Introduction
Osteosarcoma, a high-grade malignant tumor
associated with a 5-year survival rate of 37% (1), is the most frequent primary bone tumor,
and occurs mainly in children and adolescents (2,3). It is a
highly malignant tumor that is often transferred via the blood
stream to the lung, liver and other vital organs. The majority of
current protocols for the treatment of osteosarcoma include a
period of preoperative (neoadjuvant) chemotherapy (4). Chemotherapy drugs, including adriamycin
(ADR), methotrexate and cyclophosphamide, are also applied in
patients with osteosarcoma to further kill osteosarcoma cells
following amputation surgery (1–3). However,
upon long-term exposure of the tumor cells to chemotherapy drugs,
the surface of the tumor cells may overexpress P-glycoprotein
(P-gp), an adenosine triphosphate-dependent drug efflux pump
encoded by the multidrug resistance protein 1 (MDR1) gene (5,6).
Overexpressed P-gp can mediate the efflux of a large number of
intracellular chemotherapy drugs, thus leading to a significant
reduction in the intracellular drug concentration (7,8), which
causes drug resistance of tumor cells. Therefore, a reduction in
the efflux of chemotherapy drugs can increase the concentration of
chemotherapeutic agents in tumor cells and reverse the phenomenon
of tumor drug resistance by inhibiting the function of P-gp or
reducing P-gp expression (9–11).
It is well known that P-gp expression is closely
associated with the nuclear factor (NF)-κB signaling pathway
(12), the mitogen-activated protein
kinase (MAPK) signaling pathway (13), cylooxygenases-2 (14) and phosphoinositide 3-kinase (15). NF-κB can bind to the NF-κB binding
sites in the MDR1 promoter region, which results in the activation
of the transcription of the MDR1 gene (16). In addition, p38 MAPK may regulate P-gp
expression through the activation of NF-κB expression (17). Thereby, the NF-κB and MAPK signaling
pathways play significant roles in the molecular mechanisms of
P-gp-mediated multidrug resistance.
Resveratrol (trans-3,4,5-trihydroxystilbene) is a
plant polyphenol present in grapes, peanuts and various other
plants, and has potent effects in reversing multidrug resistance
(18). Quan et al has reported
that resveratrol successfully reversed multidrug resistance in
KBv200 cells by downregulation of MDR1/P-gp (19). However, the reversal mechanism of
multidrug resistance is still unknown. The present study aimed to
investigate whether resveratrol could reverse the phenomenon of
multidrug resistance in U2OS/ADR cells, an ADR-resistant human
osteosarcoma cell line, and to investigate the molecular
mechanisms.
Materials and methods
Chemicals
Resveratrol of >99% purity was purchased from
Dalian Meilun Biotech Co., Ltd. (Dalian, China). ADR was purchased
from Shenzhen Main Luck Pharmaceuticals, Inc. (Shenzhen, China),
while 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
(MTT) was obtained from USB Corporation (Cleveland, OH, USA).
Anti-p38 (phosphorylated and total; catalog nos. sc-7972 and
sc-7973, respectively) and anti-p65 (total; catalog no. sc-8008)
antibodies were purchased from Santa Cruz Biotechnology, Inc.
(Dallas, TX, USA). Anti-p65 (acetylate; catalog no. A16567) was
purchased from Thermo Fisher Scientific, Inc. (Waltham, MA, USA).
Antibodies against β-actin (catalog no. ab8226) and MDR1 (catalog
no. ab3366) were purchased from Abcam (Cambridge, MA, USA). High
glucose Dulbecco's modified Eagle (DMEM) medium and fetal bovine
serum (FBS) were provided by Gibco (Thermo Fisher Scientific,
Inc.). All other analytical grade chemicals used in the present
study were readily available from commercial sources.
Cell culture
U2OS cells were purchased from Nanjing KeyGen
Biotech Co., Ltd. (Nanjing, China) and were cultured in high
glucose DMEM supplemented with 10% FBS, 100 U/ml penicillin and 100
µg/ml streptomycin. Upon culture of U2OS cells in DMEM with 0.01,
0.04, 0.1, 0.4, 1.0 and 4.0 µg/ml ADR for 6 months, U2OS/ADR cells
were successfully induced. Then, U2OS/ADR cells steadily grew in
high DMEM containing ADR (4.0 µg/ml). All cells were kept in an
incubator at 37°C with 95% humidity and 5% CO2.
Cytotoxicity assay and multidrug
resistance reversal assay
Chemosensitivity in vitro was measured by
means of MTT colorimetric assay performed in 96-well plates. U2OS
and U2OS/ADR cells (1×104 cells/ml) were inoculated into
each well with 90 µl culture medium. Following overnight
incubation, various concentrations of ADR (10 µl) with or without
resveratrol were added to the cultures. Upon incubation for 48 h,
10 µl of MTT reagent [5 mg/ml in phosphate-buffered saline (PBS)]
was added to each well, and left to incubate for an additional 4 h.
A 100 µl aliquot of sodium dodecyl sulfate (SDS)-isobutanol-HCl
solution (5% isobutanol, 10% SDS and 12 µM HCl) was added and left
to incubate overnight. Relative cell viability was obtained on a
microplate reader (Bio-Rad Laboratories, Inc., Hercules, CA, USA)
with a 570-nm filter.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted using TRIzol®
reagent (Invitrogen; Thermo Fisher Scientific, Inc.), according to
the manufacturer's protocol. RNA pellets were resuspended in
diethyl pyrocarbonate-treated deionized water. RNA samples were
analyzed by 15% agarose gel electrophoresis, and integrity was
examined by visualization of intact 18S and 28S ribosomal RNA under
ultraviolet light. Total RNA (1 µg) was used to prepare
complementary (c)DNA by RT using a PrimeScript™ RT Reagent kit
(Takara Biotechnology Co., Ltd., Dalian, China). The primer
sequences were as follows: MDR1, forward (F)
5′-GGAGCCTACTTGGTGGCACATAA-3′ and reverse (R)
5′-TGGCATAGTCAGGAGCAAATGAAC-3′ (20);
and β-actin, F 5′-ATTGAACACGGCATTGTCAC-3′ and R
5′-CATCGGAACCGCTCATTG-3′. The cDNA was amplified using
SYBR® Premix Ex Taq kit (Takara Biotechnology Co., Ltd.)
in a M×3000P instrument (Agilent Technologies, Inc., Santa Clara,
CA, USA). The PCR conditions were as follows: 1 cycle of
denaturation at 95°C for 30 sec, followed by 40 cycles of
denaturation at 95°C for 5 sec and annealing at 60°C for 34 sec.
The PCR products were analyzed using the ΔΔCq method by ABI
PRISM® 7500 Real-Time PCR System (Applied Biosystems;
Thermo Fisher Scientific, Inc.), and compared to the housekeeping
gene β-actin (20).
