Introduction
Traditional theories have supported that schwannomas
originate from the optic and olfactory nerve, which, however, is
rather unlikely, since these two nerves do not have a Schwann cell
layer (1). Olfactory ensheathing cell
tumor (OECT) is considered to originate from OECs of the olfactory
mucosa or bulb. Not until Yasuda et al reported a case of
OECT in 2006 did researchers start to focus on this rare type of
tumor (2). Despite its rarity, ~50
cases of subfrontal olfactory groove schwannoma (OGS) and 8 cases
of OECT, with clinical and radiological features similar to those
of meningiomas and neuroblastomas in the midline of the anterior
cranial fossa, have been published worldwide to date (2–11). The
biological behaviour of OECT is currently unknown; The previous
cases reported a benign course and a short follow-up time (2,5–11). OECs resemble Schwann cells on light
and electron microscopy; however, the presence of cluster of
differentiation 57, also known as Leu-7, is considered to
differentiate OGS from OECT (2). OECT
may be treated surgically using the endoscopic transnasal approach
or craniotomy. OECT has an extremely good prognosis, with a
reported 2-year survival rate of 100% (2,5–11). The present study reports the ninth
case of OECT, which included confusing radiographic features, and
presents a review of the literature.
Case report
On March 10, 2014, a 34-year-old woman was admitted
