Introduction
Extrahepatic cholangiocarcinoma (ECC) is a type of
primary malignancy with a poor prognosis originating from
extrahepatic biliary epithelial cells (1,2). At
present, there is no effective therapy other than surgical
resection for ECC. Furthermore, despite having received complete
tumor resection, the majority of ECC patients still develop local
recurrence or distant metastasis (3).
Therefore, novel approaches to treat ECC are urgently required in
order to improve patient prognosis.
With the further understanding of the regulation of
the immune system and the development of immunotherapies, immune
checkpoint blockade has emerged as one of the most promising
strategies for treating solid tumors (4,5).
Programmed cell death ligand-1 (PD-L1, also known as B7 homolog 1)
is a member of the B7 family of molecules that is expressed on the
surface of malignant cells in numerous tumor and tumor-associated
antigen-presenting cells, and facilitates immune evasion via its
interaction with programmed cell death protein 1 (PD-1) (6–9). High
PD-L1 expression has been detected in a number of human
malignancies, including gastric cancer (10), breast cancer (11), ovarian carcinomas (12), malignant melanoma (13), renal cell carcinomas (14), pancreatic carcinomas (15,16),
urothelial carcinomas (17–19), non-small cell lung cancer (20), intrahepatic cholangiocarcinoma (ICC)
(21) and esophageal cancer (22). The PD-1 receptor is expressed on the
surface of activated T cells and overexpressed in a large
proportion of tumor-infiltrating lymphocytes (TILs) from various
tumors, and leads to an intracellular inhibitory signal when bound
to one of its ligands, namely PD-L1 (8,23–26).
Multiple tumors have been shown to express PD-L1 and
PD-1 as a mechanism to achieve tumor immune evasion and
immunotolerance (27–29); thus, it can be hypothesized that by
blocking the binding of PD-1 to PD-1, activated T cells will retain
the capacity for tumor surveillance and targeted destruction.
Targeting the immune checkpoint can boost the anticancer responses
of T cells and restore their ability to detect and attack cancer
cells (30). This rationale has been
clinically validated by a novel class of immunotherapeutic agents
called checkpoint inhibitors, which include agents that target PD-1
and PD-L1 (31,32).
With the aim of exploring the feasibility of ECC
immunotherapy and understanding the prognosis of PD-1 and PD-L1
expression in EEC patients, which has not been reported previously,
the present study firstly detected the expression of PD-L1 and PD-1
in ECC patients, and analyzed the association of PD-L1 and PD-1
expression with clinicopathological characteristics and prognosis
in ECC patients.
Materials and methods
Collection of clinical samples
Clinical data, including age, sex,
tumor-node-metastasis (TNM) stage and pathological results, were
retrospectively collected from 70 patients who underwent surgical
resection and were pathologically confirmed to have ECC from
February 2009 to March 2013 at the First Affiliated Hospital of
Xi'an Jiaotong University (Xi'an, China). The group included 38
males and 32 females aged between 33 and 83 years (mean age, 62.5
years). Patients with ICC and/or ampullary carcinoma, or with other
organ primary malignant tumors were excluded from the study. All
the patients were staged according to the TNM stage classification
system of the 2010 American Joint Committee on Cancer (AJCC)
staging criteria (33). Survival time
was calculated from the date of diagnosis to the end of follow-up.
The patients were followed up until mortality or until the deadline
date of the study (March 2015), and those whose information was
incomplete were not included in the analysis. The present study was
approved by the Ethics Committee of the First Affiliated Hospital
of Xi'an Jiaotong University and written informed consent was
obtained from all patients.
Immunohistochemical staining and
scoring
All tissue specimens were formalin fixed, paraffin
embedded and cut in 4-µm-thick serial sections. Besides, 50
para-carcinoma tissues were assessed as the control group. To
detect the expression of PD-L1 and PD-1, immunohistochemical
staining was performed using an immunohistochemical kit (SP-9001;
Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd., Beijing,
China) according to the manufacturer's protocol. Briefly, sections
were dried for 30 min at 60°C prior to being deparaffinized in
xylene and rehydrated through a series of graded ethanol solutions.
Antigen retrieval was performed in 10 mM citrate buffer (pH 6.0) at
95°C for 5 min. Then, the sections were treated with 3% hydrogen
peroxide in methanol for 10 min at room temperature to quench
endogenous peroxidase activity, which was followed by incubation
with reagent A from the IHC kit for 15 min at room temperature.