Western blotting
Upon incubation with or without resveratrol
solutions for 48 h, U2OS and U2OS/ADR cells were collected and
washed with PBS. Proteins were extracted using a total protein
extraction kit (Nanjing KeyGen Biotech Co., Ltd.), according to the
manufacturer's protocol. The proteins were separated by
centrifugation at 12,000 × g for 30 min. Protein concentrations
were measured using a bicinchoninic acid protein assay kit (Nanjing
KeyGen Biotech Co., Ltd.). Proteins (60 µg) were resuspended in
electrophoresis sample buffer containing β-mercaptoethanol and
subjected to 10% SDS-polyacrylamide gel electrophoresis. Proteins
were transferred onto a polyvinylidene difluoride membrane (EMD
Millipore, Billerica, MA, USA), and then membranes were blocked
using 5% non-fat milk in Tris-buffered saline with 0.1% Tween 20
(TBST) for 2 h at 37°C. β-actin served as a loading control.
Membranes were incubated overnight at 4°C with a 1:1,500 dilution
of polyclonal antibodies against MDR1 and β-actin (Abcam), and with
a 1:500 dilution of monoclonal antibodies against p38
(phosphorylated and total) and p65 (acetylated and total) (Santa
Cruz Biotechnology, Inc.). Upon incubation with the primary
antibody, membranes were rinsed three times with TBST and incubated
for 2 h at 37°C with a 1:1,500 dilution of anti-mouse horseradish
peroxidase-conjugated secondary antibody (catalog no. sc-8008;
Invitrogen, Thermo Fisher Scientific, Inc.). According to the
manufacturer's protocol, membranes were exposed to Enhanced
Chemiluminescence Plus reagent from Beyotime Institute of
Biotechnology (Haimen, China), following extensive washing with
TBST. The emitted light was documented with a BioSpectrum 410
multispectral imaging system with a Chemi HR 410 camera (UVP, LLC,
Upland, CA, USA). Protein bands were visualized and photographed
under transmitted ultraviolet light. Images were used for
semiquantitative measurements based on band densitometry.
Accumulation of ADR
U2OS and U2OS/ADR cells (5×105 cells/ml)
were seeded in 6-well plates. Following incubation in DMEM
containing resveratrol (100 µM) at 37°C for 48 h, U2OS and U2OS/ADR
cells were incubated with 10 µM ADR for 1 h at 37°C, and then
washed three times with ice-cold PBS. The fluorescence intensity of
intracellular ADR was determined by flow cytometry with an
excitation wavelength of 488 nm and an emission wavelength of 575
nm (BD Biosciences, Franklin Lakes, NJ, USA) (21).
Small interfering (si)RNA
transfection
According to the manufacturer's protocol, U2OS/ADR
cells (5×105 cells/ml) were seeded in 6-well plates and
transfected with specific siRNAs against p65 and p38 (Shanghai
GenePharma Co., Ltd., Shanghai, China) at a concentration of 100 nM
using Lipofectamine™ 2000 reagent (Invitrogen; Thermo Fisher
Scientific, Inc.). Cells transfected with control siRNA were pooled
and used as a negative control. The sequences of the siRNA
targeting human p65 were: Sense, 5′-GAUUGAGGAGAAACGUAAA-3′ and
antisense, 5′-UUUACGUUUCUCCUCAAUC-3′ (22). The siRNA sequences used to target
human p38 (23) were: Sense,
5′-CCCUGUAAAGCUUUCAGAA-3′ and antisense, 5′-UUCUGAAAGCUUUACAGGG-3′.
The transfected cells were incubated at 37°C in serum-free DMEM.
After transfection for 6 h, cells were cultured in DMEM with 10%
FBS. After growing for additional 48 h, cells were collected for
western blot analysis to determine the levels of the indicated
proteins.
Statistical analysis
Statistical analysis was performed using SPSS
version 11.0 software (SPSS, Inc., Chicago, IL, USA). Group data
were expressed as the mean ± standard deviation. Statistically
significant differences of data from two sets were compared using
one-way analysis of variance. P<0.05 was considered to indicate
a statistically significant difference.
Results
Multidrug resistance of U2OS/ADR
cells
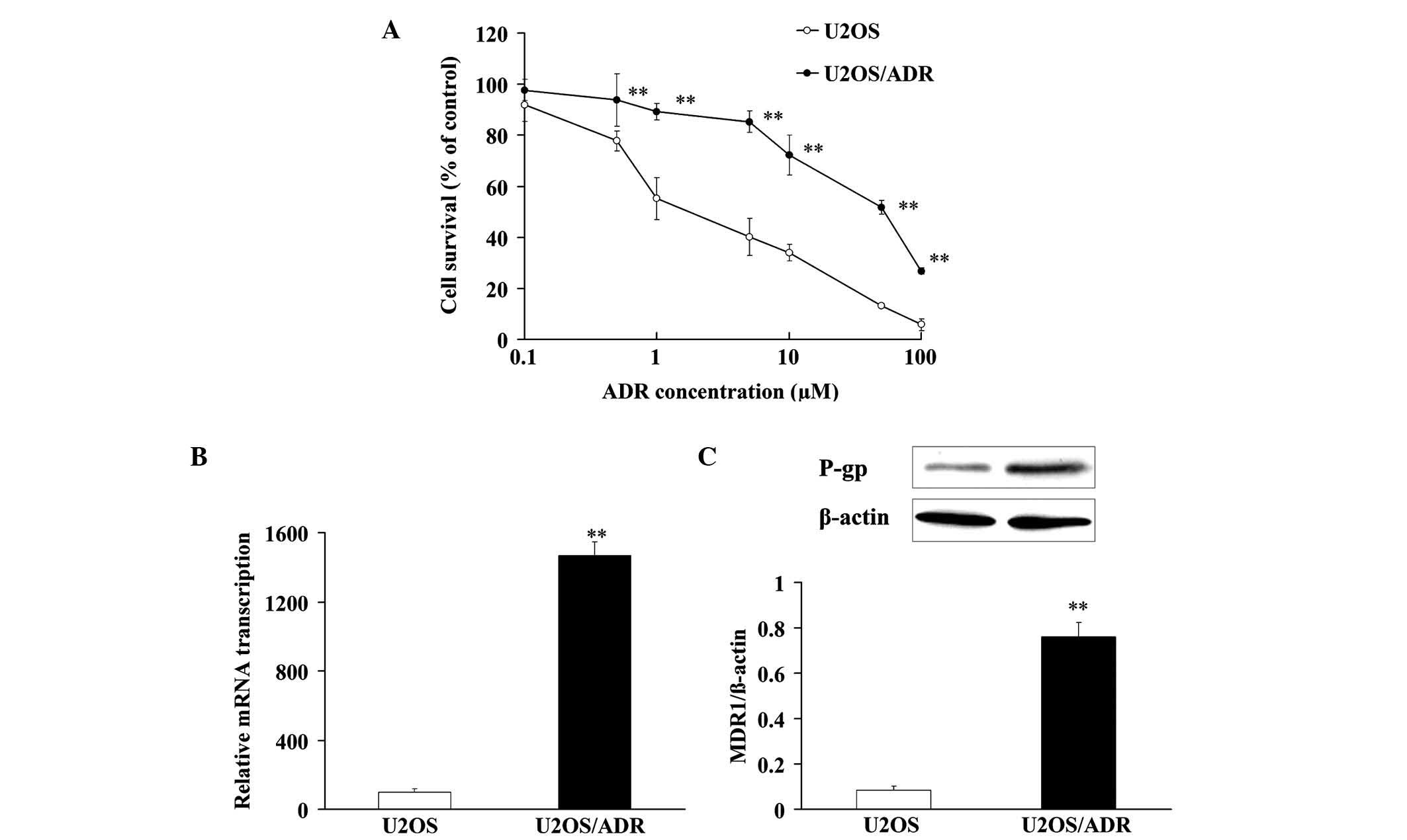
In order to verify the drug resistance of U2OS/ADR
cells, MTT assay was applied to analyze the cell viability, once
U2OS and U2OS/ADR cells had been incubated with various
concentrations of ADR. ADR exerted cytotoxicity against U2OS and
U2OS/ADR cells with IC50 values of 2.7±0.4 and 31.7±2.9
µM, respectively (Fig. 1A). These
results indicated that there was a remarkable drug resistance to
ADR in U2OS/ADR cells.
