required. The present study aimed to examine further biological
effects and potential pathways of PBX3 in GC progression.
Materials and methods
Cell lines and tumor samples
The human GC cell lines, SGC-7901, AGS, BGC-823 and
MKN-45, and human umbilical vein endothelial cells (HUVECs) were
purchased from the Shanghai Institutes for Biological Sciences,
Chinese Academy of Sciences (Shanghai, China). All cells were
cultured at 37°C in 5% CO2 and at saturation humidity in
RPMI-1640 medium containing 10% fetal bovine serum.
Gastric tumor and adjacent non-tumorous tissues (≥3
cm from tumor) were obtained from 25 patients with GC who underwent
curative surgery (D2 radical resection) at the Second Affiliated
Hospital of Soochow University (Suzhou, China) between January 2014
and December 2015. Patients that had undergone
radiotherapy/chemotherapy prior to surgery were excluded from the
study. These tissues were used for reverse transcription polymerase
chain reaction (RT-PCR). None of the patients had received
radiotherapy or chemotherapy prior to surgery. This study was
approved by the Ethics Committee of the Second Affiliated Hospital
of Soochow University, and all patients were fully informed of the
experimental procedures.
RNA isolation and RT-quantitative PCR
(RT-qPCR)
Total RNA was extracted and isolated from tissue
samples using TRIzol reagent (Invitrogen; Thermo Fisher Scientific,
Inc., Waltham, MA, USA), according to the manufacturer's protocol.
Reverse transcription of RNA was performed using a reverse
transcription kit (Promega, Madison, WI, USA).
In brief, 1 µg of total RNA from each sample was
reverse transcribed following the manufacturer's protocol. SYBR
Green reagent (Applied Biosystems; Thermo Fisher Scientific, Inc.)
was used for RT-qPCR to analyze mRNA expression. The PCR primers
were designed according to the human PBX3 and GAPDH cDNA sequences
in GenBank, as follows: PBX3 forward, 5′-GAGCTGGCCAAGAAATGCAG-3′
and reverse, 5′-GGGCGAATTGGTCTGGTTG-3′; and GAPDH forward,
5′-GGACCTGACCTGCCGTCTAG-3′ and reverse, 5′-GTAGCCCAGGATGCCCTTGA-3′.
GAPDH acted as the constitutive control. Relative expression ratios
of PBX3 in each paired tumor to non-tumor tissue sample were
calculated using the 2-ΔΔCq method (20).
Vector construction and
transfection
The pCMV6-PBX3 plasmid was purchased from Origene
(Origene Technologies, Inc., Rockville, MD, USA). The pCMV6-PBX3 or
pCMV6-vector was then transfected into SGC-7901 cells using
Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.), in
accordance with the manufacturer's protocol.
For depletion of endogenous PBX3 expression, short
hairpin RNA (shRNA) targeting 592–613 bp was inserted into a
lentivirus vector as previously described (6). Lentiviral vectors were then transfected
into MKN-45 GC cells. Stably transfected cells were selected by
treatment with 5 µg/ml blasticidin and were used for identification
and additional research.
Western blotting
GC cells were harvested and lysed using
radioimmunoprecipitation assay buffer (Solarbio, Beijing, China)
containing 1% phenylmethylsulfonyl fluoride protease inhibitors. A
bicinchoninic acid assay kit (Pierce Biotechnology, Inc., Rockford,
IL, USA) was used to measure the total protein concentration.
Equivalent amounts of protein (50 µg) were separated by 10%
SDS-PAGE, and the resolved proteins were transferred to
polyvinylidene difluoride membranes. The membranes were blocked
with 5% skim milk for 2 h and then incubated with primary
antibodies overnight at 4°C. Primary antibodies were as follows:
PBX3 (polyclonal rabbit; cat. no. 12571-1-AP; dilution, 1:500;
Proteintech Group, Inc., Rosemont, IL, USA); E-cadherin (monoclonal
rabbit; cat. no. 3195; dilution, 1:500; Cell Signaling Technology,
Inc., Danvers, MA, USA); N-cadherin (monoclonal rabbit; cat. no.
13116; dilution, 1:500; Cell Signaling Technology, Inc.); vimentin
(monoclonal rabbit; cat. no. 5741; dilution, 1:1,000; Cell
Signaling Technology, Inc.); p-AKT (Ser473) (monoclonal rabbit;
cat. no. 4060; dilution, 1:500; Cell Signaling Technology, Inc.);
total AKT (monoclonal rabbit; cat. no. 4691; dilution, 1:500; Cell
Signaling Technology, Inc.) and GAPDH (monoclonal mouse; cat. no.
ab8245; dilution, 1:10,000; Abcam, Cambridge, UK). Membranes were
then incubated with secondary antibody for 2 h at room temperature
and were visualized using an enhanced chemiluminescence detection
system (GE Healthcare Life Sciences, Chalfont, UK) in accordance
with the manufacturer's protocol.
