Introduction
Oral cancer is one of the 10 most commonly occurring
types of malignant tumor worldwide, particularly in Southeast Asian
countries (1). The habit of betel
quid chewing is implicated in the high prevalence of oral cancer in
these countries. Betel quid consists of a mixture of fresh areca
nut, slaked lime from seashells, fresh betel leaf and partially
dried tobacco (2–4). The association of these components with
oral carcinogenesis remains unclear. Areca nut and its component
arecoline, a nicotinic acid-based alkaloid, have been reported to
promote oral cancer and precancerous lesions (5). Areca nut extract and arecoline induce
the upregulation of various growth factors, enzymes and other
molecules associated with oral submucous fibrosis, a precancerous
condition (6), and stimulate oral
cancer cells and immortalized cell lines to express molecules
associated with cancer progression (7,8). Our
previous studies analyzed p14, p15, p16 and p53 genes in
precancerous oral lesions associated with betel quid chewing in Sri
Lanka (1,9,10). A high
frequency of hypermethyaltion of p14, p15 and p16 was detected in
the pre-cancerous lesions. The frequency was much higher than that
of p53 mutation.
Matrix metalloproteinase (MMP) and tissue inhibitor
of metalloproteinase (TIMP) may be key molecules in oral submucous
fibrosis (11) and cancer (7). The exact mechanism for the malignant
transformation of healthy oral epithelium remains unclear. MMPs
serve an important function in the degradation of the extracellular
matrix (ECM), a process crucial for tumor growth, invasion and
metastasis. The MMP family includes ≥28 members, which may be
classified as gelatinases, collagenases, membrane-type MMPs,
stromelysins or matrilysins, based on substrate specificity and
their sequence homology (12,13). The association of the gelatinases,
including MMP-2 and MMP-9, with the development and progression of
cancer is well documented (12,13). The
enzymatic activity of MMPs is controlled by TIMPs. A total of 4
types of TIMP have been characterized, including TIMP-1, −2, −3 and
−4. TIMP-1 and TIMP-2 are capable of inhibiting the activity of all
non-membrane-type MMPs, including MMP-2 and MMP-9 (12,13). The
increased MMPs and the decreased inhibitors may facilitate tumor
development and progression (12,13). In
order to determine the mechanism of arecoline-induced malignant
transformation, it is crucial to observe the expression pattern of
MMP-2, MMP-9, TIMP-1 and TIMP-2.
Malignant transformation may occur following chronic
stimulation with carcinogenic agents. However, in previous in
vitro experiments cells were stimulated with arecoline for
<30 h, which elicited an acute response as an experimental model
for arecoline stimulation (7,14). Since the malignant transformation
occurs as a chronic event induced by the stimulation with
arecoline, cells should be stimulated for a prolonged period in
vitro. Cell death may be induced by stimulation for a prolonged
period with arecoline, since arecoline has a cytotoxic effect
(7,8,11,15,16). In
our previous study, we developed a novel in vitro model to
simulate chronic cell stimulation over a prolonged period (17).
In the present study, this in vitro modeling
method from our previous study was used to simulate chronic
arecoline stimulation and the expression of MMP-2, MMP-9, TIMP-1
and TIMP-2 was measured. The pathway of aberrant expression of
molecules involved in carcinogenesis often can be a therapeutic
target (12,13). Therefore, the present study also
observed the pathway of the aberrant expression of MMPs and
TIMPs.
Materials and methods
Cell culture
Human gingival epithelium progenitors (HGEPs),
primary keratinocytes derived from healthy gingival epithelium,
were purchased from CELLnTEC Advanced Cell Systems AG (Basel,
Switzerland) and were cultured in CnT-Prime epithelial culture
medium (CELLnTEC Advanced Cell Systems AG) at 37°C in a humidified
atmosphere of 95% air and 5% CO2. HGEPs were spread onto 100 mm
tissue culture plates at a density of 4×104 cells/ml.
Following incubation overnight, the cells were cultured with
arecoline hydrobromide (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) and the following inhibitors: 50 µM nuclear factor
(NF)-κB/inhibitor of NF-κB (IκB) inhibitor PDTC, 10 µM
mitogen-activated protein kinase (MAPK) kinase inhibitor PD98059,
25 µM p38 MAP kinase inhibitor SB203580, and 10 µM signal
transducer and activator of transcription (STAT) 3 inhibitor VIII
5,15-DPP (all from Sigma-Aldrich; Merck KGaA).
Cell viability assays
Cell viability was determined using the cell
proliferation reagent WST-1 (Sigma-Aldrich; Merck KGaA). HGEPs were
seeded in 96-well plates in epithelial culture medium and cultured
overnight as previously described. The cells were treated with a
range of arecoline concentrations (including 0, 0.5, 1, 5, 10, 50,
100, 500, 1,000, 5,000 and 10,000 µg/ml). The arecoline was
dissolved in distilled water. Following incubation for 24, 48 or 72
h, 10 µl of WST-1 was added to each well and cultured for 1 h. The
absorbance at 450 nm was determined using a Model 680 Microplate
Reader (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
RNA extraction
Cell culture medium was replaced every 3 days,
alternating with and without 50 µg/ml arecoline for 1 month.
