Introduction
Uveal melanoma, with an incidence of 6–7 cases per
million individuals annually in the United States, is the most
common intraocular malignant tumor in adults (1). It develops in one of the most
capillary-rich tissues of the body and has a high mortality due to
a strong preference for metastases in the liver (2). Uveal melanoma responds poorly to
chemotherapy (2). Therefore, the
mortality of uveal melanoma is high, at 31% within 5 years of
diagnosis, 45% within 15 years and 49% within 25 years (2). The majority of uveal melanoma patients
die within 6 months of liver metastasis, with a median survival
time of 3.6 months (2,3). Therefore, it is urgently required to
identify a novel treatment to inhibit the proliferation, migration,
invasion and metastasis of uveal melanoma cells.
Flavonoids are a family of polyphenolic compounds
synthesized by plants with a similar structure, which are
categorized into anthocyanidins, flavanols, flavanones, flavonols,
flavones and isoflavones according to their functional groups
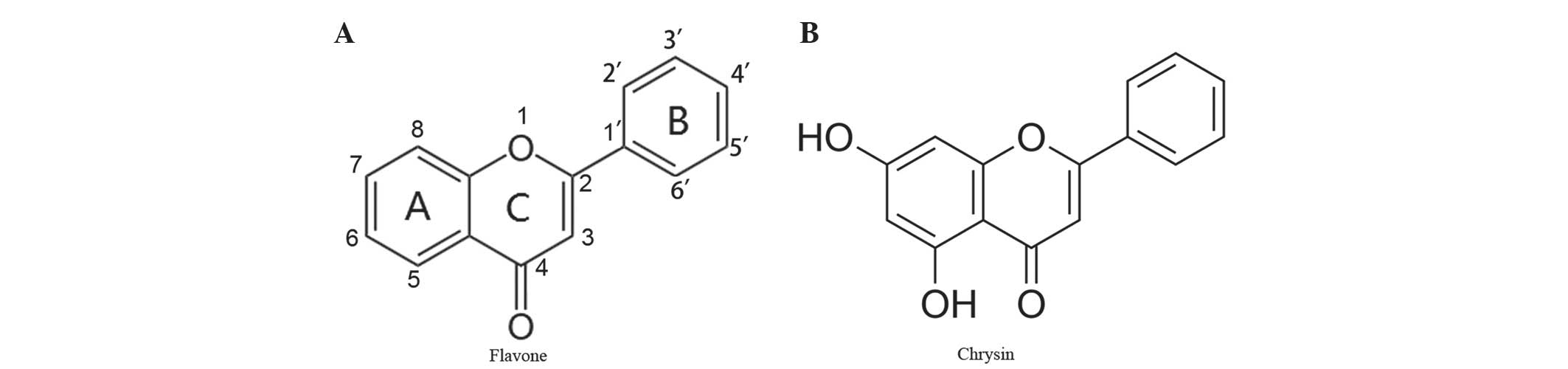
(4). Chrysin, a naturally active
compound of the flavone group, shares a common flavone structure
with additional hydroxyls at positions 5 and 7 of the A ring
(Fig. 1A and B), and the presence of
at least two hydroxyl groups in positions 3, 5 and 7 of the A ring
is reported to be necessary to confer pro-apoptotic activity
(4). Chrysin is present in honey,
propolis, and other plants and herbs (5). Chrysin was initially identified for its
anti-oxidant effects and has been shown to possess cancer
chemopreventive and anti-tumor effects (6–8). It
inhibits proliferation and induces apoptosis in a variety of cancer
cells in vitro, including cells from the prostate, skin,
breast, lung, cervix, thyroid cancer and leukemia (5,8–22). However, to the best of our knowledge,
the effects of chrysin on uveal melanoma cells have not been
previously studied.
The purpose of the present study was to investigate
the cytotoxic effects of chrysin on human uveal melanoma cells
in vitro and compare to those on normal human scleral
fibroblasts and retinal pigment epithelial (RPE) cells. The effects
of chrysin on mitochondrial permeability, cytochrome c, and
the activities of caspase-3, −8 and −9 were also studied to
elucidate the signaling pathway involved in chrysin-induced
apoptosis of human uveal melanoma cells.
Materials and methods
Reagents
Chrysin,
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
and dimethyl sulfoxide (DMSO) were purchased from Sigma-Aldrich
(EMD Millipore, Billerica, MA, USA). Dulbecco's modified Eagle's
medium (DMEM), fetal bovine serum (FBS), phosphate-buffered saline
(PBS), 0.05% trypsin-0.02% EDTA solution, and gentamicin were
purchased from Gibco (Thermo Fisher Scientific, Inc., Waltham, MA,
USA). Cytochrome c enzyme-linked immunosorbent assay (ELISA)
and caspase-3 colorimetric assay kits were purchased from EMD
Millipore. Caspase-8 and −9 colorimetric activity assay kits were
purchased from R&D Systems, Inc. (Minneapolis, MN, USA).
Cell culture
The effects of chrysin were investigated in two
human uveal melanoma cell lines (SP6.5 and M17) and compared to the
effects on two distinct normal cell lines [human retinal pigment
epithelial (RPE) cells and scleral fibroblasts]. The human M17
melanoma cell line, RPE cells and scleral fibroblasts were
established in the Tissue Culture Center of the New York Eye and
Ear Infirmary (New York, NY, USA) as previously reported (23). The SP6.5 melanoma cell line was
isolated from a primary choroidal melanoma patient and was provided
by Dr. Guy Pelletier (Research Center of Immunology, Quebec,
Canada) (24). All melanoma cells,
RPE cells and fibroblasts were cultured with DMEM, supplemented
with 10% FBS and gentamicin (50 µg/ml). Cells were incubated at
37°C in a CO2 regulated incubator in a humidified 95%
air/5% CO2 atmosphere. When cultures reached confluence,
cells were detached with trypsin-EDTA solution and passaged. All
tissues were obtained with premortem consent in accordance with the
laws and regulations in place in the various jurisdictions.
MTT assay for cell viability
MTT assay was performed as previously described
(25). Cells (6×103
cells/well) were plated into 96-well plates. Cells were incubated
overnight at 37°C in a CO2 regulated incubator in a
humidified 95% air/5% CO2 atmosphere. Chrysin was
dissolved in DMSO at a concentration of 20 mM (stock solution). In
dose-response studies, 24 h following plating, chrysin was applied
to the cultures at final concentrations of 0, 10, 30 and 100 µM and
cultured for 48 h. A total of 50µl of MTT solution at a
concentration of 1 mg/ml was added to each well. MTT solution was
aspirated following 4 h of incubation at 37°C and the produced
formazan blue was dissolved in 100 µl of DMSO. Absorbance at 540 nm
was measured using a microplate reader (Multiskan MCC/340; Thermo
Fisher Scientific, Inc.). The control group measurement was
standardized as 100% viability. The concentration at which cell
growth was inhibited by 50% (the 50% inhibitory concentration,
IC50) was determined by linear interpolation.