to The Second Affiliated Hospital of Dalian Medical University
(Dalian, China) presenting with a history of hyposmia for 1 year,
accompanied by a gradual dizziness and emotional lability for 2
months. No seizures, visual disorder, cerebrospinal fluid
rhinorrhea or neurofibromatosis-related family history was
recorded. Physical examination detected neither focal neurological
deficits nor abnormal pigmentation of neurofibromatosis, with the
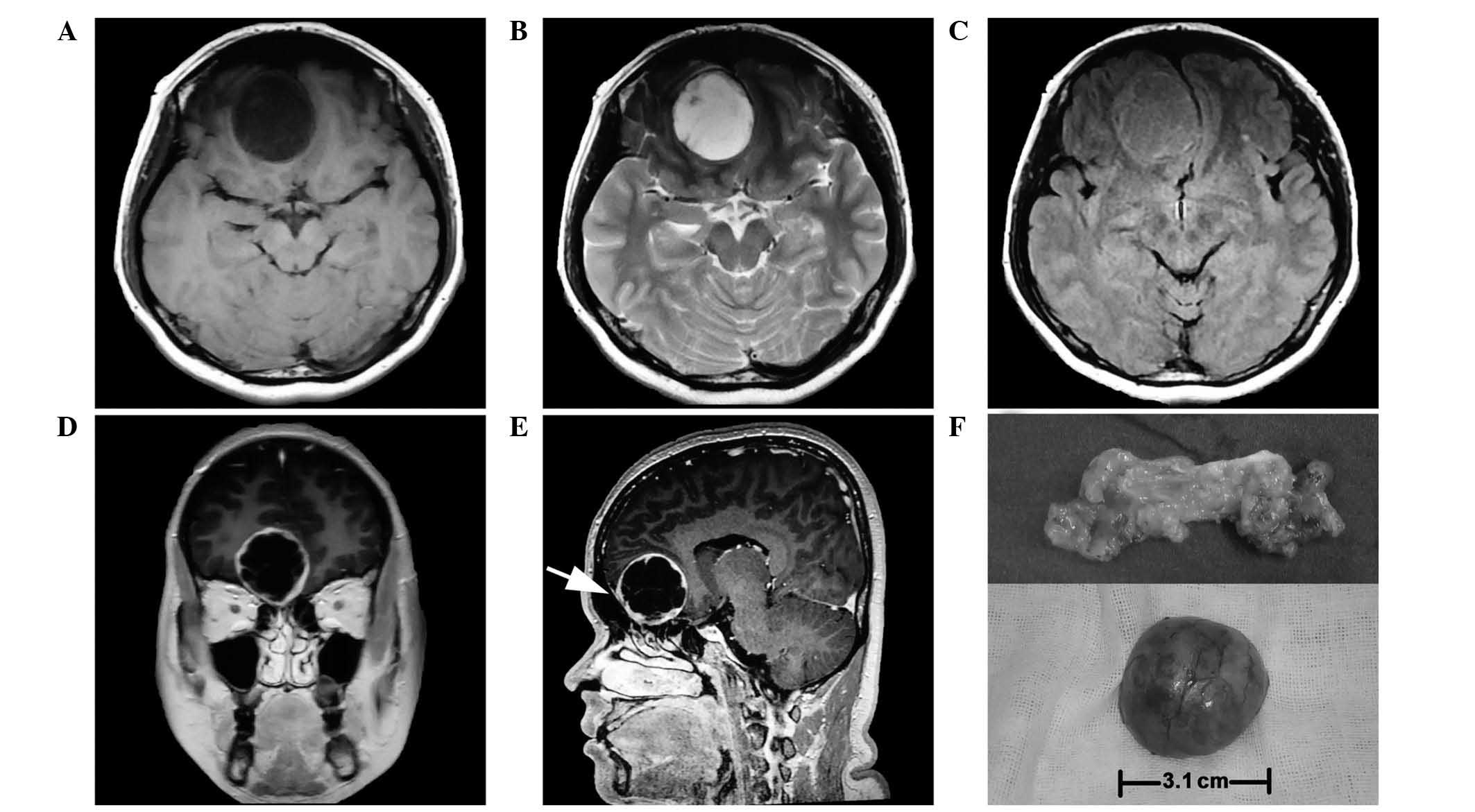
exception of right hyposmia. Magnetic resonance imaging (MRI) scan
(MR Signa 3.0T Excite; GE Healthcare Bio-Sciences, Pittsburgh, PA,
USA) revealed a 3.0×3.0×3.1-cm extra-axial, globose, well-defined
mass at the midline of the anterior cranial fossa, which deviated
to the right. The tumor displayed homogeneous hypointensity on
T1-weighted images, hyperintensity on T2-weighted images and
isointensity on fluid-attenuated inversion recovery, and was shown
to have caused brain parenchyma deformation without obvious
peritumoral edema (Fig. 1A, B and C).
Following the administration of intravenous gadolinium
(Sigma-Aldrich, St. Louis, MO, USA), the tumor was heterogeneously
enhanced and a peculiar membrane was observed inside the cystic
wall. The tumor boundary appeared clear and smooth, while the main
part was tightly connected to the endocranium (Fig. 1D and E).
The patient underwent bifrontal craniotomy. When the
right frontal lobe was softly lifted, a grayish red tumor with a