Subsequently, the sections were incubated with rabbit monoclonal
anti-PD-L1 antibody (ab174838; Abcam, Cambridge, MA, USA) at 1:250
dilution and rabbit monoclonal anti-PD-1 antibody (ab137132; Abcam)
at 1:200 dilution in a humidified chamber at 4°C overnight. Upon
being washed in PBS, the sections were sequentially incubated with
reagents B and C for 15 min at 37°C. Then, the sections were
visualized by adding 3,3′-diaminobenzidine (ZLI-9018; Beijing
Zhongshan Golden Bridge Biotechnology Co., Ltd.) according to the
manufacturer's protocol. Finally, the sections were rinsed with
water and counterstained with Harris hematoxylin. For the negative
control, PBS was used instead of the primary antibody.
The staining results were scored with regard to the
percentage of positive tumor cells and the intensity of overall
staining. The sections were observed and independently scored by
two pathologists. The judgment standards were as follows: i)
PD-L1-positive result criterion in cancer cells: The proportion of
stained cells in each field was assessed as 0, <5% stained
cells; 1, 5–25% stained cells; 2, 26–50% stained cells; and 3,
>50% stained cells. Staining intensity was graded as 0, negative
staining; 1, weak staining; 2, moderate staining; and 3, intense
staining. The staining-intensity-distribution (SID), which was
obtained by multiplying the score of the percentage of stained
cells by the score of the staining intensity, was judged as 0–2 for
negative staining and ≥3 for positive staining; ii) PD-L1-positive
result criterion in TILs: Negative, <1% stained cells; and
positive ≥1% stained cells (34); and
iii) PD-1-positive result criterion in TILs: Five randomly selected
different high-power fields were observed. The number of TILs in
300 lymphocytes in each field was counted. The mean number, i.e.,
40 of the 70 specimens was considered as the threshold. If TIL
>40, the staining was considered positive; if TIL ≤40, the
staining was considered negative.
Statistical analysis
Statistical analysis was performed using SPSS
version 18.0 (SPPS, Inc., Chicago, IL, USA). Differences between
two groups of measurement data were analyzed using the Student's
t-test. The χ2 test was used to analyze the differences
between groups of enumeration data. Kaplan-Meier plots and log-rank
tests were used for survival analysis. Multivariate analyses were
based on the Cox proportional hazards regression model. All
statistical tests were two sided, and P<0.05 was considered to