Experiments were performed in triplicate.
To investigate the time-effect of chrysin on uveal
melanoma cells, melanoma cells (SP6.5 cell line, 6×103
cells/well) were plated into 96-well plates and divided into two
groups: Chrysin-treated group and untreated group (control group).
Subsequently, chrysin was added to the cultures in the treated
groups at the concentrations of 30 µM following 24 h of incubation
at 37°C. MTT assay was subsequently performed in treated and
untreated groups at 0, 6, 12, 24 and 48 h following the addition of
chrysin. The results of each treated group were compared to the
controls at the identical time point. All measurements were
performed in three independent experiments, and each time point was
performed in triplicate.
Apoptotic cells detection assay
Cell apoptosis was determined by the terminal
deoxynucleotidyl transferase (TdT) mediated dUTP nick end-labeling
(TUNEL) assay (26,27) using a TACS.XL In Situ Apoptosis
Detection kit (cat. no. 4828-30-DK; R & D Systems, Inc.). The
assay was performed according to the manufacturer's protocol.
Briefly, cells were cultured on chamber slides and analyzed 48 h
following treatment with 10, 30 and 100 µM chrysin. Cells were
fixed, incubated with 3% H2O2/methanol and
permeabilized. Slides were covered by Labeling Reaction mix (R
& D Systems, Inc.) and incubated for 1 h at 37°C in a dark
humidified chamber, followed by incubation with anti-BrdU antibody
(1:50; cat. no. 4828-30-DK; TACS.XL In Situ Apoptosis Detection
kit; R & D Systems, Inc.) at 37°C for 1 h. The slides were
treated with streptavidin-horseradish peroxidase solution(R & D
Systems, Inc.) for 10 min, followed by staining with
3,3′-diaminobenzidine (DAB) substrate for 5 min. The samples were
mounted and analyzed under a light microscope, where the apoptotic
cells could be detected as highly condensed shrunken dark brown
cells. A total of 200 cells were counted in each group (0, 10, 30
and 100 µM chrysin treatment groups). The experiment was repeated
independently a total of three times.
Mitochondrial transmembrane potential
assessment (MTP) by JC-1 staining
MTP was measured by a Mitochondria Staining kit
(cat. no. T4069; Sigma-Aldrich; EMD Millipore), which employed JC-1
(5,5′,6,6′-tetrachloro-1,1′,3,3′-etraethylbenzimidazol-carbocyanine
iodine), a sensitive fluorescent dye, as the probe. Uveal melanoma
cells (SP6.5 cell line) were plated into 96-well plates with black
wells (Corning Incorporated, Corning, NY, USA) at a density of
8×103 cells/well. Following 24 h of incubation at 37°C,
cells were treated with chrysin at various concentrations (0, 10,
30 and 100 µM) at 37°C for 48 h. JC-1 (at final concentration 12.5
µg/ml) was added and incubated at 37°C in the dark for 15 min. JC-1
and culture medium were aspirated and cells were washed three times
with PBS. Cells were immediately observed by fluorescence
microplate reader (Spectraflour Plus; Tecan Group Ltd., Männedorf,
Switzerland). Settings of 485-nm (excitation) and 590-nm (emission)
were used to measure red fluorescence; 485-nm (excitation) and
535-nm (emission) were used to measure green fluorescence. The
detection is based on the accumulation of the dye and its
fluorescence in the mitochondria. The accumulated dye appears as
red fluorescence located in the undamaged mitochondria, whereas the
dye remains as monomers in the cytoplasm with diffuse green
fluorescence in cells with damaged MTP (28). The results (red fluorescence/green
fluorescence ratio, R/G ratio) were expressed as a percentage of
the controls (cells not treated with chrysin). This experiment was
performed a total of three independent times.
Cytochrome c release assay
To detect the release of mitochondrial cytochrome
c, uveal melanoma cells (SP6.5 cell line, 4×105
cells/well) were plated into 6-well plates. A total of 24 h later,
chrysin was added at various concentrations (0, 10, 30 and 100 µM).
Following 2 h of incubation at 37°C, the cells were harvested. The
cells were washed with cold PBS three times, treated with 0.02%
EDTA solution and scraped from the well. Subsequently, cells were
counted and centrifuged at 400 × g at 4°C for 5 min, and the
cell pellets were collected. Following cell lysis and
centrifugation at 1,000 × g at 4°C for 10 min, the
supernatant (cytosol and mitochondria) was collected and
centrifuged at 10,000 × g at 4°C for 20 min. The pellets
(mitochondria) and the supernatant (cytosol) were then collected
separately. A cytochrome c ELISA kit (EMD Millipore) was
used to measure the level of cytochrome c in the cytosol and
the procedure was performed according to the manufacturer's
protocol. The cytochrome c level was demonstrated as a
percentage of the controls (cells not treated with chrysin).
Caspase-3, caspase-8 and caspase-9
colorimetric assay
Melanoma cells (SP6.5 cell line, 4×105
cells/well) were plated into 6-well plates, and chrysin was added
at various concentrations (0, 10, 30 and 100 µM) following 24 h of
incubation at 37°C. A total of 2 h later, cells were washed with
cold PBS and collected. Subsequently, the cells were counted and
centrifuged at 400 × g at 4°C for 5 min, and the cell
pellets were collected. The cells were lysed using cell extraction
buffer (BioSource; Thermo Fisher Scientific, Inc.) with protease
inhibitor cocktail (Sigma-Aldrich; EMD Millipore) and phenylmethane
sulfonyl fluoride (BioSource; Thermo Fisher Scientific, Inc.),
incubated on ice for 30 min, and vortexed for 30 sec. The lysates
were centrifuged at 15,000 × g for 10 min at 4°C. The
supernatant was stored at −70°C until analysis. The levels of
caspase-3, −8,and −9 in cell lysates were measured by specific
colorimetric kits in accordance with the manufacturer's protocol. A
microplate reader at 405 nm was used to measure the optical
density. Caspase-3, caspase-8 and caspase-9 activities were
demonstrated as a percentage of the controls (cells not treated
with chrysin). This measurement was performed independently three
times.