Cell migration and invasion
assays
For cell migration and invasion assays, a total
number of 1×105 GC cells (SGC-7901-PBX3, SGC-7901-NC, MKN-45-NC and
MKN-45-PBX3/sh) were suspended in serum-free RPMI-1640 medium
(HUVEC cells were suspended in supernatant from GC cells) and
plated in Transwell chambers (8 µm for 24-well plate; Costar;
Corning Incorporated, Corning, NY, USA) with or without Matrigel
(BD Biosciences, San Jose, CA, USA), according to the
manufacturer's protocol. For each assay, medium containing 10% FBS
was added to the lower chamber as a chemoattractant. Subsequent to
24 h culture, the cells were fixed by 10% formalin and stained by
0.5% crystal violet. Finally, images of cells in the lower chamber
were captured and the number of cells was counted by inverted
microscopy (×200 magnification).
Endothelial tube formation assay
HUVEC cells were cultured in tumor supernatant and
plated in a 96-well plate with Matrigel (BD Biosciences) at a
concentration 3×104 cells/well. Tumor supernatant was collected
from PBX3-overexpressing groups and PBX3-silenced groups subsequent
to 24 h culture in RPMI-1640 medium. Following 12 h incubation at
37°C in a 5% CO2 atmosphere, tubes were photographed by
microscopy and evaluated by Image Pro Plus software (Media
Cybernetics, Inc., Rockville, MD, USA).
Gelatin zymography
To examine matrix metallopeptidase (MMP)-9 activity,
gelatin zymography was performed using 10% SDS-PAGE gels containing
1 mg/ml gelatin (Sigma-Aldrich; Merck Millipore, Darmstadt,
Germany). In brief, GC cells were cultured in serum-free RPMI-1640
medium for 24 h and the supernatant was then collected and
centrifuged at 201 × g for 5 min. The gels were washed twice in
renaturation buffer (2.5% Triton X-100) for 30 min each time to
remove SDS subsequent to electrophoresis and then incubated at 37°C
for 24 h in a reaction buffer consisting of 50 mM Tris-HCl (pH
7.5), 5 mM CaCl2 and 150 mM NaCl. The gels were then stained with
0.5% Coomassie brilliant blue R-250 (Sigma-Aldrich; Merck
Millipore) for 2 h and destained in buffer (30% methanol; 10%
acetic acid). Clear transparent bands in the background of blue
staining represented gelatinase activity.
Nude mouse xenograft model
Four-week-old male BALB/c nude mice (n=10; Institute
of Zoology, Chinese Academy of Sciences, Beijing, China) were used
to evaluate the role of PBX3 in peritoneal spreading in
vivo. Nude mice were housed at a specific pathogen-free
environment in the Animal Laboratory Unit, School of Medicine,
Soochow University. Mice received humane care and the study
protocols were performed according to a protocol approved by the
Institutional Animal Care and Use Committee (IACUC) at Soochow
University. A total number of 2×106 SGC-7901-PBX3 or SGC-7901-NC
cells were injected into the abdominal cavity of the 5 mice of the
PBX3 and NC groups, respectively. Mice were euthanized on the 30th
day subsequent to injection, and the abdominal masses were imaged
and photographed. All experiments were performed in accordance with
the official recommendations of the Chinese Animal Community at
Soochow University School of Medicine (Suzhou, China).