In addition, the messenger (m)RNA expression of MDR1
in U2OS/ADR cells was ~14.7 times higher than that in U2OS cells
(Fig. 1B). Compared with U2OS cells,
the protein expression of P-gp in U2OS/ADR cells was notably
upregulated (~8.94-fold) (Fig. 1C).
These findings demonstrated that the phenomenon of drug resistance
was at least partly associated with the overexpression of P-gp in
U2OS/ADR cells.
Reversal effect of resveratrol on
U2OS/ADR cells
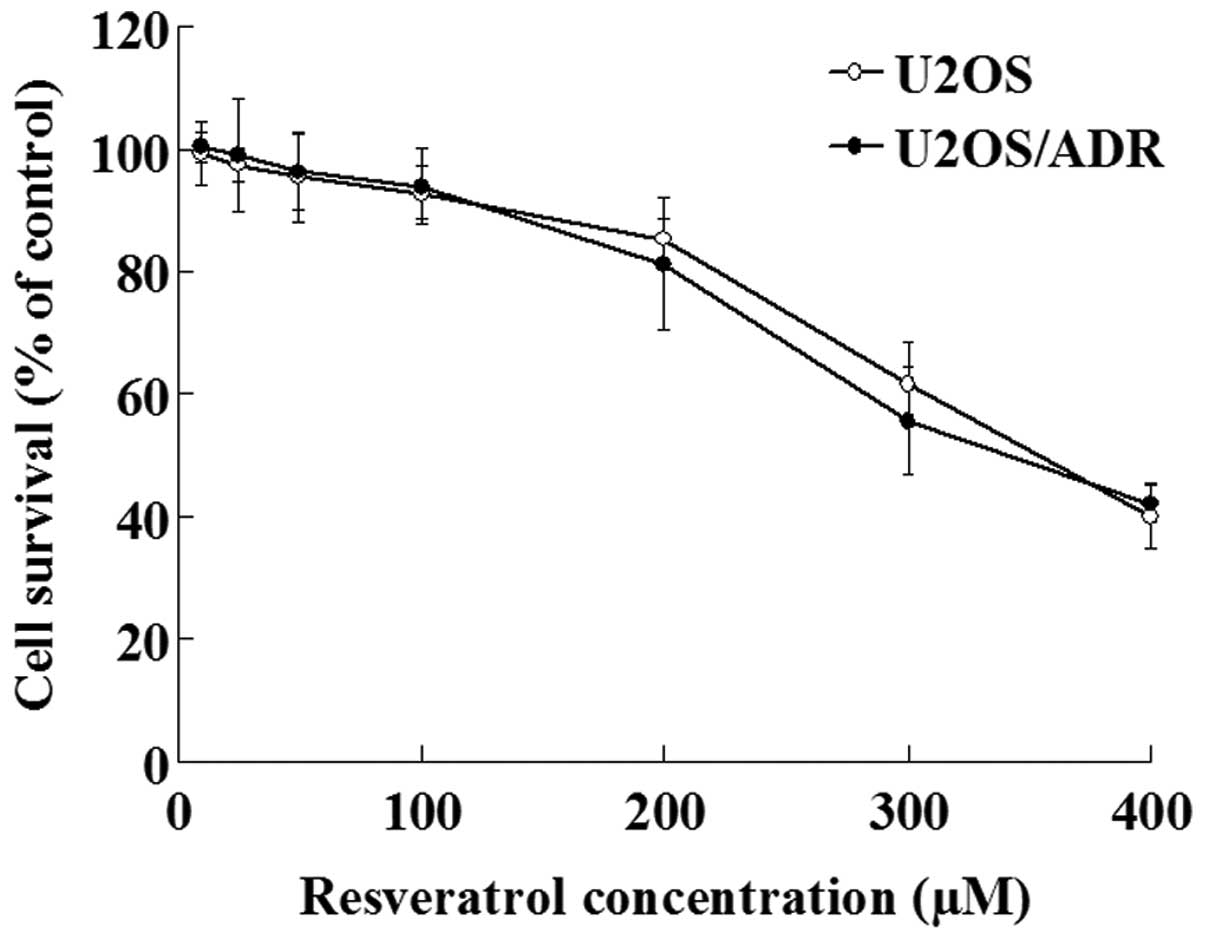
The effect of resveratrol on U2OS/ADR cells growth
was determined with MTT assay. The results demonstrated that
concentrations of resveratrol ranging from 10 to 100 µmol/l did not
exert inhibitory effects on the growth of U2OS/ADR cells, since the
cell survival rate was >90% (Fig.
2). However, higher concentrations of resveratrol (200–400
µmol/l) exhibited significant anti-proliferative effects on these
cells (P<0.01; Fig. 2). Therefore,
100 µmol/l was selected as the concentration of resveratrol
required to reverse multidrug resistance in U2OS/ADR cells.