glistening appearance was observed to be attached to the olfactory
groove. The tumor was located in the intradural, extra-axial space,
attached to the right anterior part of the cribriform plate. A
large volume of a clear, non-congealable yellow liquid was
extracted from inside the tumor. In addition, the right olfactory
tract could not be identified, and the left olfactory tract had
been squeezed by the tumor. The tumor was completely resected, and
when dissected, it appeared to contain fat, as it was soft and
yellow (Fig. 1F), however subsequent
pathology revealed that fat was not present. The cribriform plate
protruded slightly towards the nasal cavity; however, the bone
cortex was intact. Using a microscope (MT4000D; Meiji Seika Kaisha,
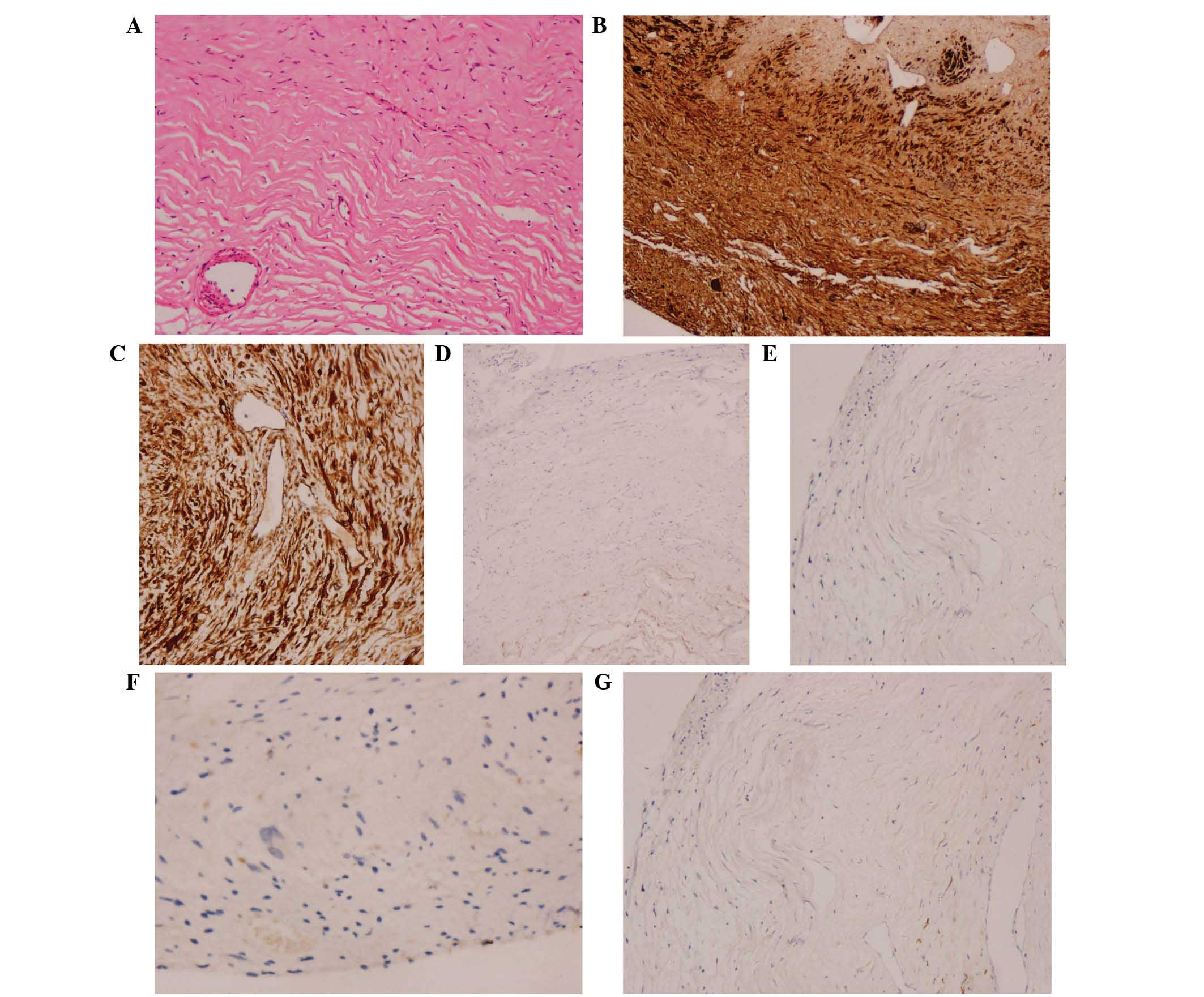
Ltd., Tokyo, Japan), the tumor tissue was examined histologically
and the findings described a tumor composed of spindle cells, with
an eosinophilic protoplasm and tadpole-shaped nucleus. No tumor
necrosis or blood vessel hyperplasia was observed (Fig. 2A). Immunostaining results revealed
positivity for S100 protein (polyclonal rabbit anti-human S100
antibody; cat. no. ENT4197; 1:10; Elabscience Biotechnology Co.,
Ltd, Wuhan, China) (Fig. 2B) and
vimentin (polyclonal rabbit anti-human antibody; cat. no. ENT4879;
1:10; Elabscience Biotechnology Co., Ltd.) (Fig. 2C), and negativity for epithelial
membrane antigen (monoclonal rat anti-human antibody; cat. no.