Statistical analysis
Student's t-test was used to examine the statistical
differences between the two groups. Data are shown as mean ±
standard deviation. Statistical analyses were performed using SPSS
19.0 software (IBM, Armonk, NY, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
PBX3 expression is upregulated in GC
tissues
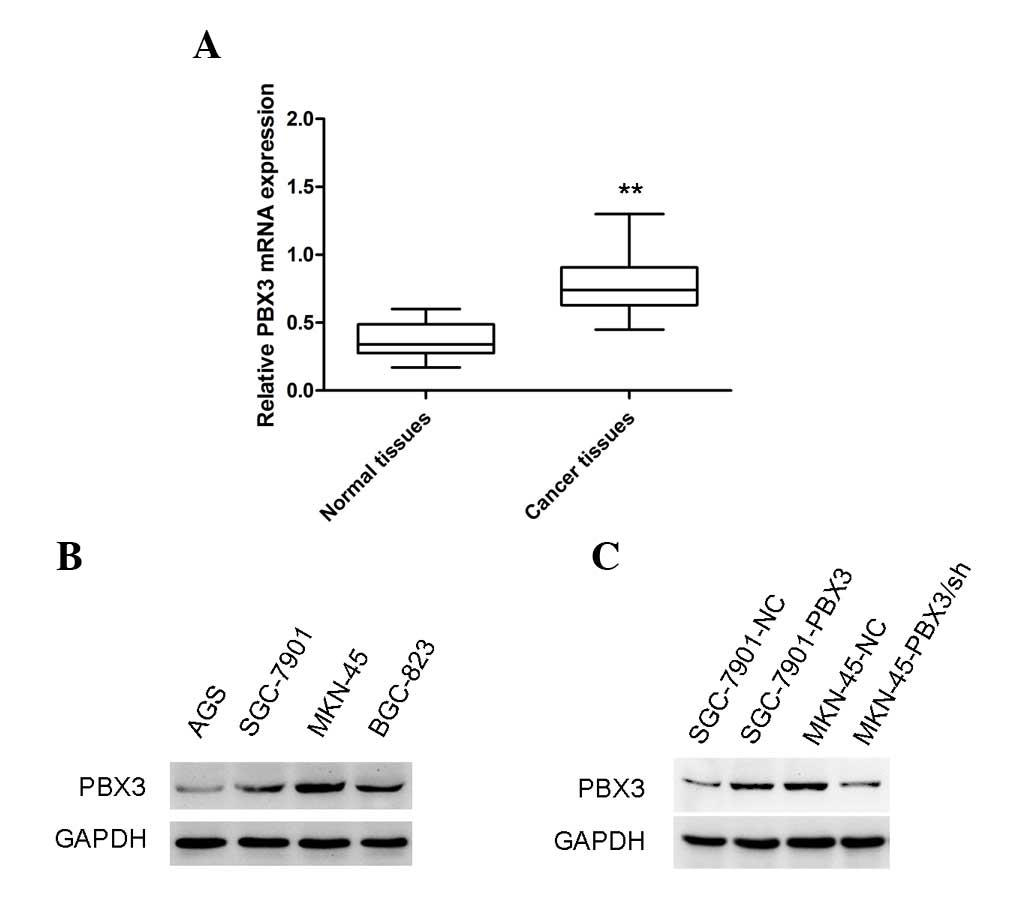
The levels of PBX3 in GC and adjacent non-tumor
tissues were examined by RT-qPCR assay. The results showed that the
PBX3 mRNA level was markedly increased in GC tissues compared with
the corresponding non-tumor tissues (Fig.
1A; P<0.01). This finding suggests that PBX3 may act as an
oncogenic gene in GC. Notably, a previous study showed that PBX3
expression was associated with poor tumor differentiation, invasion
depth and clinical stage in GC (16).
Western blot analysis was then used to examine the
expression of PBX3 at the protein level in GC cell lines. It was
found that the expression levels were different in different GC
cell lines (Fig. 1B). The results
indicated that MKN-45 GC cells inherently express high levels of
PBX3, whereas SGC-7901 GC cells express low levels of PBX3.
Overexpression of PBX3 in SGC-7901 and attenuation of PBX3
expression in MKN-45 GC cells were confirmed at the protein level
by western blot analysis (Fig. 1C).
Stably transfected cells were used for further experiments.