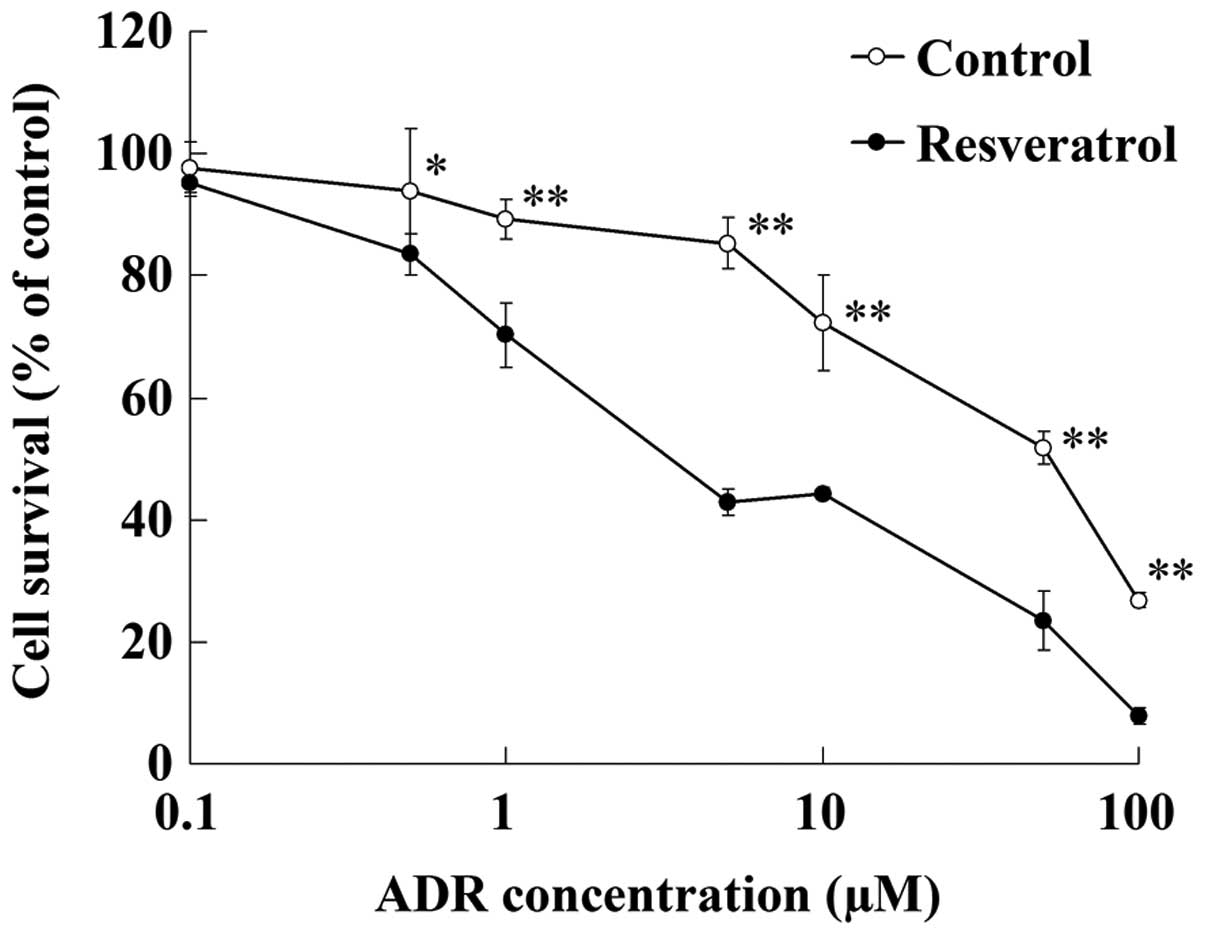
After being incubated with resveratrol (100 µmol/l)
for 48 h, U2OS/ADR cells displayed increased sensitivity to ADR
(Fig. 3). The IC50 value
of ADR in U2OS/ADR cells was reduced to 4.7±0.5 µM by resveratrol
(100 µmol/l). This result indicated that resveratrol could reverse
the drug resistance of U2OS/ADR cells towards ADR.
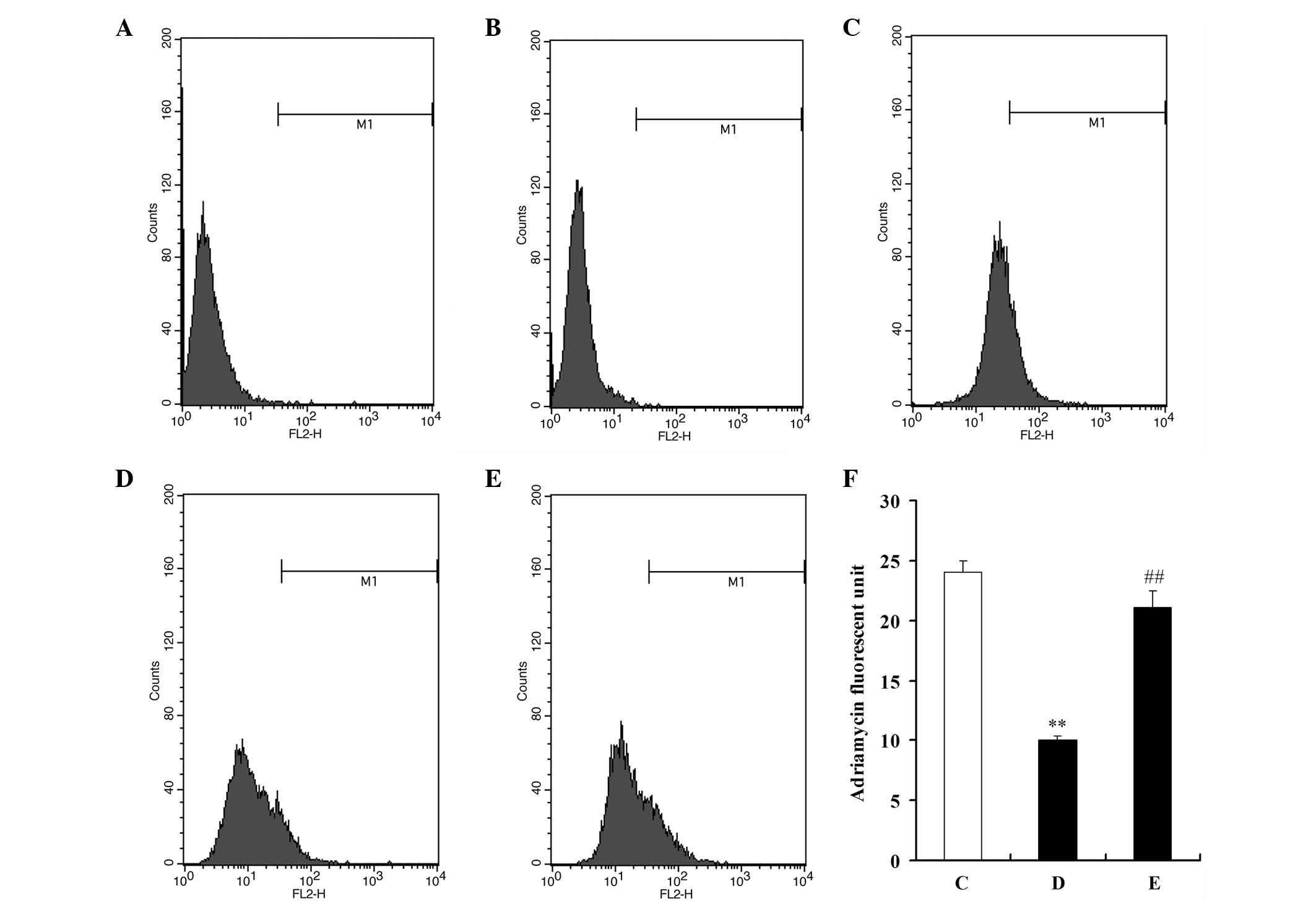
Accumulation of ADR in U2OS and
U2OS/ADR cells
Due to the autofluorescence capacity of ADR,
fluorescence intensity was used as an indicator of ADR
intracellular accumulation. After U2OS and U2OS/ADR cells had been
pre-incubated with 100 µM resveratrol for 48 h, cells were
incubated with 10 µM ADR for 1 h, and flow cytometry was then
performed to detect the fluorescence intensity of ADR in the cells.
The mean fluorescence intensity of ADR in U2OS/ADR cells was 58.1%
lower than that in U2OS cells (Fig.
4). However, the intracellular accumulation of ADR in U2OS/ADR
cells was significantly increased by resveratrol (P<0.01), and
the fluorescence intensity of ADR in resveratrol-treated U2OS/ADR
cells increased by 1.99-fold compared with untreated U2OS/ADR cells
(P<0.01; Fig. 4). These findings
indicated that resveratrol could significantly increase the
intracellular accumulation of ADR in U2OS/ADR cells.
