Introduction
Breast cancer is the most frequent type of
malignancy among women worldwide (1).
Although current treatments are often associated with excellent
short-term prognoses, ≤13% of women develop recurrence within 9
years of the initial treatment. In addition, >60% of women with
localized breast cancer eventually develop distant, late-stage
disease (2). In China, >20% of
patients with breast cancer are diagnosed at an advance stage of
the disease, typically with metastatic invasion (3). Although a large number of successful
treatment options are available for early breast cancer, standard
treatment protocols for advanced breast cancer cannot currently
circumvent patient relapse (4).
Genetic reprogramming during the metastatic process enables cancer
cells to invade distant organs and to acquire resistance to
numerous types of chemo- and radiotherapy, contributing to the high
mortality of advanced-stage breast cancer (5). Thus, improved therapeutic options for
the affected patients are required.
Complementary therapies such as traditional Chinese
medicine (TCM) have increased in popularity as a less intensive and
more ‘natural’ approach to achieving health or improving quality of
life, particularly for patients with advanced-stage cancer
(6). In the Yellow Emperor's Classic
of Internal Medicine, written circa 250 BC, the first clinical
description and phytotherapeutic treatment of breast cancer was
recorded. Besides anecdotal evidence of a cure for breast cancer
using TCM as a sole therapy (6),
previous studies suggest that TCM may be effective in breast cancer
management. For example, Huaier (Trametes robiniophila Murr)
sensitizes breast cancer cells to radiotherapy through the
regulation of cell cycle and DNA repair pathways (7). In vitro and in vivo
testing of Scutellaria barbata in breast cancer has led to
an ongoing clinical trial (8); and a
recent population-based study suggests that adjunctive TCM therapy
may lower the risk of mortality in patients with advanced breast
cancer (9).
Ruanjian Sanjie (RJSJ), composed of Ban xia
(Pinellia ternata), Xia ku cao (Prunella vulgaris),
Shan ci gu (Cremastra appendiculata) and Hai zao
(Sargassum pallidum), is an empirical traditional Chinese
herbal decoction used for softening hard lumps and resolving hard
masses (10). Although the active
principles and the mechanisms of action remain unknown, extracts
from certain individual herbal components of RJSJ exhibit
anticancer properties. For example, Prunella vulgaris
extracts exhibit growth-suppressive activity on gastric cancer
(11) and migration-suppressive
activity on liver cancer cells (12),
whereas Sargassum pallidum extracts exhibit cytotoxic
effects in breast cancer MCF-7 cells in vitro (13) and inhibit pancreatic cancer relapse
in vivo (14). The present
study demonstrates that RJSJ exhibits antitumor activity against
Ehrlich ascites carcinoma (EAC) in Swiss albino mice and breast
cancer xenografts in nude mice. RJSJ shows potent cytotoxicity
against breast cancer cells in vitro by the suppression of
the anti-apoptotic proteins B-cell lymphoma 2 (Bcl-2) and survivin,
leading to the activation of caspase-3/7 and caspase-9, and the
apoptotic cascade. The present findings offer a clear rationale to
additionally explore the therapeutic strategy of using RJSJ alone
or in combination with chemotherapeutic agents for patients with
breast cancer and the characterization of its active
principles.
Materials and methods
Herbs and preparation of aqueous
extracts
All herbs in the RJSJ formula were obtained from
Tianjin Zhong Xin Pharmaceutical Group Corporation Ltd. (Tianjin,
China). The dry weights of the four herbs were at fixed ratios
(Pinellia ternate: Prunella vulgaris: Cremastra
appendiculata: Sargassum pallidum=3:3:2:2). Whole herbs
(900 g) were ground to a powder and extracted twice with hot
distilled water, first with 10.5 liters and next with 15 liters, at
100°C for 30 min each. The solutions were combined and centrifuged
at 3,000 × g for 10 min at room temperature. The resulting
supernatant was filtered through 0.45-µm cellulose acetate filters,
concentrated using a rotary evaporator and spray-dried to obtain a
brown fine powder. This residue was dissolved in PBS at the desired
concentration and stored at −20°C as the stock extract RJSJ
solution.
Reagents, cell lines and mice
The breast cancer MDA-MB-231 and MCF-7 cell lines
were obtained from the American Type Culture Collection (Manassas,
VA, USA) through the Cell Resource Center of the Tianjin Cancer
Hospital (Tianjin, China) and were authenticated by short tandem
repeat profiling. The cells were cultured in Dulbecco's modified
Eagle's medium (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) with 10% fetal calf serum and 2 mM L-glutamine
(Gibco; Thermo Fisher Scientific, Inc.). The cultures were
maintained at 37°C in an air-5% CO2 incubator at
constant humidity. Doxorubicin, 5-flurouracil (5-Fu) and MTT were
purchased from Sigma-Aldrich; Merck Millipore (Darmstadt, Germany).
All animals, including 120 Swiss albino mice (60 female and 60
male; age, 5–6 weeks; weight, 25–30 g) and 24 female nude mice
(age, 5–6 weeks; weight, 20–25 g) were obtained from Vital River
Laboratories Co., Ltd. (Beijing, China) and used according to the
National Institutes of Health guidelines for animal care (15). Animals were maintained at between 21
and 24°C with an 8 h light/16 h dark cycle, and were allowed to
feed ad libitum. All in vivo studies were performed
in the Central Laboratory of the Tianjin Institute of Medical and
Pharmaceutical Sciences (Tianjin, China), and approved by the
Committee on Ethics of the Tianjin Institute of Medical and
Pharmaceutical Sciences.
Acute toxicity study
An acute oral toxicity assay was performed using
healthy, non-pregnant, adult female, Swiss albino mice (weight
range, 25–30 g) divided into six groups. Increasing oral doses of
RJSJ (312.5, 625, 1,250, 2,500 and 5,000 mg/kg body weight) in
distilled water were administered by intragastric (i.g.)
administration at 20 ml/kg to the different test groups. The
control group received distilled water only. Following treatment,
the mice were allowed to feed ad libitum and monitored for
mortality or any behavioral changes for 14 days (16).
EAC tumor model and RJSJ
treatment
The EAC cells were obtained from Tianjin Institute
of Medical and Pharmaceutical Sciences (Tianjin, China) and were
maintained in vivo in the Swiss albino mice by
intraperitoneal (i.p.) transplantation of 2×106 cells
per mouse every 10 days. For treatments, mice were divided into
four groups of 12 animals each as follows: Group 1, control group;
group 2, treated every other day with 20 mg/kg 5-Fu by i.p.
injection; group 3, treated daily with 500 mg/kg RJSJ by i.g.
administration; and group 4, treated with 20 mg/kg 5-Fu by i.p.
injection combined with i.g. 500 mg/kg RJSJ. The experiment was
started by inoculating EAC cells (2×106 cells per mouse)
subcutaneously to all animals, and the treatments were initiated 24
h subsequently. On day 8, subsequent to the administration of the
last dose followed by 18 h fasting, all animals were sacrificed by
cervical dislocation. Thymi and spleens were collected for thymus
and spleen index determination calculated according to the
following formulae: Spleen index=spleen weight (mg)/body weight
(g); and thymus index=thymus weight (mg)/body weight (g).
