Introduction
Maslinic acid is a natural triterpene from Olea
europaea L. (1), which acts as an
antitumor, antibacterial (2) and
anti-HIV substance (3), and exhibits
antiallodynic and analgesic properties by regulating cell
metabolism and immune function (4).
Maslinic acid has been revealed to exert therapeutic effects on a
variety of solid tumors, including in bladder, prostate, colon,
esophageal, colorectal, cervical and ovarian cancer (5–8). However,
the molecular mechanisms underlying the antitumor functions of
maslinic acid remain elusive.
Interleukin-6 (IL-6) is a pleiotropic cytokine,
which serves an important role in cell proliferation,
differentiation, apoptosis and metastasis by participating in tumor
pathogenesis (9–11). IL-6 may activate the Janus kinase
(JAK)/signal transducer and activator of transcription 3 (STAT3)
signaling pathway and the Ras/mitogen-activated protein kinase
(MAPK) signaling pathway (12,13). It
has previously been demonstrated that STAT3 is constitutively
phosphorylated in MKN28 cells, and it has been revealed that the
inhibition of IL-6 by IL-6 receptor (R) antagonists, JAK inhibitors
or the expression of a dominant-negative STAT3 mutant, can induce
apoptosis in MKN28 cell lines in vitro (14–16).
Therefore, it has been suggested that the IL-6/JAK/STAT3 signaling
pathway provides an important antiapoptotic signal in tumor cells,
and may be a promising target for the development of novel
therapeutic strategies for gastric cancer. To the best of our
knowledge, no previous studies have provided data investigating the
effect and underlying mechanisms of maslinic acid in gastric
cancer.
The present study demonstrated that maslinic acid
inhibits the proliferation and induces apoptosis of MKN28 cells.
These findings were associated with the downregulation of
phosphorylated (p)-STAT3 protein and its upstream kinase, JAK.
Inhibition of IL-6 production in MKN28 cells may account for the
inhibition of STAT3 mediated by maslinic acid. Overall, the results
of the present study provided evidence for the potential clinical
application of maslinic acid as a novel therapeutic agent against
gastric cancer.
Materials and methods
Cell culture and reagents
The MNK28 human gastric cancer cell line was
obtained from the American Type Culture Collection (Manassas, VA,
USA.) and maintained in RPMI-1640 supplemented with 10% (v/v) heat
inactivated fetal bovine serum (Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA), 100 U/ml penicillin and 100 µg/ml
streptomycin (Gibco; Thermo Fisher Scientific, Inc.) at 37°C in a
humidified incubator with 5% CO2.
Maslinic acid was obtained from Sigma-Aldrich (Merck
Millipore, Darmstadt, Germany) and stock solution was prepared in
dimethyl sulfoxide at 1 mM. Cell Counting Kit-8 (CCK-8) was
purchased from Dojindo Molecular Technologies, Inc. (Kumamoto,
Japan). The bicinchoninic acid assay (BCA) kit (71285–3) was
purchased from Beyotime Institute of Biotechnology (Haimen, China).
Antibodies against STAT3 (cat. no. ab119352), p-STAT3 (Tyr705; cat.
no. ab76315), JAK2 (cat. no. ab108596) and p-JAK2 (cat. no.
ab32101) were purchased from Abcam (Cambridge, UK). B-cell lymphoma
2 (Bcl-2; cat. no. 2870), Bcl-2 associated agonist of cell death
(Bad; cat. no. 9292), Bcl-2 associated X protein (Bax; cat. no.
2772) and β-actin (cat. no. 3700) antibodies were purchased from
Cell Signaling Technology, Inc. (Danvers, MA, USA). Enhanced
chemiluminescence (ECL) reagent was purchased from EMD Millipore
(Billerica, MA, USA). A Human IL-6 Quantikine ELISA kit (D6050),
recombinant human IL-6 protein (cat. no. 206-IL-050/CF) and human
IL-6 antibody (cat. no. MAB206-100) were obtained from R&D
Systems, Inc. (Minneapolis, MN, USA).
Cytotoxicity assay
MKN28 cells were seeded into 24-well plates at a
density of 1×104 cells/well and treated with various
concentrations of maslinic acid (0, 0.1, 1 or 10 µM) at 37°C for 24
h; subsequently CCK-8 reagent was added for a further 2-h
incubation at 37°C. Optical density was evaluated at 450 nm using a
microplate reader (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Cell number was determined using the trypan blue dye exclusion
method (17).
Apoptosis analysis by Annexin
V-propidium iodide (PI) double staining
The apoptotic rate of MNK28 cells was evaluated by
flow cytometry using the Annexin V-fluorescein isothiocyanate
(FITC)/PI double staining method. MNK28 cells were seeded in 6-well
plates and treated with maslinic acid, as described above. Cells
were trypsinized with 0.25% EDTA-free trypsin, then washed with PBS
and centrifuged at 300 × g for 3 min, prior to incubation with 1
µg/ml Annexin V-FITC and 10 µg/ml PI for 15 min at room temperature
in the dark. Samples were analyzed using a flow cytometer (BD
Biosciences, Franklin Lakes, NJ, USA) and presented as two
parameter dot-plots.