Untreated samples were used as controls. The inhibitors (50 µM of
the NF-κB/IκB inhibitor PDTC, 10 µM of the MAPK kinase inhibitor
PD98059, 25 µM of the p38 MAPK inhibitor SB203580 or 10 µM of the
STAT 3 inhibitor VIII 5,15-DPP) were added to the culture medium
for 18 days (added on day 3, 9 and 15). The culture was replaced by
alternating 3 days with arecoline and 3 days with the inhibitors.
Total RNA was extracted from the HGEPs using RNeasy®
Mini kit (Qiagen GmbH, Hilden, Germany) according to the
manufacturer's protocol. Total RNA of 2 µg was reverse transcribed
into cDNA using an ReverTra Ace® qPCR RT Master Mix
(Toyobo Co., Ltd., Osaka, Japan), following the manufacturer's
protocol.
Quantitative polymerase chain reaction
(qPCR)
For PCR, cDNA (1 µl) was mixed with FastStart
Essential DNA Green Master (Roche Diagnostics) and the relevant
primers. The cDNA levels were measured using the
LightCycler® Nano System (Roche Diagnostics, Basel,
Switzerland). The PCR primers (Eurofins Genomics, Tokyo, Japan) for
GAPDH, MMP-2, MMP-9, TIMP-1 and TIMP-2 are exhibited in Table I. The PCR was performed using the
LightCycler® Nano System (Roche Diagnostics), and
conditions included an initial incubation at 50°C for 2 min,
denaturing at 95°C for 10 min, then 40 cycles of denaturing at 95°C
for 15 sec and annealing at 60°C for 1 min. The relative expression
of each mRNA was calculated by comparison to GADPH mRNA using the
2−∆∆Cq method (18). Data
are expressed as the relative amount of target mRNA compared with
GAPDH mRNA. A total of 3 repetitions of the experimental culture
system were performed in order to extract RNA. RT-qPCR of each
experimental culture system was carried out 3 times and the mean
was used.
 | Table I.Primer sequences (5′ to 3′). |
Table I.
Primer sequences (5′ to 3′).
| Gene | Forward | Reverse | (Refs.) |
|---|
| GAPDH |
GTGAAGGTCGGAGTCAAC |
GTTGAGGTCAATGAAGGG | (31) |
| MMP-2 |
CAAGGACCGGTTTATTTGGC |
ATTCCCTGCGAAGAACACAGC | (32) |
| MMP-9 |
TTGACAGCGACAAGAAGTGG |
GCCATTCACGTCGTCCTTAT | (33) |
| TIMP-1 |
GGGACACCAGAAGTCAACCA |
GGCTTGGAACCCTTTATACATC | (34) |
| TIMP-2 |
AAGCGGTCAGTGAGAAGGAA |
TCTCAGGCCCTTTGAACATC | (34) |
Supernatant MMP-2 and MMP-9 activity
assay
MMP-2 and MMP-9 activity were measured using an
MMP-2 and MMP-9 activity assay kits (QuickZyme Biosciences, Leiden,
Netherlands) according to the manufacturer's protocol. Absorbance
values at 405 nm were determined with a Model 680 Microplate Reader
(Bio-Rad Laboratories, Inc.), and standard curves relating
concentration (MMP-9, 0.01–16 ng/ml; MMP-2, 0.02–16 ng/ml) in ng/ml
to absorbance values were plotted. In total, 3 experimental culture
systems were performed to measure MMP-2 and MMP-9 activity. The
MMP-2 and MMP-9 activity assays for each experimental culture
system were carried out 3 times and the mean was used.
Statistical analysis
Statistical analysis was performed using SPSS
version 23 (IBM SPSS, Armonk, NY, USA). Results were compared using
the Mann-Whitney U test. Data are presented as the mean ± standard
deviation. P<0.05 was considered to represent a statistically
significant difference.
Results
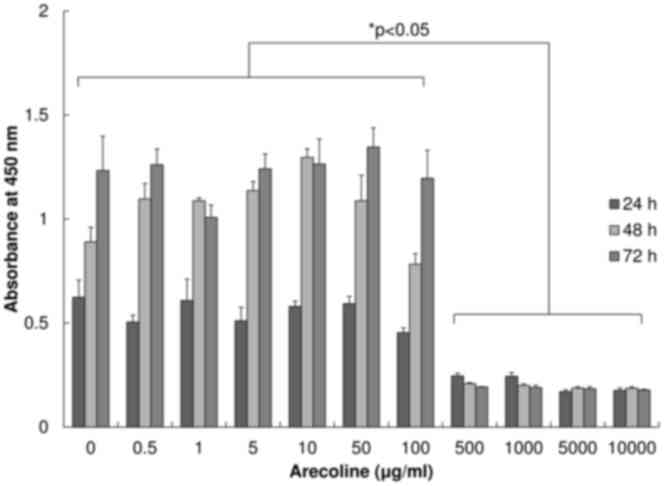
Cell viability at a range of arecoline
concentrations
To determine a concentration of arecoline that was
suitable for the treatment of cells over a prolonged period, cell
viability in different concentrations of arecoline [including 0
(control), 0.5, 1, 5, 10, 50, 100, 500, 1,000, 5,000 and 10,000
µg/ml] was initially observed. The number of viable cells was
estimated by a WST-1 assay after 24, 48 or 72 h of arecoline
treatment (Fig. 1). At doses of ≤100
µg/ml of arecoline, cell numbers increased in a time-dependent
manner. No significant differences were observed in cell numbers at
the same time point between any concentration of arecoline at ≤100
µg/ml and the control. Cell viability was significantly lower in
the ≥500 µg/ml arecoline groups compared with the ≤100 µg/ml group
(P<0.05). Since arecoline at the concentration of 50 µg/ml had
no cytotoxic effect on the cells stimulated, even for a prolonged
period, the method of alternating between 3 days with 50 µg/ml of
arecoline and 3 days without arecoline for 1 month was selected.