PBX3 promotes the migration and
invasion of GC cells
Cancer metastasis is the leading cause of
cancer-associated mortality and it has been challenging to study
due to a series of rare, stochastic events involved in cancer
metastasis (21). Therefore, the
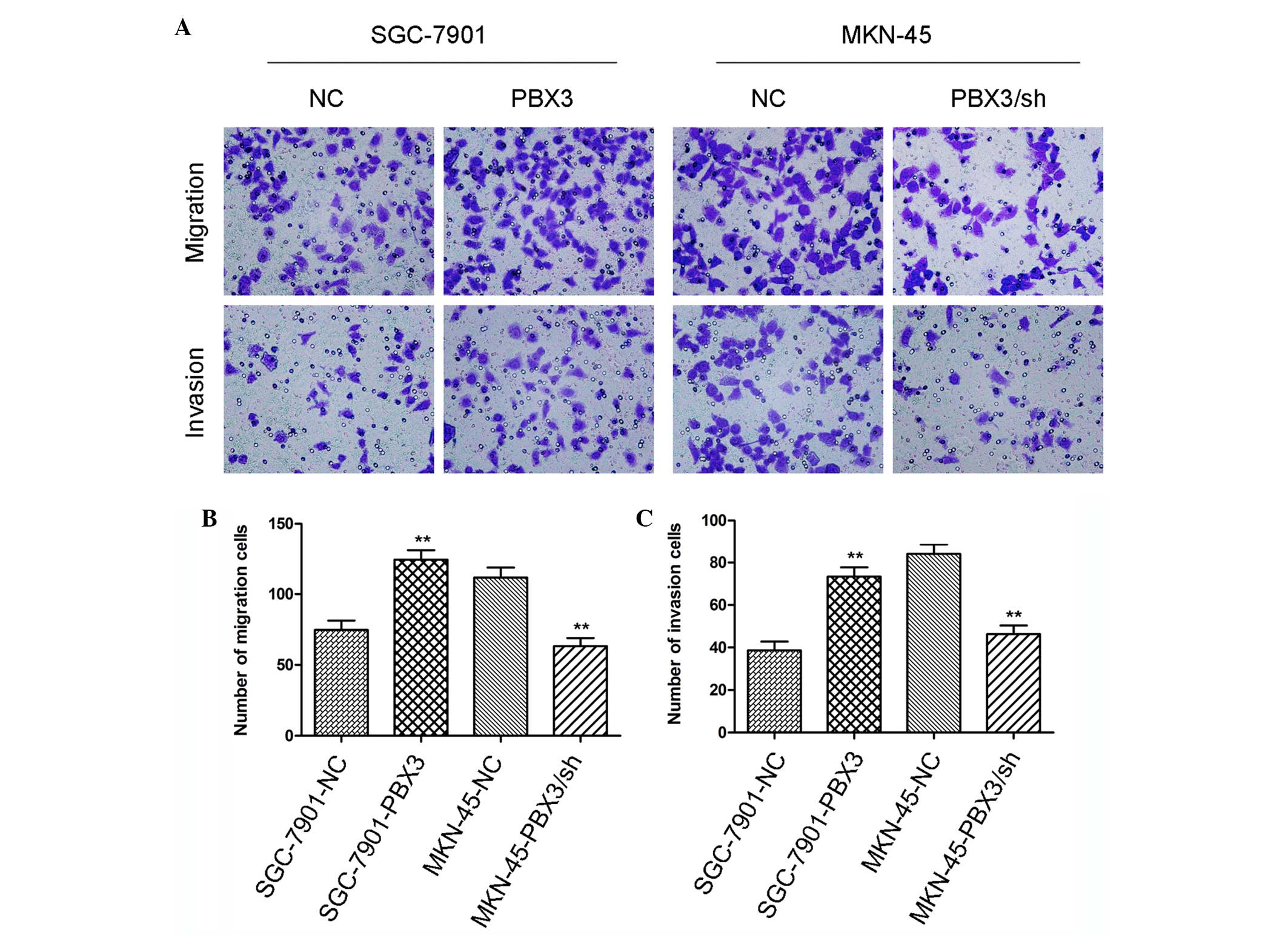
present study examined the effects of PBX3 on GC mobility by
Transwell assay. It was observed that the cell migration rate and
invasion rate were each increased in PBX3 overexpressing groups
compared with their respective control groups (Fig. 2A-C; P<0.01). The opposing results
were obtained subsequent to silencing of PBX3 in MKN-45 cells
(Fig. 2A-C; P<0.01). This finding
suggests that the mobility of GC cells changed with the expression
of PBX3.
Overexpression of PBX3 in GC promotes
tubular formation of HUVEC cells
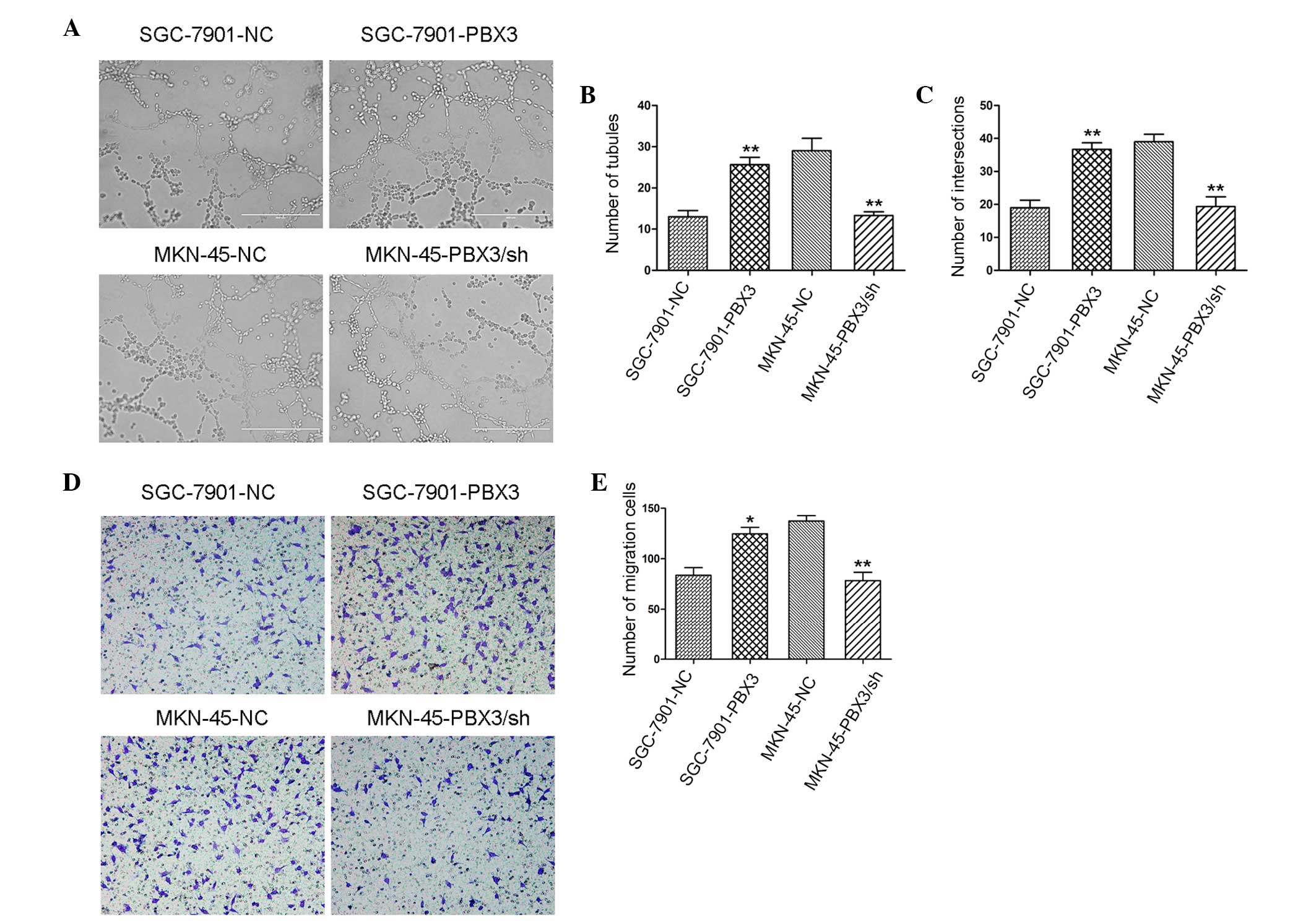
It is well known that tumor angiogenesis is a key
role in cancer progression. To examine the effects of PBX3 on
tubular formation of HUVEC cells, tumor supernatant was collected
from GC cells to suspend HUVEC cells. In total, 3×104 HUVEC cells
were plated onto a 96-well plate that was coated with Matrigel. The
96-well plate was then incubated for 12 h at 37°C in 5%
CO2. Following 12 h incubation, increased tubule forming
ability was observed in PBX3-overexpressing groups compared with
the control groups (Fig. 3A), such as
number of tubules (Fig. 3B), and
number of intersections (Fig. 3C).
Converse results were achieved in PBX3-knockdown groups compared
with the control groups (Fig.
3A-C).
Tubular formation of HUVEC cells was
associated with the migration of HUVEC cells
Therefore, the effect of PBX3-overexpressing GC
cells on the migration of HUVEC cells. It was observed that
supernatant from PBX3-overexpressing GC cells could stimulate the
migration of HUVEC cells. The mechanism of the promotion of HUVEC
cell migration by PBX3-overexpressing GC cells remains unclear.
