Introduction
Corosolic acid (CRA), a triterpenoid named
2α-hydroxyursolic acid, is a natural compound derived from
traditional Chinese medicinal herbs (1). CRA exists in various plants, including
Schisandra chinensis (2),
Lagerstroemia speciosa (3),
Orthosiphon stamineus (4) and
Eriobotrya japonica (5). CRA
has been reported to possess numerous biological activities,
including anti-diabetic (4,5), antioxidant (6), anti-atherosclerotic (7), cholesterol-reducing (8) and anti-inflammatory (9), which suggested the potential therapeutic
value of CRA. Previous studies have reported that CRA could
suppresses the growth of various types of tumors, including
glioblastoma (10), leukemia
(11), gastric cancer (12) and lung cancer (1). However, the effect of CRA on
osteosarcoma remains unclear.
Osteosarcoma is the most common malignant primary
bone tumor that occurs in children and adolescents, which comprises
20% of all bone tumors and ~5% of all pediatric tumors (13). The highest incidence of osteosarcoma
appears in the second decade of life, implying an association
between bone growth and tumor development (14). In recent years, due to multimodal
therapeutic approaches combining high-dose chemotherapy,
significant improvements in patient survival rates have been
achieved (15). However, the overall
relapse free-survival rate over 5 years has stagnated at 65–75%
(16), being distant metastases the
leading cause of mortality in osteosarcoma patients (17). Since chemotherapy is still the major
therapeutic option for osteosarcoma, the exploration and
development of more effective therapeutic agents is required.
Apoptosis, the major form of cell suicide, is
critical to various physiological processes and to the maintenance
of homeostasis in multicellular organisms (18). It is clear that apoptosis is critical
for the cytotoxicity induced by anticancer drugs (19). Over the years, accumulating evidence
has clearly indicated that anticancer drugs are able to induce
apoptosis, and that this process is involved in the mediation of
their cytotoxic effects (20). In
addition, the selective regulation of the apoptotic pathway in
cancer cells has been the goal of cancer researchers (21). However, the effect of CRA on the
apoptosis of osteosarcoma cells remains unknown.
In the present study, the effects of CRA on the cell
proliferation and tumor growth of osteosarcoma were assessed in
vitro, and the impact of CRA on the apoptosis of osteosarcoma
cells was investigated. The present study demonstrated that CRA
treatment resulted in a significant inhibition of cell
proliferation and tumor growth of osteosarcoma, which was closely
associated with the cell apoptosis induced by CRA through the
mitochondrial pathway. Therefore, our results not only provide a
potential chemotherapeutic agent for osteosarcoma, but also an
insight into the mechanism underlying the anticancer efficacy of
CRA.
Materials and methods
Antibodies and reagents
Primary antibodies specific for cytochrome c
(sc-8385), complex (COX) IV (sc-69359) and β-actin (sc-47778) were
purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA,
USA). Secondary antibodies were purchased from Cell Signaling
Technology, Inc. (Beverly, MA, USA).
3-(4,5-Dimethyl-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) and
propidium iodide (PI) were obtained from Sigma-Aldrich (Merck
Millipore, Darmstadt, Germany). The Annexin V-FITC Apoptosis
Detection kit was purchased from BD Pharmingen™ (BD Biosciences,
Franklin Lakes, NJ, USA). The Caspase-3 Activity Assay kit and the
Caspase-9 Activity Assay kit were purchased from NanJing KeyGen
Biotech Co., Ltd. (Nanjing, China). CRA was purchased from Jianfeng
Natural Product R&D Co., Ltd. (Tianjin, China).
Cells and culture conditions
Human osteosarcoma cells MG-63 were obtained from
the American Type Culture Collection (Rockville, MD, USA) and
cultured in Dulbecco's modified Eagle's medium (Sigma-Aldrich;
Merck Millipore) supplemented with 10% heat-inactivated fetal
bovine serum (HyClone; GE Healthcare Life Sciences, Logan, UT,
USA), 100 mg/ml penicillin and 100 mg/ml streptomycin (Invitrogen;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) in a humidified
incubator at 37°C in a 5% CO2 atmosphere. CRA was
dissolved in 100 µl dimethylsulfoxide (DMSO) prior to addition to
the medium. The maximum concentration of DMSO in the medium did not
exceed 0.1% (v/v). Cells treated with DMSO only served as a vehicle
control.
Cell viability assay
Cell viability was detected by MTT assay as
described previously (22). Briefly,
cells were seeded in 96-well culture plates at a density of
1×104 cells/well and incubated for 12 h at 37°C. Then,
cells were treated with several concentrations of CRA and further
incubated for 12, 24, 36 and 48 h. Finally, MTT was added to each
well and incubated for 4 h at 37°C. The resulting formazan product
was then dissolved in 100 µl DMSO, and the absorbance was
determined at 540 nm using a Bio-Rad 3350 microplate reader
(Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Detection of apoptosis
Once the cells were harvested, double staining with
annexin V and PI was conducted using the BD Pharmingen™ Annexin
V-FITC Apoptosis Detection kit according to the manufacturer's
protocol. Then, the stained cells were detected using a FACSCalibur
flow cytometer (BD Biosciences, San Jose, CA, USA).
DNA gel electrophoresis
DNA was isolated from MG-63 cells treated with CRA
according to the method described by Ariffin et al (23). DNA samples were subjected to
electrophoresis in a 1% (w/v) agarose gel for 1 h at 100 V. The gel
was examined under ultraviolet transillumination following ethidium
bromide staining to determine the extent of apoptotic DNA
fragmentation.
Detection of caspase-3 and −9
activities
The activities of caspase-3 and −9 in cell lysates
were determined using a colorimetric assay kit according to the
manufacturer's protocol. Briefly, the cells were harvested in cell
lysis buffer (NanJing KeyGen Biotech Co., Ltd.), and cell lysates
were prepared following the manufacturer's protocol. The protein
concentrations were determined using the BCA Protein Assay reagent
(Pierce Biotechnology, Inc., Rockford, IL, USA). Samples of the
cell lysates (100 µg protein/sample) were mixed with reaction
buffer and substrate, and incubated for 4 h at 37°C in the dark.
The absorbance was measured at 405 nm, and the sample readings were
calculated by subtracting the absorbance of the blank samples.