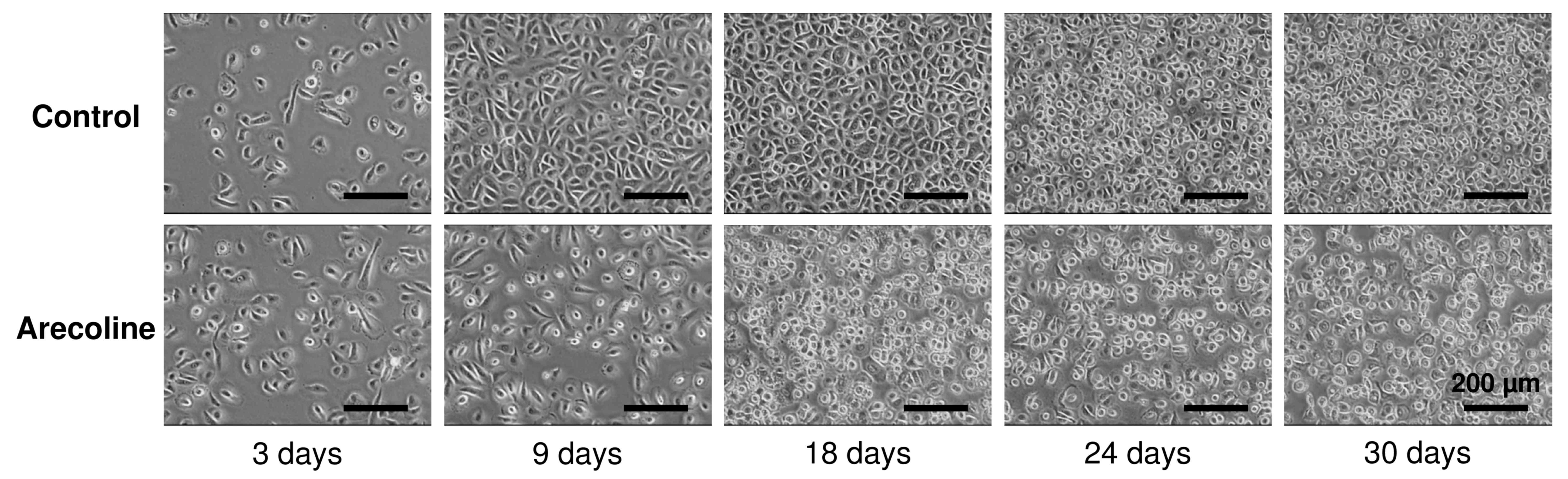
Additionally, the cells were observed using phase contrast
microscope (magnification, ×400). No significant morphological
changes were observed in cells stimulated with arecoline for 1
month, compared with the controls (Fig.
2). A number of rounded cells were often observed in the cells
stimulated with arecoline after 9 days (Fig. 2).
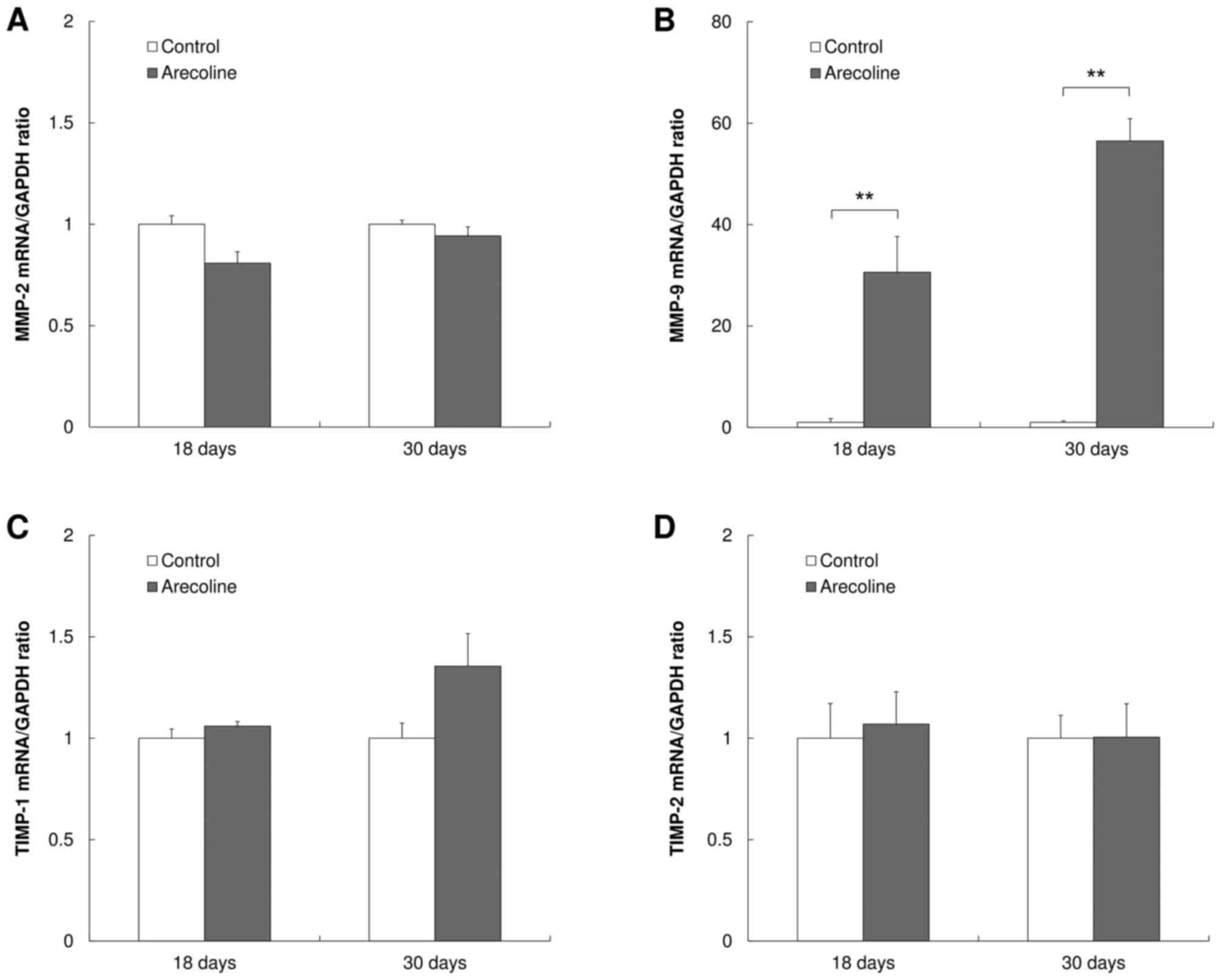
MMP-9 is significantly upregulated in
cells treated with arecoline
The expression levels of MMP-2, MMP-9, TIMP-1 and
TIMP-2 in cells treated with 50 µg/ml arecoline were evaluated by
quantitative RT-PCR at 18 and 30 days. No significant differences
in the expression of MMP-2 mRNA was observed between the
experimental and control groups (Fig.
