Introduction
The World Health Organization classifies Langerhans
cell tumors into two types: Langerhans cell sarcoma (LCS) and
Langerhans cell histiocytosis (LCH) (1). LCS is a neoplastic proliferation of
Langerhans cells with markedly malignant cytological features,
whereas LCH is proliferative disorder of Langerhans cells (1). LCS may occur in individuals of a broad
age range, and is highly metastatic (2). The disease may involve multiple organs
or tissues (3), including bone, lung,
skin, lymph nodes, gallbladder, tonsil and other soft tissues. In
the clinic, LCS is an extremely rare disease that is unfamiliar to
dermatologists (2). The clinical
behavior of the disease is aggressive and the overall survival rate
for affected patient's is <50% (3). Several therapeutic regimens have been
investigated for the treatment of LCS, including surgery,
chemotherapy, radiation therapy and combined therapy (3). However, due to the limited number of
cases reported in the literature to date, no standard effective
therapy has been suggested for the treatment of LCS (3). Among LCS patients, the therapeutic
outcomes vary depending on the extent of the disease. In addition,
the prognosis of the disease is poor and relapses usually occur
within the first 3 years after excision (3).
The current study reports one case of LCS that
originated from the subcutaneous tissue of the left knee of a male
patient. In addition, all reported cases of LCS available in the
English literature are summarized.
Case report
A 75-year-old male patient was admitted to Beijing
Hospital (Beijing, China) on November 4, 2010, complaining of an
egg-sized mass located at the medial part of his left knee. The
patient reported that the mass had gradually enlarged during the
past two months and caused mild-to-moderate pain at night. The pain
would somehow be relieved during the daytime and after taking
non-steroidal anti-inflammatory drugs. The patient had a medical
history that included tonsillectomy 55 years previously, subtotal
thyroidectomy 40 years previously (pathology indicated a thyroid
adenoma), partial prostatectomy 15 years previously (benign
pathology), radical operation for colorectal cancer 5 years
previously (pathology indicated intramucosal carcinoma) and a
resection of mass of the buttock 4 years previously (pathology
indicated an abscess).
Physical examination revealed a firm mass of ~3×3 cm
on the patient's left knee. Inguinal lymph nodes were palpated on
both sides and were found to be small, round and soft. X-ray
imaging indicated hyperosteogeny in the knee joint and an
ultrasound of the mass revealed a low-echo region in the
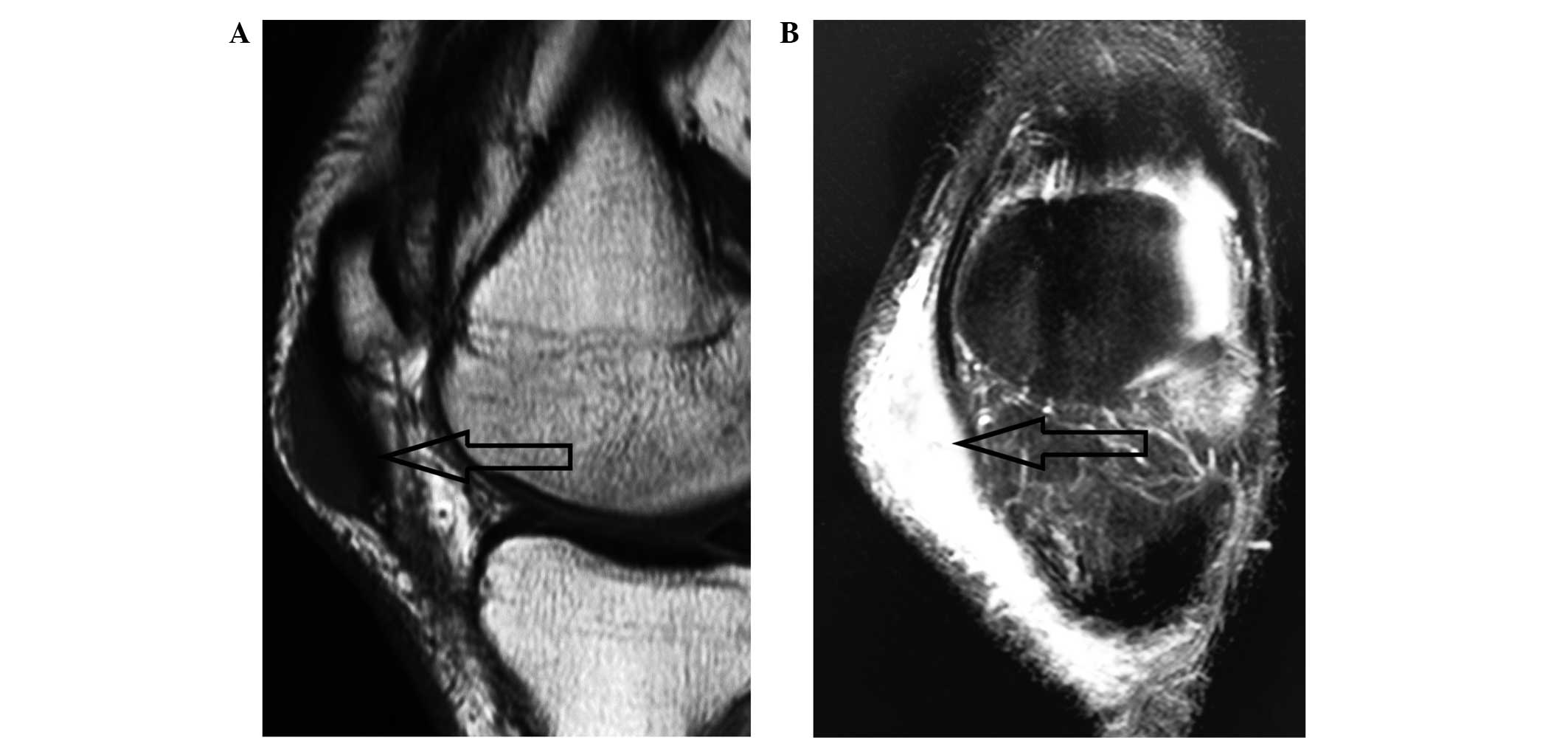
subcutaneous tissue. Magnetic resonance imaging (MRI) revealed a
mass measuring ~40×12×15 mm with a clear boundary in the
subcutaneous tissue on the anterior-medial side of the patient's
left patella (Fig. 1). The mass
exhibited homogeneous signal isointensity relative to that of
muscle on T1-weighted imaging (Fig.