indicate a statistically significant difference.
Results
Immunohistochemical analysis of PD-L1
or PD-1 expression and their association with clinicopathological
characteristics
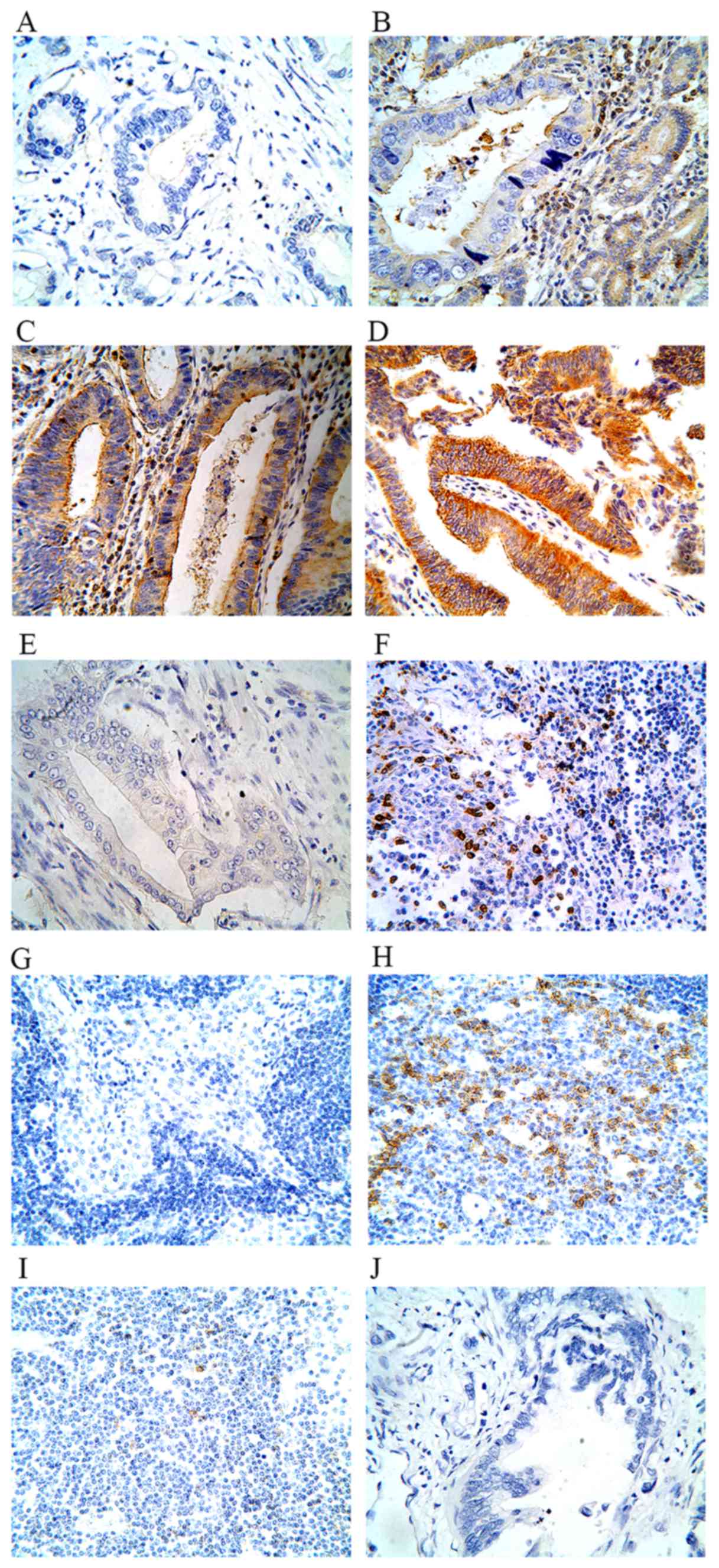
In ECC tissues, immunostaining of PD-L1 was observed
in the plasma membrane and cytoplasm of cancer cells and TILs. PD-1
expression was observed in TILs. Representative images are shown in
Fig. 1.
The positive rate of PD-L1 expression in bile duct
cells of ECC tissues was 42.86% (30/70), while the positive rate in
para-carcinoma tissues was 16.00% (8/50). The difference was
statistically significant (P=0.002). The mean SID score of PD-L1
staining was 2.8841±2.1181 [mean ± standard deviation (SD)] in ECC
tissues and 1.8400±2.1346 (mean ± SD) in para-carcinoma tissues
(P=0.038). The positive rate of PD-L1 expression in TILs of ECC
tissues was 37.10% (26/70). By contrast, only 20.00% (10/50) of
para-carcinoma tissues exhibited positive PD-L1 expression
(P=0.043).
Of 70 ECC tissues specimens, 37 cases (52.86%)
demonstrated positive PD-I expression in TILs. The positive rate
was significantly different between the ECC tissues and the
para-carcinoma tissues (P=0.002). It was also observed that PD-L1
expression correlated with PD-1 expression (P<0.05) (Table I). The association between PD-L1 or
PD-1 expression and various clinicopathological characteristics of
ECC patients is listed in Table II.
The results revealed that the expression levels of PD-LI and PD-I
were significantly associated with the AJCC TNM stage of disease
(P<0.05) and lymphatic metastasis (P<0.05).
 | Table I.Correlation between PD-L1 and PD-1
expression in extrahepatic cholangiocarcinoma tissues. |
Table I.
Correlation between PD-L1 and PD-1
expression in extrahepatic cholangiocarcinoma tissues.
|
|
| PD-L1 in cancer
cells | PD-L1 in TILs |
|---|
|
|
|---|
| PD-1 expression in
TILs | No. | Negative | Positive | χ2 | P-value | Cramér's V | Negative | Positive | χ2 | P-value | Cramér's V |
|---|
| Negative | 33 | 26 | 7 | 11.944 | 0.001 | 0.413 | 27 | 6 | 9.614 | 0.002 | 0.371 |
| Positive | 37 | 14 | 23 |
|
|
| 17 | 20 |
|
|
|
| Total | 70 | 40 | 30 |
|
|
| 44 | 26 |
|
|
|
 | Table II.Association between PD-L1 or PD-1
expression and clinicopathological characteristics of extrahepatic
cholangiocarcinoma patients. |
Table II.
Association between PD-L1 or PD-1
expression and clinicopathological characteristics of extrahepatic
cholangiocarcinoma patients.