3A). The expression of MMP-9 mRNA in the experimental group was
significantly increased compared with the control group
(P<0.001; Fig. 3B). No significant
difference in the expression of TIMP-1 (Fig. 3C) or TIMP-2 (Fig. 3D) mRNA was observed between the
experimental and control groups.
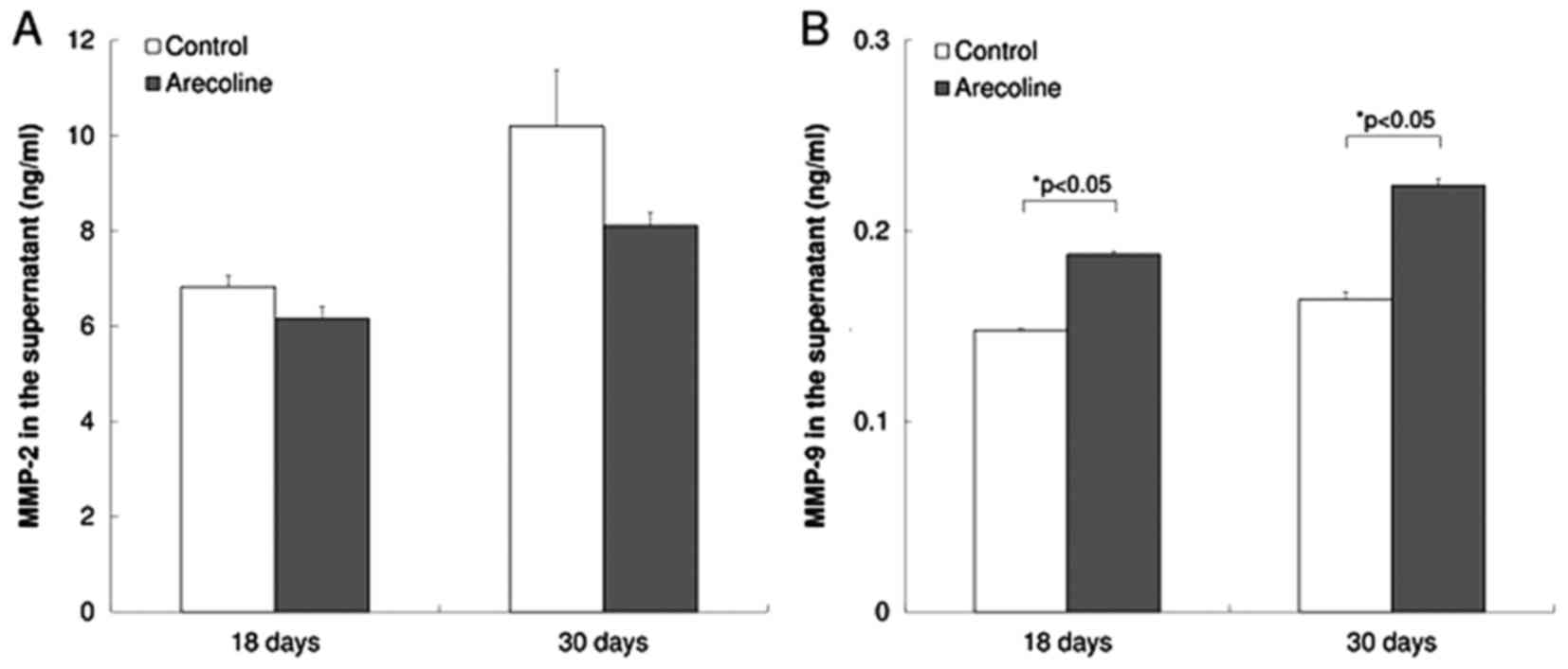
MMP-9 activity is significantly
increased in cells treated with arecoline
MMP-2 and MMP-9 activity was assessed with MMP-2 and
MMP-9 activity assays. No significant difference in the levels of
MMP-2 activity was observed between the experimental and control
groups (Fig. 4A). The levels of MMP-9
activity in the experimental group were significantly increased
compare with the control group (P<0.05; Fig. 4B).