In vivo breast cancer xenografts
A total of 1×107 MDA-MB-231 cells were
suspended in 0.1 ml PBS containing 50% Matrigel (BD Biosciences,
Franklin Lakes, NJ, USA) and injected into the mammary fat pad of
4-5-week-old female nude mice. Tumor size was measured every other
day in two dimensions using a caliper, and the tumor volume was
calculated with the following formula: Tumor volume
(mm3)=0.5xab2 (with a and b being the longest
and shortest diameter of the tumor, respectively). When the average
tumor volume reached 400 mm3, the tumor-bearing mice
were randomly divided into four groups with 6 animals/group: Group
1, control group, received normal saline; group 2 was treated every
other day with 1 mg/kg doxorubicin by i.p. injection; group 3 was
treated daily with 500 mg/kg RJSJ by i.g. administration; and group
4 was treated with a combination of 1 mg/kg doxorubicin and 500
mg/kg RJSJ as aforementioned. Tumor volume was monitored until the
mice were sacrificed by cervical dislocation when the tumor size
was >18 mm in diameter in either direction, and the tumors were
collected for RNA extraction.
Cell viability analysis
MTT assays were performed to evaluate the cell
viability in response to drug treatments and to determine the
concentration of drug that inhibited cell growth by 50%
(IC50) subsequent to 3 days of treatment (17).
Caspase activity measurement
A total of 1×104 cells were incubated
with RJSJ in a 96-well plate for 24 h. The caspase-3/7 and
caspase-9 activities were measured using the Caspase-Glo 3/7 Assay
and Caspase-Glo 9 Assay kits, respectively (Promega Corporation,
Madison, WI, USA), according to the manufacturer's protocol.
Fluorescence was measured using a GloMax 20/20 Luminometer (Promega
Corporation).
Hoechst 33258 staining assay
The nuclear morphology of the cells treated with
RJSJ was observed subsequent to Hoechst 33258 staining. The MCF-7
and MDA-MB-231 cells, at a density of 2.5×105/well, were
seeded in 6-well plates, cultured for 24 h, and treated with
different concentrations of RJSJ for 48 h. The cells were then
washed twice with PBS, fixed in 4% paraformaldehyde at 4°C for 30
min, and stained with 10 µg/ml Hoechst 33258 for 15 min at 37°C.
Changes in nuclear morphology were monitored with an Olympus IX51
inverted microscope (Olympus Corporation of the Americas, Inc.,
Central Valley, PA, USA).
Flow cytometry
For cell apoptosis analysis, an Annexin
V-fluorescein isothiocyanate (FITC) and propidium iodide (PI)
double-staining apoptosis detection kit (BD Biosciences) were used
according to the procedure suggested by the manufacturer. In total,
2×105 cells were washed twice with PBS and suspended in
100 µl binding buffer, followed by staining with 5 µl Annexin
V-FITC and 5 µl PI for 15 min in the dark at room temperature. The
fluorescence intensity was measured with a flow cytometer (BD
FACSCanto II; BD Biosciences) and the average percentage of Annexin
V-positive cells was used as a measure of apoptosis, both in the
early and late stages, according to Hu et al (18).
Drug resistance clonogenic assay
A total of 1×105 cells/well in a 6-well
plate were treated with different concentrations of RJSJ for 1
week. Resistant clones were fixed with 4% paraformaldehyde, stained
with 0.2% crystal violet and counted. The crystal violet retained
in the cells was quantified by solubilization with 0.5% acetic acid
and measurement of optical density at 592 nm.
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was isolated using TRIzol (Invitrogen;
Thermo Fisher Scientific, Inc.) following the procedure suggested
by the manufacturer. The complementary(c) DNA was generated using
oligo(dT) primers and SuperScript III Reverse Transcriptase
(Invitrogen; Thermo Fisher Scientific, Inc.) using 2 µg total RNA.
Specific primers for each gene (Table
I) were designed using Primer Express software (version 3.0;
Applied Biosystems; Thermo Fisher Scientific, Inc.). qPCR was
carried out using SYBR-Green I (Takara Biotechnology Co., Ltd.,
Dalian, China) and detected using an ABI Prism® SDS 7900
Real-Time PCR System (Applied Biosystems; Thermo Fisher Scientific,
Inc.). The cycling conditions were 95°C for 15 sec and 60°C for 1
min, for 40 cycles. A standard curve for each gene was included in
each PCR amplification for the calculation of the quantification
cycle value (19). Relative
transcript levels were normalized with ribosomal protein S14.
 | Table I.Oligonucleotides used for
quantitative polymerase chain reaction. |
Table I.
Oligonucleotides used for
quantitative polymerase chain reaction.
| Name | Sequence (5′ to
3′) |
|---|
| RPS14-forward |
TCACCGCCCTACACATCAAACT |
| RPS14-reverse |
CTGCGAGTGCTGTCAGAGG |
| Bcl-2-forward |
CAGTTGGGCAACAGAGAACCAT |
| Bcl-2-reverse |
AGCCCTTGTCCCCAATTTGGAA |
|
Survivin-forward |
GGACCACCGCATCTCTACAT |
|
Survivin-reverse |
GACAGAAAGGAAAGCGCAAC |
Protein extraction and western
blotting
A modified radioimmunoprecipitation assay buffer (50
mM Tris-HCl, 150 mM NaCl, 0.25% SDS, 1% Triton X-100, 0.25% sodium
deoxycholate, 1 mM EDTA, 1 mM ethylene glycol-bis (β-aminoethyl
ether)-N,N,N',N'-tetraacetic acid and 1 mM dithiothreitol) with a
mixture of protease inhibitors (EMD Millipore, Billerica, MA, USA)
was used for protein isolation from whole cells or nuclear
fraction. Protein concentrations were determined using a
bicinchoninic acid protein assay kit (Pierce; Thermo Fisher
Scientific, Inc.). An equal quantity, 50 µg, of protein was
resolved on 12% polyacrylamide gels and transferred onto
nitrocellulose membranes (EMD Millipore), and blocked with 5%
blotting-grade milk (Bio-Rad Laboratories, Inc., Hercules, CA, USA)
in PBS containing 0.1% Tween-20. The membranes were incubated with
primary antibodies against Bcl-2 (1:1,000 dilution; cat. no., 50E3;
Cell Signaling Technology Inc., Danvers, MA, USA) or survivin
(1:1,000 dilution; cat. no. 71G4B7; Cell Signaling Technology,
Inc.) at 4°C overnight. A horseradish peroxidase-conjugated
anti-rabbit immunoglobulin G secondary antibody (1:2,000 dilution;
cat. no. 7074; Cell Signaling Technology, Inc.) was incubated with
the membranes for 2 h at room temperature. Immunoblotting signals
were detected using the SuperSignal West Pico Chemiluminescent
Substrate (Pierce; Thermo Fisher Scientific, Inc.), according to
the manufacturer's protocol. All the membranes were re-probed with
anti-β-actin antibody (1:200 dilution; cat. no. sc47778; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA), which served as a loading
control.