BM0042; 1:10; Boster Inc., Wuhan, China) (Fig. 2D), glial fibrillary acidic protein
(polyclonal chicken anti-mouse cat. no. ab4674; 1:10; Abcam,
Cambridge, MA, USA) (Fig. 2E) and
Leu-7 (monoclonal mouse anti-human antibody; cat. no. ab187274;
1:10; Abcam) (Fig. 2F). The Ki-67
(polyclonal rabbit anti-human antibody; cat. no. EPP14636; 1:10;
Elabscience Biotechnology Co., Ltd.) index was 1% (Fig. 2G); therefore, the final pathological
diagnosis was OECT. The postoperative course of the patient was
uneventful, without adjuvant radiation and chemotherapy. No
evidence of tumor recurrence was observed during the 6-month
radiographic follow-ups.
Discussion
A literature search for relevant publications was
performed in the following databases: PubMed (www.ncbi.nlm.nih.gov/pubmed), Web of Science
(http://isiknowledge.com), MEDLINE (http://ovidsp.ovid.com/), Excerpta Medica dataBASE
(www.elsevier.com/solutions/embase-biomedical-research)
and Chinese Biomedical Database (www.sinomed.ac.cn/zh/), using the keywords ‘olfactory
ensheathing cell tumor’ and ‘olfactory groove schwannoma’ in titles
and/or abstracts. The search identified 8 cases of OECT (Table I). A review of these 8 cases in
addition to the present one revealed that the initial symptoms of
the patients included hyposmia or anosmia (6/7 patients; 86%;
initial symptoms were not reported in 2 cases), seizures (4/9
patients; 44%), emotional lability (2/9 patients; 22%), headache
and dizziness (3/9 patients; 33%), visual impairment (1/9 patients;
11%) and foreign body sensation (1/9 patients; 11%). The studies
did not mention whether the patients that suffered the seizures
were suspected of having OECT. A possible explanation for the
seizures is dualism, since OECT is an extra-axial tumor, which
makes it less likely for patients to develop seizures and thus,
OECT and seizures may be derived from two separate pathologies. The
mean age of the 9 patients reviewed was 31.9 years, which was
consistent with that of patients with OGS (32.7±14.0 years)
(12), and a female predominance was
observed (females, 67%; males, 33%). In addition, radiographic
evaluation revealed that 2/9 OECTs (22%) were cystic, 3/9 (33%)
cystic-solid and 4/9 (44%) solid. Of note, the proportion of septum
in cystic tumors was 60% (n=3/5), which was quite different from
the majority of the cystic OGSs. If further studies confirm this
finding, it may serve as an important radiographic clue for the
diagnosis of OECTs. Furthermore, 7/9 OECTs (78%) were
heterogeneously enhanced, while 2/9 (22%) were homogeneously
enhanced. Bone erosion was a rather common finding (6/9 cases;
67%), whereas none of the patients presented with peritumoral
edema. Intraoperative findings revealed that, out of the 9 OECTs, 8
originated from the olfactory bulb or tract, and 1 originated from
the olfactory mucosa (9).
 | Table I.Summary of 9 reported cases of
olfactory ensheathing cell tumor. |
Table I.