Inhibition of signaling pathways
reduces the upregulation of MMP-9 in cells treated with
arecoline
To investigate cellular signaling pathways that may
mediate the observed effect of arecoline on MMP-9 expression and
activity, signal pathway inhibitors were employed, including the
NF-κB/IκB inhibitor PDTC, the MAPK kinase inhibitor PD98059, the
p38 MAPK inhibitor SB203580 and the STAT3 inhibitor 5,15-DPP. All
inhibitors decreased the extent of MMP-9 upregulation induced by
stimulation with arecoline. PDTC and PD98059 partially inhibited
the upregulation of MMP-9, whereas SB203580 and 5,15-DPP completely
abolished the upregulation (P<0.01; Fig. 5).
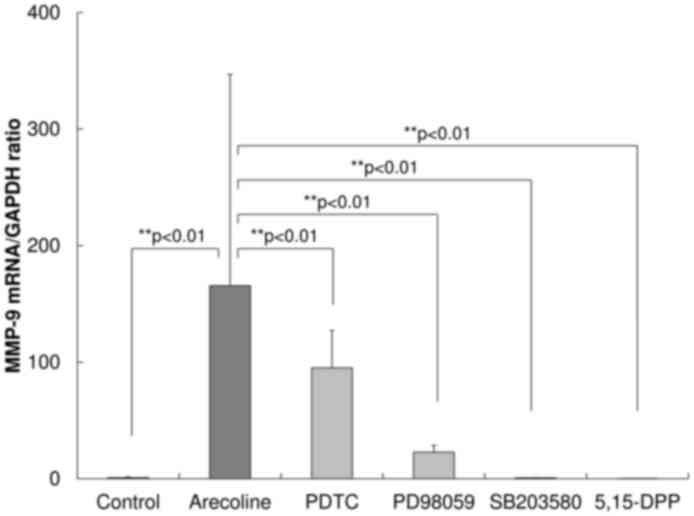 | Figure 5.Effect of inhibitors on the
expression of MMP-9 mRNA. To investigate which cellular signaling
pathways were associated with the upregulation of MMP-9 following
treatment with arecoline, inhibitors of signaling pathway were
used, including the NF-κB/IκB inhibitor PDTC, the MAPK kinase
inhibitor PD98059, the p38 MAPK inhibitor SB203580 and the STAT3
inhibitor 5,15-DPP. All inhibitors decreased the extent of the
upregulation of MMP-9 induced by stimulation with arecoline. PDTC
and PD98059 partially inhibited the upregulated expression of
MMP-9, whereas SB203580 and 5,15-DPP treatments completely
prevented the upregulation. MMP-9, matrix metalloproteinase-9;
NF-κB, nuclear factor-κB; IκB, inhibitor of NF-κB; MAPK,
mitogen-activated protein kinase; STAT3, signal transduction and
activator of transcription 3. |
Discussion
The present study investigated the expression of
MMP-2, MMP-9, TIMP-1 and TIMP-2 in primary keratinocytes derived
from healthy gingival epithelium stimulated with arecoline for a
prolonged period. MMP-9 mRNA expression and activity were
upregulated by stimulation with arecoline. MMP-9 activity may be
involved in an initial stage of the transformation of normal cells
to oral cancer and the development of precancerous lesions induced
by betel quid chewing. Arecoline was previously demonstrated to
promote fibrosis in the oral submucosa and the progression to oral
cancer (19). The majority of the
other in vitro studies have used oral cancer-derived cells
and immortalized cells to observe the effect of arecoline on oral
epithelial cells, not primary cells (7,8). These
studies exhibited the effect of arecoline on cancer progression,
but could not examine the initial alterations to normal cells that
lead to the transformation to oral cancer and the development of
precancerous lesions. In one study, normal keratinocytes were
stimulated with arecoline for ≤24 h (14). Increased expression of
O6-methylguanine-DNA methyltransferase was observed in primary
human oral keratinocytes following stimulation with arecoline for
1–24 h (14). In a second study,
activation of MMP-9 was observed in normal gingival epithelial
cells stimulated with areca nut extract, also for 24 h (20). Since betel quid chewing is a daily
habit, keratinocytes may need to be stimulated with arecoline for a
prolonged period in in vitro models to simulate the habit.