Statistical analysis
All statistical analyses were performed using SPSS
software (version 22.0; IBM SPSS, Armonk, NY, USA). All in
vitro experiments were performed in triplicate and the results
are presented as the mean ± standard deviation. Analysis of
variance followed by a Tukey's test was performed in the present
study to evaluate significant differences among groups. Two-sided
P<0.05 was considered to indicate a statistically significant
difference.
Results
Acute toxicity studies
In order to evaluate the potential RJSJ toxicity,
the present study performed an acute toxicity study. For this
purpose, the mortality of animals treated with the highest RJSJ
dose (≤5,000 mg/kg) was monitored. The present study observed that
RJSJ was safe at oral doses as high as 5,000 mg/kg body weight,
causing no mortality, behavioral change, locomotor ataxia, diarrhea
or weight loss in mice during 14 days of observation. Additionally,
food and water intake did not differ between the groups studied
(Table II).
 | Table II.Results of acute toxicity
studies. |
Table II.
Results of acute toxicity
studies.
|
| Animals (n) | Body weight
(g) |
|---|
|
|
|
|
|---|
| Groups | Day 1 | Day 14 | Day 1 | Day 14 |
|---|
| Control RJSJ
(mg/kg) | 12 | 12 | 20.88±0.80 | 30.96±2.04 |
|
312.5 | 12 | 12 | 20.88±0.80 |
28.96±2.52a |
|
625 | 12 | 12 | 20.88±0.80 |
30.26±1.94a |
|
1,250 | 12 | 12 | 20.88±0.80 |
29.57±2.26a |
|
2,500 | 12 | 12 | 20.88±0.80 |
31.06±2.57a |
|
5,000 | 12 | 12 | 20.88±0.80 |
29.71±2.83a |
Administration of RJSJ in combination
with 5-Fu is more effective and safer than the chemotherapeutic
treatment alone in EAC tumor models
To test the RJSJ antitumor efficacy in vivo,
EAC tumor models were established. The animals were divided into
different groups and treated with 5-Fu, RJSJ or 5-Fu plus RJSJ.
Tumor growth steadily progressed during the following 8 days in the
control group, whereas tumor volume increased at similar rates in
the 5-Fu and RJSJ groups (P=0.86). The animals receiving 5-Fu alone
exhibited significant body weight loss (P=0.037), which was absent
in those animals treated with RJSJ alone. Notably, the group
receiving 5-Fu and RJSJ exhibited the slowest tumor growth, with
body weight loss not greater than that obtained with 5-Fu alone
(Fig. 1A and B).
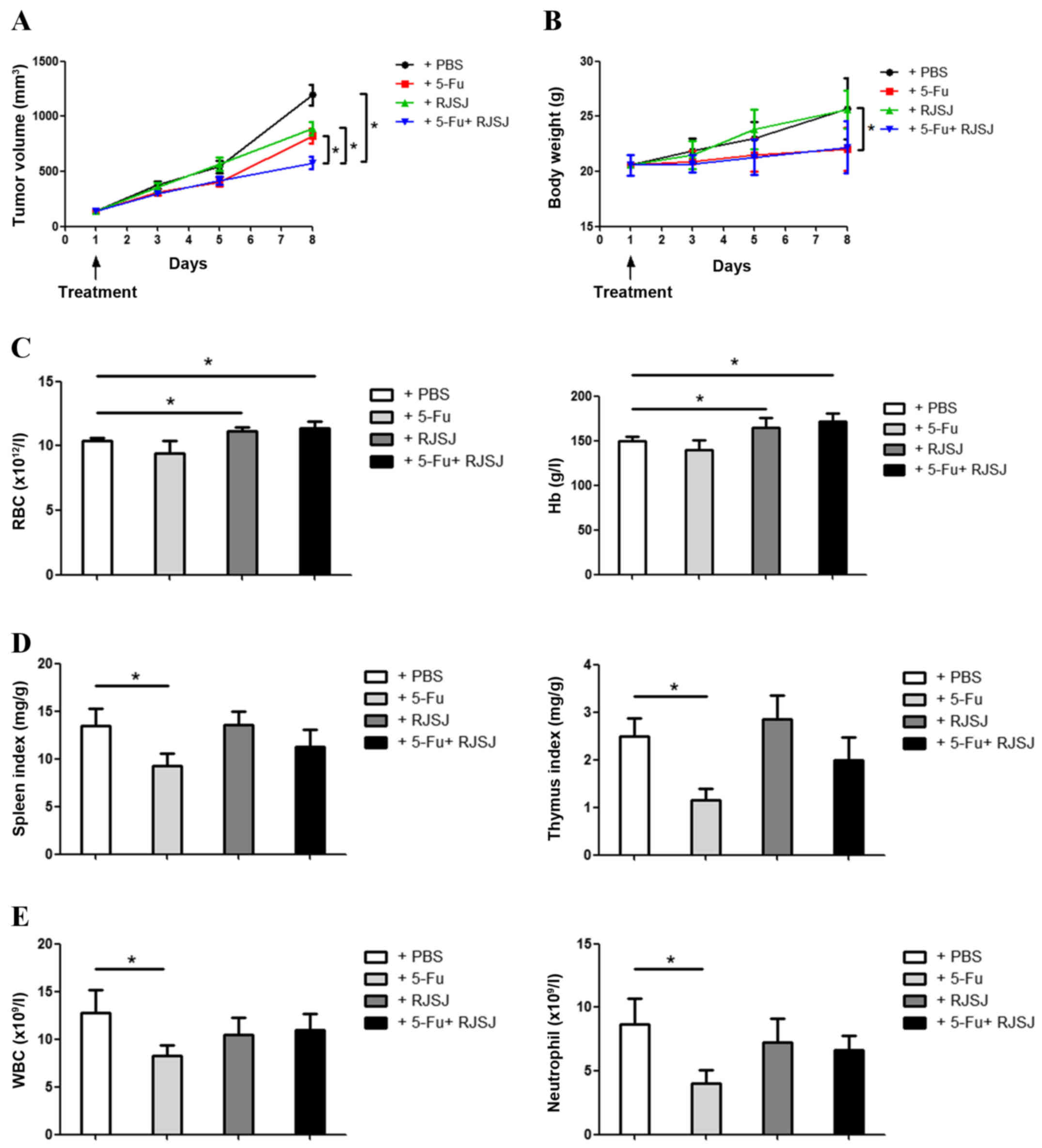 | Figure 1.RJSJ treatment is effective and well
tolerated in EAC tumor models. (A) Tumor size and (B) body weight
of EAC-bearing mice subsequent to treatment with PBS (control),
5-Fu, RJSJ or 5-Fu plus RJSJ. (C) Effect of different drug
treatments on RBC count, left panel, and Hb content, right panel.