PBX3 induces epithelial mesenchymal
transition (EMT) in GC cells
EMT is known to play important roles in the
progression of tumors, particularly in cancer metastasis. It was
found that overexpression of PBX3 elevated the migration and
invasion rates of GC cells. EMT has an unelucidated effect on these
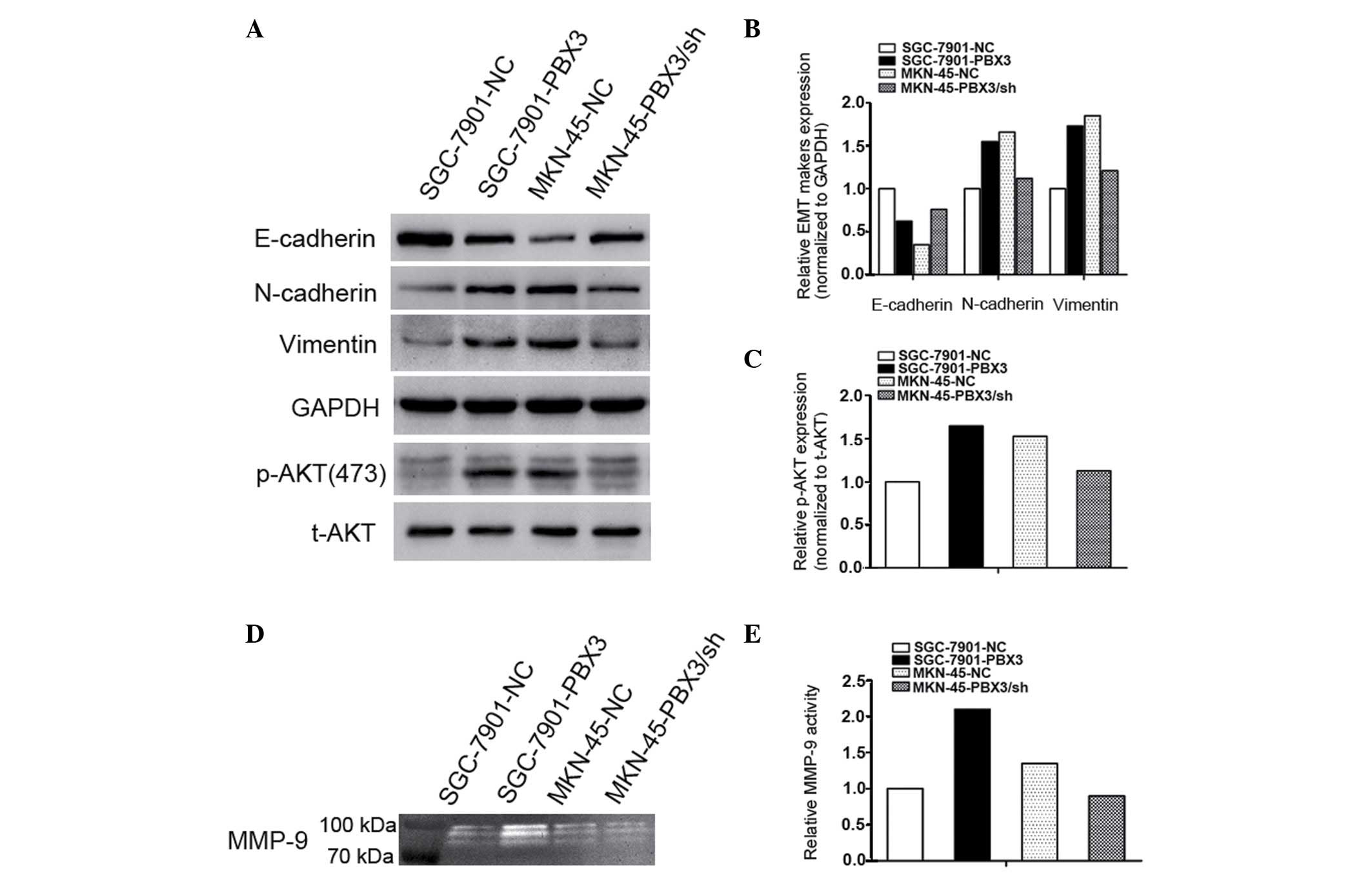
processes. To clarify this, the EMT-associated proteins in GC cells
were examined by western blot analysis. It was observed that the
N-cadherin and vimentin protein levels were increased, while
E-cadherin was decreased in PBX3-overexpressing GC cells compared
with their respective control cells (Fig.
4A and B). Converse findings were observed after PBX3 was
knocked down in MKN-45 GC cells (Fig. 4A
and B).
Furthermore, AKT signaling is strongly associated
with EMT, invasion and metastasis in a variety of human tumors.
Therefore, AKT activity was evaluated in PBX3-overexpressing GC
cells. Western blot analysis showed that the level of p-AKT
(ser473) was increased in PBX3-overexpressing GC cells, whereas
opposing results were observed in PBX3-silencing GC cells (Fig. 4A and C). Additionally, it was observed
that overexpression of PBX3 elevated the activity of MMP-9 in GC
cells by gelatin zymography assay (Fig.
4D and E). However knockdown of PBX3 made no significant change
in the activity of MMP-9 in GC cells. These findings suggest that
PBX3-induced EMT may be via the AKT signaling pathway in GC
cells.
PBX3 promotes peritoneal spreading in
vivo
To examine the effects of PBX3 on peritoneal
spreading and metastasis in vivo, SGC-7901-PBX3 and
SGC-7901-NC GC cells were injected into the abdomens of nude mice.
