Introduction
Gallbladder carcinoma (GBC) is the most common
malignancy of the biliary system and the fifth most common
gastrointestinal cancer worldwide, with an annual incidence of
>10,000 and mortality rate of ~3,300 individuals. Its morbidity
varies with racial, ethnic and regional factors (1). In recent years, numerous studies have
reported an increase in the incidence of GBC in certain areas,
including north India, Pakistan and Korea (2,3). As the
majority of GBC patients are diagnosed when the cancer has reached
an advanced stage, they have an extremely poor prognosis, with a
median 5-year survival rate of 2–5% (4). Chemotherapy is considered to be the most
valuable treatment to prolong the survival time and improve the
quality of life of these patients. However, GBC shows resistance to
the majority of currently used chemotherapeutic agents, and the
real clinical effect of such agents is unsatisfactory (5). With rapid developments in cancer
molecular and cell biology, targeted cancer therapies, which are
drugs that interfere with specific molecules involved in cancer
cell growth and survival, bring new hope for cancer patients. The
growth and development of most tumors involves multiple genetic
abnormalities, which diminishes the antitumor activities of the
majority of single-targeted drugs in clinical applications. To
date, GBC clinical trials have demonstrated that most
single-targeted drugs for GBC have poor specificity and sensitivity
(6). Network models suggest that
partial inhibition of a surprisingly small number of targets can be
more efficient than the complete inhibition of a single target.
Therefore, researchers have begun to explore novel multi-targeted
drugs and strategies for the combination of targeted drugs or
traditional chemotherapy drugs (7).
The overexpression of cyclooxygenase-2 (COX-2),
which is a rate-limiting enzyme in prostaglandin production, is
involved in the tumorigenesis of various types of tumors, including
GBC (8,9). Extracellular signal-regulated kinase 1/2
(ERK1/2) is a member of the mitogen-activated protein kinase (MAPK)
cascade, which is involved in regulating biological processes such
as cell growth and division, as well as apoptosis. Several studies
have reported that phospho (p-)ERK1/2 expression is abnormally
elevated in GBC tissues (10,11), and that aberrant ERK1/2 activation is
closely associated with tumorigenesis and progression in various
tumor types (12,13). It has also been demonstrated that
COX-2 and ERK1/2 may serve as potential therapeutic targets for
certain tumors, and their specific inhibitors have shown
encouraging outcomes for cancer patients (14). For instance, patients with EGFR
wild-type non-small cell lung cancer showed an increased
progression-free survival following treatment with a combination of
erlotinib and celecoxib (15).
However, the biological functions of COX-2 inhibitors and ERK1/2
inhibitors against the growth of GBC cells have not yet been
investigated.
In the present study, the biological effects of the
COX-2 inhibitor celecoxib and the ERK1/2 inhibitor PD184161 on the
growth, cell cycle and apoptosis of the GBC cell lines GBC-SD and
NOZ were examined in vitro and in vivo. Additionally,
the molecular mechanisms underlying these activities were
preliminarily investigated. This data may provide a theoretical
foundation and initial evidence for exploring novel therapeutic
regimens for GBC patients.
Materials and methods
Sample collection
The present study was approved by the ethics
committee of The First Affiliated Hospital of Bengbu Medical
College (Bengbu, China), and all patients provided informed
consent. Cancer tissue specimens were obtained from 24 patients
with GBC who underwent radical cholecystectomy without prior
radiotherapy or chemotherapy between June 2006 and May 2009 at the
First Affiliated Hospital of Bengbu Medical College. Normal tissues
were reserved in 10 GBC cases (all at stage I and II) and were used
as negative controls. The specimens were stored in liquid nitrogen
immediately following surgery. COX-2 and p-ERK1/2 expression levels
in tissues were examined using western blotting.
Cell culture
The human GBC cell line GBC-SD was obtained from the
Cell Bank of the Chinese Academy of Sciences (Shanghai, China). The
human GBC cell line NOZ was obtained from the Japanese Collection
of Research Bioresources Cell Bank (Osaka, Japan). The
non-immortalized human biliary epithelial cell line HIBEpiC was
obtained from the ScienCell Research Laboratories (Carlsbad, CA,
USA). All cell lines were cultured in Gibco Dulbecco's modified
Eagle's medium (Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% fetal bovine serum (GE Healthcare Life
Sciences, Logan, UT, USA) and 1% penicillin-streptomycin
(Sigma-Aldrich; EMD Millipore, Billerica, MA, USA). Cells were
cultured in a humidified atmosphere of 5% CO2 at 37°C.
Trypsin (0.25%) was used to detach the cells from the culture
flask.
Western blotting
Total protein was extracted from cells using a
Novagen Protein Extraction Kit (EMD Millipore). The protein
extracts were denatured by boiling at 95°C for 5 min and equal
amounts of proteins were separated on 10% SDS-PAGE gels and
transferred to polyvinylidene difluoride membranes (EMD Millipore).
Blots were blocked with 5% non-fat dry milk and then incubated with
the primary antibodies at 4°C overnight. The primary antibodies
included anti-COX-2 (1:100; sc-514489), anti-p-ERK1/2 (1:200;
sc-23759-R), anti-p21 (1:200; sc-6246), anti-p27 (1:200; sc-71813),
anti-β-actin (1:200; sc-47778), anti-caspase-3 (1:200; sc-7148),
anti-cytochrome c (1:200; sc-65396) and anti-COX IV (1:100;
sc-376731) antibodies (all Santa Cruz Biotechnology, Inc., Dallas,
TX, USA). COX IV was used as the loading control for the
mitochondrial fraction and β-actin was used as the loading control
for the cytosolic fraction. Subsequently, the membranes were
incubated with horseradish peroxidase-conjugated anti-mouse
(1:2,000; sc-2005; Santa Cruz Biotechnology, Inc.) and anti-rabbit
(1:5,000; sc-2004; Santa Cruz Biotechnology, Inc.) secondary
antibodies at room temperature for 1 h. Subsequently, the blots
were visualized using Pierce ECL Western Blotting Substrate (Thermo
Fisher Scientific, Inc.). β-actin was used as a loading
control.
Cell viability assay
The viability of GBC-SD and NOZ cells was determined
by the water soluble tetrazolium (WST)-1 method using a WST-1 Cell
Proliferation and Cytotoxicity Assay kit (Roche, Mannheim, Germany)
according to the manufacturer's instructions. In brief,
5×103 cells were seeded in 96-well plates and cultured
overnight. Cells were treated with celecoxib (0, 1, 2, 4, 8, 16 or
32 µM), PD184161 (0, 5, 10, 20, 40, 80 or 160 µM) or a combination
of the two drugs (both from Pfizer, Inc., New York, NY, USA). After
72 h, the cells were incubated with WST-1 reagent for 2 h at 37°C.
