Introduction
Hemangiopericytoma (HPC) was first reported and
named by Stout and Murray in 1942 (1). It is a rare soft tissue tumor and may
occur in any part of the body, but it occurs less commonly in the
central nervous system (CNS). In the past, HPC was believed to
originate from the meninges and was thus considered a subtype of
meningioma. In the 2000 World Health Organization (WHO)
classification of CNS tumors, HPC was categorized as a
non-meningeal epithelial cell tumor. It originates from Zimmerman
meningeal cells in interstitial capillaries, has high cell density
and multi-differentiation potential, and is highly vascularized.
HPCs are classified as WHO II–III grade tumors according to the
2000 classification system. In the 2007 WHO classification of CNS
tumors, HPC was divided into WHO III grade anaplastic
hemangiopericytoma (AHPC) and WHO II grade HPC (2–4). AHPC is
more aggressive and exhibits common recurrence and extracranial
metastasis. Pathological examinations revealed a great number of
irregularly arranged tumor cells that commonly have nuclear atypia
and mitotic properties. In this report, we present the imaging and
pathological features of 18 cases of AHPC and analyze the features
of AHPC that were visualized by MRI to improve the knowledge on
AHPC.
Patients and methods
Clinical data
This study included 18 cases of AHPC that were
confirmed by surgery and pathology at the Lanzhou University Second
Hospital from July 2001 to July 2013. Twelve male and 6 female
patients participated to the study, and were from 29 to 61 years of
age (mean age, 44 years). This study was approved by the Ethics
Committee of the First Affiliated Hospital of Chongqing Medical
University. Signed written informed consents were obtained from all
participants before the study. The patients agreed to the use of
their samples in scientific research.
Magnetic resonance imaging (MRI)
technique
MRI scans were performed with a 1.0T scanner
(Magnetom Harmony; Siemens Healthineers, Erlangen, Germany). The
imaging protocol included unenhanced axial and sagittal T1-weighted
sequences, axial and coronal T2-weighted sequences, and
contrast-enhanced axial, sagittal, and coronal T1-weighted
sequences. The scanning parameters were as follows: T1WI (TR/TE,
550 msec/12 msec); T2WI (TR/TE, 2,200 msec/90 msec); thickness, 5.0
mm; spacing, 1.5 mm; FOV, 320×320; matrix, 256×256; sagittal and
coronal slice, 8.0 mm; and layer spacing, 2.0 mm. An enhanced scan
bolus Gd-DTPA (DTPA magnetic display) was given intravenously at a
concentration of 0.1 mmol/kg body weight with a flow rate of 3
ml/sec. The MRI characteristics of all cases were analyzed by two
radiologists according to location, shape, signal, bone
destruction, edema and enhancing characteristics.
Pathological examination
The tumor was collected and fixed in 4% buffered
formalin for 24 h. Each fixed sample was then cut into 3–4 µm
slices, to make 5–6 sections, and tissue blocks were selected from
representative areas. After processing and paraffin wax embedding,
the sections were separately stained with hematoxylin and eosin
(H&E), CD34, Ki-67, vimentin and epithelial membrane antigen
(EMA). All slides were reviewed by two neuropathologists.
Results
General data
Nine patients presented with intracranial
hypertension and physical signs, and 2 of those patients had two
symptoms. Thirteen cases presented with headaches and/or dizziness.
Three cases exhibited homonymy hearing loss. Four cases exhibited
olfactory dysfunction. Psychiatric symptoms were present in 2
cases. Epilepsy was found in 10 cases, and hemi-vision loss was
found in 3 cases (Table I).
 | Table I.General data of 18 patients with
AHPC. |
Table I.