|
|
| PD-L1 in cancer
cells | PD-L1 in TILs | PD-1 in TILs |
|---|
|
|
|
|---|
| Variable | No. | Positive (%) | χ2 | P-value | Positive (%) | χ2 | P-value | Positive (%) | χ2 | P-value |
|---|
| Sex |
|
|
|
|
|
|
|
|
|
|
|
Male | 38 | 17 (44.7) |
0.120 | 0.729 | 11 (28.9) | 2.391 | 0.122 | 19 (50.0) | 0.272 | 0.602 |
|
Female | 32 | 13 (40.6) |
|
| 15 (46.8) |
|
| 18 (56.3) |
|
|
| Age, years |
|
|
|
|
|
|
|
|
|
|
|
≤60 | 26 | 12 (46.2) |
0.184 | 0.668 | 12 (46.2) | 1.439 | 0.230 | 16 (61.5) | 1.251 | 0.263 |
|
>60 | 44 | 18 (40.9) |
|
| 14 (31.8) |
|
| 21 (47.7) |
|
|
| AJCC stage |
|
|
|
|
|
|
|
|
|
|
| I/II | 64 | 24 (39.1) |
6.384 | 0.012a | 21 (32.8) | 4.028 | 0.045a | 31 (48.4) | 3.967 | 0.046a |
| III/IV | 6 | 6 (100.0) |
|
| 5 (83.3) |
|
| 6 (100.0) |
|
|
| Lymphatic
metastasis |
|
|
|
|
|
|
|
|
|
|
|
Yes | 21 | 15 (71.4) | 10.000 | 0.002 | 13 (61.9) | 7.879 | 0.005 | 16 (76.2) | 6.182 | 0.013 |
| No | 49 | 15 (30.6) |
|
| 13 (26.5) |
|
| 21 (42.8) |
|
|
| Histological
grade |
|
|
|
|
|
|
|
|
|
|
|
1/2 | 55 | 22 (40.0) |
0.856 | 0.355 | 18 (32.7) | 2.143 | 0.143 | 29 (52.7) | 0.002 | 0.967 |
| 3 | 15 | 8 (53.3) |
|
| 8 (53.3) |
|
| 8 (53.3) |
|
|
Survival analysis for ECC with the
Kaplan-Meier method and Cox proportional hazards regression
model
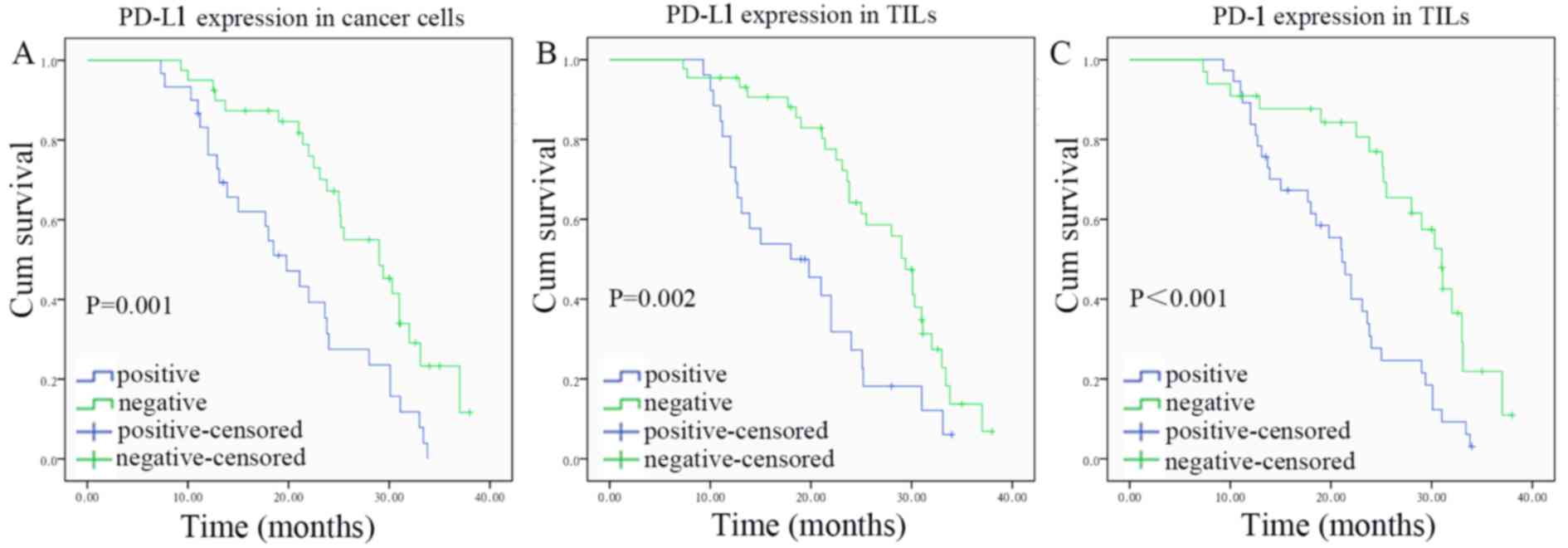
Univariate analyses revealed that the overall
survival time of ECC patients was significantly associated with
PD-L1 or PD-1 expression, TNM stage and lymphatic metastasis
(Table III). The Kaplan-Meier
survival curves for PD-L1- and PD-1-positive and -negative cases
are shown in Fig. 2. The prognostic
factors considered significant on univariate analysis were
subjected to multivariate analysis using a Cox proportional hazards
model. Further multivariate analysis revealed that PD-L1 expression
in cancer cells [hazard ratio (HR), 2.314; 95% confidence interval
(CI), 1.129–4.747; P=0.022] or TILs (HR, 2.468; 95% CI,
1.229–4.956; P=0.011) of ECC patients remained independently
associated with survival time (Table
IV).
 | Table III.Univariate analysis of prognosis for
extrahepatic cholangiocarcinoma patients (n=70). |
Table III.