Statistical analysis
Data was analyzed using SPSS version 17.0 (SPSS
Inc., Chicago, IL, USA). Significance was evaluated using one-way
analysis of variance and the least significant difference method in
comparison of data of more than two groups and Student's
t-test for of comparison data between two groups. P<0.05
was considered to indicate a statistically significant
difference.
Results
Effects of chrysin on viability of
melanoma and normal cells
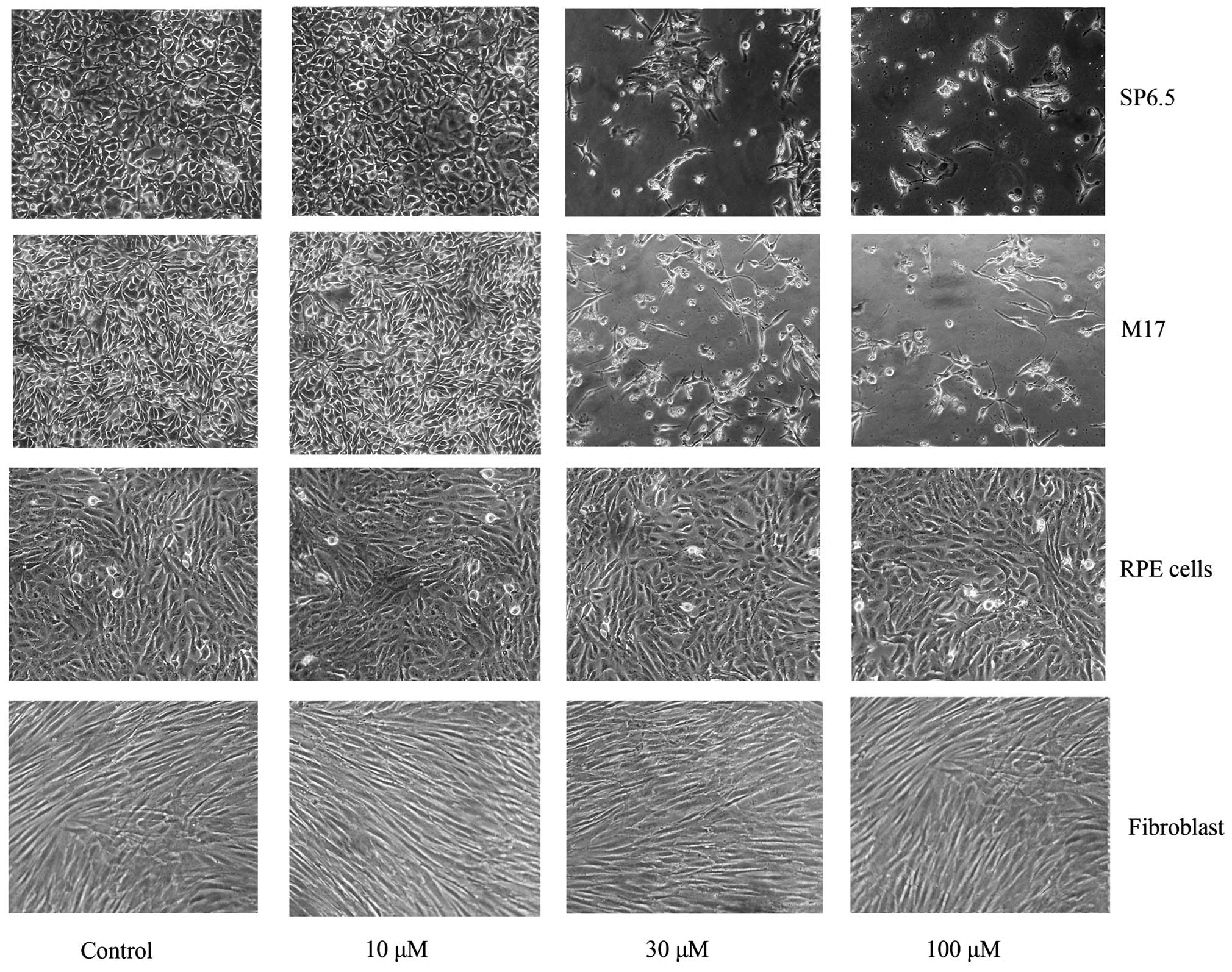
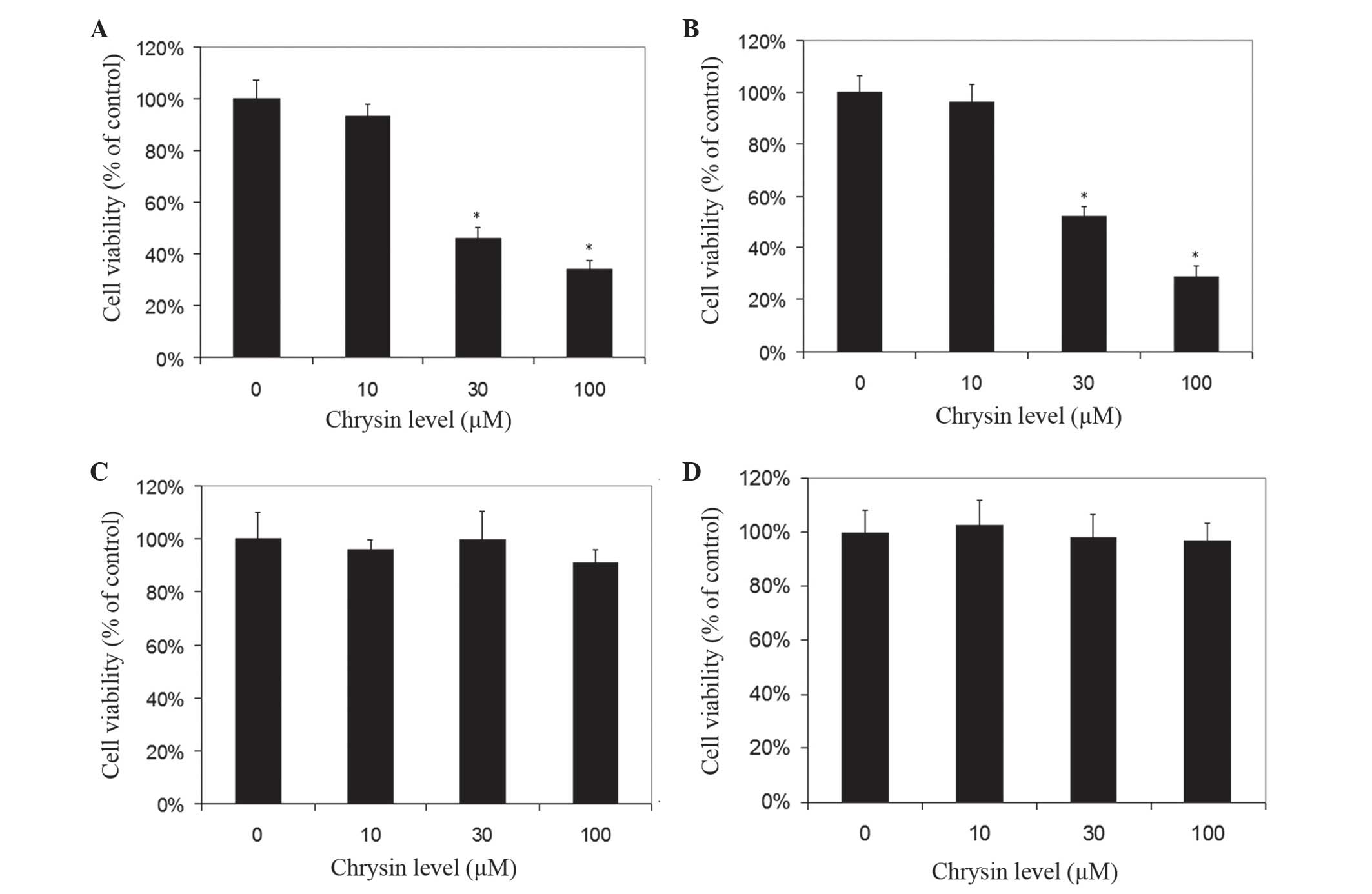
Chrysin induced cell death in cultured human uveal
melanoma cells in a dose-dependent manner (Figs. 2 and 3).