General data of 18 patients with
AHPC.
| Presenting symptom or
sign | No. of patients | Average age
(years) | Males | Females |
|---|
| Intracranial
hypertension and physical signs | 9 | 52 | 5 | 4 |
| Headaches and/or
dizziness | 13 | 48 | 10 | 3 |
| Hearing loss | 3 | 44 | 1 | 2 |
| Olfactory
dysfunction | 4 | 51 | 3 | 1 |
| Psychiatric
symptoms | 2 | 60 | 0 | 2 |
| Epilepsy | 10 | 35 | 7 | 3 |
| Hemi-vision loss | 3 | 42 | 2 | 1 |
MRI findings
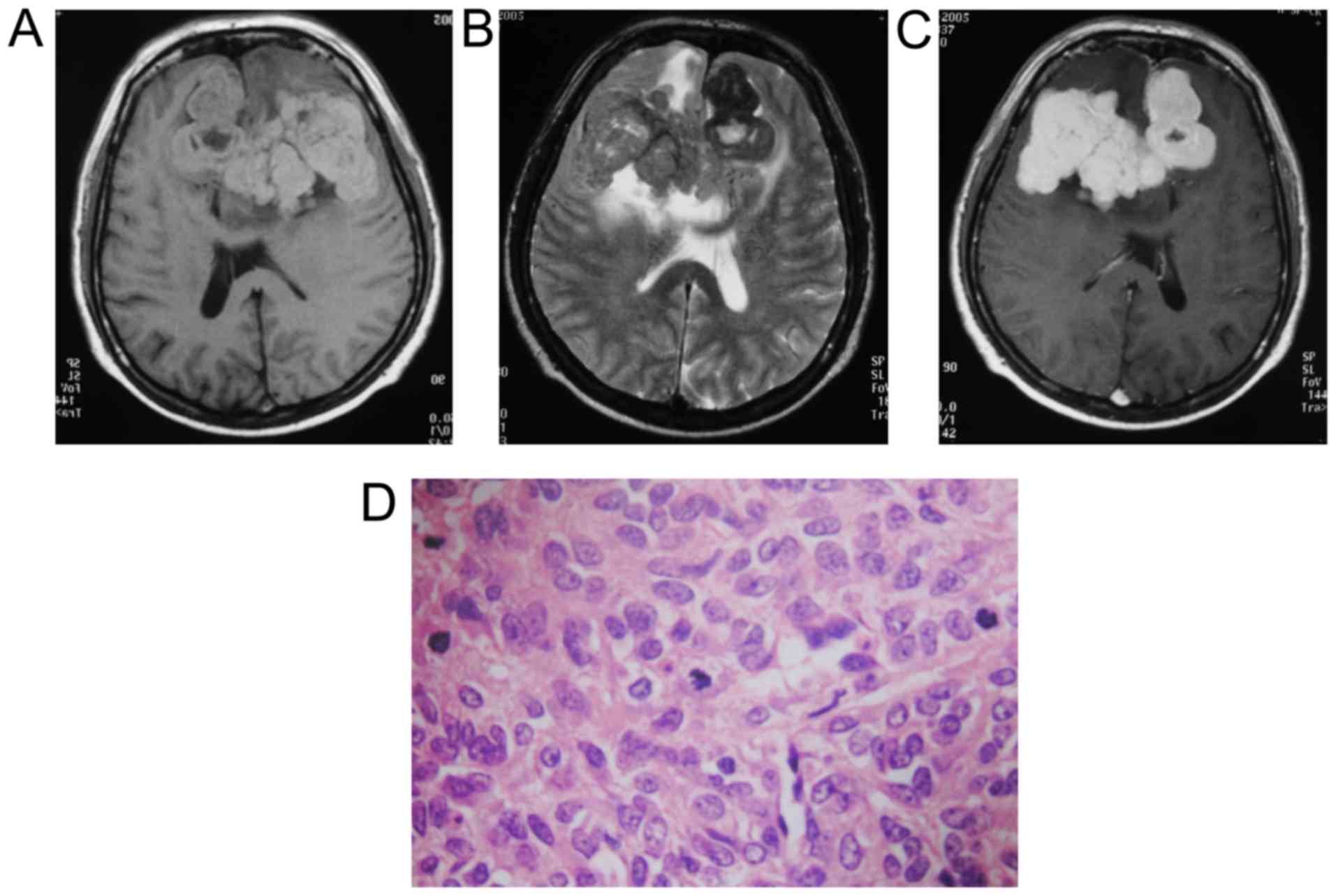
In all 18 cases, the tumor was positioned in the
cortex; in 12 cases, it was located in the frontal falx, and in 3
cases, it was located in the parietal falx. In 2 cases, the tumor
was located in the middle cranial fossa, and in 1 case, it was
located in the cerebellar hemispheres. The diameter of the tumors
ranged from 3.1 to 6.5 cm, with an average of 4.9 cm. The tumor
mass was lobulated in 12 cases, irregular-shaped in 5 cases, and
oval-shaped in 1 case. In 9 cases, the tumor was connected to the
dura or skull with a narrow base. On MRI, the lesions showed a
mixed high-low signal in 12 cases, an iso-signal in 6 cases on
plain T2WI, a mixed iso-low signal in 11 cases, an iso-mild
high-low signal in 1 case, and an iso-signal in 6 cases on plain
T1WI. After contrast injection, different levels of enhancement
were observed in all cases. In 3 cases, skull bone damage was