Univariate analysis of prognosis for
extrahepatic cholangiocarcinoma patients (n=70).
| Variable | mOS, months | HR (95% CI) | P-value |
|---|
| Sex |
|
|
|
| Female
vs. male | 25.1 vs. 23.8 | 0.921
(0.530–1.600) | 0.770 |
| Age at diagnosis,
years |
|
|
|
| ≤60 vs.
>60 | 23.1 vs. 29.0 | 0.635
(0.362–1.117) | 0.111 |
| AJCC stage |
|
|
|
| /II vs.
III/IV | 25.5 vs. 19.8 | 2.701
(1.109–6.582) | 0.022 |
| Histological
grade |
|
|
|
| 1/2 vs.
3 | 25.0 vs. 22.0 | 1.247
(0.662–2.351) | 0.491 |
| Lymphatic
metastasis |
|
|
|
| Yes vs.
no | 19.8 vs. 28.0 | 0.507
(0.284–0.905) | 0.019 |
| PD-L1 in cancer
cells |
|
|
|
|
Positive vs. negative | 19.8 vs. 29.0 | 0.413
(0.237–0.719) | 0.001 |
| PD-L1 in TILs |
|
|
|
|
Positive vs. negative | 18.0 vs. 29.4 | 0.424
(0.241–0.746) | 0.002 |
| PD-1 in TILs |
|
|
|
|
Positive vs. negative | 21.1 vs. 31.0 | 0.368
(0.205–0.659) | <0.001 |
 | Table IV.Multivariate analysis of prognosis
for extrahepatic cholangiocarcinoma patients (n=70). |
Table IV.
Multivariate analysis of prognosis
for extrahepatic cholangiocarcinoma patients (n=70).
| Variable | P-value | HR (95% CI) |
|---|
| AJCC stage | 0.764 | 1.169
(0.422–3.236) |
| Lymphatic
metastasis | 0.959 | 1.016
(0.543–1.901) |
| PD-L1 in cancer
cells | 0.022 | 2.314
(1.129–4.747) |
| PD-L1 in TILs | 0.011 | 2.468
(1.229–4.956) |
| PD-1 in TILs | 0.209 | 1.568
(0.778–3.160) |
Discussion
In the past two decades, much attention has been
paid to PD-L1 and its receptor PD-1 due to their roles in tumor
immune evasion and immunotolerance mechanisms (27–29). The
activation of the PD-L1/PD-1 pathway can lead to an inhibitory
immune tumor microenvironment, which results in the cancer cells
evading immune surveillance and destruction (7). On the contrary, blocking this pathway
can enhance endogenous antitumor immune effects, which is the
theoretical basis of the promising strategy known as immune
checkpoint blockade for targeting solid tumors (4,5).
Currently, numerous checkpoint inhibitors targeting PD-1 (such as
nivolumab and pembrolizumab) and PD-L1 (such as MPDL3280A and
BMS-936559) have achieved promising antitumor effects without
serious adverse reactions in clinical trials (31,32,35). Thus,
pembrolizumab and nivolumab were approved for the treatment of
advanced melanoma and NSCLC by the USA Food and Drug Administration
in 2014 and 2015, respectively (36).
Considering the rising morbidity and poor prognosis
of ECC as well as the benefits to clinical patients exerted by
immune therapies, and with the aim of exploring the feasibility of
ECC immunotherapy, the present study detected the expression of
PD-L1 and PD-1 by immunohistochemical staining in ECC patients, and
further analyzed the association between PD-L1 or PD-1 expression
with clinicopathological characteristics, as well as the ability of
predicting the prognosis of patients with ECC. The present study
provides the first evidence that the positive rate of PD-L1
expression as well as that of its receptor PD-1 in ECC tissues were
increased compared with those in para-carcinoma tissues. PD-L1 was
highly expressed in both cancer cells and TILs, while PD-1 was
highly expressed in TILs of ECC tissues. To better understand the
role of PD-L1 expression in ECC patients and the association
between PD-L1 and PD1 expression, the significance of PD-L1
expression was separately analyzed according to the expression
localization.