1A), and predominantly signal hyperintensity on T2-weighted
imaging (Fig. 1B). A number of small
patchy hyperintense signals were observed within the mass (Fig. 1B).
The patient received a knee arthroscopic surgery on
November 10, 2010. Grossly, a 4×3×2-cm, gray mass was identified,
located subcutaneously and with a clear border. No infiltration
into the joint space was observed. Thus, the patient underwent a
surgical resection of the mass. A 4.8×3.6×1.2-cm specimen was
obtained. The cut surface of the resected specimen was gray, soft,
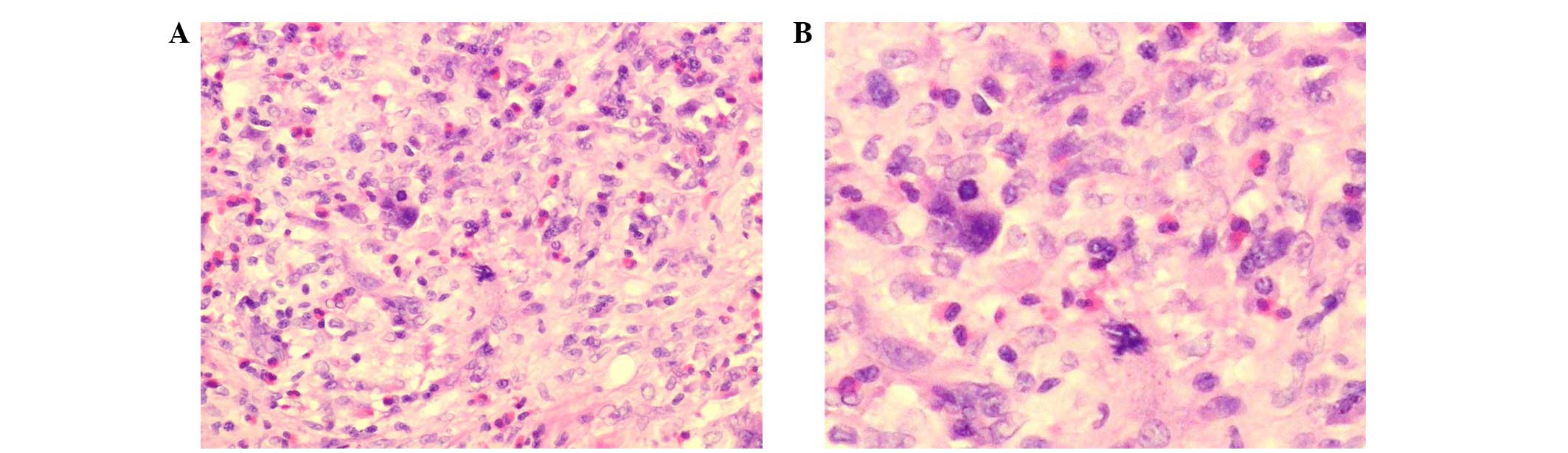
and had a pseudo-membrane. Histological examination of the specimen
buy hematoxylin and eosin staining indicated an infiltrative tumor
mass. The tumor cells were surrounded by abundant lymphocytes,
plasma cells and eosinophils (Fig.