found, and the peripheral edemas in those cases had high signals on
T2WI. For the MRI enhanced scans, a substantial part of the tumor
was significantly enhanced, and the neighboring meninges were
linearly enhanced (Table II)
(Figs. 1–3). Preoperative imaging diagnosed HPC in 4
cases, meningioma in 9 cases, malignant meningioma in 8 cases, a
metastatic tumor in 1 case, and glioma in 1 case. All 18 cases were
AHPC, as confirmed by pathological analysis after surgery.
 | Table II.MRI features of 18 cases with
AHPC. |
Table II.
MRI features of 18 cases with
AHPC.
| MRI features | No. of patients |
|---|
| Position |
| Frontal
falx | 12 |
| Parietal
falx | 3 |
| Middle
cranial fossa | 2 |
|
Cerebellar hemispheres | 1 |
| Shape |
|
Lobulated | 12 |
| Irregular
in shape | 5 |
|
Oval-shaped | 1 |
| Narrow
base connected to dura | 9 |
| T2WI |
| Mixed
high-low signal | 12 |
|
Iso-signal | 6 |
| T1WI |
| Mixed
iso-low signal | 11 |
| Iso-mild
high-low signal | 1 |
|
Iso-signal | 6 |
| Bony
destruction | 3 |
|
Peritumoral edema | 15 |
Pathological findings
The cut surfaces were grey or grey-red and fish
meat-like in texture. Microscopic examination of the tumors showed
cystic and necrotic foci in 14 cases, bleeding in 7 cases, and
calcification in 1 case. The tumor cells were densely and
irregularly arranged, abundant slit-like blood vessels were
observed, and nuclear atypia and mitosis were common.