There was a significant difference in cell viability between
melanoma cells (M17 and SP6.5) treated or untreated with chrysin
(P<0.001) at 30–100 µM levels. The IC50 dose of
chrysin for M17 and SP6.5 at 48 h was 35.8 and 28.3 µM,
respectively. Chrysin at 10–100 µM levels did not affect the cell
viability of cultured human RPE cells or fibroblasts. These results
demonstrated that chrysin at 30–100 µM selectively reduced the
viability of melanoma cells without affecting RPE cells or
fibroblasts.
Time-effect study demonstrated that chrysin at 30 µM
decreased the viability of uveal melanoma cells (SP6.5) in a
time-dependent manner from 6 to 48 h (data not shown). There was a
significant difference in cell viability at various time points
between the treated and untreated group (P<0.001).
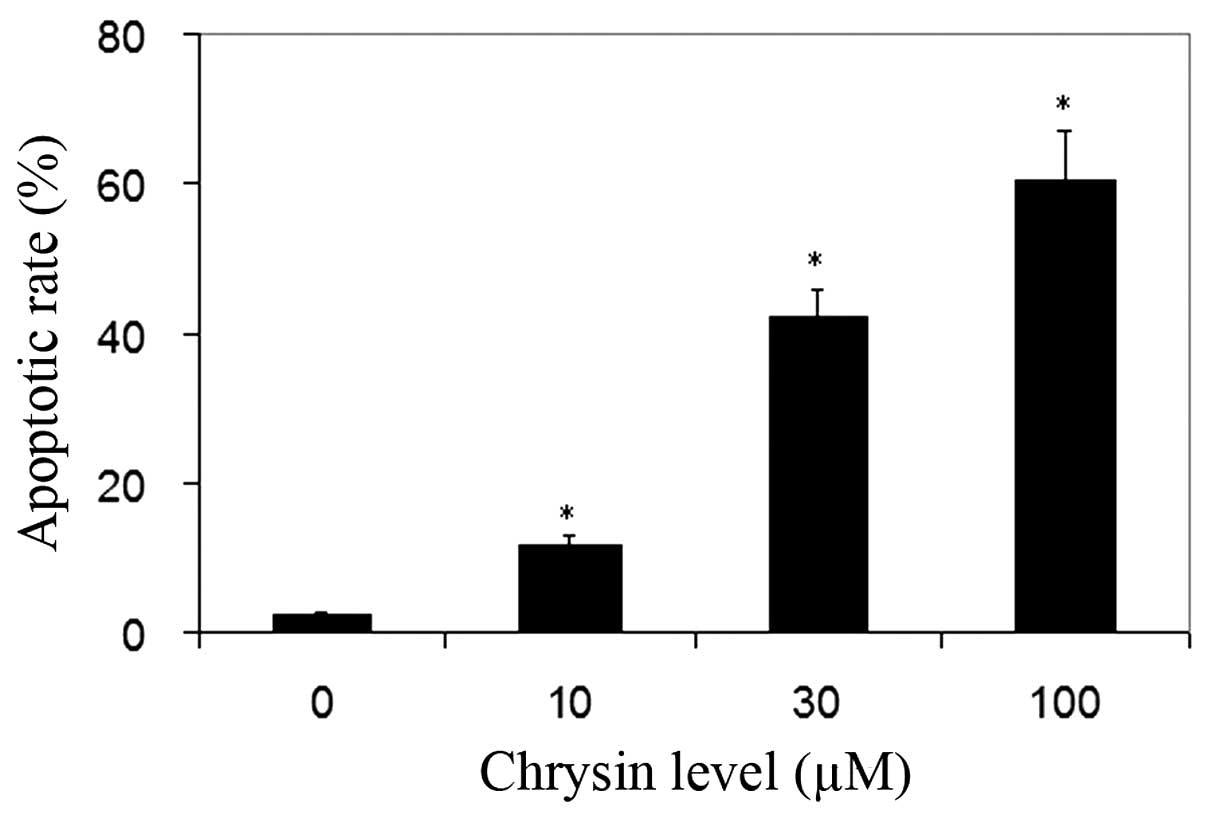
Chrysin induced apoptosis of melanoma
cells
TUNEL analysis was used for the detection of cell
apoptosis. Normal cells were stained by methyl green and were green
in color. The nuclei of apoptotic cells were stained by DAB and
were dark-brown in color. Chrysin significantly increased the
apoptotic rate of uveal melanoma cells in all treated cultures
compared to the control (P<0.001; Fig.
4).