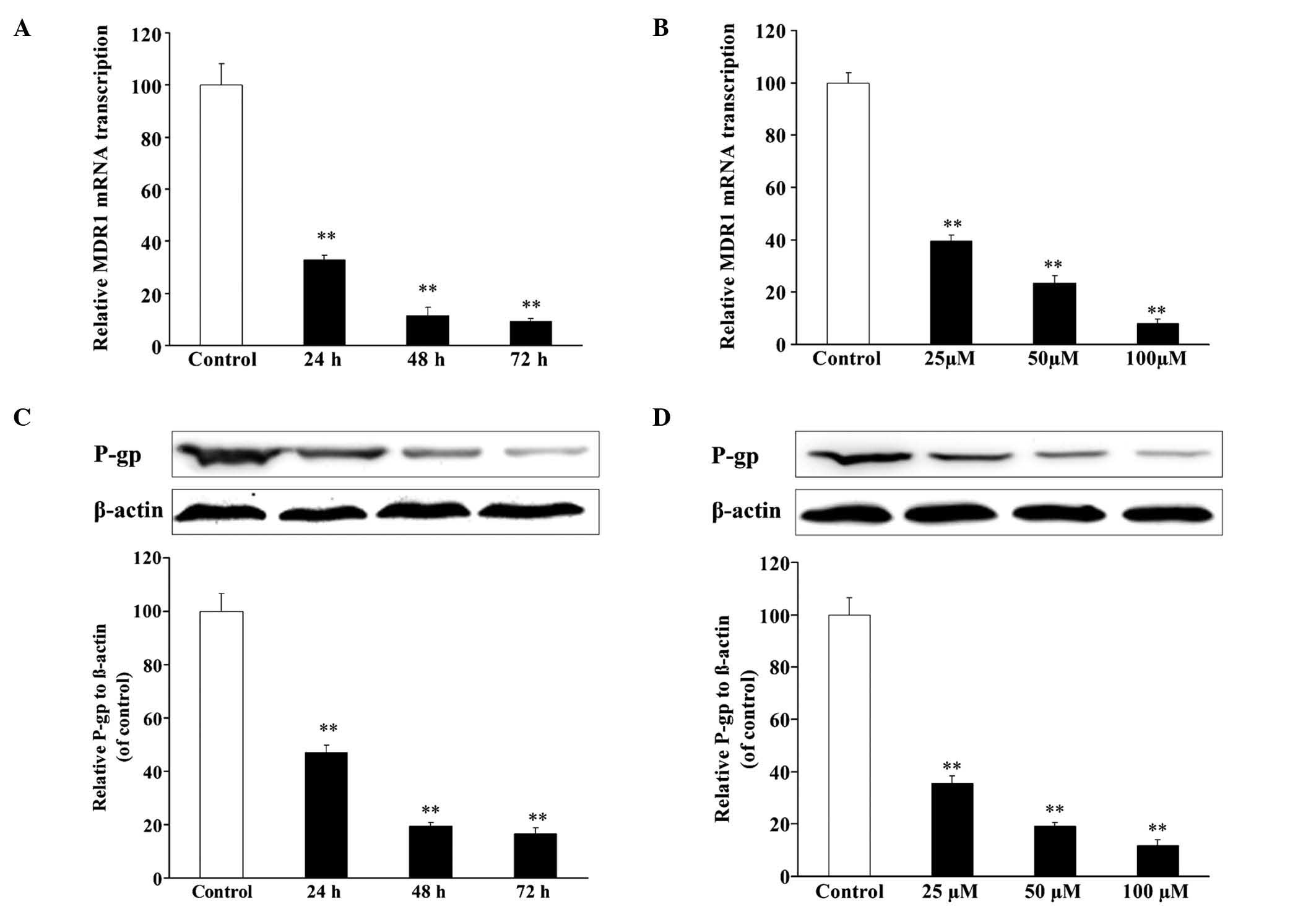
Resveratrol decreases the expression
of MDR1/P-gp in U2OS/ADR cells
In the reversal experiment, resveratrol could
reverse the drug resistance of U2OS/ADR cells towards ADR. To
explore the molecular mechanisms, the expression levels of
MDR1/P-gp were detected by RT-qPCR and western blotting. After
incubation with resveratrol (100 µmol/l) for 24, 48 and 72 h, the
mRNA expression level of MDR1 in U2OS/ADR cells decreased to 32.8,
11.1 and 9.1%, respectively, in comparison with untreated U2OS/ADR
cells (Fig. 5A). In addition,
compared with untreated U2OS/ADR cells, treatment with 25, 50 and
100 µmol/l of resveratrol for 48 h led to a reduction in the mRNA
expression level of MDR1 (40.0, 23.4 and 9.2%, respectively) in
U2OS/ADR cells (Fig. 5B). The protein
expression of P-gp was consistent with the results of RT-qPCR
(Fig. 5C and D). The findings
indicated that resveratrol could downregulate the mRNA and protein
expression of MDR1/P-gp.
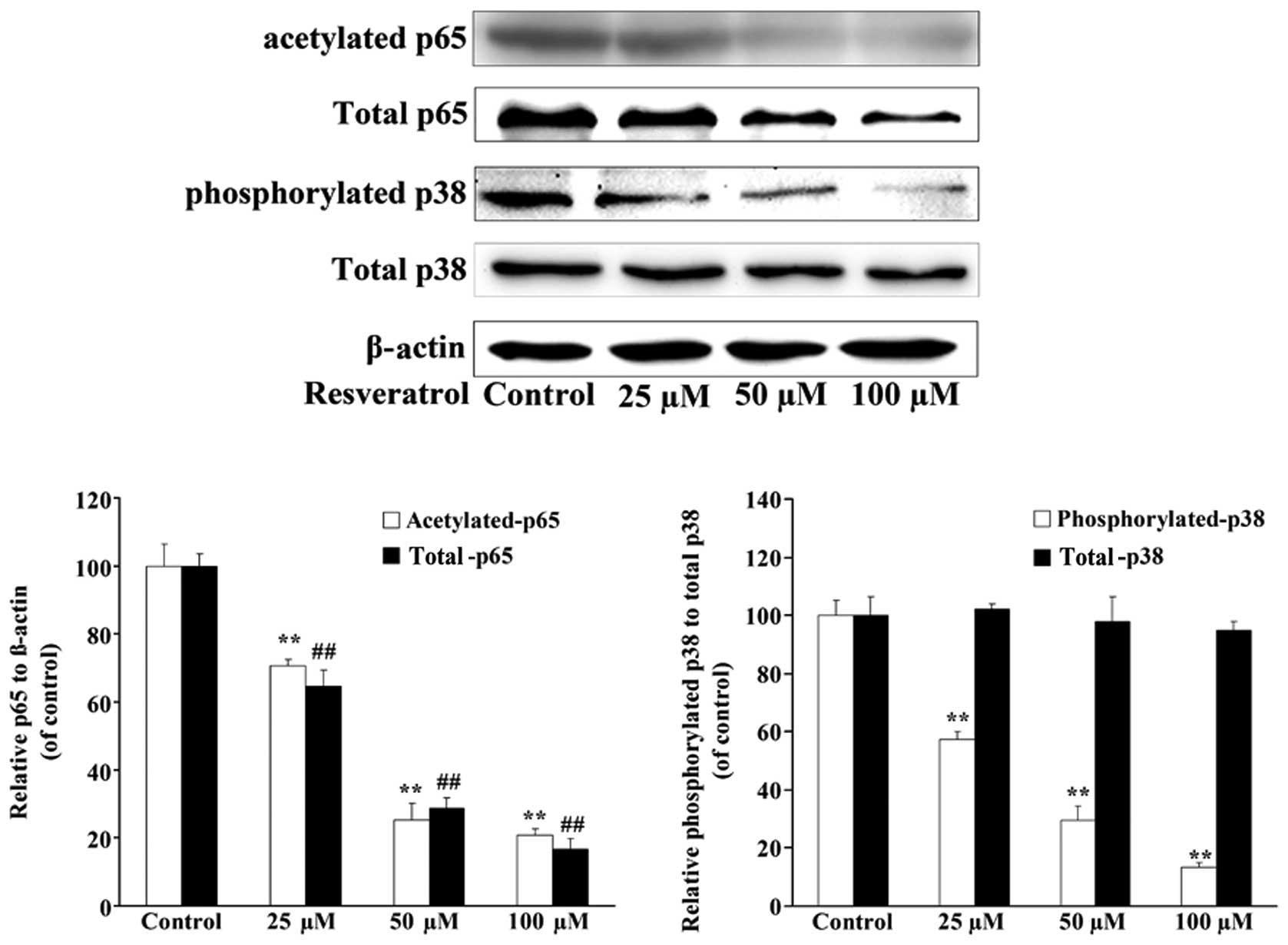
Resveratrol decreases the expression
of p38 and p65 in U2OS/ADR cells
In order to investigate whether the downregulation
of the expression of P-gp by resveratrol in U2OS/ADR cells was
associated with p38 and p65, the expression levels of p38 and p65
in U2OS/ADR cells were investigated. Although the results of
western blotting analysis demonstrated that the expression levels
of total p38 were not changed, the expression levels of p38
(phosphorylated) and p65 (acetylated and total) in U2OS/ADR cells
were significantly suppressed in comparison with untreated U2OS/ADR
cells after 48-h incubation with 25, 50 and 100 µmol/l of
resveratrol (Fig. 6). These results
indicated that resveratrol downregulated the expression of P-gp at