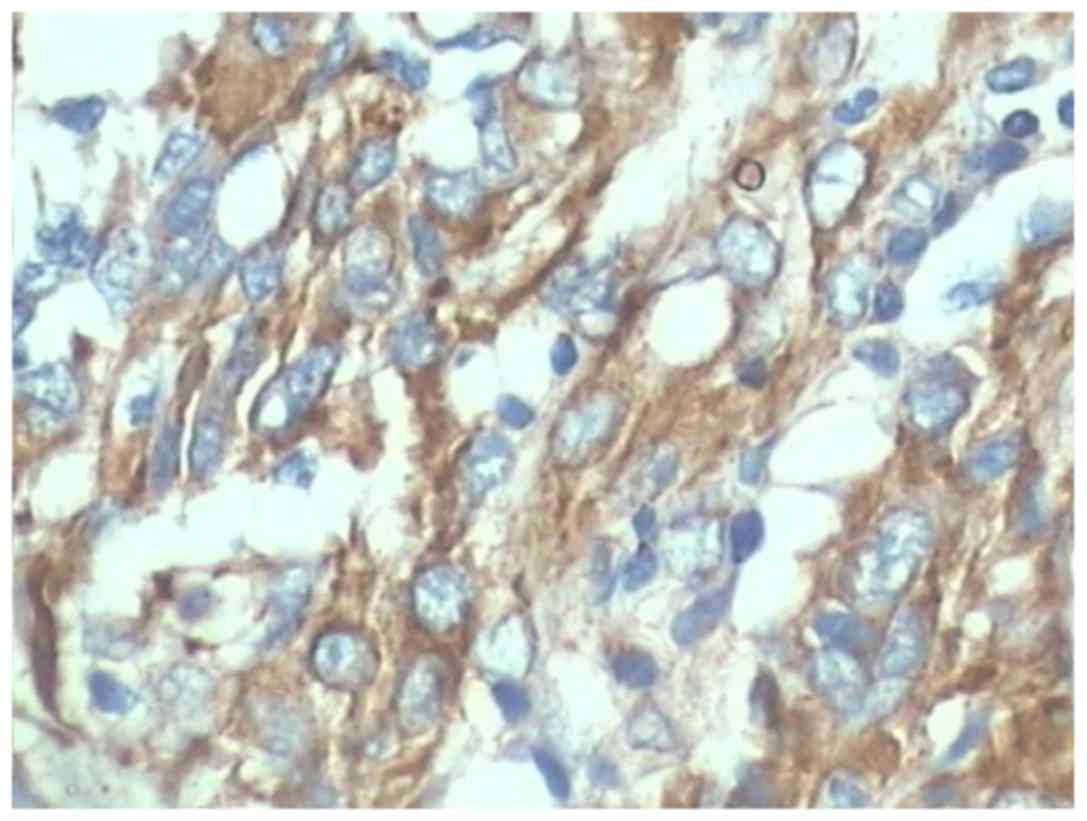
Immunohistochemical staining showed that vimentin was positive in
all cases (Fig. 3). EMA showed a
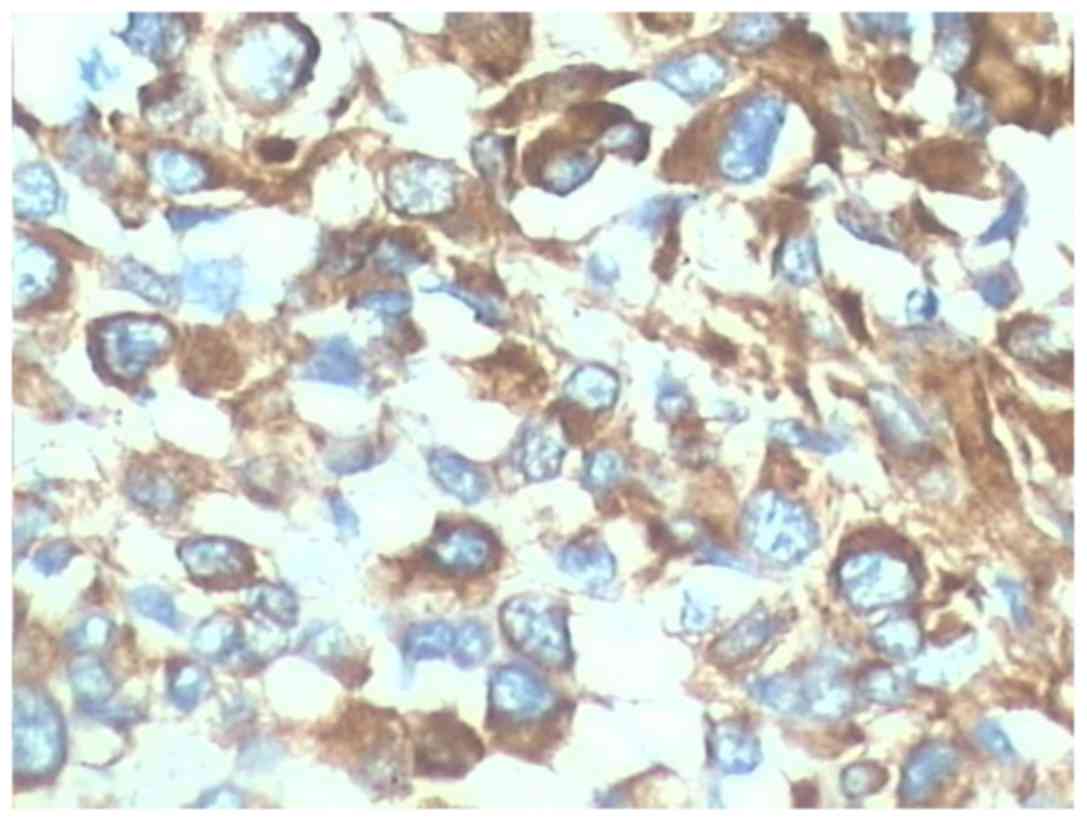