Summary of 9 reported cases of
olfactory ensheathing cell tumor.
| Case | Author (year) | Agea/gender | Clinical
features | Olfaction
disorder | Tumor appearance | Bone erosion | Septum (if
cystic) | Enhancement | Edema | Neurof. | Detection of
olfactory nerves | Ref |
|---|
| 1 | Yasuda et al
(2006) | 31/F | Seizures | Right hyposmia | Round,
cystic-solid | Yes | Yes | Heterogenous | No | No | Right absent | (2) |
| 2 | Ippili et al
(2009) | 42/M | Seizures | Normal | Irregular, solid | No | N.A. | Heterogenous | No | No | Left absent | (5) |
| 3 | Darie et al
(2010) | 28/F | Seizures, emotional
lability | Anosmia | Irregular, cloudy,
solid | Yes | N.A. | Heterogenous | No | No | Left adherent to
tumor | (6) |
| 4 | Lin et al
(2010) | 32/M | Seizures | N.M. | Round, solid | No | N.A. | Heterogenous | No | No | Left absent | (7) |
| 5 | Yamaguchi et
al (2010) | 30/F | Headache | Right anosmia | Round, solid | Yes | N.A. | Homogenous | No | N.M. | Right absent | (8) |
| 6 | Ogino-Nishimura et
al (2012) | 41/F | Headache | Anosmia | Irregular,
cystic | Yes | No | Heterogenous | No | N.M. | Both sides
detected | (9) |
| 7 | Al-Ghanem et
al (2013) | 49/M | Visual
impairment | Hyposmia | Round,
cystic-solid | Yes | No | Homogenous | No | N.M. | N.M. | (10) |
| 8 | Qi et al
(2015) | 45/F | Foreign body
sensation | N.M. | Irregular,
cystic | No | Yes | Heterogenous | No | No | N.M. | (11) |
| 9 | Liu et al
(2016) | 34/F | Dizziness, emotional
lability | Right hyposmia | Round,
cystic-solid | Yes | Yes | Heterogenous | No | No | Right absent | Present |
OECs are specialized glial cells capable of
migrating even in astrocyte-rich environments; they ensheathe
olfactory axons in order to facilitate axonal growth. Due to these
characteristics, OECs have attracted considerable attention
(11,13). Notably, OECs resemble Schwann cells in
their appearance on light and electron microscopy, and in the
majority of immunohistochemical staining features. The
differentiation between OGS and OECT is mainly based on the
presence of Leu-7 (2,5,14). All the
cases of OECT reviewed in the present study were negative for Leu-7
(2,5–11);
however, in the majority of OGS cases, testing for Leu-7 was either
not performed or not mentioned in the corresponding studies
(4,12,15).
Furthermore, information on the Leu-7 reactivity in OGS is limited;
~20% of the tumors that were considered to be schwannomas were
negative for Leu-7 (9,16); therefore, certain reported cases of
OGS could have originated from OECs rather than Schwann cells.
In the present case, the radiological manifestations
of the OECT were confusing, as a dural tail sign was evident on
enhanced MRI and bone sclerosis and calcification were identified
on computed tomography, which are similar to those observed with
OGM (9). The differential diagnosis
of tumors involving the extra-axial anterior cranial fossa, with or
without bulging into the ethmoid sinus, should include OGM, OGS,
OECT, low-grade astrocytoma, olfactory neuroblastoma and metastatic
tumors. In the present case, the extra-axial tumor with the clear
boundary that was observed to be connected to the endocranium,
could have potentially been misdiagnosed as a dural tail sign on
sagittal view MRI following contrast-enhancement. The presence of
bone scalloping, as well as the absence of bone sclerosis and
calcification, may assist the differentiation between OECTs and
meningiomas (17). By contrast, OGMs
exhibit a propensity to spread into the paranasal sinuses,
particularly in recurrent cases, without displaying evidence of
calcification. Furthermore, multiple foci of T2-hypointense MRI
signals (associated with microbleeds) may suggest schwannoma
(5). Table
II comprises a list of the main differences among OGM, OGS,
OECT, low-grade astrocytoma, olfactory neuroblastoma and metastatic
tumors, based on radiologic manifestations, immunostaining results
and possible origin.
 | Table II.List of main differences among OGM,
OGS, OECT, ON, Ast and Met, based on the radiologic manifestations
and immunostaining results of the present and previous reports. |
Table II.