2). The neoplastic cells exhibited cytological atypia,
hyperchromatic nuclei and prominent nucleoli. Nuclear grooving was
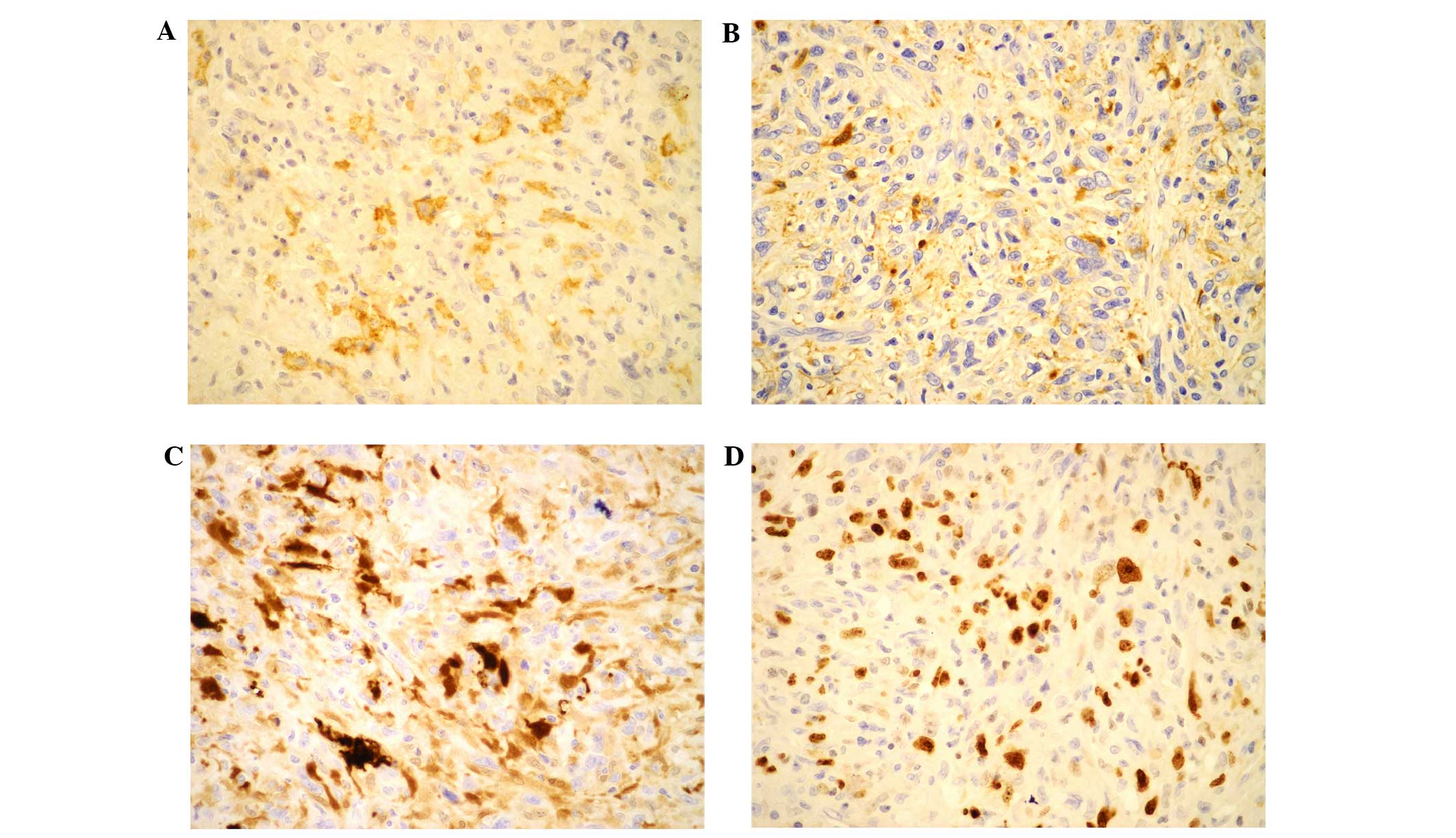
also observed in some of the neoplastic cells (Fig. 2). Immunohistochemical stains were
positive for cluster of differentiation (CD)1a (+; rabbit
monoclonal antibody; catalog no. AC-0078; 1:200 dilution; Baili
Biotechnology Co., Ltd., Changhun, China; Fig. 3A), CD68 (+; mouse monoclonal antibody;
catalog no. Z-2071; 1:200 dilution; Zeta Corporation, Los Angeles,
CA, USA; Fig. 3B), S-100 (++; rabbit
polyclonal antibody; catalog no. NCL-L-S100p; 1:150 dilution; Leica
Microsystems, Ltd., Milton Keynes, UK; Fig. 3C) and vimentin (+++; mouse monoclonal
antibody; catalog no. 202M-96; 1:150 dilution; Cell Marque,
Sigma-Aldrich, St. Louis, MO, USA). The tests were negative for
desmin (mouse monoclonal antibody; catalog no. 243M-16; 1:100
dilution; Cell Marque, Sigma-Aldrich), CD30 (mouse monoclonal
antibody; catalog no. UM800033; 1:200 dilution; Origene
Techynologies Inc., Rockville, MD, USA), CD15 (mouse monoclonal
antibody; catalog no. CM073C; 1:200 dilution; Biocare Medical LLC,
Concord, CA, USA), actin (mouse monoclonal antibody; catalog no.
202M-96; 1:150 dilution; Cell Marque, Sigma-Aldrich), HMB-45 (mouse
monoclonal antibody; catalog no. Z-2088; 1:150 dilution; Zeta Co.,
Ltd.), melan-A (mouse monoclonal antibody; catalog no. Z-2052;
1:200 dilution; Zeta Co., Ltd.) and myoglobin (mouse monoclonal
antibody; catalog no. NCL-MYOGLOBIN; 1:150 dilution; Leica
Microsystems, Ltd.). The Ki-67 index (mouse monoclonal antibody;
catalog no. UM800033; 1:200 dilution; Origene Techynologies Inc.)
was ~70% (Fig. 3D). Taken together,
the histomorphological, immunohistochemical and MRI findings
supported a diagnosis of LCS.