In the present study, an in vitro model was successfully
established, in which keratinocytes derived from normal gingival
epithelium were intermittently stimulated with arecoline at a
concentration of 50 µg/ml for a month without cytotoxic effects.
Immortalized keratinocytes (HaCaT cells) treated with arecoline for
24, 48 or 72 h underwent cell death at concentrations of >50
µg/ml (8). The arecoline
concentration used in the present study is not inconsistent with
this study.
The in vitro data of the present study
suggest that increased expression of MMP-9 may occur in the oral
mucosa of betel quid chewers. MMPs can degrade virtually all
components of the ECM and connective tissue surrounding tumor cells
and the basement membrane (21).
Among MMP type IV collagen digestive enzymes, MMP-9 is particularly
important in the process of tumor invasion and metastasis (7,22). High
expression of MMP-9 was previously demonstrated to be associated
with a poor prognosis in oral cancer (7). Increased expression levels of MMP-9 have
been observed in oral precancerous lesions as well as in oral
cancer (23). The increased
expression may be associated with the transformation of oral
precancerous lesions to oral cancer (24,25) and in
oral carcinogenesis (11). A previous
in vitro study revealed that TNF-α stimulated the production
of MMP-9 in a precancerous keratinocyte cell line (26). The transformation of oral precancerous
lesions to cancer with micro-invasion was hypothesized as induced
by inflammatory stimulation (26). In
the present study, arecoline-induced MMP-9 in the keratinocytes may
have caused the cells to develop the potential for micro-invasion.
The production of MMP-9 from gingival epithelium was previously
demonstrated to be associated with the destruction of periodontal
tissues (20). Therefore, the data of
the present study may explain the association of betel quid chewing
with periodontal disease as well as carcinogenesis. One recent
study is inconsistent with the data of the present study; in the
study, although areca nut extract stimulated MMP-9 production in
cancer cells, arecoline alone inhibited MMP-9 production at
concentrations lower than 0.2 mM (50 µg/ml) in the oral carcinoma
SAS cell line (7). The different
types of cells that were used in the studies may explain this
discrepancy. Arecoline induced significant cell death in SAS cells
at a concentration of 0.4 mM (100 µg/ml) for 24 h in the previous
study (7). Whereas, the present study
did not identify a significant reduction in cell viability at the
same concentration of arecoline at time points including 72 h,
demonstrating the extent of the differences between the cell types
used. Another type of gelatinase, MMP-2, is also often upregulated
in malignancies and performs an important role in tumor invasion
(12,13). The present results demonstrated no
significant difference in the expression of MMP-2 between arecoline
stimulation and the control. It is consistent with a previous study
indicating that areca nut extract induces activation of MMP-9 but
does not induce MMP-2 in gingival epithelial cells (20). The elevated expression of MMP-2 was,
however, shown in the saliva collected from areca nut chewers
(27). Keratinocytes mainly express
MMP-9 and to a lesser extent MMP-2 while fibroblasts express only
MMP-2 in in vitro skin models (28). The salivary MMP-2 may be mainly
derived from fibroblasts in the oral mucosa. Arecoline may not
affect MMP-2 activation in the gingival epithelium.
The mechanism by which arecoline may have induced
the upregulation of MMP-9 was investigated using inhibitors of
intercellular signaling pathways. Although all inhibitors decreased
upregulated expression of MMP-9, the inhibitory effects differed.
NF-κB/IκB and MAPK kinase inhibitors partially inhibited the
upregulation of MMP-9 following treatment with arecoline, whereas
p38 MAPK and STAT3 inhibitors completely abolished the
upregulation. Although all these pathways may be involved in the
upregulation of MMP-9, the p38 MAPK and STAT3 pathways may be more
crucial than NF-κB/IκB and MAPK kinase pathways. A number of
signaling pathways, including MAPKs, NF-κB, PI3K/Akt and STAT3,
were previously implicated in the production of MMP-9 by epithelial
cells (29). NF-κB activation was
identified to be involved in MMP-9 upregulation in normal gingival
epithelial cells stimulated with areca nut extract at a
concentration of 2.5–10 µg/ml for 2 h (20). Although areca nut extract may activate
the NF-κB pathway for MMP-9 production, arecoline alone may act on
p38 MAPK and STAT3 to a greater extent than NF-κB. Further
investigations are required to determine the pathway of
upregulation of MMP-9 induced by arecoline.
The activity of >20 types of MMPs are inhibited
by just 4 types of TIMP (12). Since
the activity of MMP-9 is inhibited by TIMP-1, the MMP-9/TIMP-1
ratio has been used as a diagnostic marker in numerous types of
cancer and inflammation-related diseases (30). A higher ratio may signify increased
degradation of the extracellular matrix in tissues related to the
development and progression of cancer and inflammation (30). In the present study, the expression of
MMP-9 was increased, whereas the expression levels of TIMP-1 and
TIMP-2 were unchanged. This higher ratio of MMP-9/TIMP-1 may form
an environment conducive to degradation of the extracellular
matrix.