The absorbance (optical density; OD) was measured at 450 nm with an
automated microplate reader (Model 550; Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). The percent viability of cells in each group
was calculated using the following equation: Cell viability = mean
OD of experimental group/mean OD of control group × 100%.
Isobologram analysis
Isobologram analysis provides a graphical
presentation for the evaluation of combined drug effects, as
described by Steel and Peckham (16).
Briefly, the half maximal inhibitory concentration
(IC50) of celecoxib and IC50 of PD184161 were
presented as ‘a’ (0, IC50 of celecoxib) and ‘b’
(IC50 of PD184161, 0) in a two-coordinate plot, and the
line of additivity was constructed by connecting the two points.
The concentrations of the two drugs used in combination produced
new IC50s, which were denoted as ‘c’ and ‘d’ in the same
plot. Points ‘c’ and ‘d’ that are below, at or above the
isobologram line for a given effect level indicate synergistic,
additive or antagonistic effects, respectively.
Mouse xenograft model
A total of 48 BALB/c female nude mice (weighing
20–25 g) were obtained from Shanghai SLAC Laboratory Animal Co.,
Ltd. (Shanghai, China) and acclimatized for 4 days. The mice were
maintained in a temperature- and humidity-controllled room (21°C
and 50% humidity) under a 12-h light/dark cycle with ad
libitum access to standard food and water. GBC-SD and NOZ cells
(1×107) were each subcutaneously injected into the
flanks of the mice. At 2 weeks after tumor cell inoculation, the
mice were treated with celecoxib (50 mg/kg/day), PD184161 (300
mg/kg/day) or combination of the two drugs by oral administration
for 24 days. Tumor size was measured every week. After 28 days, the
mice were sacrificed by cervical dislocation and the xenografted
tumors were weighed. Tumor volumes were determined according to the
formula L × W2 / 2, where L is the largest diameter of
the tumor and W is the smallest diameter perpendicular to L.
Apoptosis assay
Apoptosis in the cell lines was examined by flow
cytometry using the Annexin V/fluorescein isothiocyanate (FITC)
Apoptosis Detection kit (BD Biosciences, Franklin Lakes, NJ, USA).
Cells were pretreated with 8 µM celecoxib and 40 µM PD184161 (alone
or in combination) and then cultured in 6-well plates. After 48 h,
≥1×105 cells (including floating cells) were collected,
centrifuged at 500 × g for 10 min at 4°C, washed twice with
cold PBS and resuspended in binding buffer. Double staining was
performed with Annexin V/PI in a dark room at room temperature for
15 min, and all samples were analyzed by flow cytometry within 1 h.
FlowJo software version 7.6.1 (Tree Star Inc., Ashland, OR, USA)
was used to analyze the flow cytometry data.
Analysis of mitochondrial membrane
potential (ΔΨm)
ΔΨm is an important parameter of mitochondrial
function that may be used as an indicator of cell health (17). JC-1
(5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolocarbocyanine
iodide) is a lipophilic, cationic dye that can selectively enter
into mitochondria and reversibly change color from red to green as
the membrane potential decreases. In healthy cells with high ΔΨm,
JC-1 spontaneously forms complexes known as J-aggregates with
intense red fluorescence. By contrast, in apoptotic or unhealthy
cells with low ΔΨm, JC-1 remains in its monomeric form, which
produces only green fluorescence. Briefly, cells were pretreated
with 8 µM celecoxib and 40 µM PD184161 (alone or in combination)
for 2, 4, 6 or 8 h, then adjusted to a density of
5×105/ml, trypsinized and washed in PBS. The cells were
resuspended in 1 ml of complete medium, and stained with 5 µg/ml
JC-1 (Beyotime Institute of Biotechnology, Shanghai, China) for 20
min at 37°C in total darkness. The cells were then washed twice and
resuspended in PBS, and analyzed by flow cytometry.
Cell cycle analysis
Following treatment with 8 µM celecoxib and 40 µM
PD184161 (alone or in combination) for 48 h, cells were washed
twice with ice-cold PBS, fixed in 70% cold ethanol, treated with
100 µg/ml ribonuclease A (Roche) and labeled with 50 µg/ml PI for 1
h at 37°C. DNA content was subsequently analyzed by flow
cytometry.
Statistical analysis
Data are presented as the mean ± standard deviation
of at least three independent experiments. Differences among
variables were assessed by the Student's t-test for two
groups and ANOVA for multiple groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
Expression of COX-2 and p-ERK1/2
protein in GBC tissues and cell lines
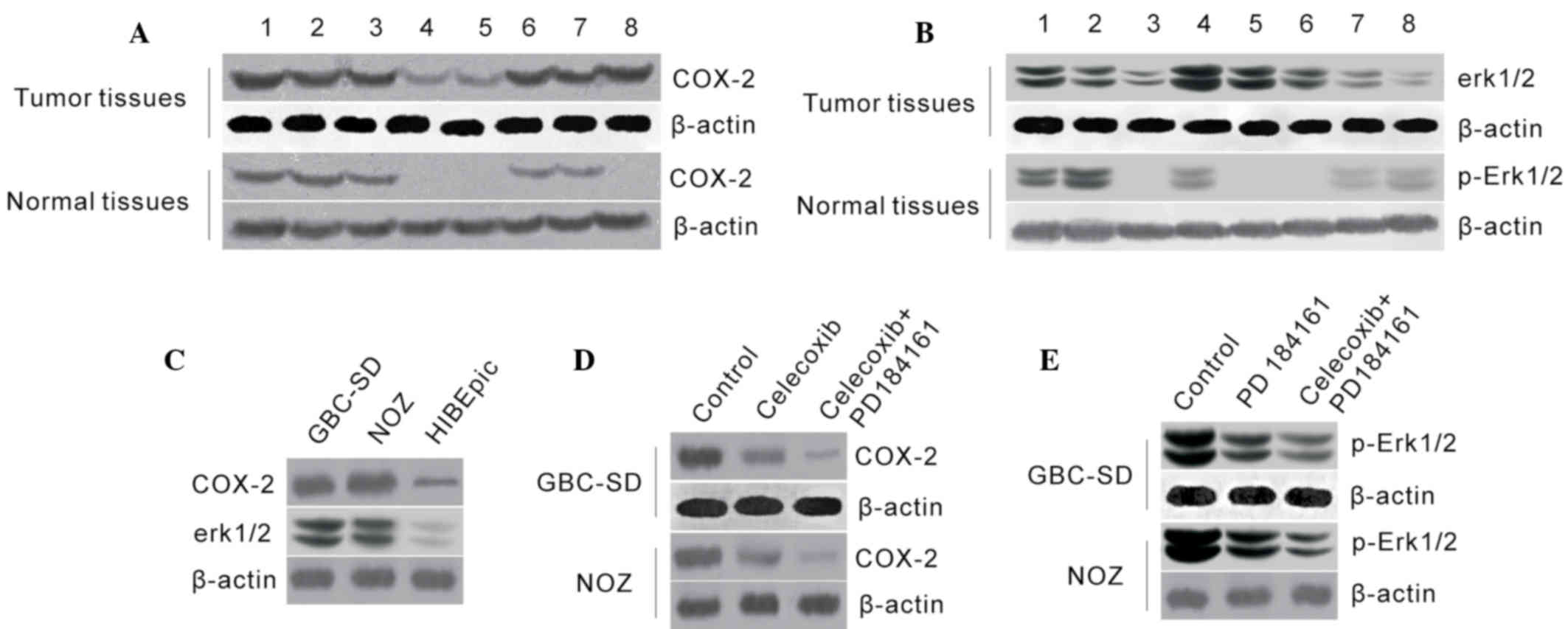
Western blot analysis revealed that COX-2 and
p-ERK1/2 protein were abundant in all GBC cases, while their
expression levels were markedly lower or undetectable in normal
tissues (Fig. 1A and B), indicating
that COX-2 and p-ERK1/2 overexpression were frequent in GBC
tissues. In addition, COX-2 and p-ERK1/2 protein levels were
significantly higher in GBC-SD and NOZ cells than that in HIBEpiC
cells (Fig. 1C and D), indicating
that COX-2 and p-ERK1/2 may participate in the development of
GBC.