Extensive peritoneal spreading was observed in the
PBX3-overexpressing group compared with the control group (Fig. 5A). There were significantly more
visible peritoneal nodules in the PBX3-overexpressing group than in
the control group (4.600±0.748 vs. 2.200±0.374; P<0.05; Fig. 5B). Thus overexpression of PBX3
promotes GC invasion and metastasis in vivo as well as in
vitro.
Discussion
GC is a major health burden in the world and
additional studies should be performed to investigate ways to
prevent gastric cancer progression. Increasing evidence indicates
that PBX3 plays an important role in human cancer progression. The
aim of the present study was to further examine the contribution of
the transcription factor PBX3 to GC progression.
In the present study, it was observed that PBX3
expression was overexpressed in GC tissues measured by RT-qPCR
assay, which was consistent with a previous study (16). PBX3 was silenced and overexpressed in
GC cells to examine the effects of PBX3 in GC. It was found that
overexpression of PBX3 promoted GC cell invasion and migration
compared with the control groups, while knockdown of PBX3 reduced
GC cell invasion and migration. These observations demonstrated
that PBX3 may induce something changing in GC that contributing to
accelerate the mobility of GC cells. To further study the effects
of PBX3 in GC metastasis in vivo, a nude mouse xenograft
model was constructed and it was observed that there were
significantly more visible peritoneal nodules in the
PBX3-overexpressing group compared with the control group.
According to these observations, it was confirmed that PBX3
promotes GC invasion and metastasis in vivo as well as in
vitro.
Tumor progression is not only associated with cancer
cell metastasis, but also with tumor angiogenesis (3). There must be angiogenesis tubular
formation in tumors with a diameter of ~2 mm (22). Notably, it was observed that
overexpression of PBX3 promoted tubular formation of HUVEC cells in
GC compared with the control groups. As an attempt to understand
the observations that promoted tubular formation through PBX3,
gelatin zymography for determining MMP-9 activity and HUVEC cells
migration assays were performed. It was detected that MMP-9
activity was increased in PBX3-overexpressing GC cells and PBX3
promoted HUVEC cell migration. MMP-9 can degrade extracellular
matrix components and plays a critical role in tissue remodeling
during development in pathological process, including inflammation,
tumor invasion and metastasis, and tumor angiogenesis (23–25). These
observations may contribute to account for the effects of PBX3 in
GC that promoting invasion, metastasis and tubular formation.
EMT is implicated in a pathological element in
cancer progression as well as in a physiological process during
embryonic development (26,27). Tumor metastasis is always associated
with EMT process in lots of tumor progression. Therefore, the
present study examined the association between PBX3 and EMT in GC
cells. It was then observed that PBX3-overexpressing in GC cells
decreased E-cadherin expression, which is a biomarker of epithelial
cells, and increased N-cadherin and vimentin expression, which are
biomarkers of mesenchymal cells. These observations suggest that
overexpression of PBX3 promotes invasion and metastasis by inducing
EMT in GC. Numerous mechanisms involved in EMT initiation have been
documented, containing the transforming growth factor β, focal
adhesion kinase, interleukin-6, phosphoinositide 3-kinase/AKT,
RAF/MAPK signaling pathways (25,28,29).
Therefore, the present study examined the effects of PBX3
overexpression on the levels of AKT phosphorylation in GC cells.
Notably, overexpression of PBX3 elevated the phosphorylation of AKT
(ser473) in GC cells. Based on the aforementioned findings, it was
concluded that PBX3 may promote GC invasion and metastasis by
inducing EMT via the AKT signaling pathway.
In conclusion, the present study illustrated that
PBX3 acts as an oncogene by promoting EMT via AKT signaling in GC.
Furthermore, PBX3 promotes GC invasion and metastasis in
vitro and in vivo, and may act as a potential new
diagnostic and prognostic marker and novel target for GC
therapy.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Piazuelo MB and Correa P: Gastric cáncer:
Overview. Colombia Med (Cali). 44:192–201. 2013.
|
|
3
|
Zang M, Zhang Y, Zhang B, Hu L, Li J, Fan
Z, Wang H, Su L, Zhu Z, Li C, et al: CEACAM6 promotes tumor
angiogenesis and vasculogenic mimicry in gastric cancer via FAK
signaling. Biochim Biophys Acta. 1852:1020–1028. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Abramovich C, Shen WF, Pineault N, Imren
S, Montpetit B, Largman C and Humphries RK: Functional cloning and
characterization of a novel nonhomeodomain protein that inhibits
the binding of PBX1-HOX complexes to DNA. J Biol Chem.