negative expression. CD34 was positive in 18 cases (Fig. 4). The proportion of Ki-67-positive
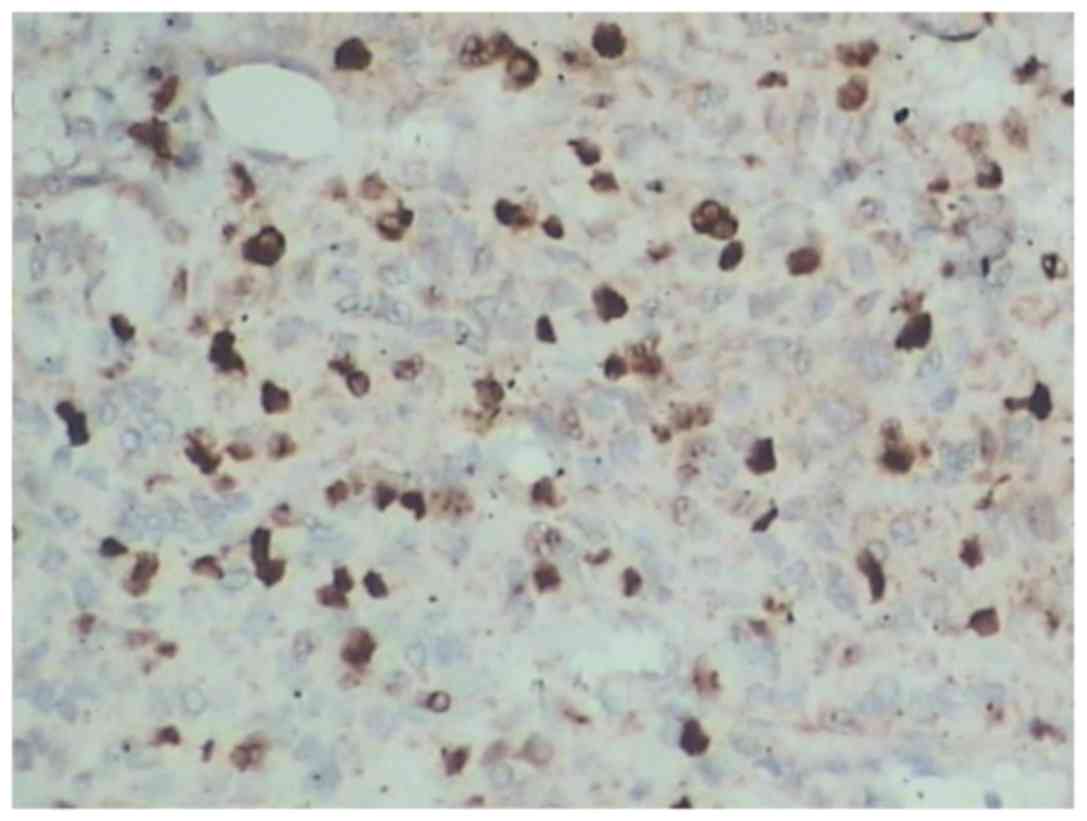
cells was between 15 and 20% in 13 cases (Fig. 5) and >20% in 5 cases.