COX-2 protein levels were decreased in GBC-SD and
NOZ cells following treatment with celecoxib, and p-ERK1/2 protein
level was also markedly decreased following treatment with
PD184161. Furthermore, compared to single treatments, COX-2 and
p-ERK1/2 protein levels were markedly lower following treatment
with a combination of celecoxib and PD184161 (Fig. 1E).
PD184161 and celecoxib co-inhibit the
growth of GB-SD and NOZ cells
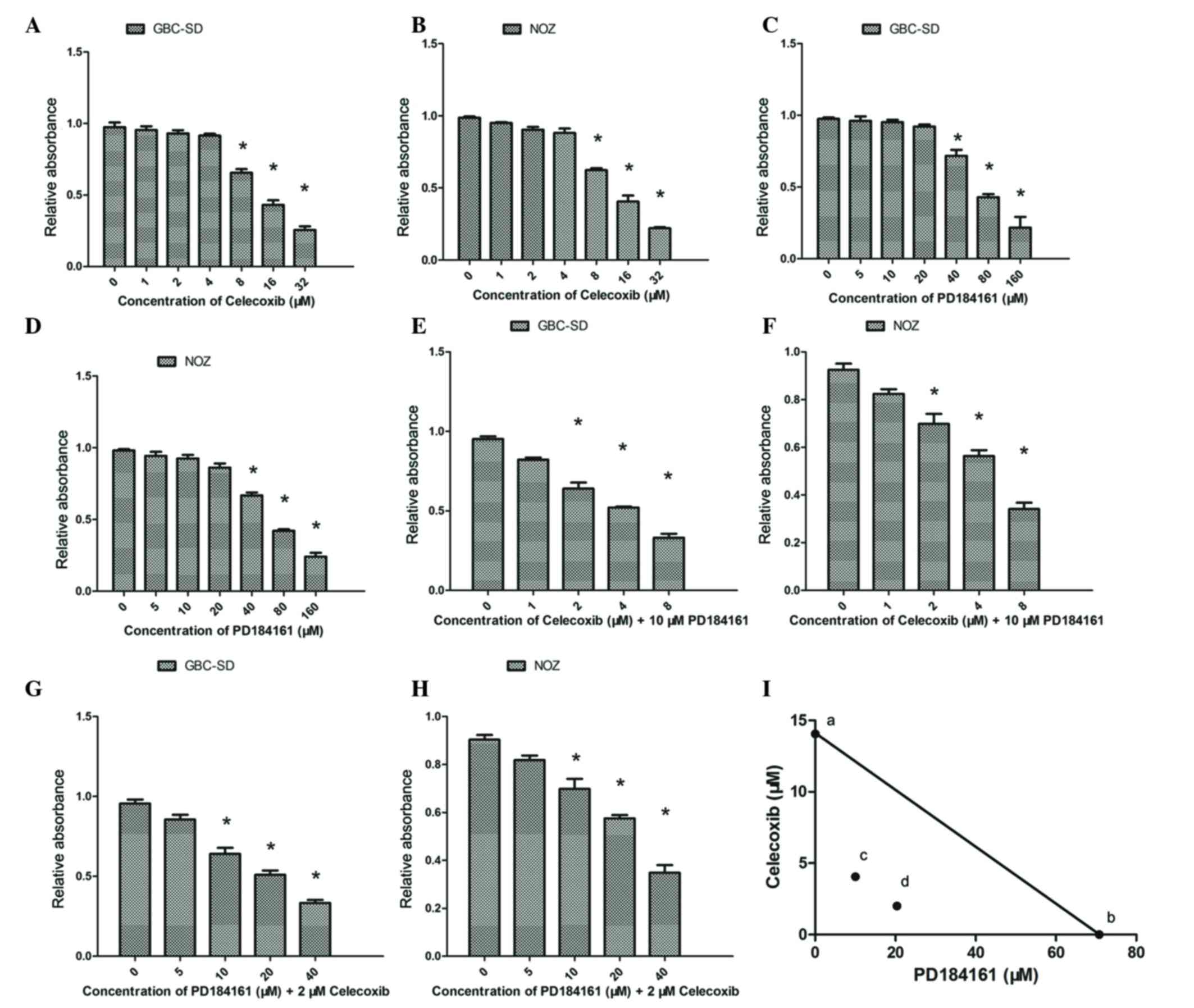
A WST-1 assay revealed that celecoxib inhibited the
growth of GBC-SD and NOZ cells in a concentration-dependent manner;
celecoxib at concentrations of 0–4 µM did not significantly
suppress the proliferation of GBC-SD and NOZ cells, but
proliferation was significantly suppressed by concentrations of
8–32 µM (Fig. 2A and B; P<0.05).
For GBC-SD and NOZ cells, the calculated IC50s were
14.05 and 12.42 µM, respectively. Similarly, PD184161 treatment led
to a concentration-dependent inhibition of growth over the
concentration range of 40–160 µM (Fig. 2C
and D), with IC50s of 70.79 and 66.77 µM for GBC-SD
and NOZ cells, respectively.
The inhibitory effects of combined treatment with
celecoxib and PD184161 were also evaluated. As shown in Fig. 2E and F, combined treatment with 2–4 µM
celecoxib and 10 µM PD184161 (sub-effective doses) resulted in
significant inhibition of cell growth in GBC-SD and NOZ cells as
compared to cells treated with the same dose of PD184161 alone.