Measurement of mitochondrial membrane
potential
The change in mitochondrial membrane potential in
osteosarcoma cells following exposure to CRA was measured by flow
cytometry using the fluorescent lipophilic cationic probe
5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolcarbocyanine
iodide (JC-1) as described previously (24). JC-1 accumulates selectively within
normal mitochondria to form multimer J-aggregates emitting red
fluorescence (25). If the
mitochondrial membrane potential is altered, JC-1 can not aggregate
in the mitochondria and remains in the cytoplasm in its monomeric
form emitting green fluorescence (26). Thus, the color of the dye changes from
orange to green depending on the mitochondrial membrane potential,
and can be analyzed by fluorescence-activated cell sorting (FACS)
with green fluorescence in channel 1 and orange emission in channel
2.
Preparation of mitochondrial and
cytosolic fractions
The extraction of the mitochondrial and cytosolic
fractions was performed using a Mitochondria/Cytosol Fractionation
kit (Abcam, Cambridge, MA, USA). Cells exposed to CRA at the
concentrations indicated were harvested and washed with ice-cold
phosphate-buffered saline (PBS) twice, and then the cells were
resuspended in cytosol extraction buffer. Upon incubation on ice,
the cells were homogenized, and the homogenates were centrifuged at
700 × g for 10 min at 4°C. The supernatants were further
centrifuged at 10,000 × g for 30 min at 4°C to separate the
cytosolic fraction. Then, the pellet, which represented the
mitochondrial fraction, was resuspended in mitochondrial extraction
buffer. All the fractions were stored at −80°C for further
detection.
Western blotting
Following treatment of MG-63 cells with CRA, the
cells were harvested, washed with cold PBS and lysed with ice-cold
lysis buffer supplemented with protease inhibitors as detailed
previously (27). For western blot
analysis of cytochrome c and COX IV, the mitochondrial and
cytosolic fractions were prepared, respectively, and proteins were
separated by 10% sodium dodecyl sulfate polyacrylamide gel
electrophoresis and transferred onto polyvinylidene fluoride
membranes. Upon blocking with 5% fat-free milk, the membrane was
incubated with the corresponding primary antibody at 1:300 dilution
at 4°C overnight. Upon washing, the membrane was incubated with the
appropriate horseradish peroxidase-conjugated secondary antibody at
1:10,000 dilution for 1 h at room temperature, and the
immunoreactive bands were visualized using enhanced
chemiluminescence reagents (EMD Millipore, Billerica, MA, USA).
Statistical analysis
All experiments were performed in triplicate and
repeated three times independently. The data were expressed as the
mean ± standard deviation. Statistical analysis was performed using
Prism 5 software (GraphPad Software, Inc., La Jolla, CA, USA).
One-way analysis of variance followed by Dunnett's test were
performed, and P<0.05 was considered to indicate a statistically
significant difference.
Results
CRA inhibits osteosarcoma cell
proliferation in vitro
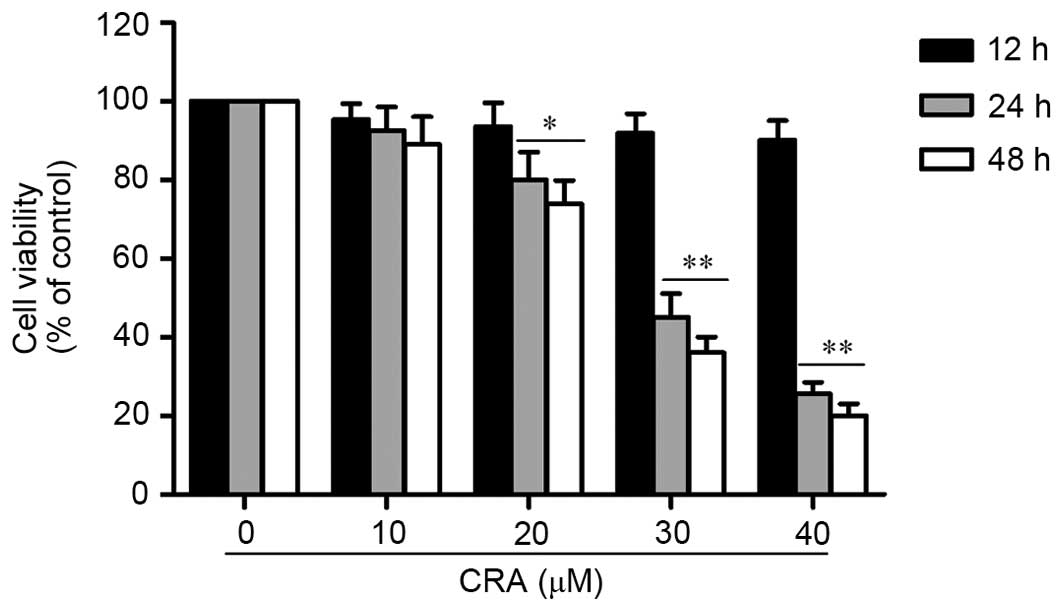
To assess the effect of CRA on the proliferation of
osteosarcoma cells, the viability of osteosarcoma cells treated
with different concentrations of CRA (10–40 µM) was detected using
MTT assay. Osteosarcoma cells (MG-63) were cultured for 12, 24 and
48 h. As shown in Fig. 1, the
treatment of MG-63 cells with CRA resulted in a significant
reduction in the viability of the cells in a dose-dependent manner
(P<0.001). Treatment with CRA for 12 h did not result in a
significant reduction in cell viability, whereas following
treatment for 24 and 48 h, a significant reduction in cell
viability was observed. In addition, it was observed that the
inhibitory effect of CRA was also time dependent. These results
indicated that CRA effectively inhibited the viability of
osteosarcoma cells, suggesting that CRA could inhibit the
proliferation of osteosarcoma cells. Since a significant decrease
in cell viability of MG-63 cells was observed after 24 h of CRA
treatment, this time point was selected for further studies.