Discussion
Intracranial HPC is a rare malignancy that usually
originates from the intracranial vasculature. It accounts for only
1% of all primary CNS tumors (5).
Intracranial HPC was previously believed to originate from the
meninges; thus, it was considered a subtype of meningioma. However,
with the development of molecular genetics, it was confirmed that
HPC has a completely different source from that of meningiomas: it
actually originates from arachnoid cap cells, as determined by the
detection of the neurofibromatosis 2 (NF2) gene in HPC tumors
(6). In 1993, WHO classified HPC as
different from meningioma (7).
However, a review of the literature and the data collected in this
study indicated that the 1993 classification of HPC did not
distinguish its subtypes (8–10). The 2007 WHO classification divided
intracranial HPC into two separate categories: WHO grade II HPC and
WHO grade III AHPC with malignant biological behaviour (11). There are differences between these
subtypes in the 5-year survival rates, recurrence rates and
transferability, and some studies have shown that AHPC recurs as
much as 6–7 years earlier than HPC does (12).
The average age of patients with AHPC is 44 in this
study. The incidence of AHPC in males is slightly higher than in
females. The symptoms of headache and intracranial pressure in
different parts of the brain are very common in AHPC patients. It
results from MRI findings that the average size of the lesions was
4.9 cm. the AHPC's malignant signs, such as tumor lobulation,
necrosis and cysts, were more common because they contained a
greater number of irregularly arranged tumor cells in which nuclear
atypia and mitotic properties were more easily found. It often
co-existed with necrosis and cystic changes, so the signal of the
MR plain scan was also mixed. Necrosis and cystic properties of the
tumor reflected a rapid tumor growth and a relative lack of
nutrition, which indicated the characteristic of high malignancy.
From Akiyama et al opinion (13), the irregular shape and ill-defined
boundary reflected the rapid growth, and with invasive growth
features of malignant tumors. In addition, such signs as skull
destruction and peritumoral edema were commonly found in AHPC. The
sign of skull destruction indicated a strong level of invasiveness.
The sign of peritumoral edema reflected the amount of infiltration
within the tumor, blood supply and pathological type as reported by
Lee et al (14). The AHPC has
a rich blood supply because pathological H&E staining showed
that there was a large number of slit-like blood vessels and an
enhanced MR scan displayed that the tumor was significantly
enhanced. Immunohistochemical staining results also showed the
malignant tendency of AHPC.
We found that intracranial AHPC should be
differentiated from malignant meningioma after literature
reviewing. Malignant meningioma has some characteristic MRI
features similar to AHPC (15). i)
Clinical features: malignant meningioma was more common in
50-year-old females, and AHPC was more common in 45-year-old males;
and ⅱ) MRI features: malignant meningioma was generally connected
to the meninges by a wide base, and AHPC was generally connected to
the meninges by a narrow base. AHPC showed a more lobulated and
irregular shape and cross-leaf growth, more necrosis and cysts,
less skull damage; and malignant meningioma showed a less lobulated
shape, less necrosis and cysts and more skull damage. Furthermore,
AHPC showed more mixed signals.
In conclusion, intracranial AHPC is a rare tumor
type that has a high degree of malignancy. Recurrence and
metastasis are common with AHPC. The location of AHPC is similar to
meningioma, but the shape of AHPC tends to be irregular. Mixed
signal is more likely to be seen and MRI enhancement often shows
heterogeneous enhancement in contrast-enhanced T1-weighted images.