275:26172–26177. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Han HB, Gu J, Ji DB, Li ZW, Zhang Y, Zhao
W, Wang LM and Zhang ZQ: PBX3 promotes migration and invasion of
colorectal cancer cells via activation of MAPK/ERK signaling
pathway. World J Gastroenterol. 20:18260–18270. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Di Giacomo G, Koss M, Capellini TD,
Brendolan A, Pöpperl H and Selleri L: Spatio-temporal expression of
Pbx3 during mouse organogenesis. Gene Expr Patterns. 6:747–757.
2006. View Article : Google Scholar
|
|
8
|
van Dijk MA, Peltenburg LT and Murre C:
Hox gene products modulate the DNA binding activity of Pbx1 and
Pbx2. Mech Dev. 52:99–108. 1995. View Article : Google Scholar
|
|
9
|
Monica K, Galili N, Nourse J, Saltman D
and Cleary ML: PBX2 and PBX3, new homeobox genes with extensive
homology to the human proto-oncogene PBX1. Mol Cell Biol.
11:6149–6157. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Milech N, Kees UR and Watt PM: Novel
alternative PBX3 isoforms in leukemia cells with distinct
interaction specificities. Genes Chromosomes Cancer. 32:275–280.
2001. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Selleri L, DiMartino J, van Deursen J,
Brendolan A, Sanyal M, Boon E, Capellini T, Smith KS, Rhee J,
Pöpperl H, et al: The TALE homeodomain protein Pbx2 is not
essential for development and long-term survival. Mol Cell Biol.
24:5324–5331. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ramberg H, Alshbib A, Berge V, Svindland A
and Taskén KA: Regulation of PBX3 expression by androgen and Let-7d
in prostate cancer. Mol Cancer. 10:502011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Han HB, Gu J, Zuo HJ, Chen ZG, Zhao W, Li
M, Ji DB, Lu YY and Zhang ZQ: Let-7c functions as a metastasis
suppressor by targeting MMP11 and PBX3 in colorectal cancer. J
Pathol. 226:544–555. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li Z, Huang H, Li Y, Jiang X, Chen P,
Arnovitz S, Radmacher MD, Maharry K, Elkahloun A, Yang X, et al:
Up-regulation of a HOXA-PBX3 homeobox-gene signature following
down-regulation of miR-181 is associated with adverse prognosis in
patients with cytogenetically abnormal AML. Blood. 119:2314–2324.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li Z, Zhang Z, Li Y, Arnovitz S, Chen P,
Huang H, Jiang X, Hong GM, Kunjamma RB, Ren H, et al: PBX3 is an
important cofactor of HOXA9 in leukemogenesis. Blood.
121:1422–1431. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li Y, Sun Z, Zhu Z, Zhang J, Sun X and Xu
H: PBX3 is overexpressed in gastric cancer and regulates cell
proliferation. Tumour Biol. 35:4363–4368. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Morgan R, Plowright L, Harrington KJ,
Michael A and Pandha HS: Targeting HOX and PBX transcription
factors in ovarian cancer. BMC cancer. 10:892010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Plowright L, Harrington KJ, Pandha HS and
Morgan R: HOX transcription factors are potential therapeutic
targets in non-small-cell lung cancer (targeting HOX genes in lung
cancer). Br J Cancer. 100:470–475. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Aulisa L, Forraz N, McGuckin C and
Hartgerink JD: Inhibition of cancer cell proliferation by designed
peptide amphiphiles. Acta Biomater. 5:842–853. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2-ΔΔCT method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Van Cutsem E, Sagaert X, Topal B,
Haustermans K and Prenen H: Gastric cancer. Lancet: Epub ahead of
print. May 5–2016.
|
|
22
|
Carmeliet P and Jain RK: Angiogenesis in
cancer and other diseases. Nature. 407:249–257. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Stetler-Stevenson WG: Matrix
metalloproteinases in angiogenesis: A moving target for therapeutic
intervention. J Clin Invest. 103:1237–1241. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Egeblad M and Werb Z: New functions for
the matrix metalloproteinases in cancer progression. Nat Rev
Cancer. 2:161–174. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zang M, Zhang B, Zhang Y, Li J, Su L, Zhu
Z, Gu Q, Liu B and Yan M: CEACAM6 promotes gastric cancer invasion
and metastasis by inducing epithelial-mesenchymal transition via
PI3K/AKT signaling pathway. PloS One. 9:e1129082014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Savagner P: The epithelial-mesenchymal
transition (EMT) phenomenon. Ann Oncol. 21:(Suppl 7). vii89–vii92.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Huber MA, Kraut N and Beug H: Molecular
requirements for epithelial-mesenchymal transition during tumor
progression. Curr Opin Cell Biol. 17:548–558. 2005. View Article : Google Scholar : PubMed/NCBI
|