(D) Effect of different drug treatments on the spleen, left panel,
and thymus, right panel, indexes. (E) Effect of different drug
treatments on WBC, left panel, and neutrophil, right panel, counts.
The results presented are the mean ± standard deviation of 12 mice
per group. *P<0.05. RBC, red blood cell; WBC, white blood cell;
RJSJ, Ruanjian Sanjie; EAC, Ehrlich ascites carcinoma; Hb,
hemoglobin; 5-Fu, 5-fluorouracil. |
The hematological parameters of mice treated with
RJSJ were observed to be significantly different to those from
5-Fu-treated mice. Although there were no differences in the
hemoglobin content or red blood cell count between control and
5-Fu-treated mice (P=0.73), treatment with RJSJ at a dose of 500
mg/kg body weight significantly increased the level of the
aforementioned hematological parameters (Fig. 1C) (P=0.04). As the thymus and spleen
are organs directly affecting the immune function, the present
study determined the effect of the aforementioned treatments on the
thymus and spleen indexes. As expected from a chemotherapeutic
drug, 5-Fu affected the two organs, with a significant reduction in
both thymus and spleen indexes (P=0.02). However, RJSJ blocked the
side effects of 5-Fu, and the thymus and spleen indexes were
indistinguishable between the combination and control groups
(Fig. 1D) (P=0.92 and 0.95,
respectively). Additionally, the white blood cell and neutrophil
counts declined in the 5-Fu treatment group, a toxic side effect
due to myelosuppression (20). By
contrast, RJSJ in combination with 5-Fu prevented these side
effects (Fig. 1E). In summary,
treatment with RJSJ at 500 mg/kg exhibited significant anticancer
activity; however, the administration of RJSJ together with 5-Fu
exhibited a greater effect in inhibiting tumor growth than either
compound alone without inducing body weight loss or reducing the
level of immune function or myelosuppression. Thus, the
administration of RJSJ in combination with 5-Fu is more effective
and safer than the chemotherapeutic treatment alone.
Toxicity of RJSJ against breast cancer
cells
As TCM has been used in the management of breast
cancer (6–9), the present study monitored the effect of
RJSJ on the growth of breast cancer MCF-7 and MDA-MB-231 cell lines
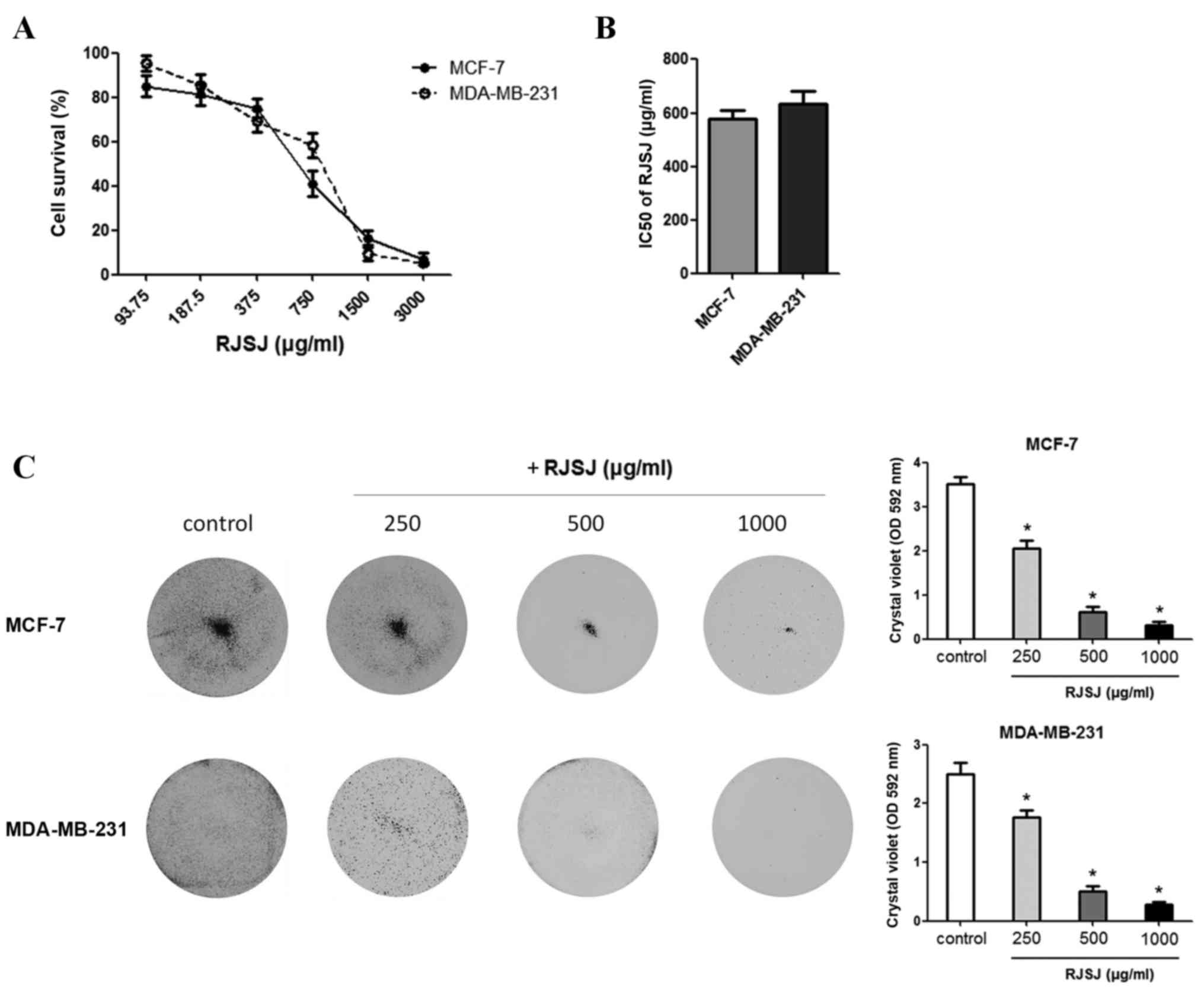
using MTT assays. RJSJ exhibited an inhibitory effect on the growth
of the cell lines, with IC50 values of 578 µg/ml for
MCF-7 and 635 µg/ml for MDA-MB-231 (Fig.
2A and B). At 1 mg/ml, RJSJ showd cytotoxicity on 80–90% of the
cancer cells. To additionally evaluate the cytotoxic effect of RJSJ
on breast cancer cells, MCF-7 and MDA-MB-231 cells were treated
with RJSJ, and crystal violet staining was used to determine the
cell mass 1 week subsequent to treatment. Consistent with the
aforementioned MTT assays, RJSJ significantly decreased the
capacity of breast cancer cells to survive in a dose-dependent
manner (Fig. 2C) (P=0.02). Thus, RJSJ
is a potent cytotoxic agent against breast cancer cells.