Similarly, combined treatment with 10–20 µM PD184161 and 2 µM
celecoxib (sub-effective doses) resulted in significant inhibition
of cell growth in GBC-SD and NOZ cells as compared to cells treated
with the same dose of celecoxib alone (Fig. 2G and H).
Isobologram analysis revealed that the growth
inhibitory effect of combined treatment with celecoxib and PD184161
was synergistic, since the data points ‘c’ and ‘d’ were located
well below the line defining an additive effect (Fig. 2I).
Celecoxib combined with PD184161
inhibits GBC-SD and NOZ cell growth in vivo
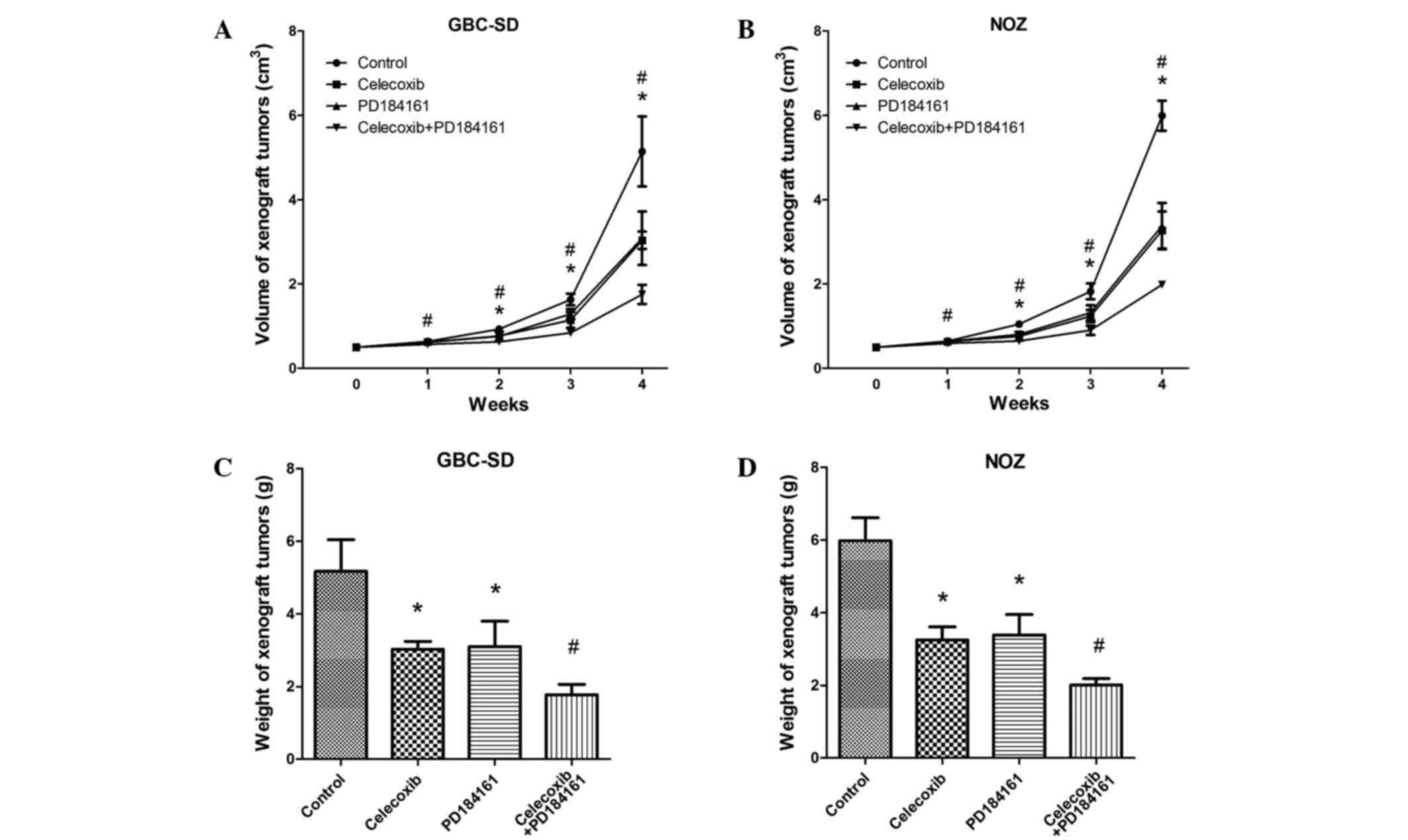
The application of celecoxib and PD184161 (alone or
combined) was found to inhibit the growth of xenograft tumors. As
shown in Fig. 3A and B, in the first
week, the volumes of the xenograft tumors treated with celecoxib
combined with PD184161 were significantly reduced compared with the
other groups (control and celecoxib and PD184161 single
treatments). From the second week, the volume of xenograft tumors
treated with celecoxib or PD184161 alone were also significantly
reduced compared with the control group (P<0.05). Similarly, the
weights of xenograft tumors treated with celecoxib combined with
PD184161 for 24 days were markedly less than that of the other
groups (P<0.05; Fig. 3C and
D).