Clone formation assay
For each treatment group, ~1×102 cells
were seeded into each well of a 6-well plate. Following incubation
with 0, 0.1, 1 and 10 µM of maslinic acid at 37°C for 12 days, the
cells were washed with PBS and images of each clone were captured
under a light microscope.
Western blotting
Following treatment with maslinic acid for 24 h,
MKN28 cells were collected and lysed in radioimmunoprecipitation
assay lysis buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1% Triton
X-100, 0.1% SDS, 1 mM EDTA, 1 mM Na3VO4, 1 mM
NaF and a protease inhibitor cocktail). The extracts were incubated
on ice for 30 min and supernatants were collected by centrifugation
at 13,400 × g for 10 min at 4°C. Subsequently, protein
concentrations were determined by BCA assay, 30 µg protein were
separated by electrophoresis on 10% SDS-PAGE gel and
electro-transferred onto a polyvinylidine fluoride membrane with
transfer buffer (25 mM Tris, 250 mM glycine and 20% methanol) at
100 V for 2 h. The membrane was blocked in 5% nonfat skimmed milk
and probed with the corresponding primary antibodies at 4°C
overnight, followed by incubation with horseradish
peroxidase-conjugated goat-anti mouse (1:5,000; cat. no., sc-2005)
and mouse-anti rabbit (1:5,000; cat. no. sc-2357) secondary
antibodies (Santa Cruz Biotechnology, Inc., Dallas, TX, USA).
Primary antibodies included anti-β-actin (1:5,000), rabbit
anti-human anti-STAT3 (1:1,000), rabbit anti-human anti-p-STAT3
(1:1,000), rabbit anti-human anti-Bad (1:1,000), rabbit anti-human
anti-Bcl-2 (1:1,000), rabbit anti-human anti-Bax (1:1,000), rabbit
anti-human anti-JAK2 (1:1,000) and rabbit anti-human anti-p-JAK2
(1:1,000). Protein expression was detected using an ECL system (GE
Healthcare Biosciences, Pittsburgh, PA, USA). For western blotting,
band density was determined with Quantity One 1-D software (Bio-Rad
Laboratories, Inc.). Western blotting was performed in
triplicate.
IL-6 analysis by ELISA
The concentration of IL-6 in the cell culture
supernatants was evaluated using the ELISA method. Briefly, MKN28
cells were plated in 24-well plates. Following treatment with
maslinic acid for 24 h, the supernatant was harvested at 13,400 × g
for 10 min at 4°C for analysis. Supernatants were analyzed using an
IL-6 ELISA protein assay kit, according to the manufacturer's
instructions. Color development was determined using a microplate
reader (MK3; Thermo Fisher Scientific, Inc.) set to 450 nm. A
standard curve was plotted using data generated by evaluation of
the absorbance of recombinant-IL-6 serial dilutions.
IL-6 and anti-IL-6 antibody
treatment
Following serum starvation for 4 h, 10 µg/ml
maslinic acid, 10 ng/ml recombinant IL-6 protein or 10 µg/ml
anti-IL-6 antibody were added to the medium for 24 h of treatment
at 37°C. Cell viability was determined using CCK-8 and the
phosphorylation status of STAT3 was evaluated by western blot
analysis as previously described.