List of main differences among OGM,
OGS, OECT, ON, Ast and Met, based on the radiologic manifestations
and immunostaining results of the present and previous reports.
|
| Typical radiologic
manifestation (CT and MRI scans) | Immunostaining
results |
|
|---|
|
|
|
|
|
|---|
| Tumor | Calcification | Tumor appearance | Enhancement | Edema | Other
manifestations | GFAP | S100 | Vim | Leu-7 | EMA | Possible
origin |
|---|
| OGM | Mostly | Solid | Homogenous | Partial | Dural tail sign,
lobulation, tendency to spread into, the paranasal sinuses | – | ± | + | – | + | Arachnoidal cap
cells |
| OGS | Rare | Cystic, solid |
Heterogenous/Homogenous | Partial when
giant | The olfactory
groove is an apparent origin but it can extend to one side, bone
erosion, lobulation | – | + | + | + | – | Aberrant Schwann
cells, Schwann cells located 0.5 mm beyond the olfactory bulb,
meningeal branches of trigeminal nerves, anterior ethmoidal nerves,
nerve plexus of dural vessels |
| OECT | Partial | Cystic, solid |
Heterogenous/Homogenous | Rare | Incomplete
olfactory nerves, bone erosion | – | + | + | – | – | OECs originating
from the olfactory mucosa and bulb |
| ON | Partial | Solid |
Heterogenous/Homogenous | Partial | Mainly grow in the
nasal cavity, infiltration, bone erosion | + | N.A. | + | – |
| Sensory neurons of
the olfactory mucosa involving the superior nasal cavity and
cribriform plate (NSE+) |
| Ast | Partial | Cystic, solid | Heterogenous | Partial | Intra-axial
lesions, infiltration into the frontal lobe | + | + | + | – | – | Neuroepithelial
cells |
| Met | Rare | Cystic, solid | Heterogenous | Mostly,
obvious | Multiple, small
lesions, clear boundary | Depends on primary
lesions | Depends on primary
lesions |
In previous reports, the aforementioned conditions
were misdiagnosed as each other preoperatively, due to their rarity
and enigmatic origin. The embryonic development of OEC is olfactory
placode-derived, while that of Schwann cells is neural
crest-derived; therefore, both tumors possess a similar peripheral
origin (1). The following five main
origins have been reported for OGS to date: i) Aberrant Schwann
cells; ii) Schwann cells located 0.5 mm beyond the olfactory bulb;
iii) meningeal branches of trigeminal nerves; iv) nerve plexus of
dural vessels; and v) anterior ethmoidal nerves (4). Glial cells originate from the central
nervous system (CNS), however, OECT has been demonstrated to
originate from OECs of the olfactory mucosa or bulb, which are part
of the peripheral nervous system (PNS). Thus, OECs may be
considered an intermediate glial cell type that originate in the
CNS, but function in the PNS. Furthermore, olfactory neuroblastoma
has been reported to originate from the sensory neurons of the
olfactory mucosa, and to involve the superior nasal cavity or the
cribriform plate, while OGM has been reported to originate from
arachnoidal cap cells as a truism (7,18).
Although the reported cases experienced a benign
course, the follow-up for these cases are too short, and the origin
of OECT, its biological behavior and the necessity for adjuvant
chemotherapy remains uncertain. In conclusion, according to the
limited number of clinical cases of OECT reported in the literature
thus far, total surgical removal of the tumor remains the suggested
treatment option, and long-term follow-up appears to be
required.