RJSJ promotes cell apoptosis, induces
caspase activity and modulates the expression of
apoptosis-associated genes
Next, the present study investigated whether the
cytotoxic effect of RJSJ on breast cancer cells is mediated by
promoting cell apoptosis using Hoechst 33258. Following apoptosis,
an increase in membrane permeability permits the dye to enter into
the cell and to stain condensed chromosomes, which is an indicator
of apoptosis (21). The present study
observed dense blue fluorescent particles within the nucleus or
cytoplasm in addition to nuclear morphological changes following 48
h of incubation with RJSJ in MCF-7 and MDA-MB-231 cells (Fig. 3A), indicating the activation of
apoptosis. Flow cytometric Annexin V/PI analysis additionally
confirmed these initial findings, as the number of apoptotic cells
increased following RJSJ treatment in a dose-dependent manner
(Fig. 3B) (P=0.011). In order to
determine whether these events were accompanied by caspase
activation, the activities of caspase-3/7 and −9 were measured. In
accordance with the Annexin V/PI analysis, RJSJ markedly induced
caspase-3/7 and caspase-9 activities in a dose-dependent manner in
the cells (Fig. 3C) (P=0.02 for
caspase-3/7 and P=0.03 for caspase-9). As the induction of
apoptotic cell death can be partly due to the alteration of
pro-survival and pro-apoptotic proteins, the present study
evaluated the messenger RNA levels of numerous survival and
apoptotic genes. RT-qPCR and western blotting results revealed that
RJSJ inhibited the expression of the pro-survival Bcl-2 and
survivin proteins in MCF-7 and MDA-MB-231 cells (Fig. 3D and E). Thus, RJSJ induces caspase
activity and the downregulation of the pro-survival proteins Bcl-2
and surviving, leading to apoptosis in breast cancer cells.
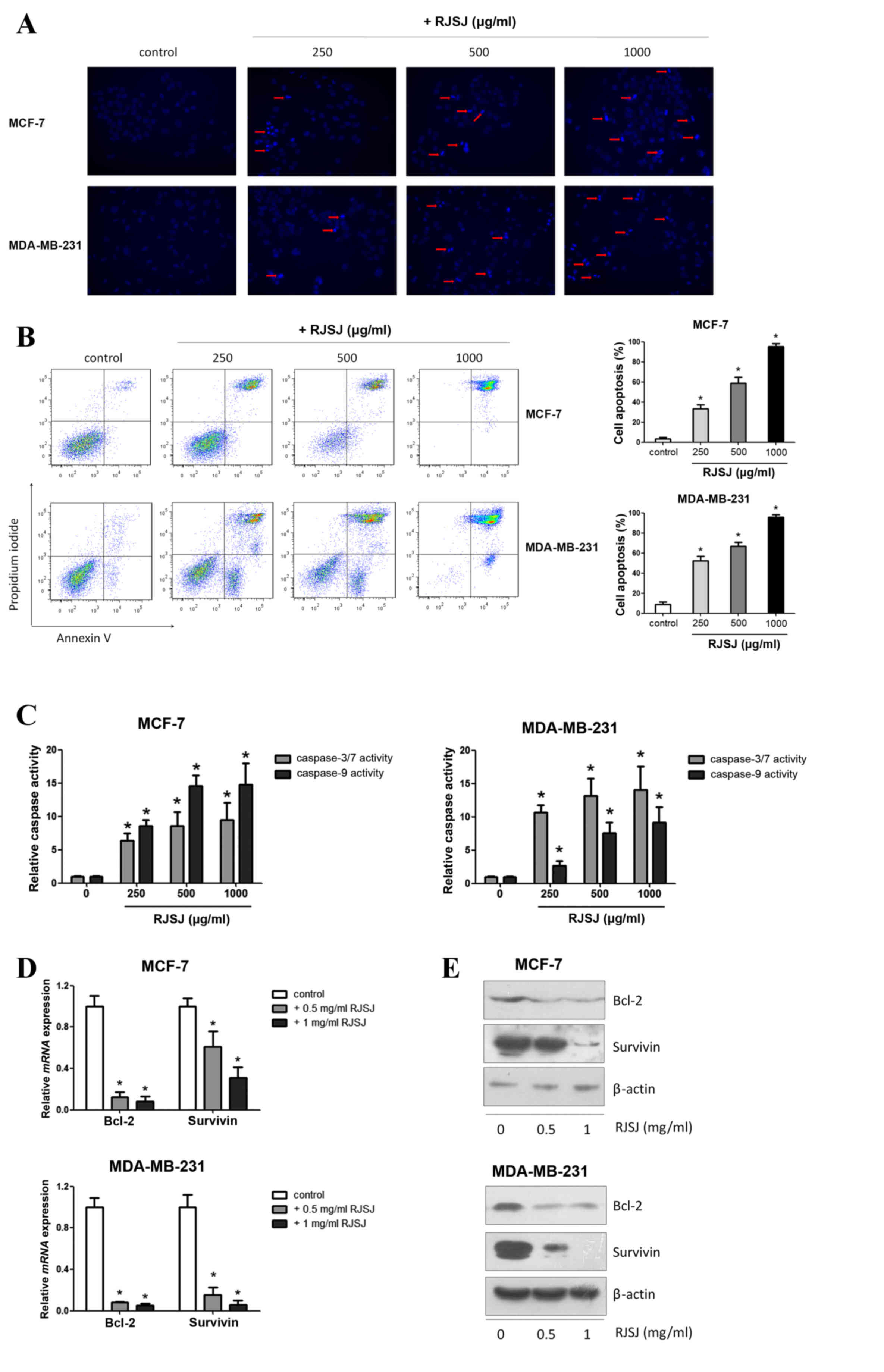 | Figure 3.RJSJ induces apoptosis in breast
cancer cells. (A) Representative fluorescence images of MCF-7
cells, upper panel, and MDA-MB-231 cells, lower panel, stained with
Hoechst 33258 subsequent to treatment with RJSJ for 24 h; red
arrows indicate cell shrinkage and nuclear fragmentation
(magnification, ×200). (B) Cells were treated with RJSJ at
different concentrations for 48 h. Annexin V/propidium iodide
staining was detected with flow cytometry. Representative plots of
3 independent experiments are shown. Quantitative data show the
average percentage of Annexin V-positive cells in early apoptosis,
lower right quadrant, and late apoptosis, upper right quadrant, of
3 independent experiments, right panel. (C) Caspase-3/7 and
caspase-9 activities in MCF-7, left histograms, and MDA-MB-231,
right histograms, cells subsequent to RJSJ treatment. (D and E)
Bcl-2 and survivin expression levels in MCF-7 and MDA-MB-231 cells
determined using (D) reverse transcription-quantitative polymerase
chain reaction and (E) western blot analysis subsequent to RJSJ
treatment for 48 h. Pictorial data show representative images of 3
independent experiments. Numerical data represent the mean ±
standard deviation of 3 independent experiments. *P<0.05. RJSJ,
Ruanjian Sanjie; Bcl-2, B-cell lymphoma 2; mRNA, messenger RNA. |
Administration of RJSJ in combination
with doxorubicin is more effective and safer than the
chemotherapeutic treatment alone in breast cancer xenografts
As the significant antitumor activity of RJSJ was
observed in both breast cancer cells and EAC tumor models, it was
investigated whether the antitumor efficacy of RJSJ may be
maintained in a breast cancer xenograft model generated with highly
metastatic MDA-MB-231 cells. As the aim of the present study was to
explore whether RJSJ may be effective in advanced breast cancer,
the tumor volume was monitored, and when the average tumor volume
reached ~400 mm3, the tumor-bearing mice were randomly
divided into four groups of 6 animals/group prior to the initiation
of the treatments. Tumor growth steadily progressed during the
following 15 days in the control group, whereas tumor volume
increased at a lower rate in the doxorubicin- and RJSJ-treated
groups. Notably, the animals receiving doxorubicin alone exhibited
a significant body weight loss, ~20% reduction compared with that
of the control group (P=0.03). By contrast, no significant body
weight loss was observed in the animals treated with RJSJ alone
(P=0.86). Additionally, the group receiving doxorubicin and RJSJ
displayed the smallest tumors (P=0.047), with no additional mouse
body weight loss (P=0.76 for combined group vs. doxorubicin group),
indicating that the RJSJ-doxorubicin combination is more
efficacious and better tolerated than doxorubicin alone (Fig. 4A and B). The expression of
anti-apoptotic Bcl-2 and pro-survival survivin in the tumors was
consistent with the in vitro results (Fig. 3D), showing a downregulation of these
genes following RJSJ administration (Fig.