Statistical analysis
Data are expressed as the mean ± standard deviation
of the mean of separate experiments (n≥3). Student's t-test was
performed for comparison of the means between the two groups, and
one-way analysis of variance was used for analyzing the means of
multiple groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
Maslinic acid inhibits cell viability
and proliferation and induces apoptosis in MKN28 cells
In order to investigate the inhibitory effects of
maslinic acid on MKN28 cell viability and proliferation, the
present study evaluated the cell number using trypan blue dye
exclusion, and determined cell viability using the CCK-8. As
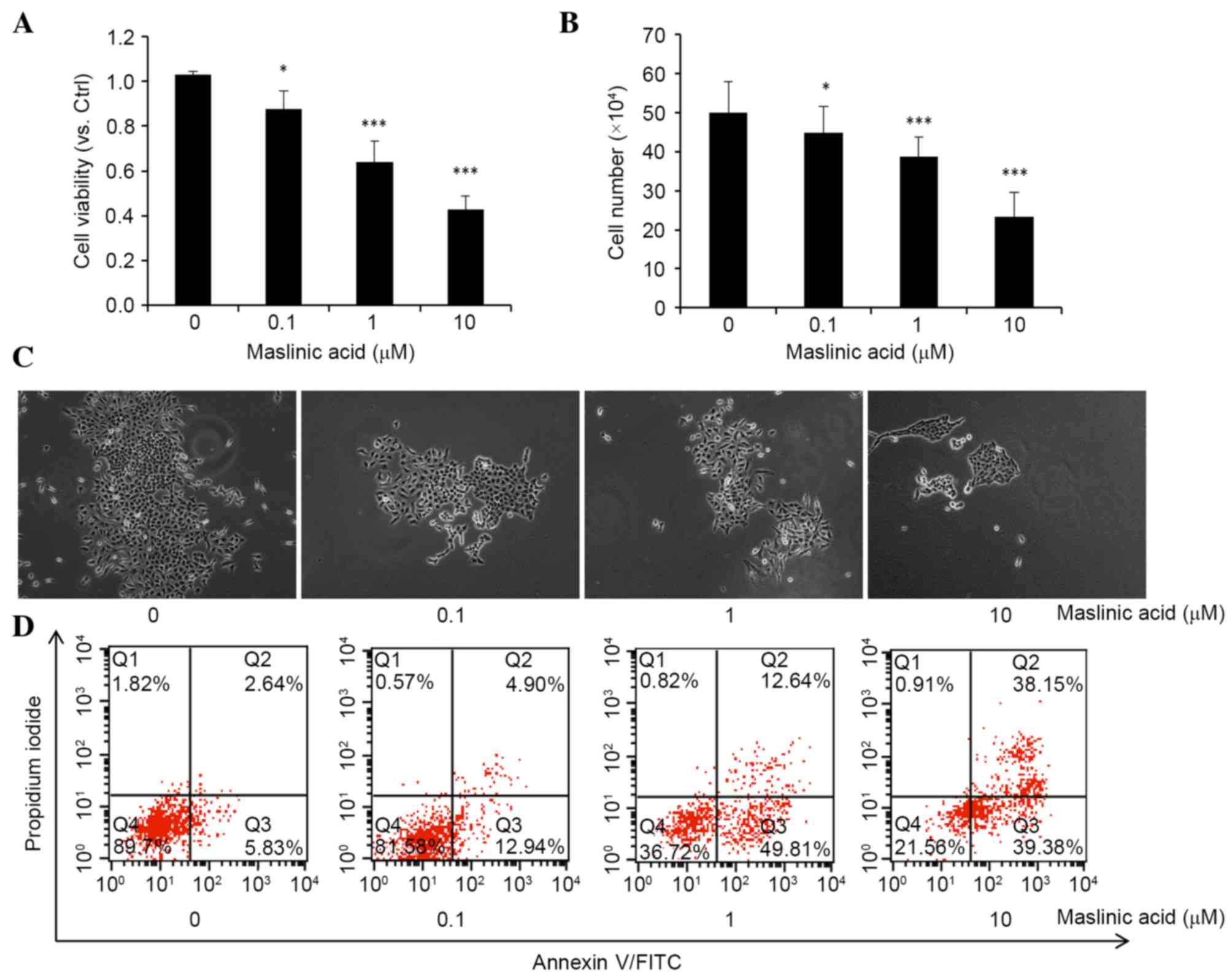
presented in Fig. 1A and B, maslinic
acid significantly reduced cell viability and proliferation in a
dose-dependent manner with a half-maximal inhibitory concentration
of 8.45 µM, P=0.032, 0.00092 and 0.000036, respectively. Colony
formation assays also demonstrated a significant reduction in the
size and number of colonies that received treatment with maslinic
acid, P=0.047, 0.00083 and 0.00006, respectively (Fig. 1C). The results revealed that maslinic
acid has an inhibitory effect on the viability and proliferation of
MKN28 cells.
Subsequently, the present study examined the
apoptosis rate of MKN28 cells following treatment with maslinic
acid for 24 h (Fig. 1D). A
significantly dose-dependent increase in the percentage of early
apoptotic (Annexin V+/PI−) and late
apoptotic/necrotic (Annexin V+/PI+) cells was
observed following culturing of MKN28 cells with maslinic acid for
24 h, P=0.048, 0.0082 and 0.00074, respectively. Totals of 5.83%
for early apoptotic and 2.64% for late apoptotic/necrotic in the
control group, 12.94% for early apoptotic and 4.90% for late
apoptotic/necrotic in the 0.1 µM maslinic acid treatment group,
49.81% for early apoptotic and 12.64% for late apoptotic/necrotic
in the 1 µM maslinic acid treatment group and 39.38% for early
apoptotic and 38.15% for late apoptotic/necrotic in the 10 µM
maslinic acid treatment group, were observed. These results
indicate that maslinic acid inhibits MKN28 cell viability by
inducing apoptosis.