Following the surgery, local radiotherapy was
proposed for the patient. Unfortunately, this planned therapy was
abolished as the patient was suffering from septic arthritis and
fever. However, a careful plan to follow up this patient was
established. At ~6 months after the first surgery, a positron
emission tomography-computed tomography (PET-CT) study of the
patient revealed a tumor mass in the left inguinal lymph nodes.
Surgical resection of the left inguinal lymph nodes was performed
in another hospital, Peking Union Medical College Hospital
(Beijing, China), due to practical reasons. LCS metastasis to the
inguinal lymph nodes was confirmed by the subsequent pathological
findings. Unexpectedly, the patient had developed septic arthritis
and fever again. Following postoperative recovery, the patient
received four cycles (21 days for one cycle) of chemotherapy with
cyclophosphamide (1.4 g on day 1), epirubicin hydrochloride (120 mg
on day 1), vindesine (4 mg on day 1) and prednisone (100 mg/day on
days 1–5) at Peking Union Medical College Hospital.
In January 2012, a follow-up PET-CT scan was
performed. A 4.2×3.3 cm soft tissue mass was detected beside the
external iliac with a maximal standardized uptake value
(SUVmax) of around 12.6. Several small lymph nodes
located close to the mass had an SUVmax of ~11.5.
Furthermore, the inguinal lymph nodes in the left groin also had
high SUVs of 5.1–7.1. Between February and March 2012, the patient
received left groin and lateral pelvis local radiotherapy (total
dose, 6,000 cGy; 30 fractions over 47 days) at Peking Union Medical
College Hospital. However, the treatment was ineffective, and the
LCS was found to have metastasized into multiple organs and tissues
(liver, omentum and ascites) 2 months later. The patient succumbed
to the disease in November 2012.
Discussion
LCS is a rare neoplastic proliferation of Langerhans
cells with notable malignant cytological features (3). Sparse reports of LCS are available in
English literature; to the best of our knowledge, only 55 cases
have been reported to date. The available reports are summarized,
together with the present case, in Table
I [(2) and references therein;
(3–39)]. A search of Pubmed was performed to
find these reports using the keyword ‘Langerhans cell sarcoma’ and
manual screening was applied. In these cases, the age at diagnosis
of LCS ranged from 11 months to 88 years, with a median age of 53
years, and a male:female ratio of 1.4:1. The collective findings
also indicate that LCS is extremely aggressive and that it
typically metastasizes to multiple organs or tissues.
 | Table I.Summary of the reported Langerhans
cell sarcoma cases. |
Table I.
Summary of the reported Langerhans
cell sarcoma cases.
| Case | Author, year | Gender/age,
years | Site | Clinical history | Diagnostic
techniques | Therapy | Outcome | Ref. |
|---|
| 1 | Wood, 1984 | M/71 | Cutaneous, lung | NA | EM, X-ray | C | Died at 2 months | (4) |
| 2 | Elleder, 1986 | F/NA | Cutaneous and mucosal
tumors | NA | EM | NA | NA | (5) |
| 3 | Delabie, 1991 | F/23 | Inguinal, iliac and
retroperitoneal adenopathies, skin lesions and interstitial lung
involvement | NA | EM, cytogenetic
study | C | Died of disease | (6) |
| 4 | Tani, 1992 | F/49 | Skin, inguinal
adenopathy | NA | EM, lab | C | Died at 4 years | (7) |
| 5 | Lauritzen, 1994 | M/38 | Skin, lymph nodes,
lung | NA | NA | C | Partial remission
after 12 months | (8) |
| 6 | Itoh, 2001 | F/74 | Skin, axillary
adenopathy | Right hand erythema
lasting a few years | CT, EM | S, R, C | Died at 14
months | (9) |
| 7 | Pileri, 2002 | F/17 | Cervical, groin,
iliac and retroperitoneal adenopathies, and systemic symptoms | NA | EM | C, R | ACR | (10) |
| 8 |
| M/46 | Submandibular and
left cervical adenopathies | NA | EM | C | AWD |
|
| 9 |
| M/28 | Fever, cytopenia,
anterior mediastinal mass and hepatosplenomegaly | NA | EM | None | Died at 3
weeks |
|
| 10 |
| F/50 | Skin | NA | EM | NA | NA |
|
| 11 |
| F/10 | Skin | NA | EM | S, R | ACR |
|
| 12 |
| F/23 | Skin, lymph nodes,
lung | NA | EM | C | Died at 2
years |
|
| 13 |
| F/65 | Generalized
adenopathies, lung lesions and hepatosplenomegaly | NA | EM | C | Died of
disease |
|
| 14 |
| M/72 | Fever, mediastinal
and axillary adenopathies, lung nodules, rib fractures and central
nervous system lesions | NA | EM | C | Died of
disease |
|
| 15 |
| F/50 | Polyostotic bone
lesions | NA | EM | S | ACR |
|
| 16 | Misery, 2005 | F/38 | Skin | NA | Lab | S | ACR | (11) |