least partly by suppressing the activation of the NF-κB and p38
MAPK signaling pathways in U2OS/ADR cells.
p38 and p65 regulate the expression of
P-gp in U2OS/ADR cells
In order to explore whether p38 and p65 could
regulate the expression of P-gp in U2OS/ADR cells, siRNAs specific
for p38 and p65 were applied to knock down p38 and p65,
respectively. U2OS/ADR cells were transfected with siRNA for p38 or
p65, and the protein level of p38 (phosphorylated and total) or p65
(acetylated and total) was significantly reduced (Fig. 7). In addition, the protein level of
P-gp was also significantly reduced (Fig.
7). These findings revealed that the expression of P-gp was at
least partly associated with p38 and p65 in U2OS/ADR cells.
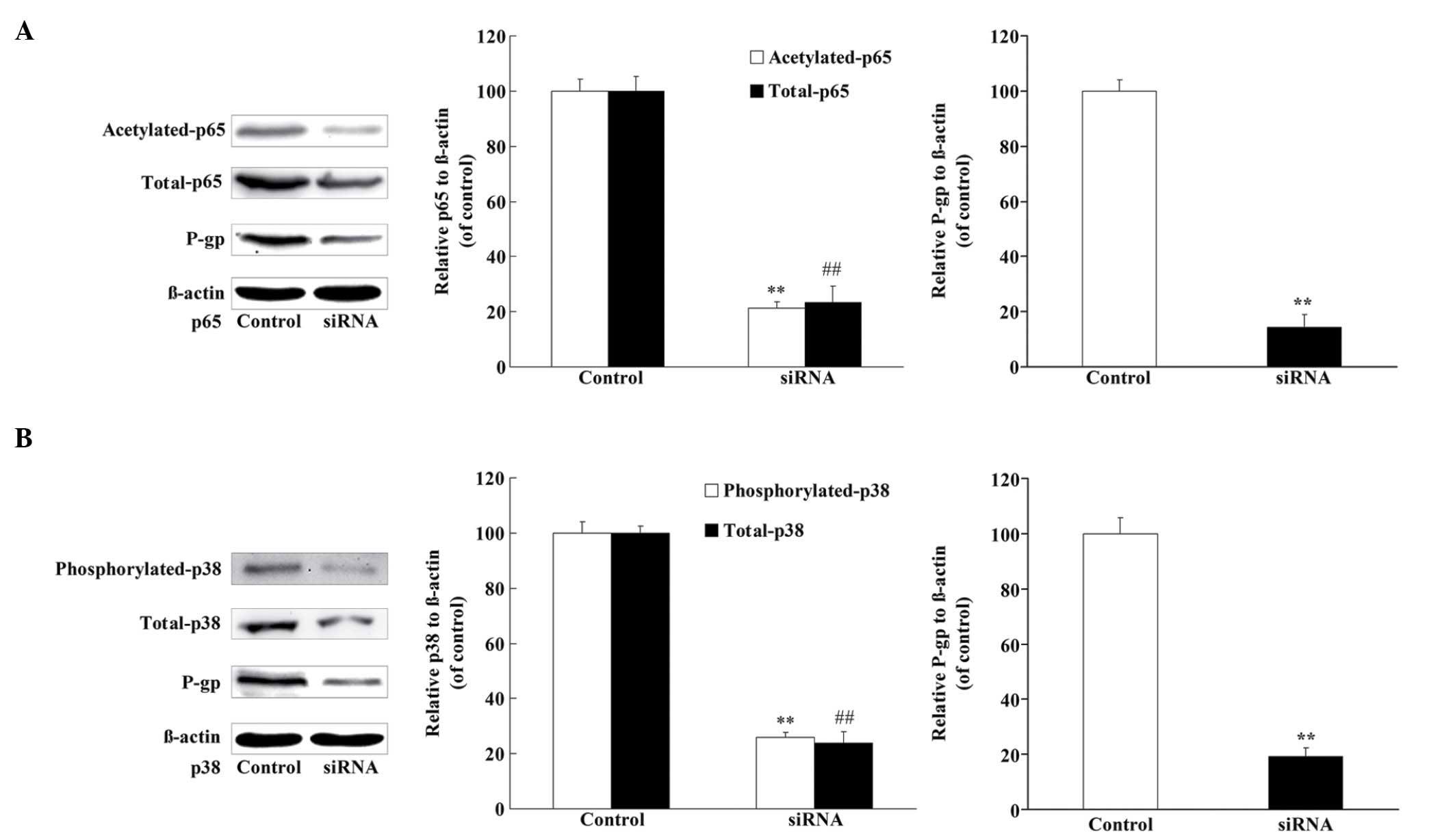 | Figure 7.P-gp protein expression was regulated
by NF-κB p65 and p38 MAPK. (A) The protein levels of p65
(acetylated and total), P-gp and β-actin were determined using
western blotting, following the knockdown of NF-κB p65 in U2OS/ADR
cells using siRNA. (B) The protein levels of p38 (phosphorylated
and total), P-gp and β-actin were determined using western
blotting, following the knockdown of p38 MAPK in U2OS/ADR cells
using siRNA. Data are expressed as the mean ± standard deviation
(**P<0.01, expression of acetylated p65 and phosphorylated p38
in treated U2OS cells vs. untreated cells; ##P<0.01,
expression of total p65 and p38 in treated U2OS cells vs. untreated
cells; n=6). siRNA, small interfering RNA; P-gp, P-glycoprotein;
NF-κB, nuclear factor-κB; MAPK, mitogen-activated protein kinase;
ADR, adriamycin. |
Discussion
Natural medicines have been widely used for
anti-oxidative stress, anti-inflammation and reversal of multidrug
resistance of tumor cells in recent years (15,19).