4C) (P=0.03 for Bcl-2 expression and P=0.013 for survivin
expression). In summary, the administration of RJSJ in combination
with doxorubicin is more effective in reducing tumor size than the
chemotherapeutic treatment alone in mouse breast cancer
xenografts.
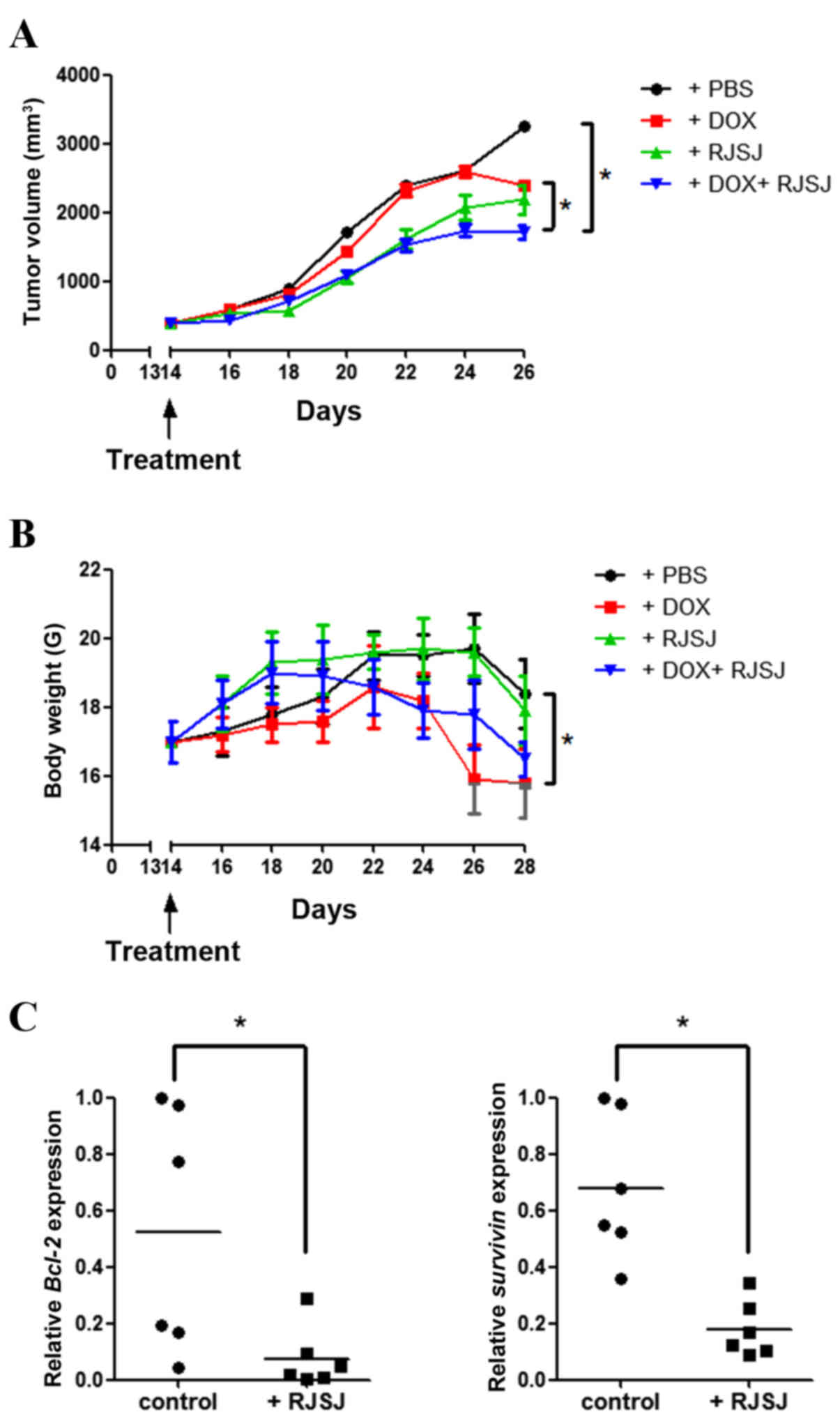 | Figure 4.Antitumor activity of RJSJ in
MDA-MB-231 advanced xenograft tumors. (A) Tumor size and (B) body
weight of nude mice subsequent to treatment with PBS (control),
DOX, RJSJ or DOX plus RJSJ. Data are presented as the mean tumor
size ± standard deviation of 6 mice per group. (C) RNA was isolated
from tumors 28 days post-implantation, and Bcl-2 and survivin
messenger RNA expression was determined by quantitative polymerase
chain reaction. The individual tumor expression data, dots, and the
mean values, lines, are indicated. *P<0.05. RJSJ, Ruanjian
Sanjie; Bcl-2, B-cell lymphoma 2; DOX, doxorubicin. |
Discussion
According to TCM, cancer is a systemic disease
formed when the internal functions of the body become imbalanced
(22). Folk Chinese tradition
attributes breast cancer to the accumulation of toxins, heat,
swelling and blood stasis in the body. This pathology is cited as
‘breast rock’ in the most ancient Chinese medical texts (6). More recently, pre-clinical and clinical
studies have shown that TCM combined with conventional Western
medicine, chemotherapy and radiotherapy, can provide effective
supportive care for patients with cancer (23–26). TCM
increases the sensitivity of chemo- and radio-therapeutics, reduces
the side effects of the aforementioned therapies, and improves
patient quality of life and survival time (23–26). One
classical model of TCM combination therapy, called formula, has
been advocated for >2,500 years. Formulae always consist of
different herbs or minerals, as their components may act on
multiple targets and show synergistic therapeutic efficacy
(27,28). For example, the Chinese medical
formula warming and relieving cold phlegm has demonstrated an
anticancer effect on hepatoma cells in vitro and on breast
cancer cells in vivo (29,30),
whereas Xiaotan Sanjie, a traditional Chinese herbal decoction
composed of 11 herbs, has been shown to be effective in gastric
cancer treatment (31,32). However, despite a long tradition and
anecdotal evidence of cancer cures using TCM as a sole therapy
(6), the key active principles in
Chinese formulae and their mechanisms of action remain unknown.