CRA induces apoptosis in osteosarcoma
cells
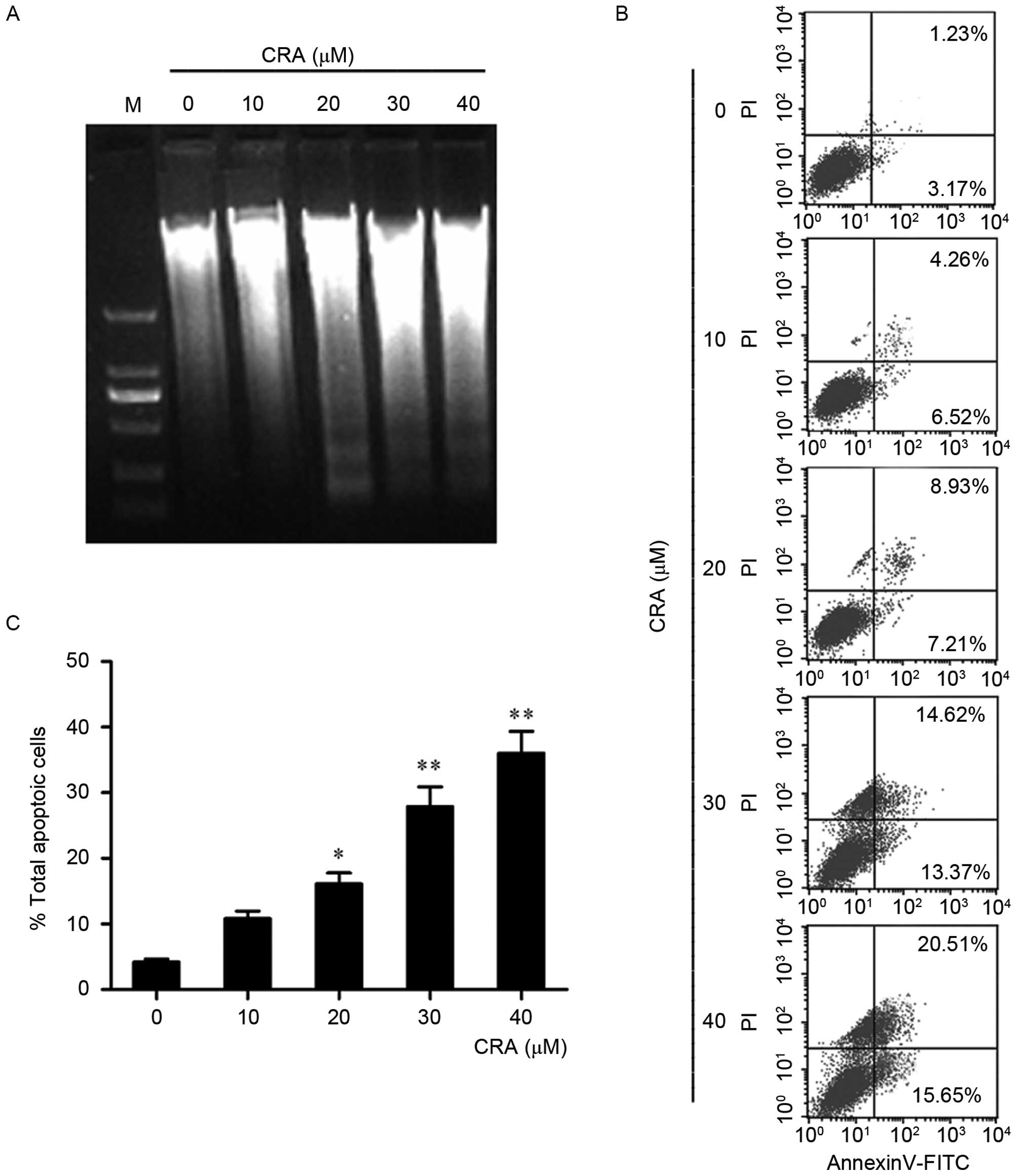
To determine whether CRA induced the apoptosis of
osteosarcoma cells, the change in DNA of MG-63 cells following CRA
treatment was measured using a DNA ladder assay (Fig. 2A). Once MG-63 cells were treated with
the indicated concentrations of CRA for 24 h, DNA was extracted.
Via agarose gel electrophoresis, an obvious DNA ladder, a classic
indicator of apoptotic changes (28),
was detected in MG-63 cells treated with CRA (20, 30 and 40 µM),
suggesting that CRA treatment may induce apoptosis in osteosarcoma
cells.
To further verify the observation of apoptosis in
MG-63 cells, cells treated with CRA were analyzed by flow
cytometry. In this assay, cells were stained with annexin V
conjugated with fluorescein isothiocyanate (FITC) and PI. The cells
in the lower right (LR) quadrant of the histogram in Fig. 2B represent the number of early
apoptotic cells, and those in the upper right quadrant represented
the cells in late apoptosis, which have taken up both FITC and PI
(29). It was observed that treatment
with CRA for 24 h significantly increased the number of early
apoptotic cells from 6.52 to 15.65% in a dose-dependent manner. The
number of late apoptotic cells also increased from 1.23% in the
untreated control group to 20.51% in the group treated with the
highest CRA concentration (40 µM). The total percentage of
apoptotic cells is summarized in Fig.
2C, and the results indicated that CRA treatment resulted in a
significant increase in the apoptosis of MG-63 cells (P=0.001).
CRA treatment activates the caspase-3
and −9 activities of osteosarcoma cells
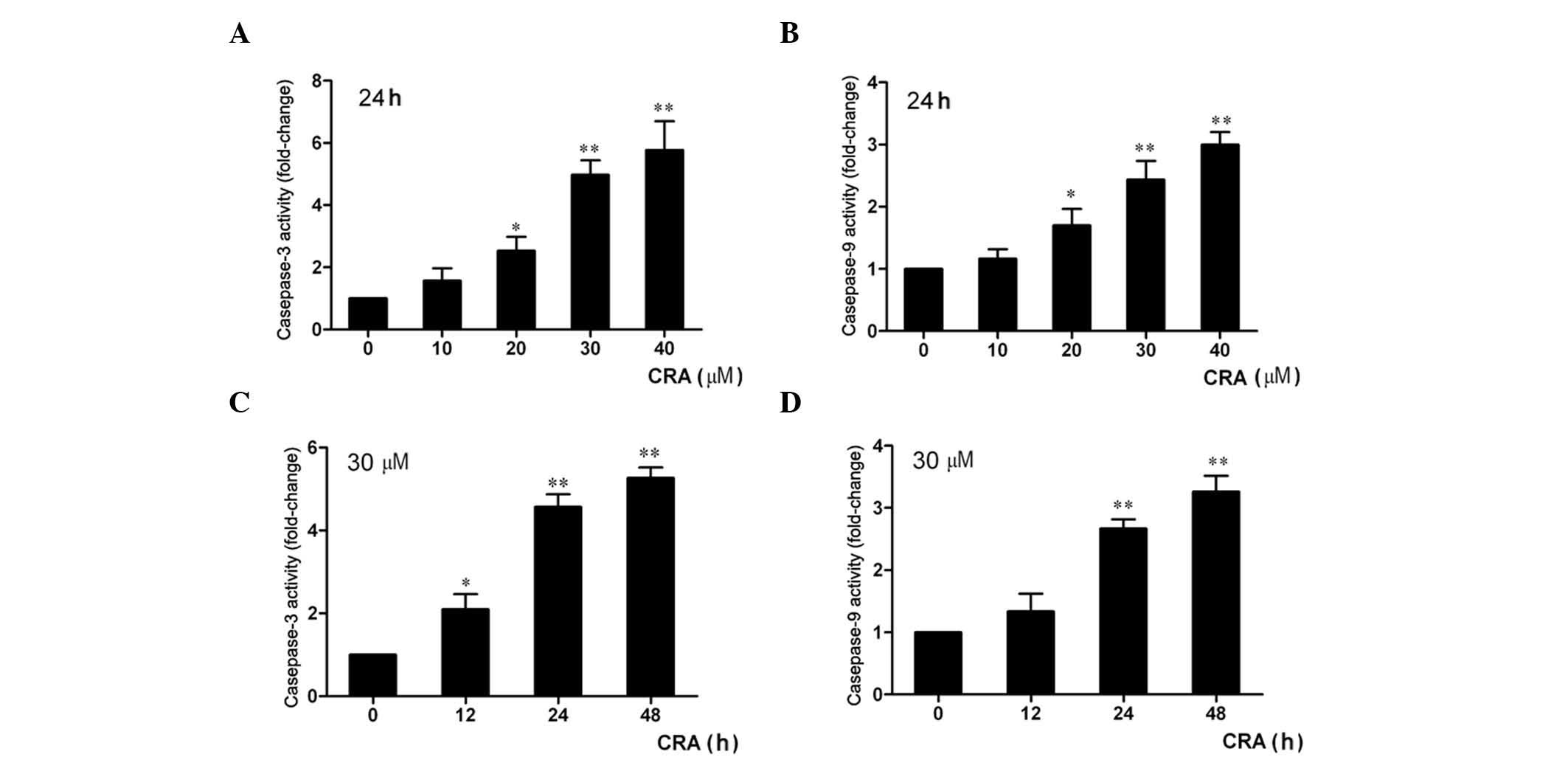
It is well known that the caspase pathway is
important in apoptosis (30). To
examine whether the apoptosis of osteosarcoma cells induced by CRA
was associated with caspase protein activation, the activities of
caspase-3 and caspase-9 were detected using a colorimetric method.