Resveratrol has an excellent effect on anti-oxidative stress and
anti-inflammation (24–26), and it has the potential to ameliorate
local inflammation by suppressing tumor necrosis factor-α-induced
interleukin-8 release (19,24–26). In
addition, resveratrol can effectively inhibit NF-κB and MAPK
pathways (27–29). It is widely reported that the NF-κB
and MAPK signaling pathways play a major role in the molecular
mechanisms of P-gp-mediated multidrug resistance, and
overexpression of P-gp is one of the important causes of multidrug
resistance of tumor cells (12,13).
Therefore, the present study investigated whether resveratrol could
significantly reduce the levels of P-gp expression and reverse the
drug resistance of U2OS/ADR cells by inhibiting the NF-κB and MAPK
signaling pathways.
In order to verify the drug resistance of U2OS/ADR
cells, the viabilities of U2OS and U2OS/ADR cells incubated with
various concentrations of ADR were analyzed by MTT assay. The
results indicated that the IC50 of U2OS/ADR cells was
increased by 11.7-fold (Fig. 1A).
Overexpression of P-gp has been reported as one of the important
causes of multidrug resistance; thus, the expression of MDR1/P-gp
was examined by RT-qPCR and western blotting. Compared with U2OS
cells, the expression levels of MDR1/P-gp were significantly
increased in U2OS/ADR cells (Fig. 1B and
C). These results suggested that U2OS/ADR cells had a
remarkable drug resistance to ADR and that overexpression of P-gp
was an important cause of multidrug resistance in U2OS/ADR
cells.
To investigate the effect of resveratrol on the
reversal of multidrug resistance, the inhibition rate of various
concentrations of resveratrol on U2OS/ADR cells was evaluated. The
results indicated that concentrations of resveratrol ranging from
10 to 100 µmol/l had no inhibitory effects on the growth of
U2OS/ADR cells (Fig. 2). Therefore, a
concentration of resveratrol of 100 µmol/l was selected to reverse
multidrug resistance in U2OS/ADR cells. After U2OS/ADR cells had
been incubated with resveratrol for 48 h, the IC50 value
of ADR that exerted cytotoxicity against U2OS/ADR cells was
remarkably reduced, and the intracellular accumulation of ADR was
significantly increased (Figs. 3 and
4). These results indicated that
resveratrol can reverse the drug resistance of U2OS/ADR cells to
ADR. To explore the reversal mechanism of drug resistance, the
levels of MDR1/P-gp expression were investigated. The results
indicated that resveratrol could decrease the expression of
MDR1/P-gp in U2OS/ADR cells in a time– and concentration-dependent
manner (Fig. 5). Therefore,
resveratrol can reverse drug resistance partly at least by reducing
the expression of MDR1/P-gp in U2OS/ADR cells.
It was previously reported that resveratrol could
inhibit cell growth and proliferation, and induce apoptosis and
arrest in the G0/G1 phase of the cell cycle by reducing the
activity of NF-κB in tumor cells (29,30).
Furthermore, resveratrol could also effectively inhibit the
activation of the p38 MAPK signaling pathway (27). Therefore, the present study focused on
the effect of resveratrol on the regulation of the activation of
the NF-κB and p38 MAPK signaling pathways. It was observed that the
expression of p65 (acetylated and total) and p38 (phosphorylated)
was suppressed upon incubation with resveratrol (Fig. 6). The NF-κB signaling pathway is
highly correlated with P-gp-mediated drug resistance (16). When the NF-κB signaling pathway was
activated, the expression of MDR1 was dramatically upregulated
(12). In addition, the p38 MAPK
signaling pathway is associated with multidrug resistance events
(17). In order to clearly verify the
phenomenon of NF-κB and MAPK signaling-mediated multidrug
resistance in U2OS/ADR cells, the expression of NF-κB p65 subunit
(acetylated and total), p38 (phosphorylated and total) and P-gp
were examined following siRNA knockdown of p65 or p38. The results
revealed that the expression of p65 (acetylated and total) and p38
(phosphorylated and total) was significantly reduced (Fig. 7). Furthermore, the expression P-gp was
consistent with that of p65 and p38 (Fig.
7). These results confirmed that resveratrol reverses
P-gp-mediated multidrug resistance of U2OS/ADR cells by suppressing
the activation of the NF-κB and p38 MAPK signaling pathways.
Numerous studies have reported that p38 MAPK can
activate NF-κB expression (31), and
thus regulate P-gp expression. The present study could not clarify
whether the expression of p65 was directly downregulated by
resveratrol or was correlated with resveratrol-induced suppression
of the p38 MAPK signaling pathway in U2OS/ADR cells. Mulakayala
et al confirmed that resveratrol may prevent the
translocation of NF-κB by interacting with it, and may also prevent
the binding of NF-κB to DNA by interacting with the residues
involved in DNA binding, according to the results of the analysis
with AutoDock 4.2 software (32). The
interaction of resveratrol and p38 MAPK would be investigated in
further studies.
In conclusion, the NF-κB and p38 MAPK signaling
pathways are correlated with ADR-induced drug resistance in
U2OS/ADR cells. Furthermore, resveratrol can downregulate the
expression of P-gp and reverse the drug resistance of U2OS/ADR
cells at least partly by suppressing the activation of the NF-κB
and p38 MAPK signaling pathways.
Acknowledgements
The present study was supported by a grant from the
National Natural Science Foundation of China (Beijing, China; grant
no. 81270052) and and the Liaoning Natural Science Foundation
(Liaoning, China; grant no. L2015159).
References
|
1
|
Lee JS, Fetsch JF, Wasdhal DA, Lee BP,
Pritchard DJ and Nascimento AG: A review of 40 patients with
extraskeletal osteosarcoma. Cancer. 76:2253–2259. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lamoureux F, Trichet V, Chipoy C,
Blanchard F, Gouin F and Redini F: Recent advances in the
management of osteosarcoma and forthcoming therapeutic strategies.
Expert Rev Anticancer Ther. 7:169–181. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Young G, Toretsky JA, Campbell AB and
Eskenazi AE: Recognition of common childhood malignancies. Am Fam
Physician. 61:2144–2154. 2000.PubMed/NCBI
|
|
4
|
Ritter J and Bielack SS: Osteosarcoma. Ann
Oncol. 21(Suppl 7): vii320–325. 2010.PubMed/NCBI
|
|
5
|
Fung KL, Pan J, Ohnuma S, Lund PE, Pixley
JN, Kimchi-Sarfaty C, Ambudkar SV and Gottesman MM: MDR1 synonymous
polymorphisms alter transporter specificity and protein stability
in a stable epithelial monolayer. Cancer Res. 74:598–608. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gromnicova R, Romero I and Male D:
Transcriptional control of the multi-drug transporter ABCB1 by
transcription factor Sp3 in different human tissues. PLoS One.