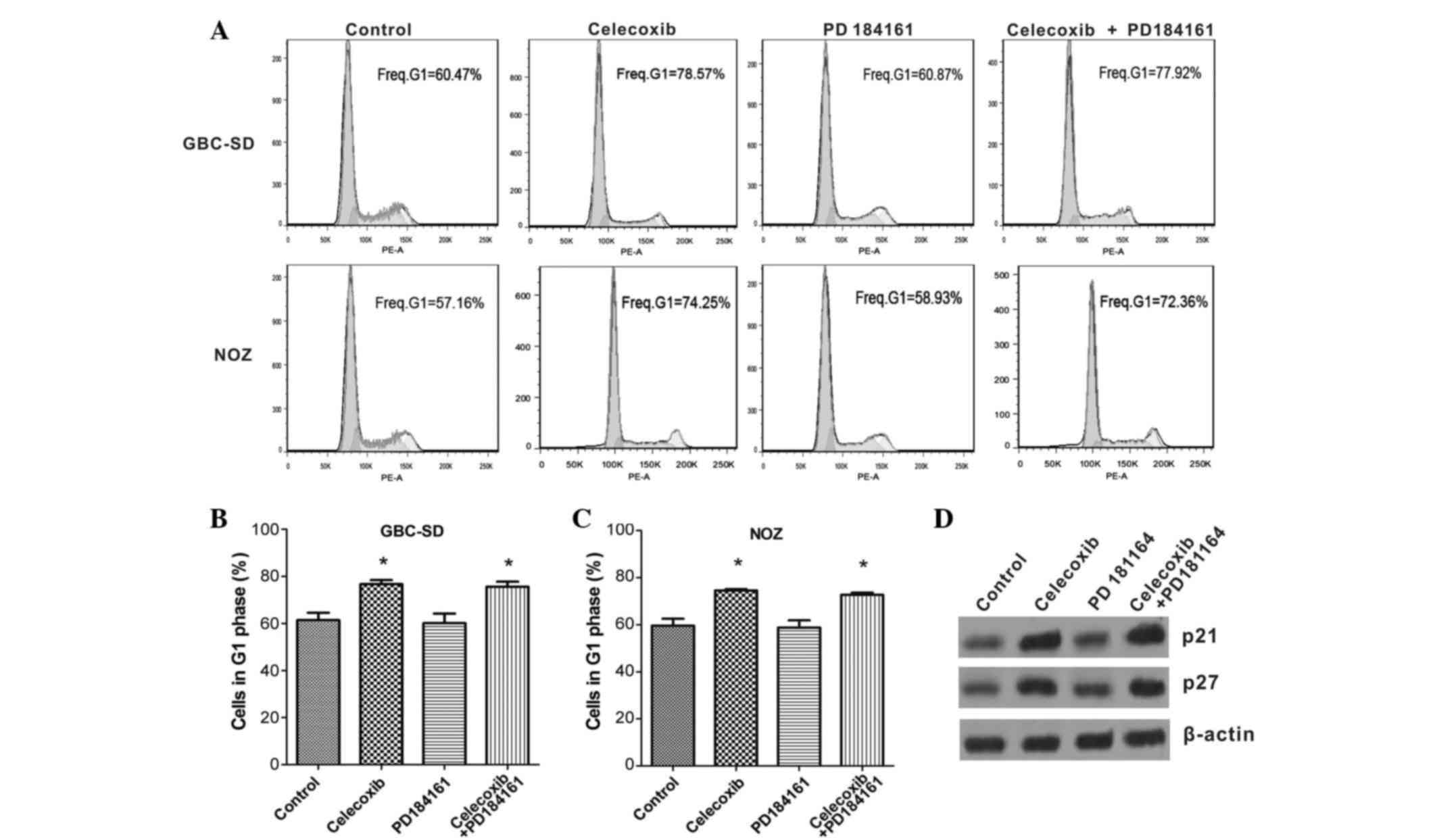
Celecoxib causes G1 arrest by
promoting p21 and p27 expression
Flow cytometric analysis (Fig. 4A-C) revealed that celecoxib alone or
the combination of celecoxib and PD184161 significantly increased
the G1 population of GBC-SD and NOZ cells compared with the
controls (both P<0.01); by contrast, the proportion of cells in
G1 phase did not alter following treatment with PD184161 alone.
Western blotting (Fig. 4D) revealed
that the expression levels of p21 and p27 were markedly elevated in
GBC cells following treatment with celecoxib, whereas p21 and p27
expression did not change following treatment with PD184161 alone.
These results indicate that the cell cycle was blocked by celecoxib
but not by PD184161.
Combination of celecoxib and PD184161
induces cell apoptosis
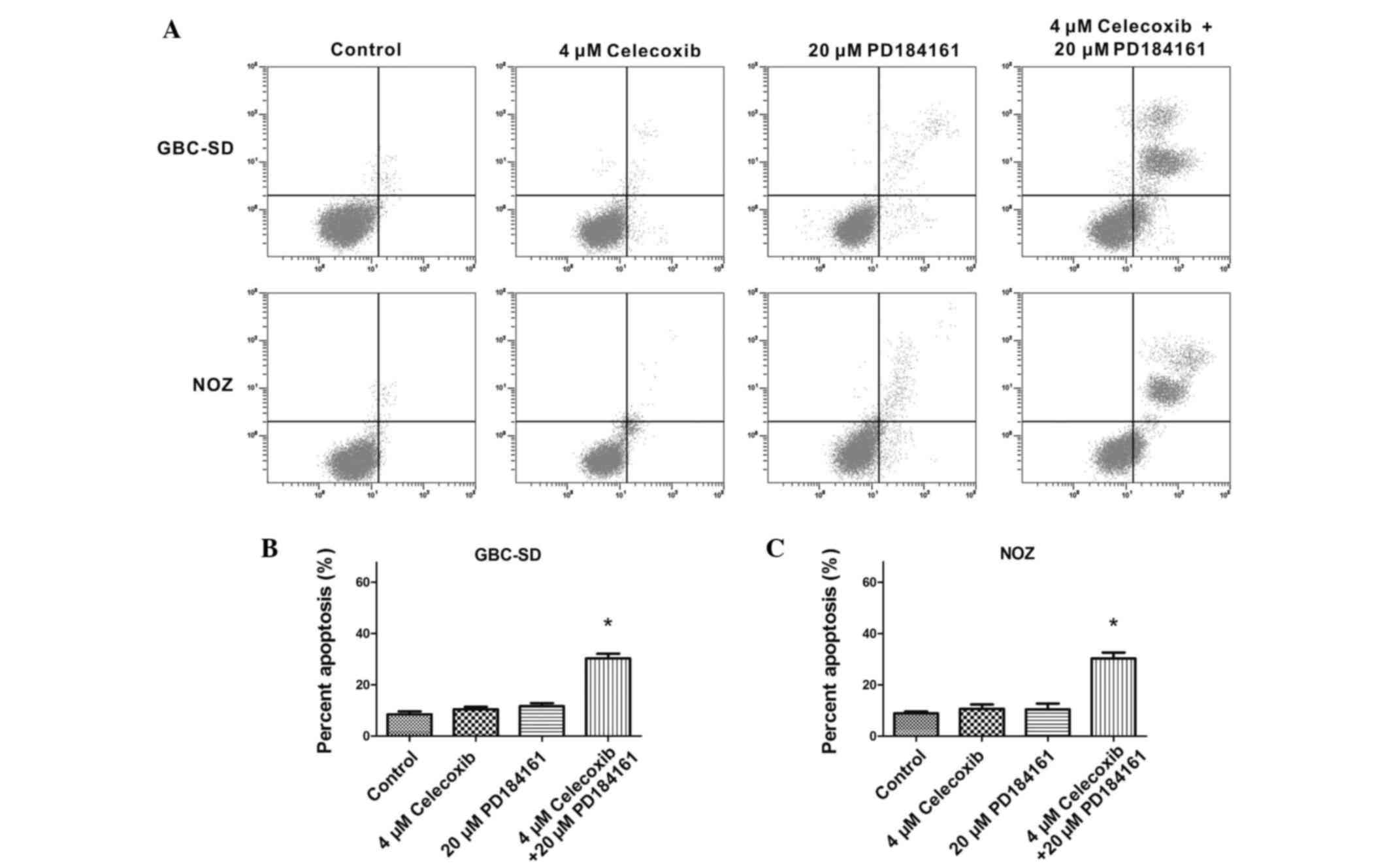
Annexin V-FITC/PI staining revealed that 4 µM
celecoxib did not promote apparent apoptosis in GBC-SD or NOZ
cells, nor did 20 µM PD184161. However, the combination of 4 µM
celecoxib and 20 µM PD184161 significantly induced apoptosis in the
two cell lines (Fig. 5).