Analysis of the associations of PD-L1 or PD-1
expression with ECC clinicopathological characteristics and
prognosis revealed that the expression levels of PD-L1 and PD-1
were significantly associated with lymphatic metastasis, tumor
stage and overall survival, which suggests that tumor-associated
PD-L1 and PD-1 expression may be relevant to tumor progression, and
that PD-L1 and PD-1 may be involved in the occurrence and
development of ECC. Thus, immunotherapy for ECC patients using
monoclonal antibodies against PD-L1 or PD-1 may benefit ECC
patients.
The high expression of PD-L1 in different cancers
and its clinical significance have been extensively studied, and
previous survival analyses demonstrated that PD-L1 was associated
with prognosis (10–12,14,16,17,22).
The present observations indicated that PD-L1 expression in cancer
cells or TILs of ECC patients was significantly correlated with
tumor stage and lymphatic metastasis, and that patients with high
expression of PD-L1 had a significantly poorer prognosis than those
with low expression, which was in line with the results from
previous reports on other cancer types (10–12,14,16,17,22).
Additionally, Muenst et al (37) explored the expression of PD-1 in TILs
on a tissue microarray encompassing 660 breast cancer cases, which
revealed that PD-1 was associated with tumor size, grade and lymph
node status, and was differentially associated with overall
survival. To summarize, the present study demonstrated that PD-L1
or PD-1 immunodetection may be a valuable prognostic marker for ECC
patients.
Notably, the present study observed that the
expression of PD-L1 exhibited a positive correlation with the
expression of PD-1. Besides, tumor-associated PD-LI expression was
inversely correlated with cluster of differentiation 8+
TILs (16,20,21).
Therefore, it is possible that overexpressed PD-L1 in ECC cancer
cells or TILs interacts with PD-1 in TILs, and these interactions
may inhibit the functions of T lymphocytes, resulting in the cancer
cells evading immune surveillance and destruction. Of note, this
assumption must be further studied, as well as the particular role
of PD-LI expression in TILs.
Regarding the regulation of PD-L1 expression, two
general mechanisms involving tumor cells have emerged: Innate
immune resistance and adaptive immune resistance (5). Certain studies indicated that the
phosphoinositide 3-kinase-AKT signaling pathway (38), anaplastic lymphoma kinase signaling
(39) and several cytokines (27,40,41)
participate in the regulation of PD-L1 expression. However, the
specific mechanism of PD-L1 increase in cancer cells and TILs, and
of PD-1 increase in TILs of ECC tissues, as well as how their
interaction leads to an immune suppressive microenvironment and
evasion of immune surveillance, must be further investigated.
Since the present study is a retrospective,
small-sample study and sampling differences may influence the
result, further prospective, large-sample studies are required to
confirm the role of PD-L1 and PD-1 in ECC. Certainly, further
investigation regarding the regulation and biological
characteristics of PD-L1 and PD-1 will help us to clarify the
clinical significance and develop new strategies of tumor
immunotherapy. The authors consider that immunotherapy will benefit
ECC patients.
Glossary
Abbreviations
Abbreviations:
|
PD-L1
|
programmed cell death ligand-1
|
|
PD-1
|
programmed cell death protein 1
|
References
|
1
|
Nakamura H, Arai Y, Totoki Y, Shirota T,
Elzawahry A, Kato M, Hama N, Hosoda F, Urushidate T, Ohashi S, et
al: Genomic spectra of biliary tract cancer. Nat Genet.
47:1003–1010. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Aljiffry M, Walsh MJ and Molinari M:
Advances in diagnosis, treatment and palliation of
cholangiocarcinoma: 1990–2009. World J Gastroenterol. 15:4240–4262.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Murakami Y, Uemura K, Sudo T, Hayashidani
Y, Hashimoto Y, Nakamura H, Nakashima A and Sueda T: Adjuvant
gemcitabine plus S-1 chemotherapy improves survival after
aggressive surgical resection for advanced biliary carcinoma. Ann
Surg. 250:950–956. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bordon Y: Tumour immunology: Checkpoint
parley. Nat Rev Immunol. 15:52015. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pardoll DM: The blockade of immune
checkpoints in cancer immunotherapy. Nat Rev Cancer. 12:252–264.
2012. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zang X and Allison JP: The B7 family and
cancer therapy: Costimulation and coinhibition. Clin Cancer Res.
13:5271–5279. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zou W and Chen L: Inhibitory B7-family
molecules in the tumour microenvironment. Nat Rev Immunol.