In an effort to shed light on the underlying
principles involved in TCM, the present study investigated the
antitumor activity of RJSJ, a decoction of Pinellia ternata,
Prunella vulgaris, Cremastra appendiculata and
Sargassum pallidum traditionally used in China (10). Although a number of preliminary
studies indicate tumor-inhibitory properties in extracts of several
individual components of RJSJ (11–14), no
studies on the effect of RJSJ on cancer have yet been published.
The acute toxicity assays of the present study indicate that RJSJ
is safe and well tolerated. However, the median lethal dose
(LD50) was not calculated, as no mortality or behavioral
changes were observed at the highest dose used (5 g/kg).
The administration of RJSJ exhibits similar efficacy
in terms of tumor growth inhibition to that of 5-Fu in EAC tumor
models and to doxorubicin in advanced breast cancer xenograft
models. RJSJ is better tolerated than these two chemotherapeutic
drugs alone, without body weight loss or toxicity due to
myelosuppression. Notably, RJSJ reduces the immunosuppressive
reaction of tumor-bearing mice treated by chemotherapeutic drugs
and strengthens the immunocompetence of the host. Having
established the excellent antiproliferative capacity of RJSJ when
used in combination with standard chemotherapeutics, the present
study demonstrated that the effect of RJSJ on breast cancer, both
in vitro and in vivo, can be at least partially
attributed to its ability to induce apoptosis. The administration
of RJSJ results in the activation of caspase-3,7 and −9, and the
downregulation of survivin and Bcl-2 expression, two pro-survival
proteins. These results are consistent with those from previous
studies indicating that Pinellia ternate extracts induce
apoptosis in human hepatoma cells (33). Prunella vulgaris also promotes
apoptosis and inhibits cell proliferation in human colorectal
cancer cells through targeting the signal transducer and activator
of transcription 3 pathway (34),
decreases Bcl-2 expression, and increases Bcl-2-like protein 4 and
Bcl-2-associated death promotor expression in human pulmonary
adenocarcinoma cells (35). In
addition, Sargassum pallidum aqueous extract enhances
antioxidant activities in rodent gastric cancer (36), whereas Cremastra appendiculata
tuber extract shows potent cytotoxicity against MCF-7 and
MDA-MB-231 cells in vitro (37). These data suggest that RJSJ may
effectively eliminate breast cancer cells through modulating a
variety of pathways in addition to apoptosis via regulating Bcl-2
and survivin expression.
In conclusion, the present study observed that the
TCM RJSJ decoction shows potent antitumor activity in vitro
and in vivo. RJSJ significantly induces cell apoptosis,
activating caspases-3,7 and 9, and suppressing Bcl-2 and survivin
expression in breast cancer cells. Additionally, considering that
i.g. RJSJ treatment is well tolerated, the combination of RJSJ and
chemotherapeutic drugs shows a synergistic effect in vivo,
particularly in advanced breast cancer xenograft models. Additional
investigation into the clinical implication of RJSJ in cancer
treatment and the characterization of its active principles is
required.
Acknowledgements
The present study was supported by the Chinese
National Natural Sciences Foundation (grant no. 81402480), the
Science and Technology Foundation of Tianjin Municipal Health
Bureau (grant no. 2014KZ078) and Tianjin Medical University Cancer
Institute and Hospital Foundation (grant no. 1416).
References
|
1
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cohen SM, Mukerji R, Cai S, Damjanov I,
Forrest ML and Cohen MS: Subcutaneous delivery of nanoconjugated
doxorubicin and cisplatin for locally advanced breast cancer
demonstrates improved efficacy and decreased toxicity at lower
doses than standard systemic combination therapy in vivo. Am J
Surg. 202:646–653. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen C, Sun S, Yuan JP, Wang YH, Cao TZ,
Zheng HM, Jiang XQ, Gong YP, Tu Y, Yao F, et al: Characteristics of
breast cancer in Central China, literature review and comparison
with USA. Breast. 30:208–213. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xu B, Hu X, Jiang Z, Li H, Chen J, Cui S,
Li Q, Liao N, Liu D, Liu J, et al: National consensus in China on
diagnosis and treatment of patients with advanced breast cancer.
Ann Transl Med. 3:2422015.PubMed/NCBI
|
|
5
|
Chaffer CL and Weinberg RA: A perspective
on cancer cell metastasis. Science. 331:1559–1564. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cohen I, Tagliaferri M and Tripathy D:
Traditional Chinese medicine in the treatment of breast cancer.
Semin Oncol. 29:563–574. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ding X, Yang Q, Kong X, Haffty BG, Gao S
and Moran MS: Radiosensitization effect of Huaier on breast cancer
cells. Oncol Rep. 35:2843–2850. 2016.PubMed/NCBI
|
|
8
|
Tao G and Balunas MJ: Current therapeutic
role and medicinal potential of Scutellaria barbata in
traditional Chinese medicine and Western research. J
Ethnopharmacol. 182:170–180. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lee YW, Chen TL, Shih YR, Tsai CL, Chang
CC, Liang HH, Tseng SH, Chien SC and Wang CC: Adjunctive
traditional Chinese medicine therapy improves survival in patients
with advanced breast cancer: A population-based study. Cancer.
120:1338–1344. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lahans T: Integrating conventional and
Chinese medicine in cancer care: A clinical guide. Churchill
Livingstone Elsevier; Edinburgh: 2007, View Article : Google Scholar
|
|
11
|
Tan J, Qi H and Ni J: Extracts of
endophytic fungus xkc-s03 from Prunella vulgaris L. Spica
inhibit gastric cancer in vitro and in vivo. Oncol Lett. 9:945–949.