7:e481892012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Johnson WW: P-glycoprotein-mediated efflux
as a major factor in the variance of absorption and distribution of
drugs: Modulation of chemotherapy resistance. Methods Find Exp Clin
Pharmacol. 24:501–514. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sorokin A: Cyclooxygenase-2: Potential
role in regulation of drug efflux and multidrug resistance
phenotype. Curr Pharm Des. 10:647–657. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Patel VA, Dunn MJ and Sorokin A:
Regulation of MDR-1 (P-glycoprotein) by cyclooxygenase-2. J Biol
Chem. 277:38915–38920. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Thomas H and Coley HM: Overcoming
multidrug resistance in cancer: An update on the clinical strategy
of inhibiting p-glycoprotein. Cancer Control. 10:159–165.
2003.PubMed/NCBI
|
|
11
|
Zhu L, Zhao L, Wang H, Wang Y, Pan D, Yao
J, Li Z, Wu G and Guo Q: Oroxylin A reverses
P-glycoprotein-mediated multidrug resistance of MCF7/ADR cells by
G2/M arrest. Toxicol Lett. 219:107–115. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ronaldson PT, Ashraf T and Bendayan R:
Regulation of multidrug resistance protein 1 by tumor necrosis
factor alpha in cultured glial cells: Involvement of nuclear
factor-kappaB and c-Jun N-terminal kinase signaling pathways. Mol
Pharmacol. 77:644–659. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Barancík M, Bohácová V, Kvackajová J,
Hudecová S, Krizanová O and Breier A: SB203580, a specific
inhibitor of p38-MAPK pathway, is a new reversal agent of
P-glycoprotein-mediated multidrug resistance. Eur J Pharm Sci.
14:29–36. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee JY, Tanabe S, Shimohira H, Kobayashi
Y, Oomachi T, Azuma S, Ogihara K and Inokuma H: Expression of
cyclooxygenase-2, P-glycoprotein and multi-drug
resistance-associated protein in canine transitional cell
carcinoma. Res Vet Sci. 83:210–216. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Choi BH, Kim CG, Lim Y, Shin SY and Lee
YH: Curcumin down-regulates the multidrug-resistance mdr1b gene by
inhibiting the PI3K/Akt/NF kappa B pathway. Cancer Lett.
259:111–118. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bentires-Alj M, Barbu V, Fillet M, Chariot
A, Relic B, Jacobs N, Gielen J, Merville MP and Bours V: NF-kappaB
transcription factor induces drug resistance through MDR1
expression in cancer cells. Oncogene. 22:90–97. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen Y, Zhao Y, Wang C, Xiao X, Zhou X and
Xu G: Inhibition of p38 MAPK diminishes doxorubicin-induced drug
resistance associated with P-glycoprotein in human leukemia K562
cells. Med Sci Monit. 18:BR383–BR388. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Torres P, Poveda A, Jimenez-Barbero J,
Ballesteros A and Plou FJ: Regioselective lipase-catalyzed
synthesis of 3-o-acyl derivatives of resveratrol and study of their
antioxidant properties. J Agric Food Chem. 58:807–813. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Quan F, Pan C, Ma Q, Zhang S and Yan L:
Reversal effect of resveratrol on multidrug resistance in KBv200
cell line. Biomed Pharmacother. 62:622–629. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Huo X, Liu Q, Wang C, Meng Q, Sun H, Peng
J, Ma X and Liu K: Enhancement effect of P-gp inhibitors on the
intestinal absorption and antiproliferative activity of bestatin.
Eur J Pharm Sci. 50:420–428. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Du J, Pan Y, Shi Y, Guo C, Jin X, Sun L,
Liu N, Qiao T and Fan D: Overexpression and significance of prion
protein in gastric cancer and multidrug-resistant gastric carcinoma
cell line SGC7901/ADR. Int J Cancer. 113:213–220. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kim K, Kim KH, Kim HY, Cho HK, Sakamoto N
and Cheong J: Curcumin inhibits hepatitis C virus replication via
suppressing the Akt-SREBP-1 pathway. FEBS Lett. 584:707–712. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bae CH, Kim JW, Ye SB, Song SY, Kim YW,
Park SY and Kim YD: AMPK induces MUC5B expression via p38 MAPK in
NCI-H292 airway epithelial cells. Biochem Biophys Res Commun.
409:669–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sinha K, Chaudhary G and Gupta YK:
Protective effect of resveratrol against oxidative stress in middle
cerebral artery occlusion model of stroke in rats. Life Sci.
71:655–665. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gupta YK, Briyal S and Chaudhary G:
Protective effect of trans-resveratrol against kainic acid-induced
seizures and oxidative stress in rats. Pharmacol Biochem Behav.
71:245–249. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bishayee A, Barnes KF, Bhatia D, Darvesh
AS and Carroll RT: Resveratrol suppresses oxidative stress and
inflammatory response in diethylnitrosamine-initiated rat
hepatocarcinogenesis. Cancer Prev Res (Phila). 3:753–763. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Huang Z, Wang C, Wei L, Wang J, Fan Y,
Wang L, Wang Y and Chen T: Resveratrol inhibits EMMPRIN expression
via P38 and ERK1/2 pathways in PMA-induced THP-1 cells. Biochem
Biophys Res Commun. 374:517–521. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
El-Mowafy AM and White RE: Resveratrol
inhibits MAPK activity and nuclear translocation in coronary artery
smooth muscle: Reversal of endothelin-1 stimulatory effects. FEBS
Lett. 451:63–67. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sun C, Hu Y, Liu X, Wu T, Wang Y, He W and
Wei W: Resveratrol downregulates the constitutional activation of
nuclear factor-kappaB in multiple myeloma cells, leading to
suppression of proliferation and invasion, arrest of cell cycle,
and induction of apoptosis. Cancer Genet Cytogenet. 165:9–19. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yu H, Pan C, Zhao S, Wang Z, Zhang H and
Wu W: Resveratrol inhibits tumor necrosis factor-alpha-mediated
matrix metalloproteinase-9 expression and invasion of human
hepatocellular carcinoma cells. Biomed Pharmacother. 62:366–372.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Berghe W Vanden, Plaisance S, Boone E, De
Bosscher K, Schmitz ML, Fiers W and Haegeman G: p38 and
extracellular signal-regulated kinase mitogen-activated protein
kinase pathways are required for nuclear factor-kappaB p65
transactivation mediated by tumor necrosis factor. J Biol Chem.
273:3285–3290. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mulakayala C, Babajan B, Madhusudana P,
Anuradha CM, Rao RM, Nune RP, Manna SK, Mulakayala N and Kumar CS:
Synthesis and evaluation of resveratrol derivatives as new chemical
entities for cancer. J Mol Graph Model. 41:43–54. 2013. View Article : Google Scholar : PubMed/NCBI
|