Maslinic acid inhibits the JAK/STAT3
pathway
JAK/STAT signaling is involved in oncogenesis and
cancer progression by upregulation of anti-apoptotic genes
(18). To explore the mechanisms
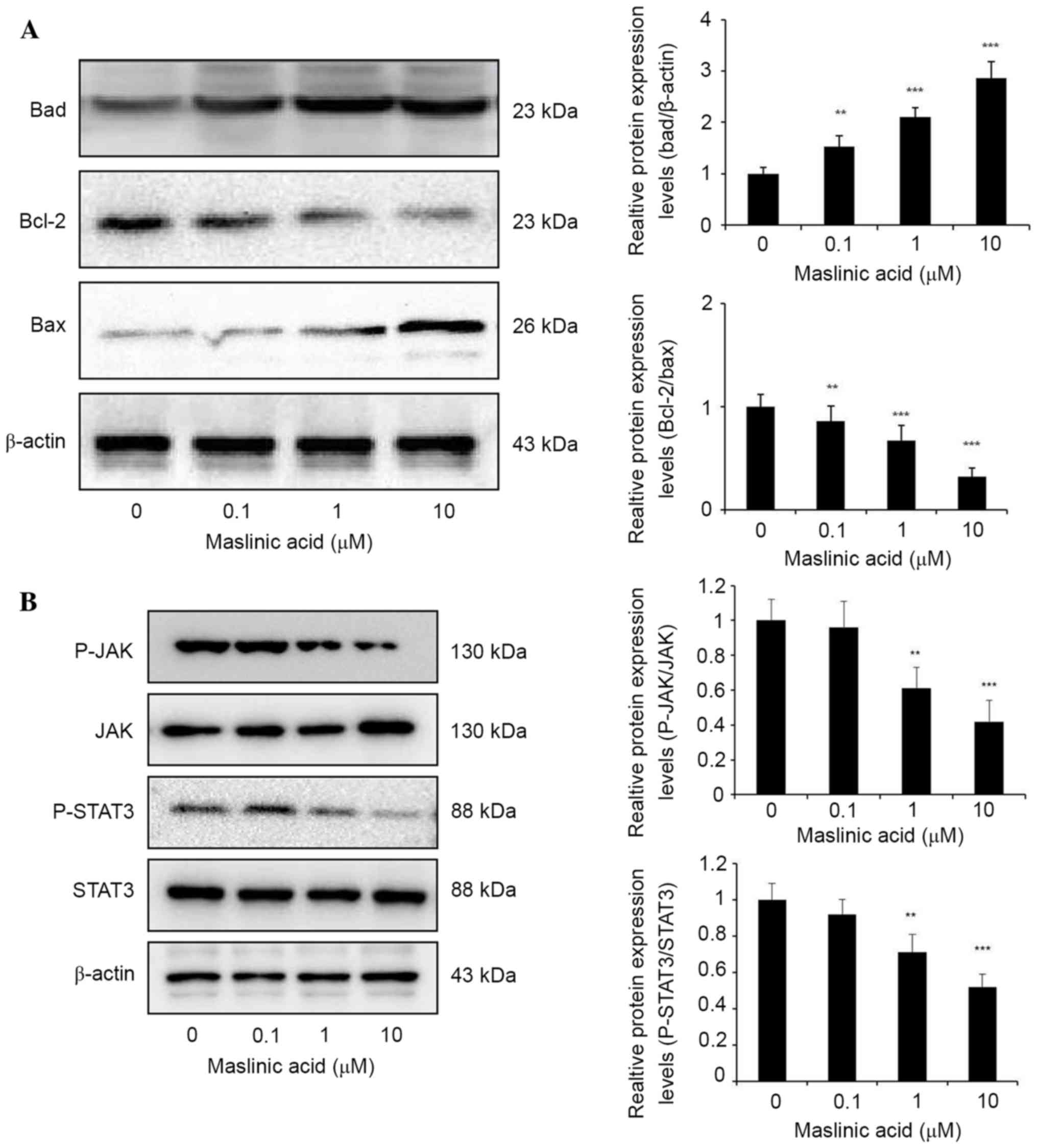
underlying maslinic acid induced apoptosis, the Bcl-2 protein
family expression was analyzed by western blotting. Maslinic acid
treatment significantly increased the expression level of Bad
compared with DMSO group, P=0.0089, 0.00035 and 0.00001,
respectively. Bcl-2/Bax expression level was significantly
decreased in MKN28 cells treated with maslinic acid for 24 h
compared with the DMSO group, P=0.0049, 0.00088 and 0.000053,
respectively (Fig. 2A). These results
indicated that the inhibition of proliferation due to maslinic acid
treatment may result from significantly attenuated expression
levels of Bcl-2 protein family products, including Bcl-2, Bax and
Bad.
JAK/STAT3 signaling regulates gene products involved
in various cellular processes including survival, proliferation and
cell cycle progression (19). Western
blot analysis revealed that maslinic acid treatment resulted in a
marked downregulation of STAT3 phosphorylation levels without
observable significant effects on the total STAT3 protein level in
MKN28 cells. Inhibition of JAK2 phosphorylation at a concentration
of 1 and 10 µM maslinic acid was observed, with negligible effects
on total JAK2 protein levels, suggesting that the inhibition of
p-JAK2 may contribute to the inhibition of STAT3 activity induced
by maslinic acid (Fig. 2B).