| 17 | Kawase, 2005 | M/59 | Spleen, skin, lymph
nodes, bone marrow | Essentially normal,
splenomegaly, no hepatomegaly | None | C | Died at 9
years | (12) |
| 18 | Kawase, 2005 | M/35 | Bone, lymph nodes,
lung, liver | NA | X-ray, MRI, CT | C | Died at 4
years | (12) |
| 19 | Kawase, 2005 | F/62 | Spleen, lymph
nodes, liver | 2-year multiple
bone pain | X-ray, CT | C | Died at 10
months | (12) |
| 20 | Kawase, 2005 | M/60 | Bone | NA | X-ray, MRI | R | ACR | (12) |
| 21 | Jülg, 2006 | M/81 | Mediastinal mass
and interstitial lung involvement | NA | CT, EM | C | Died at 1
month | (13) |
| 22 | Ferringer,
2006 | M/33 | Posterior thigh
skin, lymph nodes | NA | EM | C | ACR | (14) |
| 23 | Lee, 2014 | M/34 | Lung | Smoker, treated and
cured pulmonary tuberculosis | CT | S | ACR | (15) |
| 24 | Diaz-Sarrio,
2007 | M/58 | Skin, lymph
nodes | Liver
transplantation and immunosuppressive therapy | X-ray, EM | S | ACR | (16) |
| 25 | Bohn, 2007 | M/47 | Skin, lymph
nodes | NA | CT, EM | S, R, C | AWD | (17) |
| 26 | López-Ferrer,
2008 | M/67 | Cervical lymph
node | NA | NA | S | NA | (18) |
| 27 | Sumida, 2008 | M/57 | Supraclavicular
lymph node | NA | Lab, EM, IGH | C | Died at 7
months | (19) |
| 28 | Yoshimi, 2008 | F/53 | Skin, lymph nodes,
lung, stomach, liver, spleen, kidneys, bone marrow | Liver failure due
to primary biliary cirrhosis | CT, Lab,
FDG-PET | C | Died at 5
months | (20) |
| 29 | Uchida, 2008 | M/72 | Skin | Otherwise
healthy | FDG-PET, MRI | C | ACR | (21) |
| 30 | Zhao, 2009 | F/74 | Gallbladder, lymph
nodes | NA | Ultrasound, MRI,
CT | S | ACR | (22) |
| 31 | Langfort, 2009 | M/47 | Lung, lymph
nodes | Smoker, 30
packs/year | CT, EM | S, C | AWD | (23) |
| 32 | Diaz-Sarrio,
2007 | M/63 | Skin, lymph
nodes | Liver
transplantation and immunosuppressive therapy | PET | C, R | Died at 5
years | (16) |
| 33 | Nakayama, 2010 | M/62 | Cervical lymph
node | 3-month neck
swelling | CT, FDG-PET | R | ACR | (24) |
| 34 | Ratei, 2010 | M/20 | Supraclavicular
lymph node | ACR from B-cell
acute lymphoblastic leukemia | IGH, CT | C, allo-PBSCT | ACR | (25) |
| 35 | Chen, 2012 | M/53 | Lung,
lymphadenopathy | Heavy smoker,
severe cough | PET-CT | NA | NA | (26) |
| 36 | Muslimani,
2012 | F/66 | Left pyriform
sinus | Hairy cell
leukemia | PET-CT, cytogenetic
study, IGH | C | Died of
disease | (27) |
| 37 | Furmanczyk,
2012 | M/75 | Skin, bone
marrow | 1-year
leukemia | MRI, IGH | S, R, C | Died of
disease | (28) |
| 38 | Shimizu, 2012 | F/67 | Inguinal lymph
node, cervical node | NA | CT, PET | C | ACR | (29) |
| 39 | Wang, 2012 | M/41 | Multifocal
cutaneous, anterior iliac spine, liver, lung | NA | NA | S, C | Died at 1 year | (30) |
| 40 | Xu, 2012 | M/86 | Lymphadenopathy and
splenomegaly | Diabetes, 25-year
arteritis and myocardial infarction | Lab, CT | S, R | Died at 1
month | (31) |
| 41 | Yang, 2013 | M/52 | Lung, lymph nodes,
rib | Healthy smoker | Lab, PET-CT | C | AWD | (32) |
| 42 | Wang, 2013 | F/77 | Lymph nodes and
nasopharyngeal recess mass | 1-month neck
swelling and pain | EM, CT | None | Died at 2
weeks | (2) |
| 43 | Chang, 2013 | F/70 | Lymph node | Chronic myelogenous
leukemia undergoing imatinib mesylate therapy | Lab, cytogenetic
study | S, C | ACR | (33) |
| 44 | Chen, 2013 | F/68 | Inguinal lymph
node | Chronic lymphocytic
leukemia | Cytogenetic
study | C | AWD | (34) |
| 45 | Chung, 2013 | F/11 mo. | Fever, lymph
nodes | NA | Lab, CT, MRI | C | AWD | (35) |
| 46 |
| F/17 | Fever,
hepatosplenomegaly, petechiae, lymph nodes, liver, skin | Unremarkable past
medical and family histories | Lab, CT, MRI | C | AWD |
|
| 47 | Keklik, 2013 | M/39 | Nasopharynx | NA | NA | C | Died | (36) |
| 48 | Li, 2013 | M/48 | Ulcerated
erythematous plaque on right knee, skin nodule | NA | Lab | C | ACR | (37) |
| 49 | Sagransky,
2013 | M/54 | Subcutaneous
nodules, neutropenic fever, Clostridium difficile
colitis | Acute myeloid
leukemia | NA | C | ACR | (38) |
| 50 |
| F/63 | Skin | Unclassifiable
myelodysplastic/myeloproliferative neoplasm, 1-week eruptive
papular violaceous rash | NA | C | Died at 3
months |
|
| 51 |
| M/61 | Scalp ulceration
nodular | Unremarkable past
medical history | NA | S | ACR |
|
| 52 |
| M/88 | Scalp skin
nodule | NA | EM | S | Died at 3
months |
| 53 | Valentin-Nogueras,
2013 | M/71 | Skin, lymph
nodes | Hypertension,
myelodysplastic syndrome | Lab, X-ray, CT | S, R, C | Died at 7
months | (3) |
| 54 | West, 2013 | M/52 |
Lymphadenopathy | NA | NA | C | Died at 8
years | (39) |
| 55 | Lee, 2014 | F/45 | Lymph nodes,