The results revealed that the treatment of MG-63 cells with the
indicated concentrations of CRA for 24 h resulted in a significant
increase in caspase-3 activity in a dose-dependent manner (Fig. 3A), and the activity of caspase-9 in
MG-63 cells treated with CRA was also significantly increased
compared with that in the untreated cells (Fig. 3B, P<0.001). In addition, it was
also observed a time-dependent increase in the activities of
caspase-3 and −9 in MG-63 cells following treatment with CRA. The
activities of caspase-3 and caspase-9 increased by ~2.1- and
1.3-fold after 12 h, by 4.1- and 2.8-fold after 24 h, and by 5.2-
and 3.3-fold after 48 h of treatment (Fig. 3C and D). These results indicated that
the apoptosis of osteosarcoma cells induced by CRA involved caspase
pathway activation.
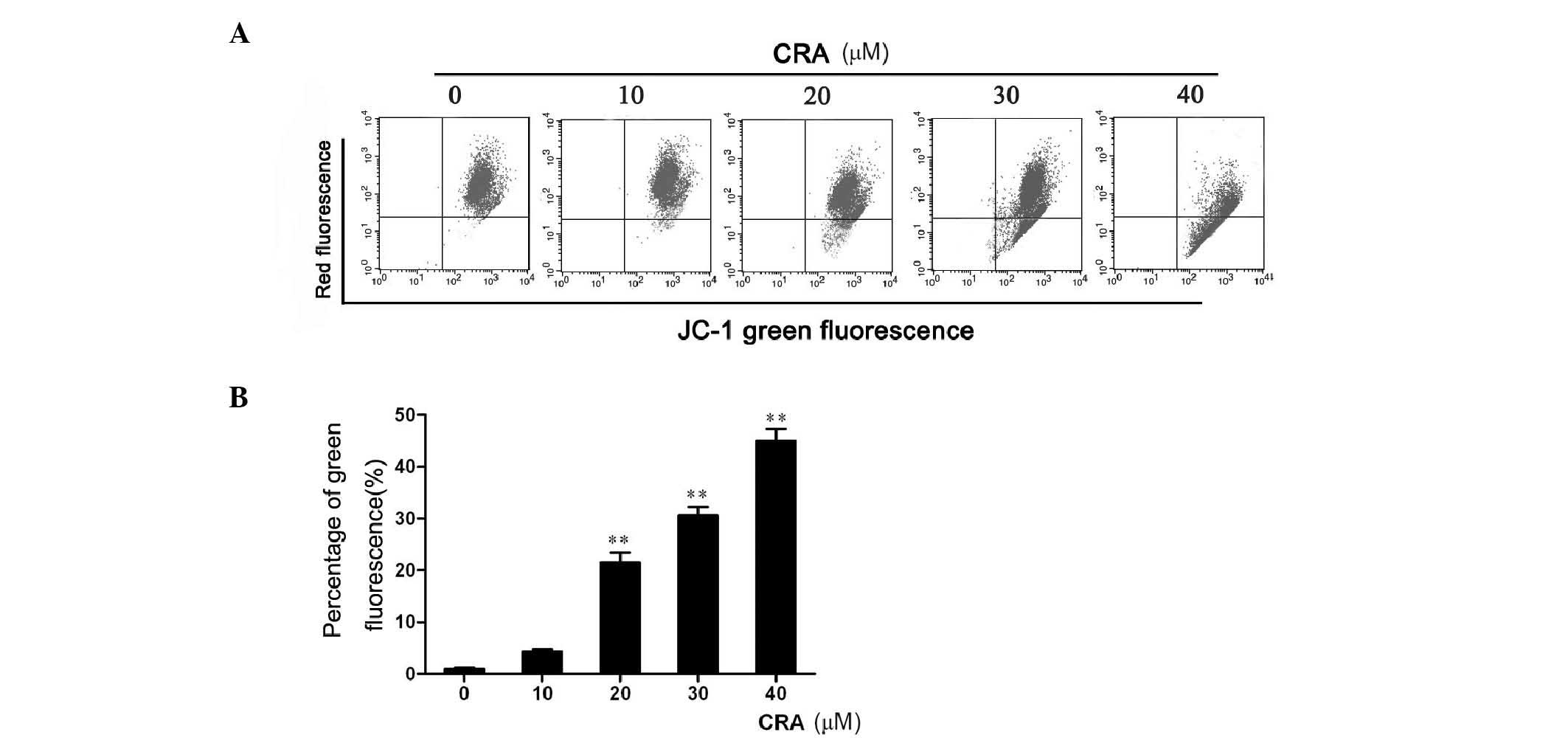
CRA induces loss of mitochondrial
membrane potential
Loss of mitochondrial membrane potential has been
associated with the initiation and activation of the apoptotic
process in cells (31), and the
variations observed for caspase-3 and caspase-9 activities prompted
us to research a potential loss in mitochondrial membrane
potential. For that purpose, a cationic lipophilic dye, JC-1, was
used, which accumulates within the mitochondria in a
potential-dependent manner, to determine the integrity of the
mitochondrial membrane. After CRA treatment for 24 h, MG-63 cells
were harvested and incubated with JC-1 dye, and the fluorescence
emission was analyzed by flow cytometry (Fig. 4A). The results revealed that CRA
treatment of MG-63 cells resulted in a significant increase in the
number of green fluorescence-positive cells (P<0.001, Fig. 4B), as shown in the LR quadrant of the
FACS histogram, suggesting that CRA could induce osteosarcoma cells
to lose mitochondrial membrane potential. Collectively, these
studies suggest that CRA treatment induces the apoptosis of
osteosarcoma cells through disruption of their mitochondrial
membrane potential.