Maslinic acid inhibits IL-6-mediated
STAT3 activation in MKN28 cells
IL-6 is one of the most prevalent cytokines that
mediates its effects via the phosphorylation of STAT3 (20). As JAK/STAT3 activation is known to be
involved in hematologic malignancies (21), it was imperative to determine whether
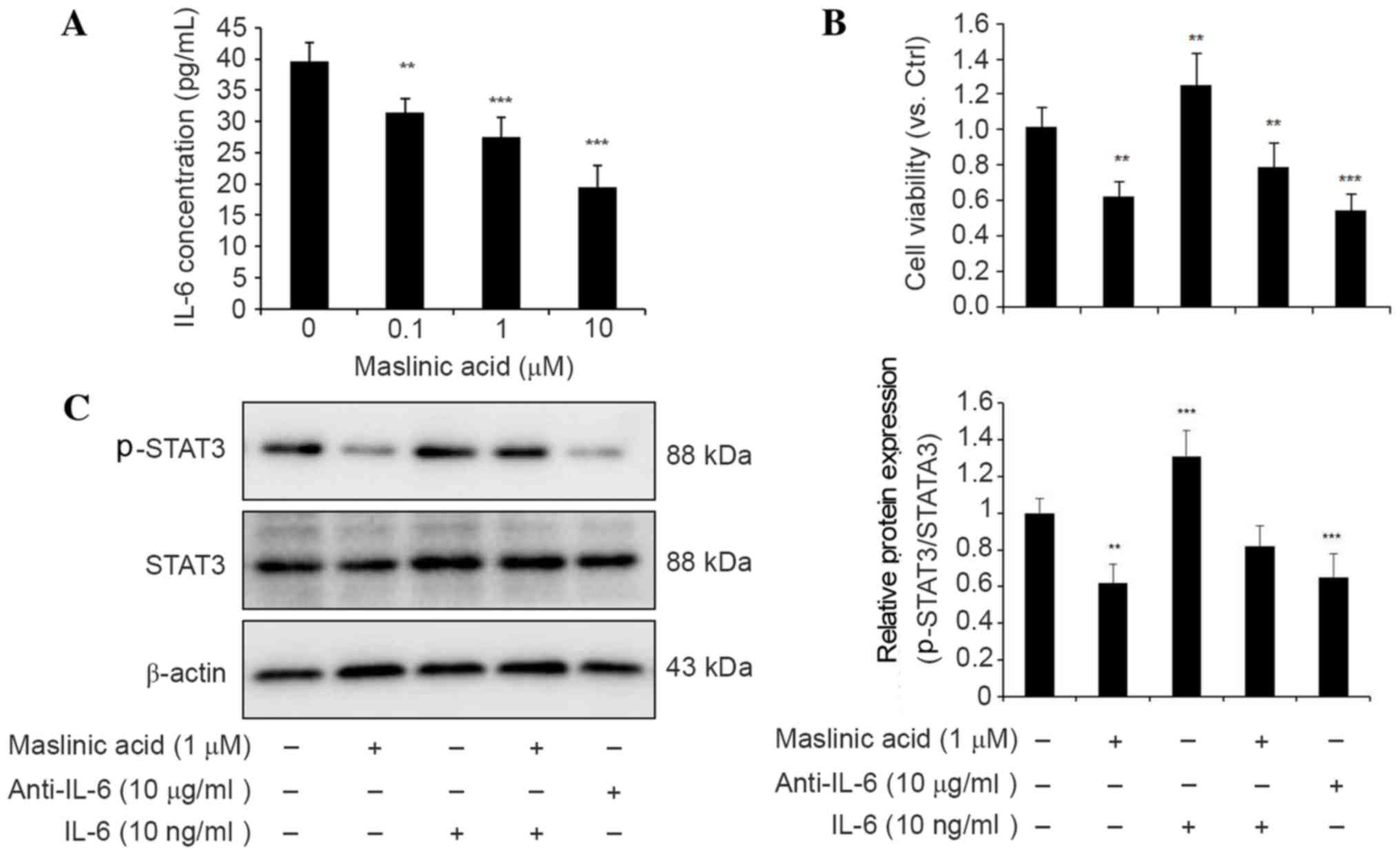
maslinic acid had deleterious effects on the production of IL-6.
The present study investigated the production of IL-6 in MKN28
cells using ELISA prior to and following maslinic acid treatment. A
decrease of IL-6 protein in the medium following maslinic acid
treatment was revealed (Fig. 3A).
Therefore, it is likely that suppression of the JAK/STAT3 signaling
pathway following maslinic acid treatment may be induced by the
upstream inhibition of IL-6 expression.