2015.PubMed/NCBI
|
|
12
|
Kim SH, Huang CY, Tsai CY, Lu SY, Chiu CC
and Fang K: The aqueous extract of Prunella vulgaris
suppresses cell invasion and migration in human liver cancer cells
by attenuating matrix metalloproteinases. Am J Chin Med.
40:643–656. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kurt O, Ozdal-Kurt F, Tuğlu MI and Akçora
CM: The cytotoxic, neurotoxic, apoptotic and antiproliferative
activities of extracts of some marine algae on the MCF-7 cell line.
Biotech Histochem. 89:568–576. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Aravindan S, Ramraj SK, Somasundaram ST,
Herman TS and Aravindan N: Polyphenols from marine brown algae
target radiotherapy-coordinated EMT and stemness-maintenance in
residual pancreatic cancer. Stem Cell Res Ther. 6:1822015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
National Research Council (US) Committee
for the Update of the Guide for the Care and Use of Laboratory
AnimalsGuide for the Care and Use of Laboratory Animals. 8th.
Washington (DC): National Academies Press (US); 2011, PubMed/NCBI
|
|
16
|
Alam B, Majumder R, Akter S and Lee SH:
Piper betle extracts exhibit antitumor activity by augmenting
antioxidant potential. Oncol Lett. 9:863–868. 2015.PubMed/NCBI
|
|
17
|
Hu Y, Guo R, Wei J, Zhou Y, Ji W, Liu J,
Zhi X and Zhang J: Effects of PI3K inhibitor NVP-BKM120 on
overcoming drug resistance and eliminating cancer stem cells in
human breast cancer cells. Cell Death Dis. 6:e20202015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hu Y, Li K, Asaduzzaman M, Cuella R, Shi
H, Raguz S, Coombes RC, Zhou Y and Yagüe E: miR-106b~25 cluster
regulates multidrug resistance in an ABC transporter-independent
manner via downregulation of EP300. Oncol Rep. 35:1170–1178.
2016.PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Peng M, Ding Y, Yu L, Deng Y, Lai W, Hu Y,
Zhang H, Wu X, Fan H, Ding H, et al: Tegafur substitution for 5-Fu
in combination with actinomycin D to treat gestational
trophoblastic neoplasm. PLoS One. 10:e01435312015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sun HY, Liu BB, Hu JY, Xu LJ, Chan SW,
Chan CO, Mok DK, Zhang DM, Ye WC and Chen SB: Novel cycloartane
triterpenoid from Cimicifuga foetida (Sheng ma) induces
mitochondrial apoptosis via inhibiting Raf/MEK/ERK pathway and Akt
phosphorylation in human breast carcinoma MCF-7 cells. Chin Med.
11:12016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chung VC, Wu X, Lu P, Hui EP, Zhang Y,
Zhang AL, Lau AY, Zhao J, Fan M, Ziea ET, et al: Chinese herbal
medicine for symptom management in cancer palliative care:
Systematic review and meta-analysis. Medicine (Baltimore).
95:e27932016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Efferth T, Kahl S, Paulus K, Adams M, Rauh
R, Boechzelt H, Hao X, Kaina B and Bauer R: Phytochemistry and
pharmacogenomics of natural products derived from traditional
Chinese medicine and Chinese materia medica with activity against
tumor cells. Mol Cancer Ther. 7:152–161. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Man YN, Liu XH and Wu XZ: Chinese medicine
herbal treatment based on syndrome differentiation improves the
overall survival of patients with unresectable hepatocellular
carcinoma. Chin J Integr Med. 21:49–57. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Konkimalla VB and Efferth T:
Evidence-based Chinese medicine for cancer therapy. J
Ethnopharmacol. 116:207–210. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lam W, Bussom S, Guan F, Jiang Z, Zhang W,
Gullen EA, Liu SH and Cheng YC: The four-herb Chinese medicine
PHY906 reduces chemotherapy-induced gastrointestinal toxicity. Sci
Transl Med. 2:45ra592010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Qi F, Li A, Inagaki Y, Gao J, Li J, Kokudo
N, Li XK and Tang W: Chinese herbal medicines as adjuvant treatment
during chemo- or radio-therapy for cancer. Biosci Trends.
4:297–307. 2010.PubMed/NCBI
|
|
28
|
Cao R, Zhang H, Guo J, Liu XH, Liu C, Zhu
CH and Wu XZ: A novel pharmacological method to study the Chinese
medicinal formula Hua-Zheng-Hui-Sheng-Dan. Evid Based Complement
Alternat Med. 2015:4368072015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yan ZC, Chen D, Wu XZ, Xie GR, Ba Y and
Yan Z: Effects of aqueous extracts of Aconitum carmichaeli,
Rhizoma bolbostemmatis, Phytolacca acinosa, Panax notoginseng
and Gekko swinhonis Guenther on Bel-7402 cells. World J
Gastroenterol. 13:2743–2746. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang XL, Ma F and Wu XZ: Anticancer
effects of 5-fluorouracil combined with warming and relieving cold
phlegm formula on human breast cancer. Chin J Integr Med.
18:599–604. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shi J and Wei PK: Xiaotan Sanjie decoction
inhibits interleukin-8-induced metastatic potency in gastric
cancer. World J Gastroenterol. 21:1479–1487. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yan B, Liu L, Zhao Y, Xiu LJ, Sun DZ, Liu
X, Lu Y, Shi J, Zhang YC, Li YJ, et al: Xiaotan Sanjie decoction
attenuates tumor angiogenesis by manipulating Notch-1-regulated
proliferation of gastric cancer stem-like cells. World J
Gastroenterol. 20:13105–13118. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhou W, Gao Y, Xu S, Yang Z and Xu T:
Purification of a mannose-binding lectin Pinellia ternata
agglutinin and its induction of apoptosis in Bel-7404 cells.
Protein Expr Purif. 93:11–17. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lin W, Zheng L, Zhuang Q, Zhao J, Cao Z,
Zeng J, Lin S, Xu W and Peng J: Spica prunellae promotes
cancer cell apoptosis, inhibits cell proliferation and tumor
angiogenesis in a mouse model of colorectal cancer via suppression
of stat3 pathway. BMC Complement Altern Med. 13:1442013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Feng L, Au-Yeung W, Xu YH, Wang SS, Zhu Q
and Xiang P: Oleanolic acid from Prunella vulgaris L.
induces SPC-A-1 cell line apoptosis via regulation of Bax, Bad and
Bcl-2 expression. Asian Pac J Cancer Prev. 12:403–408.
2011.PubMed/NCBI
|
|
36
|
Zhang RL, Luo WD, Bi TN and Zhou SK:
Evaluation of antioxidant and immunity-enhancing activities of
Sargassum pallidum aqueous extract in gastric cancer rats.
Molecules. 17:8419–8429. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Liu L, Li J, Zeng KW, Jiang Y and Tu PF:
Five new benzylphenanthrenes from Cremastra appendiculata.
Fitoterapia. 103:27–32. 2015. View Article : Google Scholar : PubMed/NCBI
|