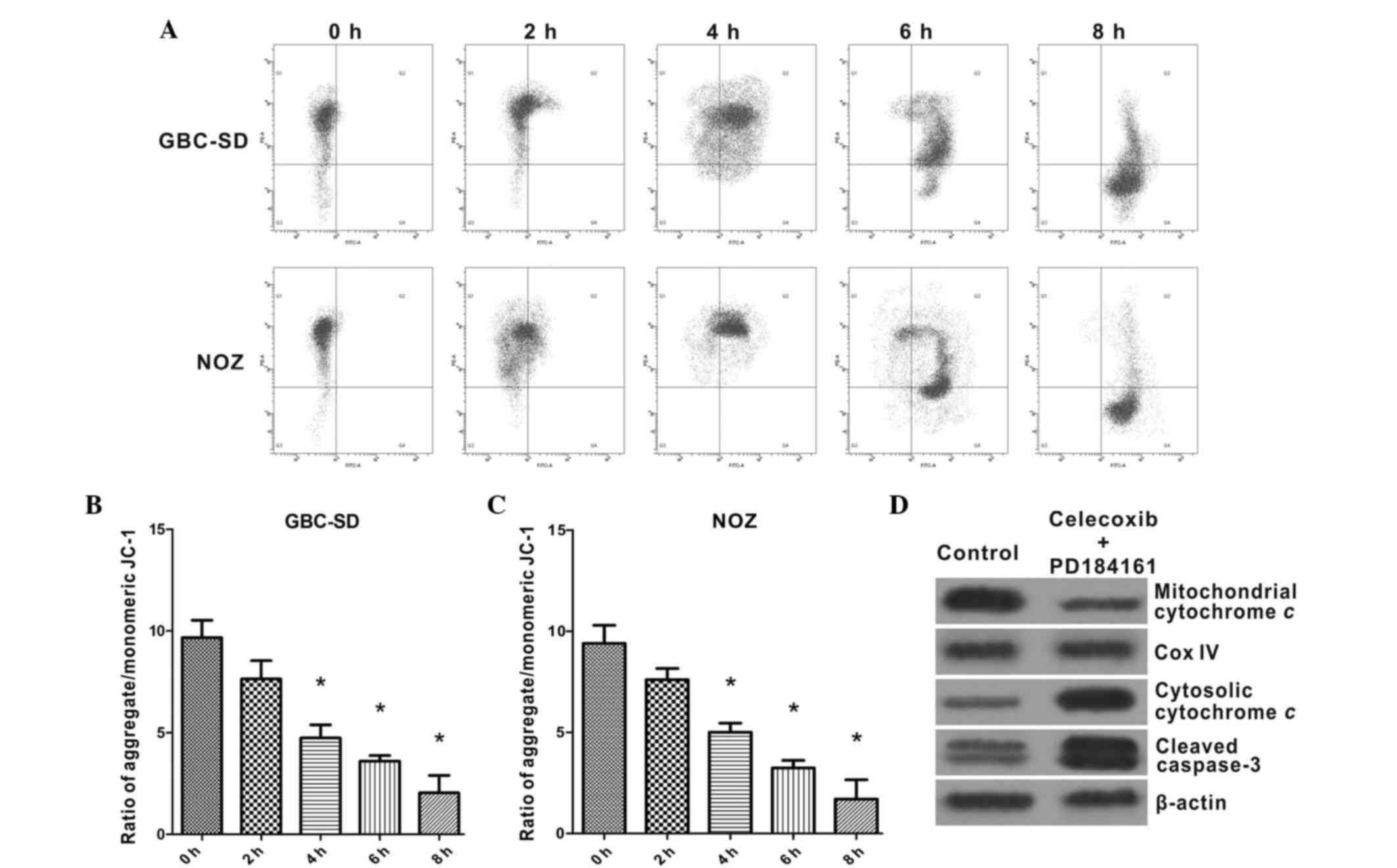
Celecoxib and PD184161 treatment
induces the collapse of ΔΨm and activates caspase-3 protein
JC-1 staining (Fig.
6A-C) revealed that GBC-SD and NOZ cells maintained high ΔΨm in
the absence of celecoxib or PD184161, whereas the majority of
GBC-SD and NOZ cells exhibited a gradual decrease in ΔΨm following
2–8 h incubation with 4 µM celecoxib and 20 µM PD184161. Western
blot analysis revealed that the amount of mitochondrial cytochrome
c was markedly decreased following treatment with celecoxib
and PD184161, whereas the amount of cytosolic cytochrome c
was increased in GBC-SD and NOZ cells, indicating that celecoxib
and PD184161 can promote the release of cytochrome c from
the mitochondria to the cytoplasm (Fig.
6D). Furthermore, levels of cleaved caspase-3 were markedly
increased in GBC-SD cells following treatment with celecoxib and
PD184161, indicating that caspase-3 was extensively activated.
Discussion
To enhance the efficacy of treatment for GBC is an
important issue that is yet to be resolved in clinical practice.
Since tumors were established to be a gene-related disease, much
attention has been paid to targeted drugs that are designed to
directly or indirectly interfere with the vital molecules involved
in tumor growth and development. An increasing number of targeted
drugs have been developed and applied in clinical practice, and
these are occasionally used as a first-line treatment for certain
tumors (18). It has been
demonstrated that COX-2 and pERK1/2 proteins, which are involved in
tumor carcinogenesis and development, are aberrantly increased in
various malignant tumors of the digestive tract, including hepatic
carcinoma (19), gastric carcinoma
(20), colon cancer (21) and pancreatic cancer (22). Therefore, they are considered to be
important therapeutic targets for these tumor types, and the
efficacy of the COX-2 inhibitor celecoxib and the ERK1/2 inhibitor
PD184161 have been confirmed in recent studies (23,24).
However, the effects of combining celecoxib with PD184161 on GBC
cell growth have not yet been reported. In the present study, COX-2
and p-ERK1/2 expression levels were examined in GBC cell lines for
the first time, and their potential as therapeutic targets for GBC
treatment was also evaluated. In addition, the effects of celecoxib
and PD184161 on the growth, cell cycle and apoptosis of GBC cells
were investigated.