Pretreatment with the anti-IL-6 antibody for 24 h
also resulted in the inhibition of cell viability and
phosphorylation of STAT3. The addition of recombinant IL-6 to the
medium resulted in an increase in cell viability and the
reactivation of STAT3 phosphorylation, suggesting that IL-6 is
responsible for STAT3 activation (Fig. 3B
and C). Taken together, these findings suggest that maslinic
acid serves an important role in the inhibition of IL-6-mediated
STAT3 activation in the MKN28 gastric cancer cell line.
Discussion
Maslinic acid is a natural triterpene from Olea
europaea L. (1). Accumulating
evidence exists that maslinic acid may inhibit the growth of
various human tumor cell lines in vitro (1,22,23). In vivo studies also demonstrated that
maslinic acid inhibits the growth of various types of cancer cells
in mice, including the HT29 human colon-cancer cell line (24), Panc-28 pancreatic cell line, Panc-1,
BxPC-3, AsPC-1 (22) and Raji B
lymphoma cell line (25). Although
the antitumor effects of maslinic acid have been demonstrated in
vitro and in vivo for various types of cancer (22–25),
little is known about its effect and underlying mechanisms in
gastric cancer.
In the present study, maslinic acid was demonstrated
to inhibit MKN28 cell viability in a dose-dependent manner using
CCK-8, and to induce cell apoptosis as determined using flow
cytometry. The western blot analysis results revelead that maslinic
acid increased the protein level of Bad and inhibited the Bcl-2/Bax
ratio, which indicated the cell survival or apoptosis grade. These
results confirmed that maslinic acid exhibits a significant
anticancer effect on MKN28 cells. Subsequently, the present study
focussed on the molecular mechansims underlying the maslinic
acid-mediated inhibition of MKN28 cell proliferation.
Inflammation is recognized to serve an important
role in the pathogenesis of numerous types of tumors, and is a
critical component of tumor progression (26). The production and release of various
survival factors, including IL-6, a major mediator of inflammation,
serves to block apoptosis in cells during the inflammatory process,
keeping them alive in toxic environments (27). IL-6 binds to the soluble IL-6R
(glycoprotein (gp)80, present either on the cell surface or in
solution), which then induces dimerization of gp130 chains
resulting in activation of the associated JAKs (28). JAKs phosphorylate gp130, leading to
the recruitment and activation of STAT3 transcription factors, as
well as other molecules (29). STAT3
is constitutively activated in various gastric cancer cells, and is
often associated with cell survival, proliferation and
transformation (30).
The present study revealed that maslinic acid
treatment inhibits IL-6 protein secretion and results in loss of
STAT3 phosphorylation, accounting for the inhibition of the
IL-6/STAT3 signaling pathway. Western blot analysis was used for
evaluation of the expression levels of JAK2, an upstream protein
tyrosine kinase, which serves an important role in STAT3 homo-dimer
formation and activation (31). The
results demonstrated that maslinic acid treatment of MKN28 cells
results in a significantly diminished expression level of p-JAK2
protein. Taken together, these results suggest that the
inactivation of upstream p-JAK2 is involved in the inhibition of
the IL-6-JAK/STAT3 signaling pathway in MKN28 cells.
A number of previous studies have indicated that
consti-tutive activation of STAT3 is mediated via
autocrine/paracrine stimulation by cytokines, including the IL-6
family of cytokines, involved in hematopoietic development
(32–35). In order to further explore the
mechanisms underlying inhibition of IL-6 induced by maslinic acid,
the present study used an anti-IL-6 antibody to block the
endogenous IL-6 or recombinant IL-6 protein, in order to restore
STAT3 phosphorylation in MKN28 cells, thus suggesting that IL-6 was
responsible for STAT3 activation. The results confirmed that the
overexpression of IL-6 promoted tumor growth and STAT3
phosphorylation, and the inhibition of IL-6 production may decrease
cell proliferation and phosphorylation of STAT3. In addition,
maslinic acid may decrease IL-6 protein levels in the culture
medium of MKN28 cells. Thus, the anticancer properties of maslinic
acid may result from its inhibition of IL-6 expression and
subsequent inhibition of downstream JAK2/STAT3 signaling.
To the best of our knowledge, no previous studies
have investigated the effect of maslinic acid on the IL-6-JAK/STAT3
signaling cascade in gastric cancer cells. The results of the
present study have demonstrated for the first time that the
underlying mechanism of maslinic acid anti-gastric tumor activity
is the inhibition of IL-6 expression and subsequent downregulation
of the JAK/STAT3 signaling pathway. It was revealed that maslinic
acid inhibited the generation and secretion of IL-6 in MKN28 cells,
induced JAK and STAT3 phosphorylation and downregulated the
expression levels of STAT3-mediated proteins involved in apoptosis
and proliferation (Bad, Bcl-2 and Bax). However, further
investigation is required in order to elucidate the direct
molecular mechanisms underlying the maslinic acid inhibition of
IL-6. It is possible that maslinic acid may be useful as a
therapeutic treatment for gastric cancer.