lung | Asymptomatic
erythematous plaques for 4 years on scalp and 1 year on
axillae | CT, PET | C, R | AWD | (15) |
| 56 | Present case | M/75 | Subcutaneous
tissue, inguinal lymph nodes, multiple organs | Tonsillectomy,
subtotal thyroidectomy, partial prostatectomy, ACR from colorectal
cancer, resection of abscess mass of buttock | MRI, PET-CT | C, R | Died at 2
years |
|
The differential diagnosis of LCS from LCH may often
be challenging due to their histological and immunohistochemical
similarities (40). Cytologically,
however, LCS exhibits a markedly higher degree of cytological
atypia and more frequent mitotic features compared with LCH
(10). In the majority of cases, LCS
and LCH are immunohistochemically positive for CD1a, S-100 protein
and langerin (CD207), while negative for CD21 and CD35; however,
LCS commonly has a higher Ki-67 index than LCH (41). Supplementary use of other diagnostic
techniques demonstrates great advantages for differentiating
between LCS and LCH (35). As shown
in Table I, laboratory
investigations, electron microscopy, ultrasound, X-ray, CT, MRI,
FDG-PET and cytogenetic analysis have been used as accessory
techniques in the diagnosis of LCS. In the current case, MRI was
utilized during diagnosis and PET-CT was used in follow-up
examinations.
Notably, the patient in the present case had an
abnormal clinical history compared with other reported cases. The
patient had experienced thyroid adenoma, benign prostate mass and
intramucosal carcinoma. Studies of immunoglobulin heavy chain
rearrangement have demonstrated that LCS may not only develop de
novo, but may originate from LCH (42) or leukemia (27). Hence, it may be of great importance to
analyze the clonal relationship between LCS and other types of
tumor cells, such as thyroid adenoma and intramucosal carcinoma as
seen in the present case. Unfortunately, such studies were not
conducted with the specimen from this patient.
Another phenomenon noted in the present case was
that, following the two surgical resections, the patient suffered
from septic arthritis and fever. The patient had undergone a
resection of a mass of the buttock 4 years prior to his
presentation with the left knee mass, and the pathology had
indicated an abscess. Whether the septic arthritis was a result of
the LCS or was due to the patient's idiosyncrasies was not clear.
Nevertheless, close attention must be paid to a patient's clinical
history in clinical practice, as such information may aid in the
evaluation of the patient's immune surveillance system. The
occurrence of LCS has been reported previously in a patient having
ongoing immunosuppression therapy following a liver transplant
(16).
Due to the rarity of LCS, no standard treatment with
good efficacy has been suggested to date (3). Local resection is commonly applied to
isolated LCS lesions (Table I).
Chemotherapies, such as a modified ESHAP (etoposide, carboplatin,
cytarabine, and methylprednisolone) (20) and MAID (mesna, doxorubicin,
ifosfamide, dacarbazine) (21)
regimens, have been demonstrated to be effective in a proportion of
patients. Radiotherapy has also been reported to be effective in
certain cases. Complete remission, without signs of recurrence or
metastasis for 45 months without adjuvant therapy, was achieved by
a total dose of 59.4 Gy radiotherapy to a cervical lymph node LCS
patient (25). In the current case,
metastasis to inguinal lymph nodes was detected at ~6 months after
the first surgical resection. Furthermore, multiple organs
metastasis was detected following four cycles of chemotherapy with
adjuvant radiotherapy. Considering the poor outcome and prognosis
of LCS, more aggressive and effective standard therapies are
urgently required, and a careful follow-up plan is necessary.
In summary, the present study reported a rare case
of LCS originating from the subcutaneous tissue of the left knee.
The diagnosis, treatment and disease progress of the case were
described, which should aid in expanding the currently available
knowledge concerning LCS.
Acknowledgements
The authors acknowledge Dr. Yan Song (Department of
Radiology, Beijing Hospital) for providing assistance in the
interpretation of the MRI data.
References
|
1
|
Swerdlow SH, Campo E, Harris NL, Jaffe ES,
Pieri SA, Stein H, Thiele J and Vardiman JW: Tumors derived from
Langerhans cellsWorld Health Organization Classification of
Tumours: Pathology and Genetics of Tumours of Haematopoietic and
Lymphoid Tissues. 4th. IARC Press; Lyon, France: pp. 278–289.
