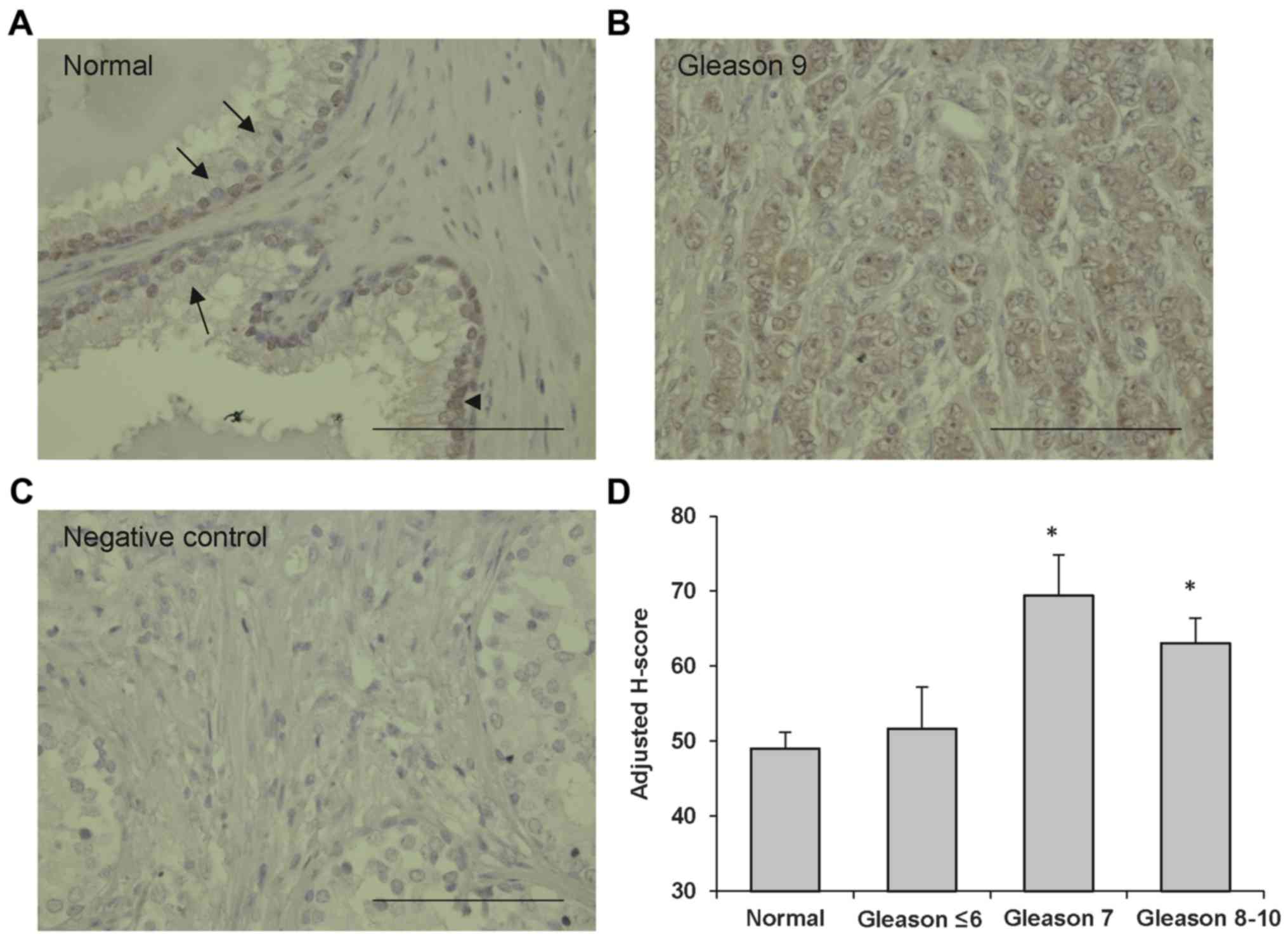
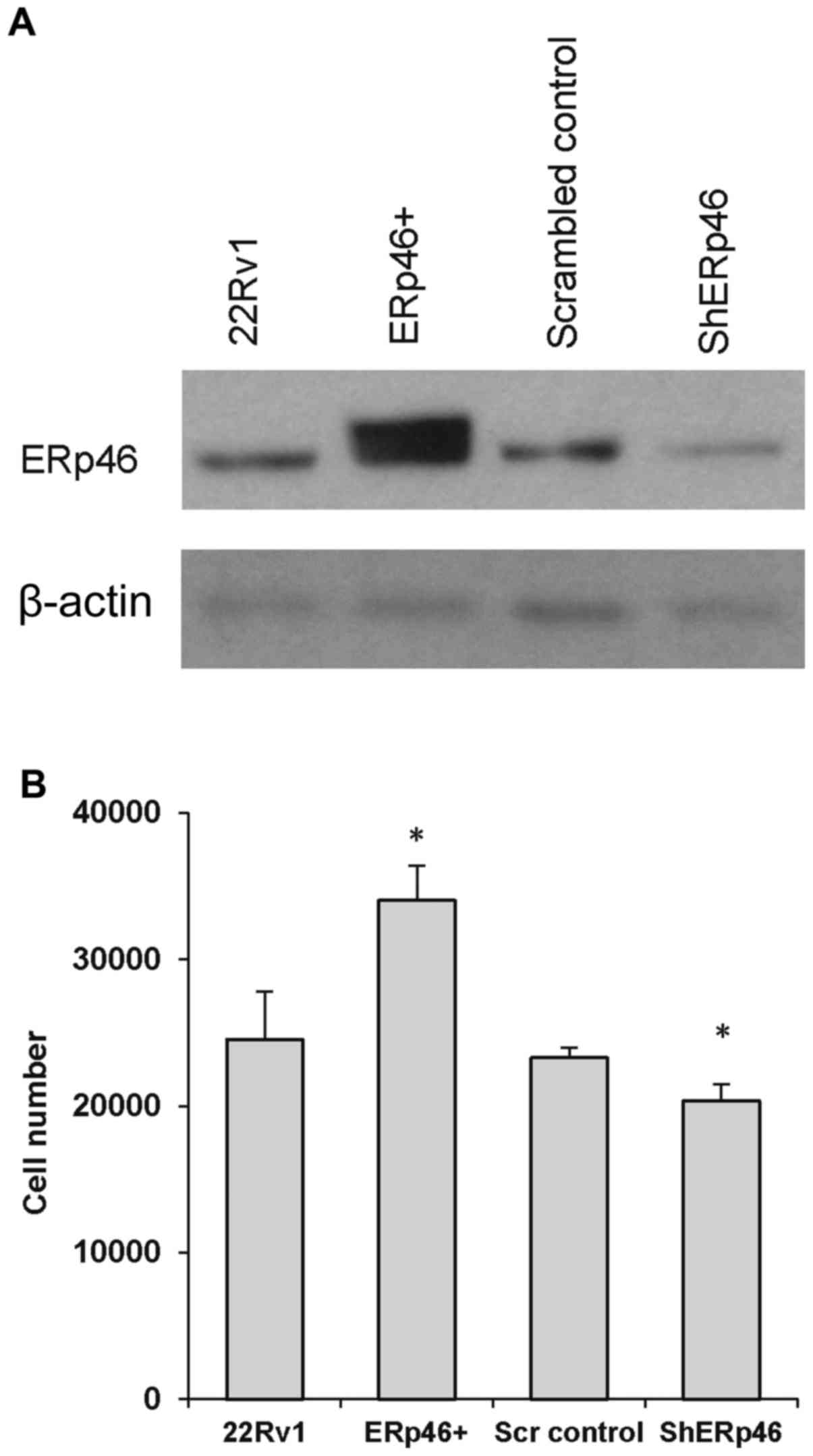
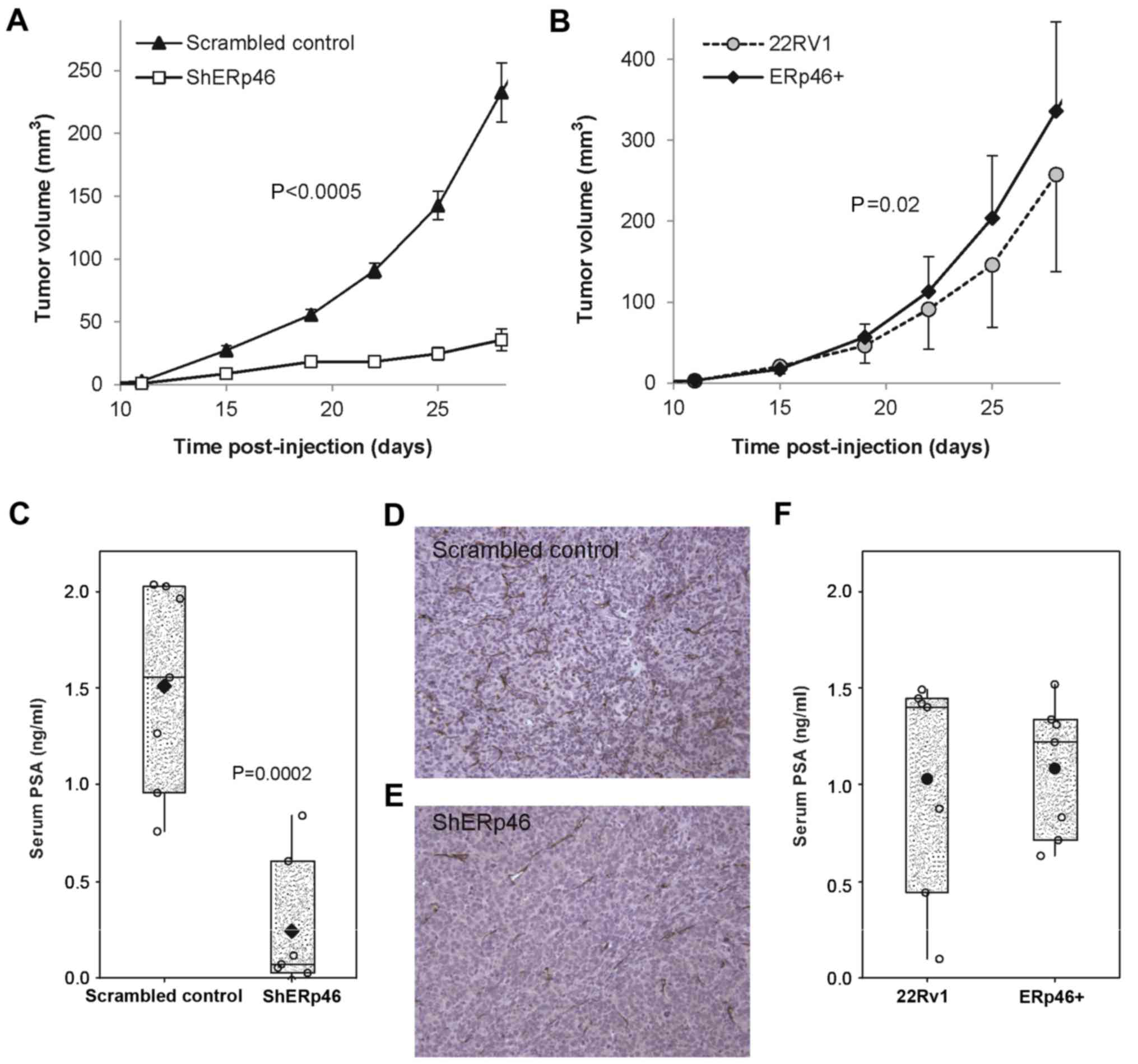
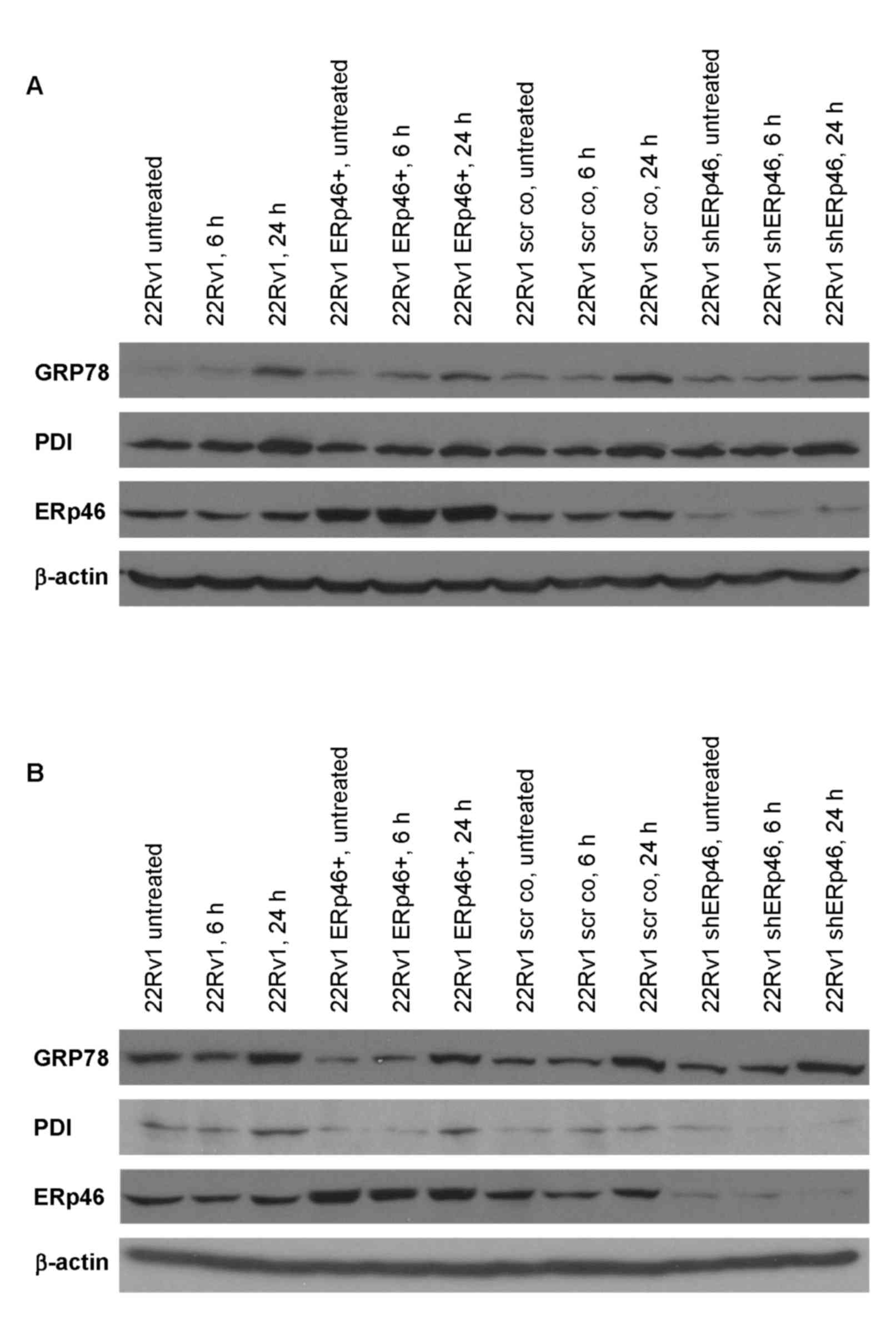
|
1
|
Hatahet F and Ruddock LW: Protein
disulfide isomerase: A critical evaluation of its function in
disulfide bond formation. Antioxid Redox Signal. 11:2807–2850.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Duivenvoorden WC, Paschos A, Hopmans SN,
Austin RC and Pinthus JH: Endoplasmic reticulum protein ERp46 in
renal cell carcinoma. PLoS One. 9:e903892014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Benham AM: The protein disulfide isomerase
family: Key players in health and disease. Antioxid Redox Signal.