COX-2 and p-ERK1/2 expression were found to be
markedly lower in normal gallbladder tissues than in GBC tissues,
and their expression levels were also lower in HIBEpiC cells than
in GBC-SD and NOZ cells. These results were consistent with
previous studies (9–11), indicating that aberrant COX-2 and
ERK1/2 activation may serve key roles in GBC tumorigenesis, and
verifying their potential as targets for GBC targeted therapy. As a
member of the MAPK family of signal transduction molecules, ERK1/2
is involved in the process of cell growth, proliferation and
differentiation. The ERK1/2 signaling pathway is often
over-activated in various tumors, and p-ERK1/2 expression is
abnormally high. COX-2 is an important rate-limiting enzyme in the
synthesis of prostaglandin E2 (PGE2). COX-2/PGE2 can activate a
variety of signaling pathways involved in tumor carcinogenesis and
development, including ERK1/2 signaling pathways. Under
physiological conditions, COX-2 protein expression is weak or
absent in the majority of normal tissues (25); by contrast, under pathological
situations, such as in tumors or inflammation, its expression may
be induced by a variety of inflammatory cytokines, which are
produced by tumors or their microenvironment. It has been
documented that interleukin (IL)-1 (26), IL-8 (27), nuclear factor κB (28) and hypoxia inducible factor 1α
(29) can stimulate the expression of
COX-2. Most of these factors are abundant in GBC tissues, which may
be the reason for the aberrant activation of COX-2 and p-ERK1/2 in
GBC tissues and cell lines.
Subsequently, the effects of celecoxib and PD184161
on the growth, cell cycle and apoptosis of GBC-SD and NOZ cells,
which exhibit high COX-2 and p-ERK1/2 expression, were examined. A
WST-1 assay revealed that celecoxib and PD184161 exert
concentration-dependent inhibitory effects on the growth of GBC-SD
and NOZ cells, and that their combination exerts synergistic
inhibitory effects on cell growth. Furthermore, p-ERK1/2 and COX-2
protein expression were significantly downregulated following
treatment with PD184161 and celecoxib alone, while co-treatment
caused a more marked decrease in p-ERK1/2 and COX-2 protein
expression compared with single treatment. PD184161 exerts its
antitumor activities predominantly by inhibiting ERK1/2 activation,
resulting in inhibition of proliferation, migration and invasion,
as well as induction of apoptosis (30). By contrast, celecoxib exerts its
antitumor activities predominantly by suppressing COX-2 protein
expression and PGE2 synthesis (31).
Recent studies demonstrated that celecoxib can block ERK1/2
phosphorylation, thereby blocking the cell proliferation signaling
pathway mediated by ERK1/2 (32,33); this
indicated that, to a certain extent, celecoxib may enhance the
PD184161-induced inhibition of ERK1/2 activity, and that this may
be a reasonable mechanism by which celecoxib combined with PD184161
could cause synergistic growth inhibition. Additionally, the
inhibitory effects of celecoxib combined with PD184161 in
vivo were confirmed by xenograft tumor experiments in the
present study, indicating their potential for clinical application
in the future.
Flow cytometric analysis revealed that celecoxib
induces G1 arrest, while PD184161 does not affect the cell cycle
distribution. Furthermore, celecoxib induced the expression of the
cyclin-dependent kinase inhibitors p21 and p27, while PD184161 did
not alter their expression levels. In accordance with another study
(34), the present results suggested
that accumulation of p21 and p27 caused by celecoxib leads to G1
arrest.
The induction of apoptosis by celecoxib and PD184161
was also examined in the present study. FITC-Annexin V/PI staining
revealed that the combination of celecoxib and PD184161 induced a
significant increase in cell apoptosis. Additionally, a JC-1
staining assay revealed that celecoxib and PD184161 induced a
collapse of the ΔΨm, and western blotting demonstrated that
celecoxib and PD184161 promote cytochrome c release from the
mitochondria to the cytoplasm as well as caspase-3 activation.
In conclusion, COX-2 and p-ERK1/2 protein, which are
aberrantly expressed in GBC tissues and cells, may serve as
effective therapeutic targets for GBC treatment. The combination of
celecoxib and PD184161 treatments leads to synergistic inhibition
of GBC cell growth through triggering cell cycle arrest and
apoptosis. It is hoped that this work will extend the investigation
of celecoxib and PD184161 to a broader extent by offering new
insight into the clinical application of the multi-targeted
treatment of GBC. In future studies, clinical trials based on
celecoxib and PD184161 alone or in combination for the treatment of
GBC patients should be performed to further validate the
effectiveness and safety of these regimens.
Acknowledgements
This study was supported by the Key Program of
Foundation for Young Talents in Colleges and Universities of Anhui
Province (Hefei, China; grant no. 2013SQRL053ZD).
References
|
1
|
Stinton LM and Shaffer EA: Epidemiology of
gallbladder disease: Cholelithiasis and cancer. Gut Liver.
6:172–187. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Eslick GD: Epidemiology of gallbladder
cancer. Gastroenterol Clin North Am. 39:307–330, ix. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hundal R and Shaffer EA: Gallbladder
cancer: Epidemiology and outcome. Clin Epidemiol. 6:99–109.
2014.PubMed/NCBI
|
|
4
|
Levy AD, Murakata LA and Rohrmann CA Jr:
Gallbladder carcinoma: Radiologic-pathologic correlation.
Radiographics. 21:295–314. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bonet B, eltrán M, Allal AS, Gich I, Solé
JM and Carrió I: Is adjuvant radiotherapy needed after curative
resection of extrahepatic biliary tract cancers? A systematic
review with a meta-analysis of observational studies. Cancer Treat
Rev. 38:111–119. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhu AX and Hezel AF: Development of
molecularly targeted therapies in biliary tract cancers:
Reassessing the challenges and opportunities. Hepatology.
53:695–704. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bottegoni G, Favia AD, Recanatini M and
Cavalli A: The role of fragment-based and computational methods in
polypharmacology. Drug Discov Today. 17:23–34. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Greenhough A, Smartt HJ, Moore AE, Roberts
HR, Williams AC, Paraskeva C and Kaidi A: The COX-2/PGE2 pathway:
Key roles in the hallmarks of cancer and adaptation to the tumour
microenvironment. Carcinogenesis. 30:377–386. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Doval DC, Azam S, Sinha R, Batra U and
Mehta A: Expression of epidermal growth factor receptor, p53, Bcl2,
vascular endothelial growth factor, cyclooxygenase-2, cyclin D1,
human epidermal receptor-2 and Ki-67: Association with
clinicopathological profiles and outcomes in gallbladder carcinoma.
J Carcinog. 13:102014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li Q and Yang Z: Expression of
phospho-ERK1/2 and PI3-K in benign and malignant gallbladder
lesions and its clinical and pathological correlations. J Exp Clin
Cancer Res. 28:652009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jinawath A, Akiyama Y, Yuasa Y and
Pairojkul C: Expression of phosphorylated ERK1/2 and homeodomain
protein CDX2 in cholangiocarcinoma. J Cancer Res Clin Oncol.