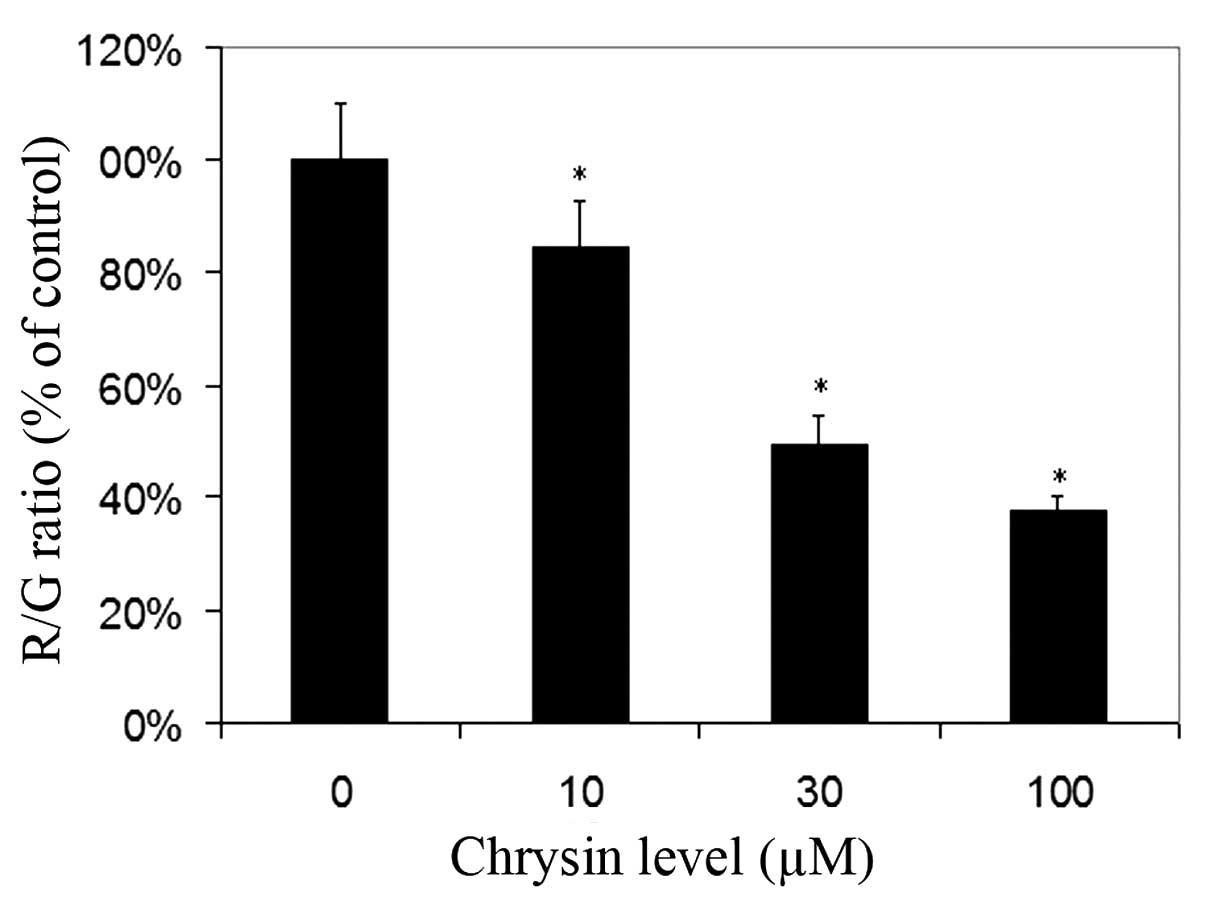
Effects of chrysin on MTP in melanoma
cells
For cells cultured with JC-1, red fluorescence was
due to a potential-dependent aggregation of JC-1 in the
mitochondria. Green fluorescence observed in the cytosol following
mitochondrial membrane depolarization reflected the monomeric form
of JC-1. Chrysin significantly decreased the R/G ratio in a
dose-dependent manner (compared to the control; P<0.001 at all
tested levels), which revealed that chrysin caused damage to the
MTP in uveal melanoma cells (Fig.
5).
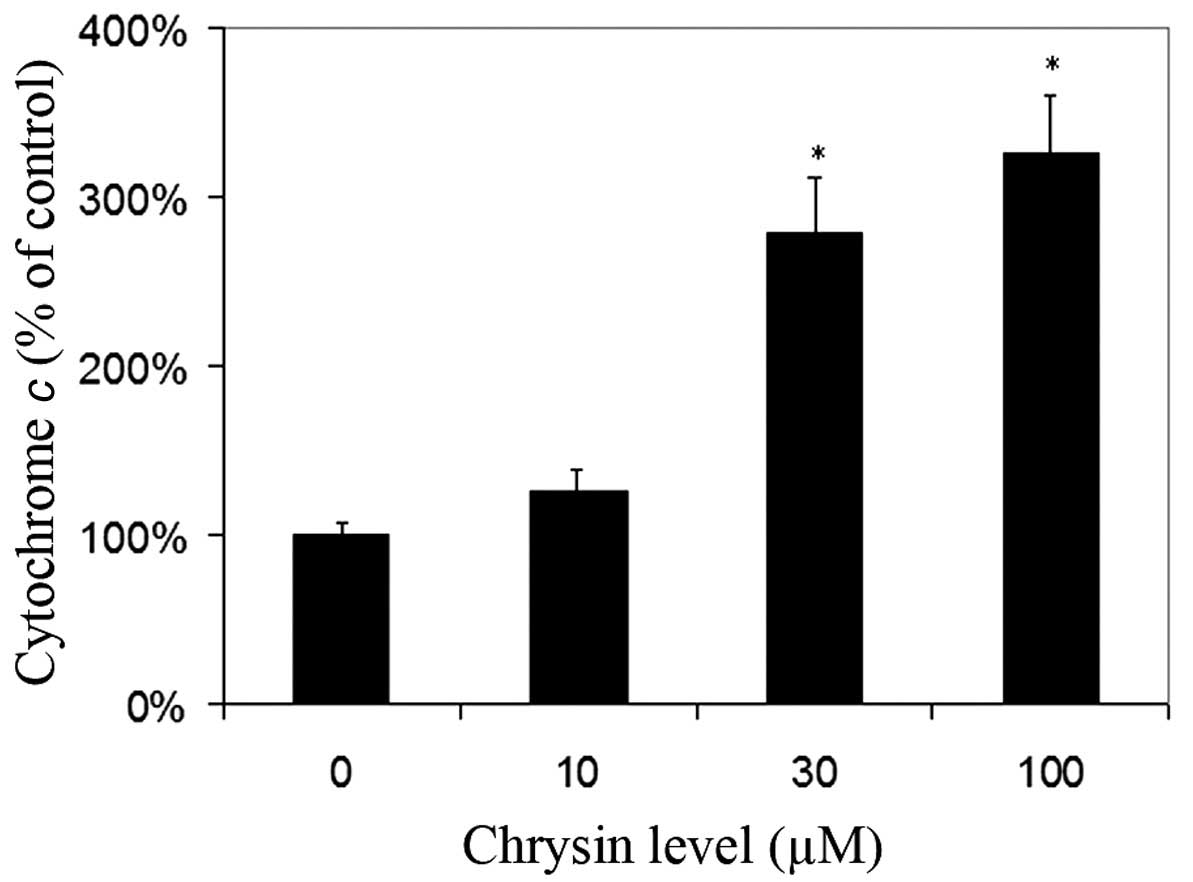
Effects of chrysin on the release of
cytochrome c in cytosol
Chrysin caused an increase of cytosol cytochrome
c levels in melanoma cells in a dose-dependent manner
(compared to the control; P=0.25 at the levels of 10 µM; P<0.001
at the levels of 30–100 µM) (Fig.