8:467–477. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Godwin JL, Zibelman M, Plimack ER and
Geynisman DM: Immune checkpoint blockade as a novel
immunotherapeutic strategy for renal cell carcinoma: A review of
clinical trials. Discov Med. 18:341–350. 2014.PubMed/NCBI
|
|
9
|
Freeman GJ, Long AJ, Iwai Y, Bourque K,
Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne
MC, et al: Engagement of the PD-1 immunoinhibitory receptor by a
novel B7 family member leads to negative regulation of lymphocyte
activation. J Exp Med. 192:1027–1034. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wu C, Zhu Y, Jiang J, Zhao J, Zhang XG and
Xu N: Immunohistochemical localization of programmed death-1
ligand-1 (PD-L1) in gastric carcinoma and its clinical
significance. Acta histochem. 108:19–24. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ghebeh H, Mohammed S, Al-Omair A, Qattan
A, Lehe C, Al-Qudaihi G, Elkum N, Alshabanah M, Bin Amer S, Tulbah
A, et al: The B7-H1 (PD-L1) T lymphocyte-inhibitory molecule is
expressed in breast cancer patients with infiltrating ductal
carcinoma: Correlation with important high-risk prognostic factors.
Neoplasia. 8:190–198. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hamanishi J, Mandai M, Iwasaki M, Okazaki
T, Tanaka Y, Yamaguchi K, Higuchi T, Yagi H, Takakura K, Minato N,
et al: Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+
T lymphocytes are prognostic factors of human ovarian cancer. Proc
Natl Acad Sci USA. 104:pp. 3360–3365. 2007; View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yang W, Chen PW, Li H, Alizadeh H and
Niederkorn JY: PD-L1: PD-1 interaction contributes to the
functional suppression of T-cell responses to human uveal melanoma
cells in vitro. Invest Ophthalmol Vis Sci. 49:2518–2525. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Thompson RH, Kuntz SM, Leibovich BC, Dong
H, Lohse CM, Webster WS, Sengupta S, Frank I, Parker AS, Zincke H,
et al: Tumor B7-H1 is associated with poor prognosis in renal cell
carcinoma patients with long-term follow-up. Cancer Res.
66:3381–3385. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Geng L, Huang D, Liu J, Qian Y, Deng J, Li
D, Hu Z, Zhang J, Jiang G and Zheng S: B7-H1 up-regulated
expression in human pancreatic carcinoma tissue associates with
tumor progression. J Cancer Res Clin Oncol. 134:1021–1027. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nomi T, Sho M, Akahori T, Hamada K, Kubo
A, Kanehiro H, Nakamura S, Enomoto K, Yagita H, Azuma M and
Nakajima Y: Clinical significance and therapeutic potential of the
programmed death-1 ligand/programmed death-1 pathway in human
pancreatic cancer. Clin Cancer Res. 13:2151–2157. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xylinas E, Robinson BD, Kluth LA, Volkmer
BG, Hautmann R, Küfer R, Zerbib M, Kwon E, Thompson RH, Boorjian SA
and Shariat SF: Association of T-cell co-regulatory protein
expression with clinical outcomes following radical cystectomy for
urothelial carcinoma of the bladder. Eur J Surg Oncol. 40:121–127.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nakanishi J, Wada Y, Matsumoto K, Azuma M,
Kikuchi K and Ueda S: Overexpression of B7-H1 (PD-L1) significantly
associates with tumor grade and postoperative prognosis in human
urothelial cancers. Cancer Immunol Immunother. 56:1173–1182. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Inman BA, Sebo TJ, Frigola X, Dong H,
Bergstralh EJ, Frank I, Fradet Y, Lacombe L and Kwon ED: PD-L1
(B7-H1) expression by urothelial carcinoma of the bladder and
BCG-induced granulomata: Associations with localized stage
progression. Cancer. 109:1499–1505. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Konishi J, Yamazaki K, Azuma M, Kinoshita
I, Dosaka-Akita H and Nishimura M: B7-H1 expression on non-small
cell lung cancer cells and its relationship with tumor-infiltrating
lymphocytes and their PD-1 expression. Clin Cancer Res.
10:5094–5100. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ye Y, Zhou L, Xie X, Jiang G, Xie H and
Zheng S: Interaction of B7-H1 on intrahepatic cholangiocarcinoma
cells with PD-1 on tumor-infiltrating T cells as a mechanism of
immune evasion. J Surg Oncol. 100:500–504. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ohigashi Y, Sho M, Yamada Y, Tsurui Y,
Hamada K, Ikeda N, Mizuno T, Yoriki R, Kashizuka H, Yane K, et al:
Clinical significance of programmed death-1 ligand-1 and programmed
death-1 ligand-2 expression in human esophageal cancer. Clin Cancer
Res. 11:2947–2953. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang Y, Huang S, Gong D, Qin Y and Shen
Q: Programmed death-1 upregulation is correlated with dysfunction
of tumor-infiltrating CD8+ T lymphocytes in human non-small cell
lung cancer. Cell Mol Immunol. 7:389–395. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lyford-Pike S, Peng S, Young GD, Taube JM,
Westra WH, Akpeng B, Bruno TC, Richmon JD, Wang H, Bishop JA, et
al: Evidence for a role of the PD-1:PD-L1 pathway in immune
resistance of HPV-associated head and neck squamous cell carcinoma.