CRA induces cytochrome c release from
mitochondria
Mitochondria are important in the apoptosis
triggered by exogenous chemical agents (32). The disruption of the mitochondrial
membrane results in the release of cytochrome c into the
cytosol (33). In the cytosol,
cytochrome c binds to apoptotic protease activating factor 1
(Apaf-1), allowing the recruitment of caspase-9 and the formation
of an apoptosome complex, resulting in caspase-3 activation and
execution of cell death (25). To
examine whether the apoptosis induced by CRA involved the release
of mitochondrial cytochrome c, the levels of cytochrome
c in the cytoplasm and mitochondria were detected using
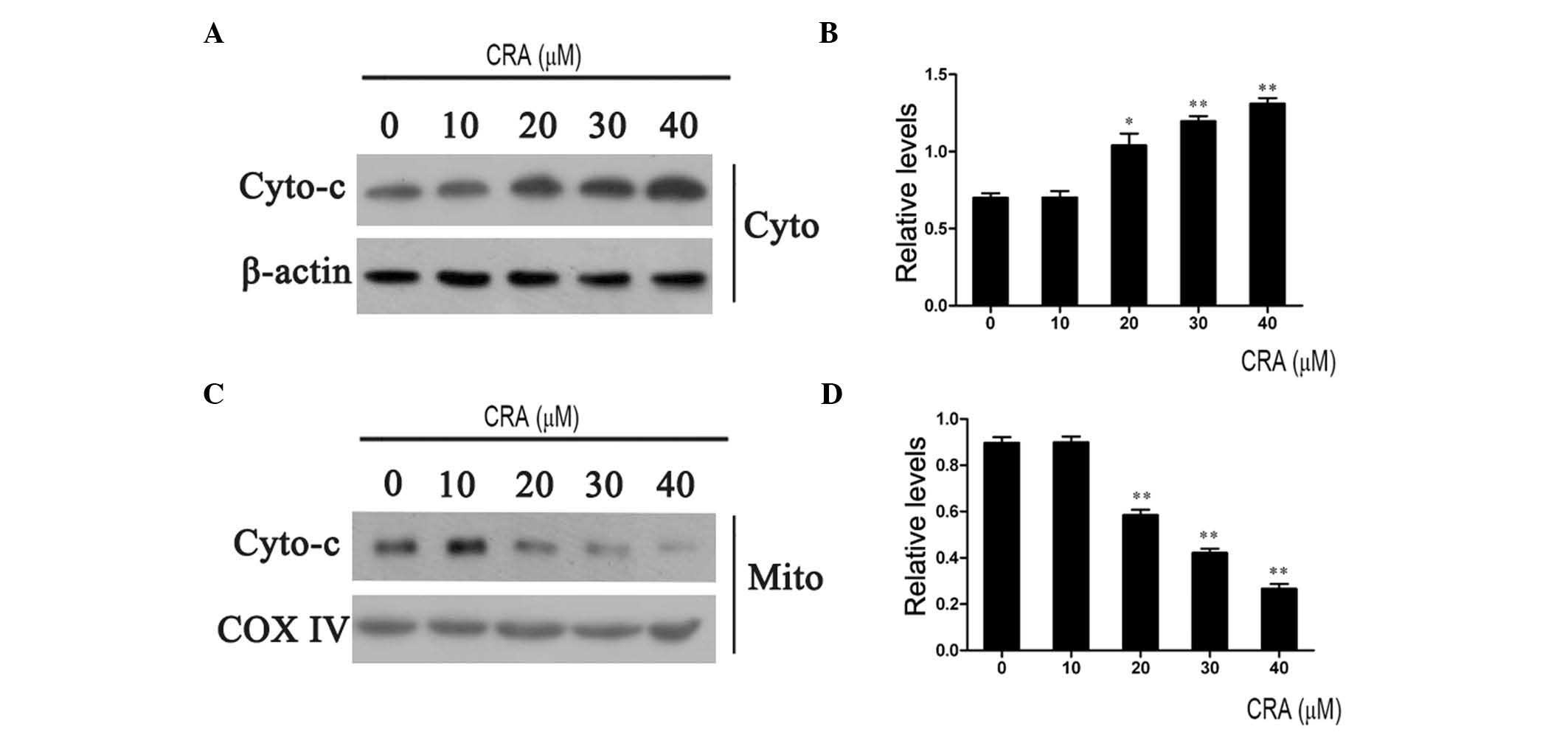
western blotting. As shown in Fig. 5,
treatment of MG-63 cells with CRA resulted in a gradual increase in
cytochrome c levels in the cytoplasm (Fig. 5A) and a dose-dependent decrease in
cytochrome c levels in the mitochondria (Fig. 5C). The relative levels of cytochrome
c in the cytoplasm and mitochondria were further calculated
through normalization with β-actin and COX IV levels (Fig. 5B and D). The results indicated that
CRA treatment provoked the release of cytochrome c from the
mitochondria to the cytoplasm, further confirming the above
observations that CRA resulted in the loss of mitochondrial
membrane potential.