References
|
1
|
Reyes-Zurita FJ, Rufino-Palomares EE,
Lupiáñez JA and Cascante M: Maslinic acid, a natural triterpene
from Olea europaea L., induces apoptosis in HT29 human colon-cancer
cells via the mitochondrial apoptotic pathway. Cancer Lett.
273:44–54. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pavel IZ, Danciu C, Oprean C, Dehelean CA,
Muntean D, Csuk R and Muntean DM: In vitro evaluation of the
antimicrobial ability and cytotoxicity on two melanoma cell lines
of a benzylamide derivative of maslinic acid. Anal Cell Pathol
(Amst). 2016:27876232016.PubMed/NCBI
|
|
3
|
Parra A, Rivas F, Lopez PE,
Garcia-Granados A, Martinez A, Albericio F, Marquez N and Muñoz E:
Solution- and solid-phase synthesis and anti-HIV activity of
maslinic acid derivatives containing amino acids and peptides.
Bioorg Med Chem. 17:1139–1145. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nieto FR, Cobos EJ, Entrena JM, Parra A,
García-Granados A and Baeyens JM: Antiallodynic and analgesic
effects of maslinic acid, a pentacyclic triterpenoid from Olea
europaea. J Nat Prod. 76:737–740. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang S, Ding D, Zhang X, Shan L and Liu
Z: Maslinic acid induced apoptosis in bladder cancer cells through
activating p38 MAPK signaling pathway. Mol Cell Biochem.
392:281–287. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Park SY, Nho CW, Kwon DY, Kang YH, Lee KW
and Park JH: Maslinic acid inhibits the metastatic capacity of
DU145 human prostate cancer cells: Possible mediation via
hypoxia-inducible factor-1α signalling. Br J Nutr. 109:210–222.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rufino-Palomares EE, Reyes-Zurita FJ,
García-Salguero L, Mokhtari K, Medina PP, Lupiáñez JA and Peragón
J: Maslinic acid, a triterpenic anti-tumoural agent, interferes
with cytoskeleton protein expression in HT29 human colon-cancer
cells. J Proteomics. 83:15–25. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Reyes-Zurita FJ, Pachón-Peña G, Lizárraga
D, Rufino-Palomares EE, Cascante M and Lupiáñez JA: The natural
triterpene maslinic acid induces apoptosis in HT29 colon cancer
cells by a JNK-p53-dependent mechanism. BMC Cancer. 11:1542011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Burger R: Impact of interleukin-6 in
hematological malignancies. Transfus Med Hemother. 40:336–343.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Reynaud D, Pietras E, Barry-Holson K, Mir
A, Binnewies M, Jeanne M, Sala-Torra O, Radich JP and Passegué E:
IL-6 controls leukemic multipotent progenitor cell fate and
contributes to chronic myelogenous leukemia development. Cancer
Cell. 20:661–673. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hirano T, Ishihara K and Hibi M: Roles of
STAT3 in mediating the cell growth, differentiation and survival
signals relayed through the IL-6 family of cytokine receptors.
Oncogene. 19:2548–2556. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Heinrich P, Behrmann I, Müller-Newen G,
Schaper F and Graeve L: Interleukin-6-type cytokine signalling
through the gp130/Jak/STAT pathway. Biochem J. 334:297–314. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ogata A, Chauhan D, Teoh G, Treon SP,
Urashima M, Schlossman RL and Anderson KC: IL-6 triggers cell
growth via the Ras-dependent mitogen-activated protein kinase
cascade. J Immunol. 159:2212–2221. 1997.PubMed/NCBI
|
|
14
|
Huang W, Yu LF, Zhong J, Wu W, Zhu JY,
Jiang FX and Wu YL: Stat3 is involved in angiotensin II-induced
expression of MMP2 in gastric cancer cells. Dig Dis Sci.
54:2056–2062. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ding SZ, Cho CH and Lam SK: Regulation of
interleukin 6 production in a human gastric epithelial cell line
MKN-28. Cytokine. 12:1129–1135. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
To KF, Chan MW, Leung WK, Ng EK, Yu J, Bai
AH, Lo AW, Chu SH, Tong JH, Lo KW, et al: Constitutional activation
of IL-6-mediated JAK/STAT pathway through hypermethylation of
SOCS-1 in human gastric cancer cell line. Br J Cancer.
91:1335–1341. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Strober W: Trypan blue exclusion test of
cell viability. Curr Protoc Immunol Appendix. 3:Appendix 3B. 2001.