References
|
1
|
Wewetzer K, Verdú E, Angelov DN and
Navarro X: Olfactory ensheathing glia and Schwann cells: Two of a
kind? Cell Tissue Res. 309:337–345. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yasuda M, Higuchi O, Takano S and
Matsumura A: Olfactory ensheathing cell tumor: A case report. J
Neurooncol. 76:111–113. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Salunke P, Patra DP, Futane S and Nada R:
Olfactory region schwannoma: Excision with preservation of
olfaction. J Neurosci Rural Pract. 5:281–283. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Figueiredo EG, Soga Y, Amorim RL, Oliveira
AM and Teixeira MJ: The puzzling olfactory groove schwannoma: A
systematic review. Skull Base. 21:31–36. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ippili K, Ratnam BG, Gowrishankar S,
Ranjan A and Lath R: Olfactory ensheathing cell tumor. Neurol
India. 57:76–78. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Darie I, Riffaud L, Saïkali S, Brassier G
and Hamlat A: Olfactory ensheathing cell tumour: Case report and
literature review. J Neurooncol. 100:285–289. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lin SC, Chen MH, Lin CF and Ho DM:
Olfactory ensheathing cell tumor with neurofibroma-like features: A
case report and review of the literature. J Neurooncol. 97:117–122.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yamaguchi T, Fujii H, Dziurzynski K,
Delashaw JB and Watanabe E: Olfactory ensheathing cell tumor: Case
report. Skull Base. 20:357–361. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ogino-Nishimura E, Nakagawa T, Mikami Y
and Ito J: Olfactory ensheathing cell tumor arising from the
olfactory mucosa. Case Rep Med. 2012:4268532012.PubMed/NCBI
|
|
10
|
Al-Ghanem R, Ramos-Pleguezuelos FM,
Pérez-Darosa SI, Galicia-Bulnes JM, Cabrerizo-Carvajal F and
El-Rubaidi OA: Olfactory ensheathing cell tumour: Case report and
literature review. Neurocirugia (Astur). 24:130–134. 2013.(In
Spanish). View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Qi X, Wan Y, Yan Q, Wang Y and Yang S:
Cystic olfactory ensheathing cell tumor: A case report. Acta Neurol
Belg. 115:191–193. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li YP, Jiang S, Zhou PZ and Ni YB:
Solitary olfactory schwannoma without olfactory dysfunction: A new
case report and literature review. Neurol Sci. 33:137–142. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Vincent AJ, Taylor JM, Choi-Lundberg DL,
West AK and Chuah MI: Genetic expression profile of olfactory
ensheathing cells is distinct from that of Schwann cells and
astrocytes. Glia. 51:132–147. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bianco JI, Perry C, Harkin DG, Mackay-Sim
A and Féron F: Neurotrophin 3 promotes purification and
proliferation of olfactory ensheathing cells from human nose. Glia.
45:111–123. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang Z, Zhang W, You G, Wang J, Li G, Gao
Z and Xie J: Olfactory schwannoma: A report of two cases and
literature review. Neurol India. 62:429–431. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Johnson MD, Glick AD and Davis BW:
Immunohistochemical evaluation of Leu-7, myelin basic-protein,
S100-protein, glial-fibrillary acidic-protein, and LN3
immunoreactivity in nerve sheath tumors and sarcomas. Arch Pathol
Lab Med. 112:155–160. 1988.PubMed/NCBI
|
|
17
|
Santhosh K, Kesavadas C, Radhakrishnan VV,
Thomas B, Kapilamoorthy TR and Gupta AK: Usefulness of T2*-weighted
MR sequence for the diagnosis of subfrontal schwannoma. J
Neuroradiol. 34:330–333. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yamahata H, Hirahara K, Tomosugi T, Yamada
M, Ishii T, Ishigami T, Uetsuhara K, Sueyoshi K, Matsukida S,
Yatsushiro K and Arita K: Subfrontal schwannoma mimicking
neuroblastoma: Case report. Skull Base Rep. 1:59–64. 2011.
View Article : Google Scholar : PubMed/NCBI
|