6).
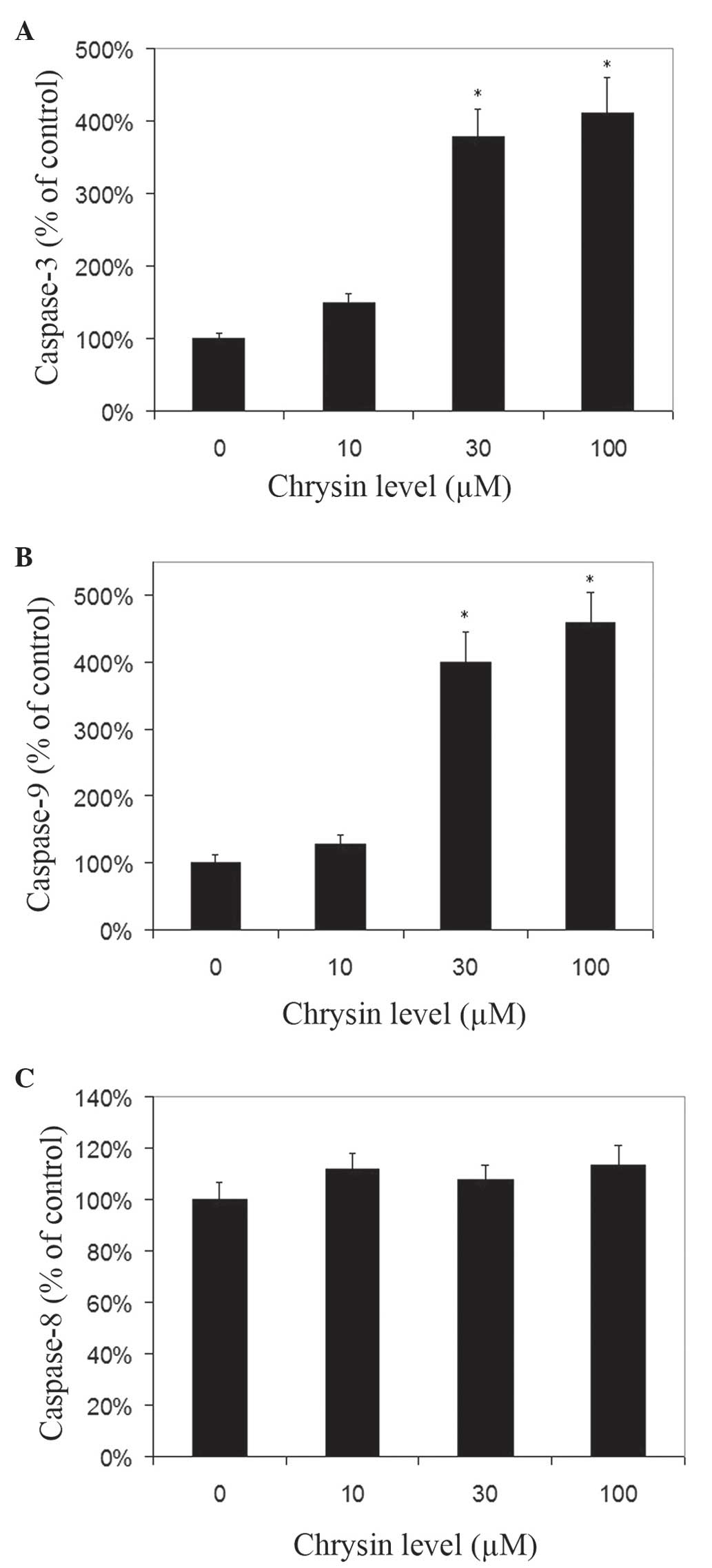
Effects of chrysin on caspase-3,
caspase-8 and caspase-9 activities in melanoma cells
Chrysin increased the activities of caspase-3 and −9
in melanoma cells in a dose-dependent manner (Fig. 7A and B). Chrysin at 30–100 µM levels
resulted in a significant increase of caspase-3 and −9 activities
in melanoma cells (P<0.001). In uveal melanoma cells treated
with 100 µM chrysin, an ~4.11-fold and 4.58-fold increase in
caspase-3 and caspase-9 activities was noted, respectively. There
was no significant change in caspase-8 activity following treatment
with chrysin at 10–100 µM levels (Fig.
7C).
Discussion
Chrysin has been demonstrated to possess cancer
chemopreventive activity through inhibition of cell proliferation
(10,18), and induce apoptosis in various
malignant cancer cells (5,11,15–17,20–22).
It has been reported that chrysin is cytotoxic in
murine and human cutaneous melanoma cells in vitro (9,10).
However, to the best of our knowledge, the effect of chrysin on
uveal melanoma cells has not been previously reported. Cutaneous
melanomas differ from uveal melanomas not only in the etiologic and
epidemiologic aspects, but also in their molecular biological
patterns. The majority of cutaneous melanomas occur in areas
exposed to sun radiation, whereas the majority of uveal melanomas
occur in the posterior segment of the eye, which is not exposed to
significant sun radiation. Chromosomal assessment and molecular
biological and epidemiological studies demonstrate that cutaneous
melanomas differ from uveal melanomas (29–34),
whereas conjunctival melanomas appear to have more similarities to
cutaneous than uveal melanomas (32,34). The
primary underlying biological differences between uveal and
cutaneous melanomas may be associated with various gene mutations
and differing microenvironments. Therefore, uveal melanomas and
cutaneous melanomas should be considered as two distinct types of
cancer, and each disease has a unique oncogenetic signaling pathway
for the development of the tumor. Additional studies are required
to investigate the pathogenesis and relevant novel treatments for
each type of melanoma.