In conclusion, the present study demonstrated
increased expression of MMP-9 caused by stimulation with arecoline
for a prolonged period in primary keratinocytes derived from normal
gingival epithelium. This phenomenon occurring in an in
vitro experimental model may reflect the conditions in the oral
mucosa of betel quid chewers. Although the increased expression
level of MMP-9 may be involved in the pathological alterations of
oral epithelium caused by betel quid chewing, it remains unclear
how MMP-9 directly affects this process, and other genes may
cooperate with MMP-9 during oral carcinogenesis. Further
investigation is needed to clarify these phenomena.
References
|
1
|
Chiba I, Muthumala M, Yamazaki Y, Uz Zaman
A, Iizuka T, Amemiya A, Shibata T, Kashiwazaki H, Sugiura C and
Fukuda H: Characteristics of mutations in the p53 gene of oral
squamous-cell carcinomas associated with betel-quid chewing in Sri
Lanka. Int J Cancer. 77:839–842. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
IARC Monographs on the Evaluation of the
Carcinogenic Risk of Chemicals to Human: Tobacco Habits Other than
Smoking; Betel-Quid and Areca-Nut Chewing; and Some Related
Nitrosamines. 37. IARC; Lyon: pp. 141–200. 1985
|
|
3
|
Ariyawardana A, Athukorala AD and
Arulanandam A: Effect of betel chewing, tobacco smoking and alcohol
consumption on oral submucous fibrosis: A case-control study in Sri
Lanka. J Oral Pathol Med. 35:197–201. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chung CH, Yang YH, Wang TY, Shieh TY and
Warnakulasuriya S: Oral precancerous disorders associated with
areca quid chewing, smoking, and alcohol drinking in southern
Taiwan. J Oral Pathol Med. 34:460–466. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gupta B and Johnson NW: Systematic review
and meta-analysis of association of smokeless tobacco and of betel
quid without tobacco with incidence of oral cancer in South Asia
and the Pacific. PLoS One. 9:e1133852014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wollina U, Verma SB, Ali FM and Patil K:
Oral submucous fibrosis: An update. Clin Cosmet Investig Dermatol.
8:193–204. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chang MC, Chan CP, Wang WT, Chang BE, Lee
JJ, Tseng SK, Yeung SY, Hahn LJ and Jeng JH: Toxicity of areca nut
ingredients: Activation of CHK1/CHK2, induction of cell cycle
arrest, and regulation of MMP-9 and TIMPs production in SAS
epithelial cells. Head Neck. 35:1295–1302. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Thangjam GS and Kondaiah P: Regulation of
oxidative-stress responsive genes by arecoline in human
keratinocytes. J Periodontal Res. 44:673–682. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Topcu Z, Chiba I, Fujieda M, Shibata T,
Ariyoshi N, Yamazaki H, Sevgican F, Muthumala M, Kobayashi H and
Kamataki T: CYP2A6 gene deletion reduces oral cancer risk in betel
quid chewers in Sri Lanka. Carcinogenesis. 23:595–598. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Takeshima M, Saitoh M, Kusano K, Nagayasu
H, Kurashige Y, Malsantha M, Arakawa T, Takuma T, Chiba I, Kaku T,
et al: High frequency of hypermethylation of p14, p15 and p16 in
oral pre-cancerous lesions associated with betel-quid chewing in
Sri Lanka. J Oral Pathol Med. 37:475–479. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shieh DH, Chiang LC and Shieh TY:
Augmented mRNA expression of tissue inhibitor of
metalloproteinase-1 in buccal mucosal fibroblasts by arecoline and
safrole as a possible pathogenesis for oral submucous fibrosis.
Oral Oncol. 39:728–735. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Groblewska M, Siewko M, Mroczko B and
Szmitkowski M: The role of matrix metalloproteinases (MMPs) and
their inhibitors (TIMPs) in the development of esophageal cancer.
Folia Histochem Cytobiol. 50:12–19. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Herszényi L, Hritz I, Lakatos G, Varga MZ
and Tulassay Z: The behavior of matrix metalloproteinases and their
inhibitors in colorectal cancer. Int J Mol Sci. 13:13240–13263.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee SS, Tsai CH, Yu CC, Ho YC, Hsu HI and
Chang YC: The expression of O(6)-methylguanine-DNA
methyltransferase in human oral keratinocytes stimulated with
arecoline. J Oral Pathol Med. 42:600–605. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen PH, Lee KW, Hsu CC, Chen JY, Wang YH,
Chen KK, Wang HM, Huang HW and Huang B: Expression of a splice
variant of CYP26B1 in betel quid-related oral cancer.