Inhibition of caspase activity
attenuates the apoptosis induced by CRA
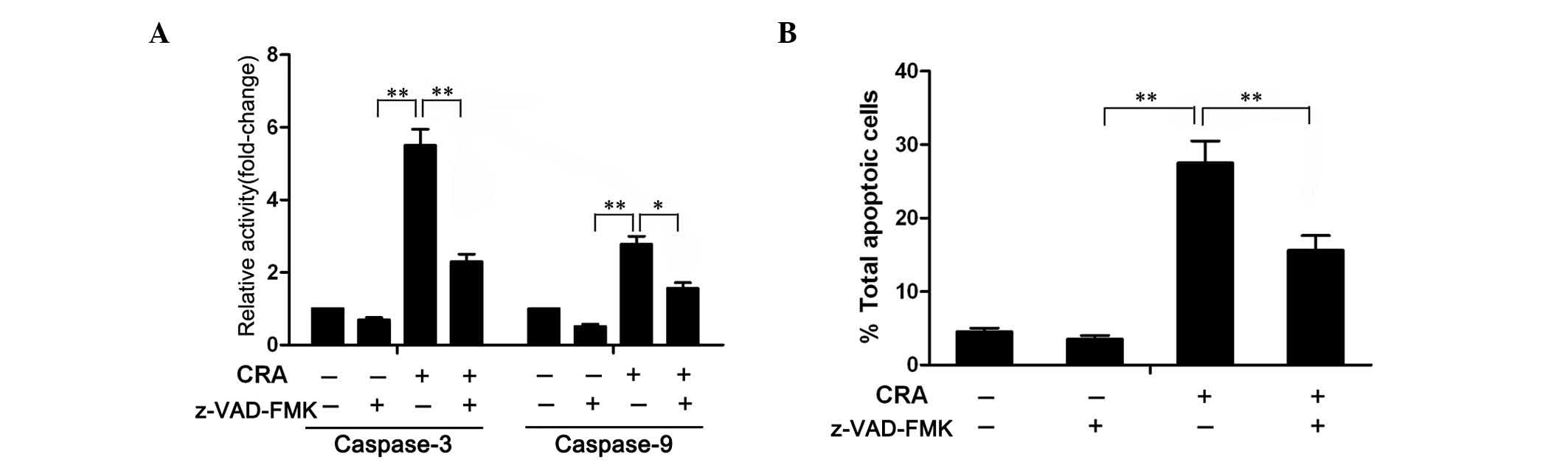
To confirm whether CRA-induced apoptosis involved
caspase cascade activation, MG-63 cells were pretreated with 100 µM
of z-VAD-FMK (Sigma-Aldrich; Merck Millipore), a general caspase
inhibitor (34), for 1 h, prior to be
treated with CRA. The colorimetric assay revealed that the
increased activity of caspase-3 and −9 induced by CRA was
significantly diminished in the presence of z-VAD-FMK (Fig. 6A), suggesting that z-VAD-FMK indeed
blocked the activity of caspase-3 and −9. Further analysis of
apoptosis demonstrated that treatment of cells with z-VAD-FMK
markedly attenuated the CRA-induced apoptosis. As shown in Fig. 6B, apoptosis was observed in ~28.5% of
the cells at 24 h following treatment with CRA in the absence of
z-VAD-FMK, but only in 17.3% of the cells in the presence of
z-VAD-FMK. These results clearly indicated that CRA-induced
apoptosis is associated with caspase activation.
Discussion
Human osteosarcoma, a primary malignant bone tumor,
is most prevalent in adolescence (35). Survival rates of osteosarcoma patients
have not improved significantly in the last 25 years (36). Aiming to increase this survival rate,
numerous studies have been carried out (37). Recently, several bioactive components
from plant origin have been reported to offer promising options for
the development of effective strategies for the therapy of cancers
(33). CRA, a natural compound
derived from apple pomace, has recently been shown to have
anticancer activity (1,38,39). To
understand the effects of CRA on osteosarcoma, the effects of CRA
on the osteosarcoma cell line MG-63 were evaluated in vitro
in the present study.
The treatment of MG-63 cells with different
concentrations of CRA resulted in a significant decrease in cell
viability in a dose- and time-dependent manner. These data
suggested that CRA could inhibit the proliferation of osteosarcoma
cells. It is known that apoptosis plays an essential role in the
development and maintenance of tissue homeostasis through
eliminating unwanted or damaged cells (40), and the aberrant suppression of
apoptosis is frequently associated with tumorigenesis (41,42). For
this reason, the induction of apoptotic cell death is an effective
strategy to inhibit cancer growth. To determine whether CRA could
induce the apoptotic cell death of osteosarcoma cells, the DNA from
MG-63 cells treated with CRA was extracted and subjected to a DNA
ladder assay. An obvious DNA ladder, which is a typical
characteristic of apoptosis (28),
was observed in the cells treated with CRA, but not in the
untreated cells, suggesting that CRA was likely to induce the
apoptotic cell death of MG-63 cells. To further confirm these
results, through double staining with annexin V and PI, the
percentage of apoptotic cells was quantitatively analyzed by flow
cytometry. The results also demonstrated that CRA treatment led to
a dose-dependent increase in the apoptosis of MG-63 cells.
Collectively, these results suggested that CRA could induce the
apoptosis of osteosarcoma cells, and that the induction of
apoptosis by CRA is likely to be an important mechanism of growth
inhibition in MG-63 cells.
To the best of our knowledge, the induction of
apoptosis is associated with two major different activation
pathways: The intrinsic pathway and the extrinsic pathway (43). The intrinsic pathway involves the loss
of mitochondrial membrane potential and the release of cytochrome
c from the mitochondria to the cytosol (44,45). The
cytosolic cytochrome c binds to Apaf-1 and then recruits
pro-caspase-9, forming an apoptosome complex, which finally results
in the activation of caspase-3, one of the key mediators of
apoptosis, and the execution of cell death (46). The extrinsic pathway is initiated with
death receptor ligation or Fas/Fas ligand interaction, resulting in
the subsequent recruitment of Fas-associated death domain protein
and the execution of apoptosis (47).
Therefore, to clearly understand the exact mechanism of the
apoptotic effect of CRA on osteosarcoma cells, the activity of
caspase proteins and the integrity of the mitochondrial membrane
were examined. In the present study, it was observed that treatment
of MG-63 cells with CRA resulted in a significant increase in the
activity of caspase-3 and −9 and in a loss in mitochondrial
membrane potential. These results suggested that the apoptosis of
MG-63 cells induced by CRA was likely to involve the mitochondrial
apoptotic pathway. Consistent with this notion, further
investigation revealed that the levels of cytochrome c in
the cytoplasm of MG-63 cells treated with CRA were significantly
increased compared with those in the untreated cells. On the
contrary, the cytochrome c levels in the mitochondria were
significantly decreased compared with those in the untreated cells,
which suggested that the treatment with CRA caused the cytochrome
c release from the mitochondria to the cytoplasm, and that
the subsequent caspase cascade activation was likely to be the
executive mechanism involved in CRA-mediated apoptosis.