In the present study, chrysin caused a significant
decrease in the viability of human melanoma cell lines (M17 and
SP6.5) at concentrations of 30–100 µM in a dose- and time-dependent
manner, whereas viability of normal cells (scleral fibroblasts and
RPE cells) was not affected. The results of the present study
suggested that chrysin may have specific anticancer activity in
uveal melanoma cells.
In the present study, cell apoptosis was determined
by TUNEL analysis. Apoptosis is characterized by a number of
intracellular phenomena, including membrane blebbing, chromatin
condensation and nuclear DNA fragmentation. Detection of DNA
fragmentation is a widely accepted method to assay for apoptosis
(26,27). Fragmentation may be visualized by and
detected in situ by incorporating labeled nucleotides onto
the free 3′OH ends of the fragments using TdT enzyme followed by
detection of the labeled molecules. This assay allows distinction
between apoptotic cells (with brown colored nuclei) and normal
cells (non-specific stained green colored nuclei). For the cells
treated with chrysin, cells stained with DAB (indicating apoptotic
changes) were significantly increased compared to the controls.
This is consistent with previous studies, which have reported an
apoptosis-induction effect of chrysin in prostate cancer, lung
cancer, leukemia and cutaneous melanoma cells (4,5,9,12).
There are two distinct apoptosis signaling pathways:
Intrinsic and extrinsic pathways (35). For the intrinsic apoptosis signaling
pathway, loss of MTP, which may be recognized in living cells using
JC-1, is an early event (28). Loss
of MTP induces the increase of mitochondrial membrane permeability,
which leads to the release of cytochrome c from the
mitochondria to the cytoplasm (36).
When cytochrome c is released to the cytoplasm, caspase-9 is
activated. Subsequently, the activated caspase-9 in turn promotes
activation of caspase-3, resulting in apoptosis of the tumor cells
(36). Therefore, the intrinsic
signaling pathway is also called the mitochondrial apoptotic
pathway. For the extrinsic apoptosis signaling pathway, various
cell death surface receptors are activated following binding to the
relevant ligands (35). Following
this, the activated death receptors activate caspase-8 by binding
to the secondary adaptor protein, which results in the cleavage of
caspase-3 and cell apoptosis (35).
Previous studies have revealed that chrysin induces
apoptosis of malignant cells through the mitochondrial signaling
pathway (9,12). However, Monasterio et al
(4) reported that chrysin induced
human leukemia U937 cell apoptosis in a manner that required the
activation of caspase-8 instead of caspase-9, which is the
extrinsic signaling pathway. This indicates that the apoptotic
signaling pathway involved in chrysin-induced apoptosis may be
cell-type specific.
In the present study, chrysin caused the loss of
MTP, increased the levels of cytosol cytochrome c and the
activities of caspase-9 and −3 in a dose-dependent manner. However,
the activities of caspase-8 were not altered following treatment
with chrysin. These observations are consistent with previous
reports (9,12), that suggested that chrysin induced
apoptosis in cutaneous melanoma cells and lung adenocarcinoma
epithelial cells primarily via the intrinsic mitochondrial
signaling pathway.
Two uveal melanoma cell lines were investigated in
the present study (M17 and SP6.5 cell lines). Both cell lines were
isolated from Caucasian primary choroidal melanoma patients, and
have comparable growth capacity and pigmentation. However,
epigenetic studies observed that the SP6.5 cell line differed from
the M17 cell line in that the decrease of miR-137 expression was
more pronounced in SP6.5 than in M17 (37).
In summary, chrysin significantly decreased cell
viability and induced apoptosis of human uveal melanoma cells
without affecting normal cell viability, which suggested that
chrysin had a selective and potent pro-apoptotic effect on human
uveal melanoma cells in vitro. This effect was primarily
through the intrinsic mitochondrial signaling pathway. These
encouraging in vitro findings suggest that chrysin may be a
promising agent worthy of being investigated for the treatment of
uveal melanoma.
Acknowledgments
The authors thank Dr. Guy Pelletier (Research Center
of Immunology, Quebec, Canada) for providing the SP6.5 melanoma
cell line.
References
|
1
|
Hu DN, Yu GP, McCormick SA, Schneider S
and Finger PT: Population-based incidence of uveal melanoma in
various races and ethnic groups. Am J Ophthalmol. 140:612–617.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kujala E, Mäkitie T and Kivelä T: Very
long-term prognosis of patients with malignant uveal melanoma.
Invest Ophthalmol Vis Sci. 44:4651–4659. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Augsburger JJ, Corrêa ZM and Shaikh AH:
Effectiveness of treatments for metastatic uveal melanoma. Am J
Ophthalmol. 148:119–127. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Monasterio A, Urdaci MC, Pinchuk IV,
López-Moratalla N and Martínez-Irujo JJ: Flavonoids induce
apoptosis in human leukemia U937 cells through caspase- and
caspase-calpain-dependent pathways. Nutr Cancer. 50:90–100. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Samarghandian S, Afshari JT and Davoodi S:
Chrysin reduces proliferation and induces apoptosis in the human
prostate cancer cell line pc-3. Clinics (Sao Paulo). 66:1073–1079.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cho H, Yun CW, Park WK, Kong JY, Kim KS,
Park Y, Lee S and Kim BK: Modulation of the activity of
pro-inflammatory enzymes, COX-2 and iNOS, by chrysin derivatives.
Pharmacol Res. 49:37–43. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Woodman OL and Chan ECh: Vascular and
anti-oxidant actions of flavonols and flavones. Clin Exp Pharmacol
Physiol. 31:786–790. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Khoo BY, Chua SL and Balaram P: Apoptotic
effects of chrysin in human cancer cell lines. Int J Mol Sci.
11:2188–2199. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pichichero E, Cicconi R, Mattei M and
Canini A: Chrysin-induced apoptosis is mediated through p38 and Bax
activation in B16-F1 and A375 melanoma cells. Int J Oncol.