View Article : Google Scholar
|
|
18
|
Catlett-Falcone R, Landowski TH, Oshiro
MM, Turkson J, Levitzki A, Savino R, Ciliberto G, Moscinski L,
Fernández-Luna JL, Nuñez G, et al: Constitutive activation of Stat3
signaling confers resistance to apoptosis in human U266 myeloma
cells. Immunity. 10:105–115. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shain KH, Yarde DN, Meads MB, Huang M,
Jove R, Hazlehurst LA and Dalton WS: Beta1 integrin adhesion
enhances IL-6-mediated STAT3 signaling in myeloma cells:
Implications for microenvironment influence on tumor survival and
proliferation. Cancer Res. 69:1009–1015. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhong Z, Wen Z and Darnell JE Jr: Stat3: A
STAT family member activated by tyrosine phosphorylation in
response to epidermal growth factor and interleukin-6. Science.
264:95–98. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Al Zaid Siddiquee K and Turkson J: STAT3
as a target for inducing apoptosis in solid and hematological
tumors. Cell Res. 18:254–267. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li C, Yang Z, Zhai C, Qiu W, Li D, Yi Z,
Wang L, Tang J, Qian M, Luo J and Liu M: Maslinic acid potentiates
the anti-tumor activity of tumor necrosis factor alpha by
inhibiting NF-kappaB signaling pathway. Mol Cancer. 9:732010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wu DM, Zhao D, Li DZ, Xu DY, Chu WF and
Wang XF: Maslinic acid induces apoptosis in salivary gland adenoid
cystic carcinoma cells by Ca2+-evoked p38 signaling pathway. Naunyn
Schmiedebergs Arch Pharmacol. 383:321–330. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Reyes-Zurita FJ, Rufino-Palomares EE,
Lupiáñez JA and Cascante M: Maslinic acid, a natural triterpene
from Olea europaea L., induces apoptosis in HT29 human colon-cancer
cells via the mitochondrial apoptotic pathway. Cancer Lett.
273:44–54. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hsum YW, Yew WT, Hong PL, Soo KK, Hoon LS,
Chieng YC and Mooi LY: Cancer chemopreventive activity of maslinic
acid: Suppression of COX-2 expression and inhibition of NF-κB and
AP-1 activation in raji cells. Planta Med. 77:152–157. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Coussens LM and Werb Z: Inflammation and
cancer. Nature. 420:860–867. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hodge DR, Hurt EM and Farrar WL: The role
of IL-6 and STAT3 in inflammation and cancer. Eur J Cancer.
41:2502–2512. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Murakami M, Hibi M, Nakagawa N, Nakagawa
T, Yasukawa K, Yamanishi K, Taga T and Kishimoto T: IL-6-induced
homodimerization of gp130 and associated activation of a tyrosine
kinase. Science. 260:1808–1810. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Heinrich PC, Behrmann I, Müller-Newen G,
Schaper F and Graeve L: Interleukin-6-type cytokine signalling
through the gp130/Jak/STAT pathway. Biochem J. 334:297–314. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kanda N, Seno H, Konda Y, Marusawa H,
Kanai M, Nakajima T, Kawashima T, Nanakin A, Sawabu T, Uenoyama Y,
et al: STAT3 is constitutively activated and supports cell survival
in association with survivin expression in gastric cancer cells.
Oncogene. 23:4921–4929. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Endo TA, Masuhara M, Yokouchi M, Suzuki R,
Sakamoto H, Mitsui K, Matsumoto A, Tanimura S, Ohtsubo M, Misawa H,
et al: A new protein containing an SH2 domain that inhibits JAK
kinases. Nature. 387:921–924. 1997. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yadav A, Kumar B, Datta J, Teknos TN and
Kumar P: IL-6 promotes head and neck tumor metastasis by inducing
epithelial-mesenchymal transition via the JAK-STAT3-SNAIL signaling
pathway. Mol Cancer Res. 9:1658–1667. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sriuranpong V, Park JI, Amornphimoltham P,
Patel V, Nelkin BD and Gutkind JS: Epidermal growth factor
receptor-independent constitutive activation of STAT3 in head and
neck squamous cell carcinoma is mediated by the autocrine/paracrine
stimulation of the interleukin 6/gp130 cytokine system. Cancer Res.
63:2948–2956. 2003.PubMed/NCBI
|
|
34
|
Alas S and Bonavida B: Rituximab
inactivates signal transducer and activation of transcription 3
(STAT3) activity in B-non-Hodgkin's lymphoma through inhibition of
the interleukin 10 autocrine/paracrine loop and results in
down-regulation of Bcl-2 and sensitization to cytotoxic drugs.
Cancer Res. 61:5137–5144. 2001.PubMed/NCBI
|
|
35
|
Sano M, Fukuda K, Kodama H, Takahashi T,
Kato T, Hakuno D, Sato T, Manabe T, Tahara S and Ogawa S:
Autocrine/Paracrine secretion of IL-6 family cytokines causes
angiotensin II-induced delayed STAT3 activation. Biochem Biophys
Res Commun. 269:798–802. 2000. View Article : Google Scholar : PubMed/NCBI
|