132:805–810. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Vicent S, López-Picazo JM, Toledo G,
Lozano MD, Torre W, Garcia-Corchón C, Quero C, Soria JC,
Martín-Algarra S, Manzano RG and Montuenga LM: ERK1/2 is activated
in non-small-cell lung cancer and associated with advanced tumours.
Br J Cancer. 90:1047–1052. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yeh JJ, Routh ED, Rubinas T, Peacock J,
Martin TD, Shen XJ, Sandler RS, Kim HJ, Keku TO and Der CJ:
KRAS/BRAF mutation status and ERK1/2 activation as biomarkers for
MEK1/2 inhibitor therapy in colorectal cancer. Mol Cancer Ther.
8:834–843. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wong DJL, Robert L, Atefi MS, Lassen A,
Avarappatt G, Cerniglia M, Avramis E, Tsoi J, Foulad D, Graeber TG,
et al: Antitumor activity of the ERK inhibitor SCH772984
[corrected] against BRAF mutant, NRAS mutant and wild-type
melanoma. Mol Cancer. 13:1942014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Reckamp KL, Koczywas M, Cristea MC, Dowell
JE, Wang HJ, Gardner BK, Milne GL, Figlin RA, Fishbein MC, Elashoff
RM and Dubinett SM: Randomized phase 2 trial of erlotinib in
combination with high-dose celecoxib or placebo in patients with
advanced non-small cell lung cancer. Cancer. 121:3298–3306. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Steel GG and Peckham MJ: Exploitable
mechanisms in combined radiotherapy-chemotherapy: The concept of
additivity. Int J Radiat Oncol Biol Phys. 5:85–91. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Smaili SS, Hsu YT, Carvalho AC, Rosenstoc
TR, Sharpe JC and Youle RJ: Mitochondria, calcium and pro-apoptotic
proteins as mediators in cell death signaling. Braz J Med Biol Res.
36:183–190. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wijesinghe P and Bollig-Fischer A: Lung
Cancer Genomics in the Era of Accelerated Targeted Drug
Development. Adv Exp Med Biol. 890:1–23. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bae SH, Jung ES, Park YM, Kim BS, Kim BK,
Kim DG and Ryu WS: Expression of cyclooxygenase-2 (COX-2) in
hepatocellular carcinoma and growth inhibition of hepatoma cell
lines by a COX-2 inhibitor, NS-398. Clin Cancer Res. 7:1410–1418.
2001.PubMed/NCBI
|
|
20
|
He XP, Shao Y, Li XL, Xu W, Chen GS, Sun
HH, Xu HC, Xu X, Tang D, Zheng XF, et al: Downregulation of miR-101
in gastric cancer correlates with cyclooxygenase-2 overexpression
and tumor growth. FEBS J. 279:4201–4212. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wendum D, Masliah J, Trugnan G and Fléjou
JF: Cyclooxygenase-2 and its role in colorectal cancer development.
Virchows Arch. 445:327–333. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pomianowska E, Schjølberg AR, Clausen OP
and Gladhaug IP: COX-2 overexpression in resected pancreatic head
adenocarcinomas correlates with favourable prognosis. BMC Cancer.
14:4582014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lev-Ari S, Strier L, Kazanov D,
Madar-Shapiro L, Dvory-Sobol H, Pinchuk I, Marian B, Lichtenberg D
and Arber N: Celecoxib and curcumin synergistically inhibit the
growth of colorectal cancer cells. Clin Cancer Res. 11:6738–6744.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Klein PJ, Schmidt CM, Wiesenauer CA, Choi
JN, Gage EA, Yip-Schneider MT, Wiebke EA, Wang Y, Omer C and
Sebolt-Leopold JS: The effects of a novel MEK inhibitor PD184161 on
MEK-ERK signaling and growth in human liver cancer. Neoplasia.
8:1–8. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen WS, Wei SJ, Liu JM, Hsiao M, Kou-Lin
J and Yang WK: Tumor invasiveness and liver metastasis of colon
cancer cells correlated with cyclooxygenase-2 (COX-2) expression
and inhibited by a COX-2-selective inhibitor, etodolac. Int J
Cancer. 91:894–899. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Huang ZF, Massey JB and Via DP:
Differential regulation of cyclooxygenase-2 (COX-2) mRNA stability
by interleukin-1 beta (IL-1 beta) and tumor necrosis factor-alpha
(TNF-alpha) in human in vitro differentiated macrophages. Biochem
Pharmacol. 59:187–194. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Manna SK and Ramesh GT: Interleukin-8
induces nuclear transcription factor-kappaB through a
TRAF6-dependent pathway. J Biol Chem. 280:7010–7021. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Choi YH, Jin GY, Li GZ and Yan GH:
Cornuside suppresses lipopolysaccharide-induced inflammatory
mediators by inhibiting nuclear factor-kappaB activation in RAW
264.7 macrophages. Biol Pharm Bull. 34:959–966. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
He J, Wang M, Jiang Y, Chen Q, Xu S, Xu Q,
Jiang BH and Liu LZ: Chronic arsenic exposure and angiogenesis in
human bronchial epithelial cells via the
ROS/miR-199a-5p/HIF-1α/COX-2 pathway. Environ Health Perspect.
122:255–261. 2014.PubMed/NCBI
|
|
30
|
Kohno M and Pouyssegur J: Targeting the
ERK signaling pathway in cancer therapy. Ann Med. 38:200–211. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ferrandina G, Ranelletti FO, Legge F,
Lauriola L, Salutari V, Gessi M, Testa AC, Werner U, Navarra P,
Tringali G, et al: Celecoxib modulates the expression of
cyclooxygenase-2, ki67, apoptosis-related marker, and microvessel
density in human cervical cancer: A pilot study. Clin Cancer Res.
9:4324–4331. 2003.PubMed/NCBI
|
|
32
|
Li F, Liu S, Ouyang Y, Fan C, Wang T,
Zhang C, Zeng B, Chai Y and Wang X: Effect of celecoxib on
proliferation, collagen expression, ERK1/2 and SMAD2/3
phosphorylation in NIH/3T3 fibroblasts. Eur J Pharmacol. 678:1–5.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li F, Fan C, Zeng B, Zhang C, Chai Y, Liu
S and Ouyang Y: Celecoxib suppresses fibroblast proliferation and
collagen expression by inhibiting ERK1/2 and SMAD2/3
phosphorylation. Mol Med Rep. 5:827–831. 2012.PubMed/NCBI
|
|
34
|
Liu H, Huang P, Xu X, Liu J and Guo C:
Anticancer effect of celecoxib via COX-2 dependent and independent
mechanisms in human gastric cancers cells. Dig Dis Sci.
54:1418–1424. 2009. View Article : Google Scholar : PubMed/NCBI
|