2008
|
|
2
|
Wang YN, Zhou XG and Wang Z: Langerhans
cell sarcoma in the cervical lymph node: A case report and
literature review. Acta Haematol. 129:114–120. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Valentín-Nogueras SM, Seijo-Montes R,
Montalván-Miró E and Sánchez JL: Langerhans cell sarcoma: A case
report. J Cutan Pathol. 40:670–675. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wood C, Wood GS, Deneau DG, Oseroff A,
Beckstead JH and Malin J: Malignant histiocytosis X: Report of a
rapidly fatal case in an elderly man. Cancer. 54:347–352. 1984.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Elleder M, Fakan F and Hula M:
Pleiomorphous histiocytic sarcoma arising in a patient with
histiocytosis X. Neoplasma. 33:117–128. 1986.PubMed/NCBI
|
|
6
|
Delabie J, De Wolf-Peeters C, De Vos R,
Vandenberghe E, Kennes K, De Jonge I and Desmet V: True histiocytic
neoplasm of Langerhans' cell type. J Pathol. 163:217–223. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tani M, Ishii N, Kumagai M, Ban M, Sasase
A and Mishima Y: Malignant Langerhans cell tumour. Br J Dermatol.
126:398–403. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lauritzen AF, Delsol G, Hansen NE, Horn T,
Ersbøll J, Hou-Jensen K and Ralfkiaer E: Histiocytic sarcomas and
monoblastic leukemias: A clinical, histologic, and
immunophenotypical study. Am J Clin Pathol. 102:45–54. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Itoh H, Miyaguni H, Kataoka H, Akiyama Y,
Tateyama S, Marutsuka K, Asada Y, Ogata K and Koono M: Primary
cutaneous Langerhans cell histiocytosis showing malignant phenotype
in an elderly woman: Report of a fatal case. J Cutan Pathol.
28:371–378. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pileri SA, Grogan TM, Harris NL, Banks P,
Campo E, Chan JK, Favera RD, Delsol G, De Wolf-Peeters C, Falini B,
et al: Tumours of histiocytes and accessory dendritic cells: An
immunohistochemical approach to classification from the
international lymphoma study group based on 61 cases.
Histopathology. 41:1–29. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Misery L, Godard W, Hamzeh H, Lévigne V,
Vincent C, Perrot JL, Gentil-Perret A, Schmitt D and Cambazard F:
Malignant Langerhans cell tumor: A case with a favorable outcome
associated with the absence of blood dendritic cell proliferation.
J Am Acad Dermatol. 49:527–529. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kawase T, Hamazaki M, Ogura M, Kawase Y,
Murayama T, Mori Y, Nagai H, Tateno M, Oyama T, Kamiya Y, et al:
CD56/NCAM-positive Langerhans cell sarcoma: A clinicopathologic
study of 4 cases. Int J Hematol. 81:323–329. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jülg B, Weidner S and Mayr D: Pulmonary
manifestation of a Langerhans cell sarcoma: Case report and review
of the literature. Virchows Arch. 448:369–374. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ferringer T, Banks PM and Metcalf JS:
Langerhans cell sarcoma. Am J Dermatopathol. 28:36–39. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lee JY, Jung KE, Kim HS, Lee JY, Kim HO
and Park YM: Langerhans cell sarcoma: A case report and review of
the literature. Int J Dermatol. 53:e84–e87. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Diaz-Sarrio C, Salvatella-Danés N,
Castro-Forns M and Nadal A: Langerhans cell sarcoma in a patient
who underwent transplantation. J Eur Acad Dermatol Venereol.
21:973–976. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bohn OL, Ruiz-Argüelles G, Navarro L,
Saldivar J and Sanchez-Sosa S: Cutaneous Langerhans cell sarcoma: A
case report and review of the literature. Int J Hematol.
85:116–120. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
López-Ferrer P, Jiménez-Heffernan JA,
Alves-Ferreira J, Vicandi B and Viguer JM: Fine needle aspiration
cytology of Langerhans cell sarcoma. Cytopathology. 19:59–61.
2008.PubMed/NCBI
|
|
19
|
Sumida K, Yoshidomi Y, Koga H, Kuwahara N,
Matsuishi E, Karube K, Oshima K and Gondo H: Leukemic
transformation of Langerhans cell sarcoma. Int J Hematol.