Cancer Res. 73:1733–1741. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sfanos KS, Bruno TC, Meeker AK, de Marzo
AM, Isaacs WB and Drake CG: Human prostate-infiltrating CD8+ T
lymphocytes are oligoclonal and PD-1+. Prostate. 69:1694–1703.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ahmadzadeh M, Johnson LA, Heemskerk B,
Wunderlich JR, Dudley ME, White DE and Rosenberg SA: Tumor
antigen-specific CD8 T cells infiltrating the tumor express high
levels of PD-1 and are functionally impaired. Blood. 114:1537–1544.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dong H, Strome SE, Salomao DR, Tamura H,
Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, et al:
Tumor-associated B7-H1 promotes T-cell apoptosis: A potential
mechanism of immune evasion. Nat Med. 8:793–800. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Blank C, Brown I, Peterson AC, Spiotto M,
Iwai Y, Honjo T and Gajewski TF: PD-L1/B7H-1 inhibits the effector
phase of tumor rejection by T cell receptor (TCR) transgenic CD8+ T
cells. Cancer Res. 64:1140–1145. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Keir ME, Butte MJ, Freeman GJ and Sharpe
AH: PD-1 and its ligands in tolerance and immunity. Annu Rev
Immunol. 26:677–704. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Flemming A: Cancer: PD1 makes waves in
anticancer immunotherapy. Nat Rev Drug Discov. 11:6012012.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Topalian SL, Hodi FS, Brahmer JR,
Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD,
Sosman JA, Atkins MB, et al: Safety, activity, and immune
correlates of anti-PD-1 antibody in cancer. N Engl J Med.
366:2443–2454. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ,
Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, et al:
Safety and activity of anti-PD-L1 antibody in patients with
advanced cancer. N Engl J Med. 366:2455–2465. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Edge SB, Byrd DR, Compton CC, Fritz AG,
Greene FL and Trotti A: AJCC Cancer Staging Manual. 7th. Springer;
New York, NY: pp. 66–71. 2010
|
|
34
|
Al-Shibli K, Al-Saad S, Donnem T, Persson
M, Bremnes RM and Busund LT: The prognostic value of
intraepithelial and stromal innate immune system cells in non-small
cell lung carcinoma. Histopathology. 55:301–312. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sunshine J and Taube JM: PD-1/PD-L1
inhibitors. Curr Opin Pharmacol. 23:32–38. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lee J, Kefford R and Carlino M: PD-1 and
PD-L1 inhibitors in melanoma treatment: Past success, present
application and future challenges. Immunotherapy. 8:733–746. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Muenst S, Soysal SD, Gao F, Obermann EC,
Oertli D and Gillanders WE: The presence of programmed death 1
(PD-1)-positive tumor-infiltrating lymphocytes is associated with
poor prognosis in human breast cancer. Breast Cancer Res Treat.
139:667–676. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Parsa AT, Waldron JS, Panner A, Crane CA,
Parney IF, Barry JJ, Cachola KE, Murray JC, Tihan T, Jensen MC, et
al: Loss of tumor suppressor PTEN function increases B7-H1
expression and immunoresistance in glioma. Nat Med. 13:84–88. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Marzec M, Zhang Q, Goradia A, Raghunath
PN, Liu X, Paessler M, Wang HY, Wysocka M, Cheng M, Ruggeri BA and
Wasik MA: Oncogenic kinase NPM/ALK induces through STAT3 expression
of immunosuppressive protein CD274 (PD-L1, B7-H1). Proc Natl Acad
Sci USA. 105:pp. 20852–20857. 2008; View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Curiel TJ, Wei S, Dong H, Alvarez X, Cheng
P, Mottram P, Krzysiek R, Knutson KL, Daniel B, Zimmermann MC, et
al: Blockade of B7-H1 improves myeloid dendritic cell-mediated
antitumor immunity. Nat Med. 9:562–567. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Brown JA, Dorfman DM, Ma FR, Sullivan EL,
Munoz O, Wood CR, Greenfield EA and Freeman GJ: Blockade of
programmed death-1 ligands on dendritic cells enhances T cell
activation and cytokine production. J Immunol. 170:1257–1266. 2003.
View Article : Google Scholar : PubMed/NCBI
|