Notably, it was also observed that the CRA-mediated
increase in activity of caspase-3 and −9 was abrogated when the
general caspase inhibitor z-VAD-FMK was employed. In addition, the
apoptosis of MG-63 cells induced by CRA was also significantly
inhibited, suggesting that the activation of the
mitochondria-mediated intrinsic death signaling pathway was
completely blocked by z-VAD-FMK. These findings provide evidence
that the apoptosis induced by CRA in MG-63 cells is mediated by the
mitochondrial pathway. Our observations regarding the caspase
cascade-mediated apoptosis-inducing properties of CRA were in
agreement with those from previous studies (1,34).
Overall, our results demonstrated that CRA could
induce the apoptosis of osteosarcoma cells, and that CRA triggered
the apoptosis of osteosarcoma cells via loss of mitochondrial
membrane potential, release of cytochrome c and activation
of the caspase cascade, suggesting that the apoptosis induced by
CRA was mediated by the mitochondrial pathway. The outcome of the
present study indicated that CRA is a potential bioactive
phytochemical for chemotherapy of osteosarcoma. However, further
studies are required to determine the safety and efficacy of CRA
in vivo.
Acknowledgements
The present study was supported by a grant from the
Department of Healthcare, General Logistics (Beijing, China; grant
number BWS 11J003).
References
|
1
|
Nho KJ, Chun JM and Kim HK: Corosolic acid
induces apoptotic cell death in human lung adenocarcinoma A549
cells in vitro. Food Chem Toxicol. 56:8–17. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li B, Meng X, Zhu L, Jiao X and Zhang J:
Application of high-speed counter-current chromatography for
isolation of triterpenes from Schisandra Chinensis (Turcz.) Baill
and induction apoptosis mechanism of HSC-T6. Biomed Mater Eng.
24:969–977. 2014.PubMed/NCBI
|
|
3
|
Miura T, Takagi S and Ishida T: Management
of diabetes and its complications with Banaba (Lagerstroemia
speciosa L.) and corosolic acid. Evid Based Complement Alternat
Med. 2012:8714952012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Caligiani A, Malavasi G, Palla G,
Marseglia A, Tognolini M and Bruni R: A simple GC-MS method for the
screening of betulinic, corosolic, maslinic, oleanolic and ursolic
acid contents in commercial botanicals used as food supplement
ingredients. Food Chem. 136:735–741. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zong W and Zhao G: Corosolic acid
isolation from the leaves of Eriobotrta japonica showing the
effects on carbohydrate metabolism and differentiation of 3T3-L1
adipocytes. Asia Pac J Clin Nutr. 16(Suppl 1): 346–352.
2007.PubMed/NCBI
|
|
6
|
Yin MC, Lin MC, Mong MC and Lin CY:
Bioavailability, distribution, and antioxidative effects of
selected triterpenes in mice. J Agric Food Chem. 60:7697–7701.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen H, Yang J, Zhang Q, Chen LH and Wang
Q: Corosolic acid ameliorates atherosclerosis in apolipoprotein
E-deficient mice by regulating the nuclear factor-kB signaling
pathway and inhibiting monocyte chemoattractant protein-1
expression. Circ J. 76:995–1003. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Takagi S, Miura T, Ishihara E, Ishida T
and Chinzei Y: Effect of corosolic acid on dietary
hypercholesterolemia and hepatic steatosis in KK-Ay diabetic mice.
Biomed Res. 31:213–218. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Klein G, Kim J, Himmeldirk K, Cao Y and
Chen X: Antidiabetes and Anti-obesity Activity of Lagerstroemia
speciosa. Evid Based Complement Alternat Med. 4:401–407. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fujiwara Y, Komohara Y, Ikeda T and Takeya
M: Corosolic acid inhibits glioblastoma cell proliferation by
suppressing the activation of signal transducer and activator of
transcription-3 and nuclear factor-kappa B in tumor cells and
tumor-associated macrophages. Cancer Sci. 102:206–211. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ahn KS, Hahm MS, Park EJ, Lee HK and Kim
IH: Corosolic acid isolated from the fruit of Crataegus pinnatifida
var. psilosa is a protein kinase C inhibitor as well as a cytotoxic
agent. Planta Med. 64:468–470. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lee MS, Cha EY, Thuong PT, Kim JY, Ahn MS
and Sul JY: Down-regulation of human epidermal growth factor
receptor 2/neu oncogene by corosolic acid induces cell cycle arrest
and apoptosis in NCI-N87 human gastric cancer cells. Biol Pharm
Bull. 33:931–937. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gatta G, Capocaccia R, Stiller C, Kaatsch
P, Berrino F and Terenziani M: EUROCARE Working Group: Childhood
cancer survival trends in Europe: A EUROCARE Working Group study. J
Clin Oncol. 23:3742–3751. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sulzbacher I, Birner P, Trieb K,
Pichlbauer E and Lang S: The expression of bone morphogenetic
proteins in osteosarcoma and its relevance as a prognostic
parameter. J Clin Pathol. 55:381–385. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Federman N, Bernthal N, Eilber FC and Tap
WD: The multidisciplinary management of osteosarcoma. Curr Treat
Options Oncol. 10:82–93. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lewis IJ, Nooij MA, Whelan J, Sydes MR,
Grimer R, Hogendoorn PC, Memon MA, Weeden S, Uscinska BM, van
Glabbeke M, et al: Improvement in histologic response but not
survival in osteosarcoma patients treated with intensified
chemotherapy: A randomized phase III trial of the European
Osteosarcoma Intergroup. J Natl Cancer Inst. 99:112–128. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Buddingh EP, Anninga JK, Versteegh MI,
Taminiau AH, Egeler RM, van Rijswijk CS, Hogendoorn PC, Lankester
AC and Gelderblom H: Prognostic factors in pulmonary metastasized
high-grade osteosarcoma. Pediatr Blood Cancer. 54:216–221.
2010.PubMed/NCBI
|
|
18
|
Fischer U and Schulze-Osthoff K: New
approaches and therapeutics targeting apoptosis in disease.
Pharmacol Rev. 57:187–215. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cotter TG: Apoptosis and cancer: The
genesis of a research field. Nat Rev Cancer. 9:501–507. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cohen Z, Maimon Y, Yoeli-Lerner M, Yang P,
Samuels N and Berger R: Selective anticancer effects and protection
from chemotherapy by the botanical compound LCS101: Implications
for cancer treatment. Int J Oncol. 46:308–316. 2015.PubMed/NCBI
|
|
21
|
Lowe SW and Lin AW: Apoptosis in cancer.