Skull destruction and peritumoral can easily be found in AHPC.
Understanding the MRI features of AHPC has great significance for
guiding clinical treatment and predicting prognosis.
Acknowledgements
The authors are indebted to the Departments of
Radiology and Pathology, Lanzhou University Second Hospital, P.R.
China, for their help in collecting materials.
References
|
1
|
Stout AP and Murray MR:
Hemangiopericytoma: a vascular tumor featuring Zimmermann's
pericytes. Ann Surg. 116:26–33. 1942. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chiechi MV, Smirniotopoulos JG and Mena H:
Intracranial hemangiopericytomas: MR and CT features. AJNR Am J
Neuroradiol. 17:1365–1371. 1996.PubMed/NCBI
|
|
3
|
Geng D, Shen T, Chen X, Xiao Q and Huang
Y: Comparing CT and MRI features with pathology in intracranial
hemangiopericytomas. Chin Computed Med Imag. 6:304–306. 2000.
|
|
4
|
Junlin Z and Ning He DC: Comparison of MRI
signs with pathological findings in intracranial
hemangiopericytomas: a report of 13 cases. J Clin Radiol.
8:631–634. 2003.
|
|
5
|
Maruya J, Seki Y, Morita K, Nishimaki K
and Minakawa T: Meningeal hemangiopericytoma manifesting as massive
intracranial hemorrhage - two case reports. Neurol Med Chir
(Tokyo). 46:92–97. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Joseph JT, Lisle DK, Jacoby LB, Paulus W,
Barone R, Cohen ML, Roggendorf WH, Bruner JM, Gusella JF and Louis
DN: NF2 gene analysis distinguishes hemangiopericytoma from
meningioma. Am J Pathol. 147:1450–1455. 1995.PubMed/NCBI
|
|
7
|
Tao J, Wang H, Chen J, Xu H and Li S:
Effects of saponin monomer 13 of dwarf lilyturf tuber on L-type
calcium currents in adult rat ventricular myocytes. Am J Chin Med.
33:797–806. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhongli J and Jizun Z: Intracranial
hemangiopericytoma 32 cases reports. Nat Med J China. 80:432–434.
2000.
|
|
9
|
Ghose A, Guha G, Kundu R, Tew J and
Chaudhary R: CNS Hemangiopericytoma: a systematic review of 523
patients. Am J Clin Oncol. Oct 27–2014.(Epub ahead of print).
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Coffey RJ, Cascino TL and Shaw EG:
Radiosurgical treatment of recurrent hemangiopericytomas of the
meninges: preliminary results. J Neurosurg. 78:903–908. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Louis DN, Ohgaki H, Wiestler OD, Cavenee
WK, Burger PC, Jouvet A, Scheithauer BW and Kleihues P: The 2007
WHO classification of tumours of the central nervous system. Acta
Neuropathol. 114:97–109. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ecker RD, Marsh WR, Pollock BE,
Kurtkaya-Yapicier O, McClelland R, Scheithauer BW and Buckner JC:
Hemangiopericytoma in the central nervous system: treatment,
pathological features, and long-term follow up in 38 patients. J
Neurosurg. 98:1182–1187. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Akiyama M, Sakai H, Onoue H, Miyazaki Y
and Abe T: Imaging intracranial haemangiopericytomas: study of
seven cases. Neuroradiology. 46:194–197. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee KJ, Joo WI, Rha HK, Park HK, Chough
JK, Hong YK and Park CK: Peritumoral brain edema in meningiomas:
correlations between magnetic resonance imaging, angiography, and
pathology. Surg Neurol. 69:350–355; discussion 355. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang YN, He N, Zhou JL and Wang HY: Value
of MRI features in preoperative diagnosis of malignant meningioma.
J China Clin Med Imaging. 18:777–780. 2007.(In Chinese).
|