ScientificWorldJournal. 2014:8105612014.PubMed/NCBI
|
|
16
|
Chen PH, Huang B, Shieh TY, Wang YH, Chen
YK, Wu JH, Huang JH, Chen CC and Lee KW: The influence of monoamine
oxidase variants on the risk of betel quid-associated oral and
pharyngeal cancer. ScientificWorldJournal. 2014:1835482014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Takai R, Uehara O, Harada F, Utsunomiya M,
Chujo T, Yoshida K, Sato J, Nishimura M, Chiba I and Abiko Y: DNA
hypermethylation of extracellular matrix-related genes in human
periodontal fibroblasts induced by stimulation for a prolonged
period with lipopolysaccharide derived from Porphyromonas
gingivalis. J Periodontal Res. 51:508–517. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rehman A, Ali S, Lone MA, Atif M, Hassona
Y, Prime SS, Pitiyage GN, James EL and Parkinson EK: Areca nut
alkaloids induce irreparable DNA damage and senescence in
fibroblasts and may create a favourable environment for tumour
progression. J Oral Pathol Med. 45:365–372. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tseng YH, Chang KW, Liu CJ, Lin CY, Yang
SC and Lin SC: Areca nut extract represses migration and
differentiation while activating matrix metalloproteinase-9 of
normal gingival epithelial cells. J Periodontal Res. 43:490–499.
2008.PubMed/NCBI
|
|
21
|
Lukaszewicz-Zając M, Mroczko B and
Szmitkowski M: Gastric cancer - The role of matrix
metalloproteinases in tumor progression. Clin Chim Acta.
412:1725–1730. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li Y, Ma J, Guo Q, Duan F, Tang F, Zheng
P, Zhao Z and Lu G: Overexpression of MMP-2 and MMP-9 in esophageal
squamous cell carcinoma. Dis Esophagus. 22:664–667. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen Y, Zhang W, Geng N, Tian K and
Windsor L Jack: MMPs, TIMP-2, and TGF-beta1 in the cancerization of
oral lichen planus. Head Neck. 30:1237–1245. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fracalossi AC, Miranda SR, Oshima CT,
Franco M and Ribeiro DA: The role of matrix metalloproteinases 2
and 9 during rat tongue carcinogenesis induced by 4-nitroquinoline
1-oxide. J Mol Histol. 41:19–25. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jordan RC, Macabeo-Ong M, Shiboski CH,
Dekker N, Ginzinger DG, Wong DT and Schmidt BL: Overexpression of
matrix metalloproteinase-1 and -9 mRNA is associated with
progression of oral dysplasia to cancer. Clin Cancer Res.
10:6460–6465. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hohberger L, Wuertz BR, Xie H, Griffin T
and Ondrey F: TNF-alpha drives matrix metalloproteinase-9 in
squamous oral carcinogenesis. Laryngoscope. 118:1395–1399. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu SY, Lin MH, Yang SC, Huang GC, Chang
L, Chang S, Yen CY, Chiang WF, Kuo YY, Chen LL, et al: Increased
expression of matrix metalloproteinase-2 in oral cells after
short-term stimulation and long-term usage of areca quid. J Formos
Med Assoc. 104:390–397. 2005.PubMed/NCBI
|
|
28
|
Sawicki G, Marcoux Y, Sarkhosh K, Tredget
EE and Ghahary A: Interaction of keratinocytes and fibroblasts
modulates the expression of matrix metalloproteinases-2 and -9 and
their inhibitors. Mol Cell Biochem. 269:209–216. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lai WW, Hsu SC, Chueh FS, Chen YY, Yang
JS, Lin JP, Lien JC, Tsai CH and Chung JG: Quercetin inhibits
migration and invasion of SAS human oral cancer cells through
inhibition of NF-κB and matrix metalloproteinase-2/−9 signaling
pathways. Anticancer Res. 33:1941–1950. 2013.PubMed/NCBI
|
|
30
|
Lu LC, Yang CW, Hsieh WY, Chuang WH, Lin
YC and Lin CS: Decreases in plasma MMP-2/TIMP-2 and MMP-9/TIMP-1
ratios in uremic patients during hemodialysis. Clin Exp Nephrol.
20:934–942. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li S, Li C, Ryu HH, Lim SH, Jang WY and
Jung S: Bacitracin inhibits the migration of U87-MG Glioma cells
via interferences of the integrin outside-in signaling pathway. J
Korean Neurosurg Soc. 59:106–116. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mukhopadhyay P, Rajesh M, Bátkai S,
Kashiwaya Y, Haskó G, Liaudet L, Szabó C and Pacher P: Role of
superoxide, nitric oxide, and peroxynitrite in doxorubicin-induced
cell death in vivo and in vitro. Am J Physiol Heart Circ Physiol.
296:H1466–H1483. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chen KC, Wang YS, Hu CY, Chang WC, Liao
YC, Dai CY and Juo SH: OxLDL up-regulates microRNA-29b, leading to
epigenetic modifications of MMP-2/MMP-9 genes: A novel mechanism
for cardiovascular diseases. FASEB J. 25:1718–1728. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Scrideli CA, Cortez MA, Yunes JA, Queiróz
RG, Valera ET, da Mata JF, Toledo SR, Pavoni-Ferreira P, Lee ML,
Petrilli AS, et al: mRNA expression of matrix metalloproteinases
(MMPs) 2 and 9 and tissue inhibitor of matrix metalloproteinases
(TIMPs) 1 and 2 in childhood acute lymphoblastic leukemia:
Potential role of TIMP1 as an adverse prognostic factor. Leuk Res.
34:32–37. 2010. View Article : Google Scholar : PubMed/NCBI
|