16:781–789. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Charlton HK, Webster J, Kruger S, Simpson
F, Richards AA and Whitehead JP: ERp46 binds to AdipoR1, but not
AdipoR2, and modulates adiponectin signalling. Biochem Biophys Res
Commun. 392:234–239. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jessop CE, Watkins RH, Simmons JJ, Tasab M
and Bulleid NJ: Protein disulphide isomerase family members show
distinct substrate specificity: P5 is targeted to BiP client
proteins. J Cell Sci. 122:4287–4295. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Havugimana PC, Hart GT, Nepusz T, Yang H,
Turinsky AL, Li Z, Wang PI, Boutz DR, Fong V, Phanse S, et al: A
census of human soluble protein complexes. Cell. 150:1068–1081.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kristensen AR, Gsponer J and Foster LJ: A
high-throughput approach for measuring temporal changes in the
interactome. Nat Methods. 9:907–909. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang L, Song G, Chang X, Tan W, Pan J, Zhu
X, Liu Z, Qi M, Yu J and Han B: The role of TXNDC5 in
castration-resistant prostate cancer-involvement of androgen
receptor signaling pathway. Oncogene. 34:4735–4745. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Duivenvoorden WC, Beatty LK, Lhotak S,
Hill B, Mak I, Paulin G, Gallino D, Popovic S, Austin RC and
Pinthus JH: Underexpression of the tumor suppressor LKB1 in clear
cell renal cell carcinoma is common and confers growth advantage in
vitro and in vivo. Br J Cancer. 108:327–333. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rago R, Mitchen J and Wilding G: DNA
fluorometric assay in 96-well tissue culture plates using Hoechst
33258 after cell lysis by freezing in distilled water. Anal
Biochem. 191:31–34. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chou TC: Theoretical basis, experimental
design and computerized simulation of synergism and antagonism in
drug combination studies. Pharmacol Rev. 58:621–681. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kleinmann N, Duivenvoorden WC, Hopmans SN,
Beatty LK, Qiao S, Gallino D, Lhotak S, Daya D, Paschos A, Austin
RC and Pinthus JH: Underactivation of the adiponectin-adiponectin
receptor 1 axis in clear cell renal cell carcinoma: Implications
for progression. Clin Exp Metastasis. 31:169–183. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Vincent EE, Elder DJ, Phillips L, Heesom
KJ, Pawade J, Luckett M, Sohail M, May MT, Hetzel MR and Tavaré JM:
Overexpression of the TXNDC5 protein in non-small cell lung
carcinoma. Anticancer Res. 31:1577–1582. 2011.PubMed/NCBI
|
|
14
|
Wang Y, Ma Y, Lü B, Xu E, Huang Q and Lai
M: Differential expression of mimecan and thioredoxin
domain-containing protein 5 in colorectal adenoma and cancer: A
proteomic study. Exp Biol Med (Maywood). 232:1152–1159. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Harding HP, Calfon M, Urano F, Novoa I and
Ron D: Transcriptional and translational control in the mammalian
unfolded protein response. Annu Rev Cell Dev Biol. 18:575–599.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Daneshmand S, Quek ML, Lin E, Lee C, Cote
RJ, Hawes D, Cai J, Groshen S, Lieskovsky G, Skinner DG, et al:
Glucose-regulated protein GRP78 is up-regulated in prostate cancer
and correlates with recurrence and survival. Hum Pathol.
38:1547–1552. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fu Y, Wey S, Wang M, Ye R, Liao CP,
Roy-Burman P and Lee AS: Pten null prostate tumorigenesis and AKT
activation are blocked by targeted knockout of ER chaperone
GRP78/BiP in prostate epithelium. Proc Natl Acad Sci USA.
105:19444–19449. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wu Y, Fabritius M and Ip C:
Chemotherapeutic sensitization by endoplasmic reticulum stress:
Increasing the efficacy of taxane against prostate cancer. Cancer
Biol Ther. 8:146–152. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tanaka S, Uehara T and Nomura Y:
Up-regulation of protein-disulfide isomerase in response to
hypoxia/brain ischemia and its protective effect against apoptotic
cell death. J Biol Chem. 275:10388–10393. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ko HS, Uehara T and Nomura Y: Role of
ubiquilin associated with protein-disulfide isomerase in the
endoplasmic reticulum in stress-induced apoptotic cell death. J
Biol Chem. 277:35386–35392. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Festuccia C, Gravina GL, D'Alessandro AM,
Muzi P, Millimaggi D, Dolo V, Ricevuto E, Vicentini C and Bologna
M: Azacitidine improves antitumor effects of docetaxel and
cisplatin in aggressive prostate cancer models. Endocr Relat
Cancer. 16:401–413. 2009. View Article : Google Scholar : PubMed/NCBI
|