87:527–531. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yoshimi A, Kumano K, Motokura T, Takazawa
Y, Oota S, Chiba S, Takahashi T, Fukayama M and Kurokawa M: ESHAP
therapy effective in a patient with Langerhans cell sarcoma. Int J
Hematol. 87:532–537. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Uchida K, Kobayashi S, Inukai T, Noriki S,
Imamura Y, Nakajima H, Yayama T, Orwotho N and Baba H: Langerhans
cell sarcoma emanating from the upper arm skin: Successful
treatment by MAID regimen. J Orthop Sci. 13:89–93. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhao G, Meng L, Wu ZY, Liu Q, Zhang B, Gao
RL and Zhang ZQ: Langerhans cell sarcoma involving gallbladder and
perineal lymph nodes: A case report. Int J Surg Pathol. 17:347–353.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Langfort R, Radzikowska E, Czarnowska E,
Wiatr E, Grajkowska W, Błasińska-Przerwa K, Giedronowicz D and
Witkiewicz I: Langerhans cell sarcoma with pulmonary manifestation,
mediastinum involvement and bronchoesophageal fistula. A rare
location and difficulties in histopathological diagnosis. Pneumonol
Alergol Pol. 77:327–334. 2009.PubMed/NCBI
|
|
24
|
Nakayama M, Takahashi K, Hori M, Okumura
T, Saito M, Yamakawa M, Tabuchi K and Hara A: Langerhans cell
sarcoma of the cervical lymph node: A case report and literature
review. Auris Nasus Larynx. 37:750–753. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ratei R, Hummel M, Anagnostopoulos I,
Jähne D, Arnold R, Dörken B, Mathas S, Benter T, Dudeck O, Ludwig
WD and Stein H: Common clonal origin of an acute B-lymphoblastic
leukemia and a Langerhans' cell sarcoma: Evidence for hematopoietic
plasticity. Haematologica. 95:1461–1466. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen YW, Chang CC, Hou PN, Yin SL, Lai YC
and Hou MF: The characteristics of FDG PET/CT imaging in pulmonary
Langerhans cell sarcoma. Clin Nucl Med. 37:495–497. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Muslimani A, Chisti MM, Blenc AM, Boxwala
I, Micale MA and Jaiyesimi I: Langerhans/dendritic cell sarcoma
arising from hairy cell leukemia: A rare phenomenon. Ann Hematol.
91:1485–1487. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Furmanczyk PS, Lisle AE, Caldwell RB,
Kraemer KG, Mercer SE, George E and Argenyi ZB: Langerhans cell
sarcoma in a patient with hairy cell leukemia: Common clonal origin
indicated by identical immunoglobulin gene rearrangements. J Cutan
Pathol. 39:644–650. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shimizu I, Takeda W, Kirihara T, Sato K,
Fujikawa Y, Ueki T, Sumi M, Ueno M, Ichikawa N, Kobayashi H, et al:
Long-term remission of Langerhans cell sarcoma by AIM regimen
combined with involved-field irradiation. Rinsho Ketsueki.
53:1911–1915. 2012.(In Japanese). PubMed/NCBI
|
|
30
|
Wang C, Chen Y, Gao C, Yin J and Li H:
Multifocal Langerhans cell sarcoma involving epidermis: A case
report and review. Diagn Pathol. 7:992012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xu Z, Padmore R, Faught C, Duffet L and
Burns BF: Langerhans cell sarcoma with an aberrant cytoplasmic CD3
expression. Diagn Pathol. 7:1282012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yang CJ, Lee JY, Wu CC, Yin HL, Lien CT
and Liu YC: An unusual pulmonary mass with mediastinal invasion and
multiple intrapulmonary nodules in a 52-year-old man. Chest.
141:253–258. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chang NY, Wang J, Wen MC and Lee FY:
Langerhans cell sarcoma in a chronic myelogenous leukemia patient
undergoing imatinib mesylate therapy: A case study and review of
the literature. Int J Surg Pathol. 22:456–463. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chen W, Jaffe R, Zhang L, Hill C, Block
AM, Sait S, Song B, Liu Y and Cai D: Langerhans cell sarcoma
arising from chronic lymphocytic lymphoma/small lymphocytic
leukemia: Lineage analysis and BRAF V600E mutation study. N Am J
Med Sci. 5:386–391. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chung WD, Im SA, Chung NG and Park GS:
Langerhans cell sarcoma in two young children: Imaging findings on
initial presentation and recurrence. Korean J Radiol. 14:520–524.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Keklik M, Sivgin S, Kontas O, Abdulrezzak
U, Kaynar L and Cetin M: Langerhans cell sarcoma of the
nasopharynx: A rare case. Scott Med J. 58:e17–e20. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li Y, Li B, Tian XY and Li Z: Unusual
cutaneous Langerhans cell sarcoma without extracutaneous
involvement. Diagn Pathol. 8:202013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sagransky MJ, Deng AU and Magro CM:
Primary cutaneous langerhans cell sarcoma: A report of four cases
and review of the literature. Am J Dermatopathol. 35:196–204. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
West DS, Dogan A, Quint PS, Tricker-Klar
ML, Porcher JC, Ketterling RP, Law ME, McPhail ED, Viswanatha DS,
Kurtin PJ, et al: Clonally related follicular lymphomas and
Langerhans cell neoplasms: Expanding the spectrum of
transdifferentiation. Am J Surg Pathol. 37:978–986. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhao G, Luo M, Wu ZY, Liu Q, Zhang B, Gao
RL and Zhang ZQ: Langerhans cell sarcoma involving gallbladder and
peritoneal lymph nodes: A case report. Int J Surg Pathol.
17:347–353. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Orii T, Takeda H, Kawata S, Maeda K and
Yamakawa M: Differential immunophenotypic analysis of dendritic
cell tumours. J Clin Pathol. 63:497–503. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lee JS, Ko GH, Kim HC, Jang IS, Jeon KN
and Lee JH: Langerhans cell sarcoma arising from Langerhans cell
histiocytosis: A case report. J Korean Med Sci. 21:577–580. 2006.
View Article : Google Scholar : PubMed/NCBI
|