Carcinogenesis. 21:485–495. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Roy AM, Baliga MS and Katiyar SK:
Epigallocatechin-3-gallate induces apoptosis in estrogen
receptor-negative human breast carcinoma cells via modulation in
protein expression of p53 and Bax and caspase-3 activation. Mol
Cancer Ther. 4:81–90. 2005.PubMed/NCBI
|
|
23
|
Ariffin SH, Yeen WW, Abidin IZ, Wahab RM
Abdul, Ariffin ZZ and Senafi S: Cytotoxicity effect of degraded and
undegraded kappa and iota carrageenan in human intestine and liver
cell lines. BMC Complement Altern Med. 14:5082014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mantena SK, Sharma SD and Katiyar SK:
Berberine inhibits growth, induces G1 arrest and apoptosis in human
epidermoid carcinoma A431 cells by regulating Cdki-Cdk-cyclin
cascade, disruption of mitochondrial membrane potential and
cleavage of caspase 3 and PARP. Carcinogenesis. 27:2018–2027. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Singh T, Sharma SD and Katiyar SK: Grape
proanthocyanidins induce apoptosis by loss of mitochondrial
membrane potential of human non-small cell lung cancer cells in
vitro and in vivo. PLoS One. 6:e274442011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang F, Franco R, Skotak M, Hu G and
Chandra N: Mechanical stretch exacerbates the cell death in SH-SY5Y
cells exposed to paraquat: Mitochondrial dysfunction and oxidative
stress. Neurotoxicology. 41:54–63. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang Y, Li B, Ji ZZ and Zheng PS: Notch1
regulates the growth of human colon cancers. Cancer. 116:5207–5218.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Song TH, Yang JY, Jeong IK, Park JS, Jee
YK, Kim YS and Lee KY: Paraquat-induced apoptotic cell death in
lung epithelial cells. Tuberc Respir Dis. 61:366–373. 2006.
View Article : Google Scholar
|
|
29
|
Zhou J, Li P, Xue X, He S, Kuang Y, Zhao
H, Chen S, Zhi Q and Guo X: Salinomycin induces apoptosis in
cisplatin-resistant colorectal cancer cells by accumulation of
reactive oxygen species. Toxicol Lett. 222:139–145. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Philchenkov A: Caspases: Potential targets
for regulating cell death. J Cell Mol Med. 8:432–444. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Reed JC: Regulation of apoptosis by bcl-2
family proteins and its role in cancer and chemoresistance. Curr
Opin Oncol. 7:541–546. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Xiao X, Chen L, Ouyang Y, Zhu W, Qiu P, Su
X, Dou Y, Tang L, Yan M, Zhang H, et al: Pregnenolone, a
cholesterol metabolite, induces glioma cell apoptosis via
activating extrinsic and intrinsic apoptotic pathways. Oncol Lett.
8:645–650. 2014.PubMed/NCBI
|
|
33
|
Chen Q, Liu XF and Zheng PS: Grape seed
proanthocyanidins (GSPs) inhibit the growth of cervical cancer by
inducing apoptosis mediated by the mitochondrial pathway. PLoS One.
9:e1070452014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Xu Y, Ge R, Du J, Xin H, Yi T, Sheng J,
Wang Y and Ling C: Corosolic acid induces apoptosis through
mitochondrial pathway and caspase activation in human cervix
adenocarcinoma HeLa cells. Cancer Lett. 284:229–237. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Shi Z, Huang X, Liu B, Tao H, Cai Y and
Tang R: Biological response of osteosarcoma cells to
size-controlled nanostructured hydroxyapatite. J Biomater Appl.
25:19–37. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kuijjer ML, Namløs HM, Hauben EI, Machado
I, Kresse SH, Serra M, Llombart-Bosch A, Hogendoorn PC, Meza-Zepeda
LA, Myklebost O and Cleton-Jansen AM: mRNA expression profiles of
primary high-grade central osteosarcoma are preserved in cell lines
and xenografts. BMC Med Genomics. 4:662011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Biao LU: Correlation between ErbB2
expression and survival rates of osteosarcoma patients. Medical
Journal of Wuhan University. 2:273–276. 2014.
|
|
38
|
Sung B, Kang YJ, Kim DH, Hwang SY, Lee Y,
Kim M, Yoon JH, Kim CM, Chung HY and Kim ND: Corosolic acid induces
apoptotic cell death in HCT116 human colon cancer cells through a
caspase-dependent pathway. Int J Mol Med. 33:943–949.
2014.PubMed/NCBI
|
|
39
|
Fujiwara Y, Takaishi K, Nakao J, Ikeda T,
Katabuchi H, Takeya M and Komohara Y: Corosolic acid enhances the
antitumor effects of chemotherapy on epithelial ovarian cancer by
inhibiting signal transducer and activator of transcription 3
signaling. Oncol Lett. 6:1619–1623. 2013.PubMed/NCBI
|
|
40
|
Thompson CB: Apoptosis in the pathogenesis
and treatment of disease. Science. 267:1456–1462. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Correa P and Miller MJ: Carcinogenesis,
apoptosis and cell proliferation. Br Med Bull. 54:151–162. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ambrosini G, Adida C and Altieri DC: A
novel anti-apoptosis gene, survivin, expressed in cancer and
lymphoma. Nat Med. 3:917–921. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zou WW, Xiao HP, Gu MN, Liu KX and Liu ZQ:
Propofol induces rat embryonic neural stem cell apoptosis by
activating both extrinsic and intrinsic pathways. Mol Med Rep.
7:1123–1128. 2013.PubMed/NCBI
|
|
44
|
Chipuk JE, Kuwana T, Bouchier-Hayes L,
Droin NM, Newmeyer DD, Schuler M and Green DR: Direct activation of
Bax by p53 mediates mitochondrial membrane permeabilization and
apoptosis. Science. 303:1010–1014. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Tan J, Zhuang L, Leong HS, Iyer NG, Liu ET
and Yu Q: Pharmacologic modulation of glycogen synthase
kinase-3beta promotes p53-dependent apoptosis through a direct
Bax-mediated mitochondrial pathway in colorectal cancer cells.
Cancer Res. 65:9012–9020. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kluck RM, Bossy-Wetzel E, Green DR and
Newmeyer DD: The release of cytochrome c from mitochondria: A
primary site for Bcl-2 regulation of apoptosis. Science.
275:1132–1136. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kischkel FC, Hellbardt S, Behrmann I,
Germer M, Pawlita M, Krammer PH and Peter ME:
Cytotoxicity-dependent APO-1 (Fas/CD95)-associated proteins form a
death-inducing signaling complex (DISC) with the receptor. EMBO J.
14:5579–5588. 1995.PubMed/NCBI
|