38:473–483. 2011.PubMed/NCBI
|
|
10
|
Pichichero E, Cicconi R, Mattei M, Muzi MG
and Canini A: Acacia honey and chrysin reduce proliferation of
melanoma cells through alterations in cell cycle progression. Int J
Oncol. 37:973–981. 2010.PubMed/NCBI
|
|
11
|
Yang B, Huang J, Xiang T, Yin X, Luo X,
Huang J, Luo F, Li H, Li H and Ren G: Chrysin inhibits metastatic
potential of human triple-negative breast cancer cells by
modulating matrix metalloproteinase-10, epithelial to mesenchymal
transition, and PI3K/Akt signaling pathway. J Appl Toxicol.
34:105–112. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Samarghandian S, Nezhad MA and Mohammadi
G: Role of caspases, Bax and Bcl-2 in chrysin-induced apoptosis in
the A549 human lung adenocarcinoma epithelial cells. Anticancer
Agents Med Chem. 14:901–909. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee SJ, Yoon JH and Song KS: Chrysin
inhibited stem cell factor (SCF)/c-Kit complex-induced cell
proliferation in human myeloid leukemia cells. Biochem Pharmacol.
74:215–225. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sak K: Cytotoxicity of dietary flavonoids
on different human cancer types. Pharmacogn Rev. 8:122–146. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yu XM, Phan T, Patel PN, Jaskula-Sztul R
and Chen H: Chrysin activates Notch1 signaling and suppresses tumor
growth of anaplastic thyroid carcinoma in vitro and in vivo.
Cancer. 119:774–781. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shao JJ, Zhang AP, Qin W, Zheng L, Zhu YF
and Chen X: AMP-activated protein kinase (AMPK) activation is
involved in chrysin-induced growth inhibition and apoptosis in
cultured A549 lung cancer cells. Biochem Biophys Res Commun.
423:448–453. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Phan T, Yu XM, Kunnimalaiyaan M and Chen
H: Antiproliferative effect of chrysin on anaplastic thyroid
cancer. J Surg Res. 170:84–88. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pilátová M, Stupáková V, Varinská L,
Sarisský M, Mirossay L, Mirossay A, Gál P, Kraus V, Dianisková K
and Mojzis J: Effect of selected flavones on cancer and endothelial
cells. Gen Physiol Biophys. 29:134–143. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hong TB, Rahumatullah A, Yogarajah T,
Ahmad M and Yin KB: Potential effects of chrysin on MDA-MB-231
cells. Int J Mol Sci. 11:1057–1069. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Weng MS, Ho YS and Lin JK: Chrysin induces
G1 phase cell cycle arrest in C6 glioma cells through inducing
p21Waf1/Cip1 expression: Involvement of p38 mitogen-activated
protein kinase. Biochem Pharmacol. 69:1815–1827. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Woo KJ, Jeong YJ, Park JW and Kwon TK:
Chrysin-induced apoptosis is mediated through caspase activation
and Akt inactivation in U937 leukemia cells. Biochem Biophys Res
Commun. 325:1215–1222. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang T, Chen X, Qu L, Wu J, Cui R and
Zhao Y: Chrysin and its phosphate ester inhibit cell proliferation
and induce apoptosis in Hela cells. Bioorg Med Chem. 12:6097–6105.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hu DN, McCormick SA, Ritch R and
Pelton-Henrion K: Studies of human uveal melanocytes in vitro:
Isolation, purification and cultivation of human uveal melanocytes.
Invest Ophthalmol Vis Sci. 34:2210–2219. 1993.PubMed/NCBI
|
|
24
|
Soulieres D, Rousseau A, Deschenes J,
Tremblay M, Tardif M and Pelletier G: Characterization of
gangliosides in human uveal melanoma cells. Int J Cancer.
49:498–503. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lu C, Song E, Hu DN, Chen M, Xue C, Rosen
R and McCormick SA: Curcumin induces cell death in human uveal
melanoma cells through mitochondrial pathway. Curr Eye Res.
35:352–360. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Parthasarathy G and Philipp MT: The
MEK/ERK pathway is the primary conduit for Borrelia
burgdorferi-induced inflammation and P53-mediated apoptosis in
oligodendrocytes. Apoptosis. 19:76–89. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Turgeman T, Kakongi N, Schneider A,
Vinokur Y, Teper-Bamnolker P, Carmeli S, Levy M, Skory CD, Lichter
A and Eshel D: Induction of Rhizopus oryzae germination under
starvation using host metabolites increases spore susceptibility to
heat stress. Phytopathology. 104:240–247. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cossarizza A, Baccarani-Contri M,
Kalashnikova G and Franceschi C: A new method for the
cytofluorimetric analysis of mitochondrial membrane potential using
the J-aggregate forming lipophilic cation
5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolcarbocyanine
iodide (JC-1). Biochem Biophys Res Commun. 197:40–45. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gilchrest BA, Eller MS, Geller AC and Yaar
M: The pathogenesis of melanoma induced by ultraviolet radiation. N
Engl J Med. 340:1341–1348. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bergman L, Seregard S, Nilsson B, Ringborg
U, Lundell G and Ragnarsson-Olding B: Incidence of uveal melanoma
in Sweden from 1960 to 1998. Invest Ophthalmol Vis Sci.
43:2579–2583. 2002.PubMed/NCBI
|
|
31
|
Yu GP, Hu DN and McCormick SA: Latitude
and incidence of ocular melanoma. Photochem Photobiol.
82:1621–1626. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Spendlove HE, Damato BE, Humphreys J,
Barker KT, Hiscott PS and Houlston RS: BRAF mutations are
detectable in conjunctival but not uveal melanomas. Melanoma Res.
14:449–452. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Vajdic CM, Hutchins AM, Kricker A, Aitken
JF, Armstrong BK, Hayward NK and Armes JE: Chromosomal gains and
losses in ocular melanoma detected by comparative genomic
hybridization in an Australian population-based study. Cancer Genet
Cytogenet. 144:12–17. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hu DN, Yu G, McCormick SA and Finger PT:
Population-based incidence of conjunctival melanoma in various
races and ethnic groups and comparison with other melanomas. Am J
Ophthalmol. 145:418–423. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
McConkey DJ: Biochemical determinants of
apoptosis and necrosis. Toxicol Lett. 99:157–168. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kroemer G, Dallaporta B and Resche-Rigon
M: The mitochondrial death/life regulator in apoptosis and
necrosis. Annu Rev Physiol. 60:619–642. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chen X, Wang J, Shen H, Lu J, Li C, Hu DN,
Dong XD, Yan D and Tu L: Epigenetics, microRNAs, and
carcinogenesis: Functional role of microRNA-137 in uveal melanoma.
Invest Ophthalmol Vis Sci. 52:1193–1199. 2011. View Article : Google Scholar : PubMed/NCBI
|