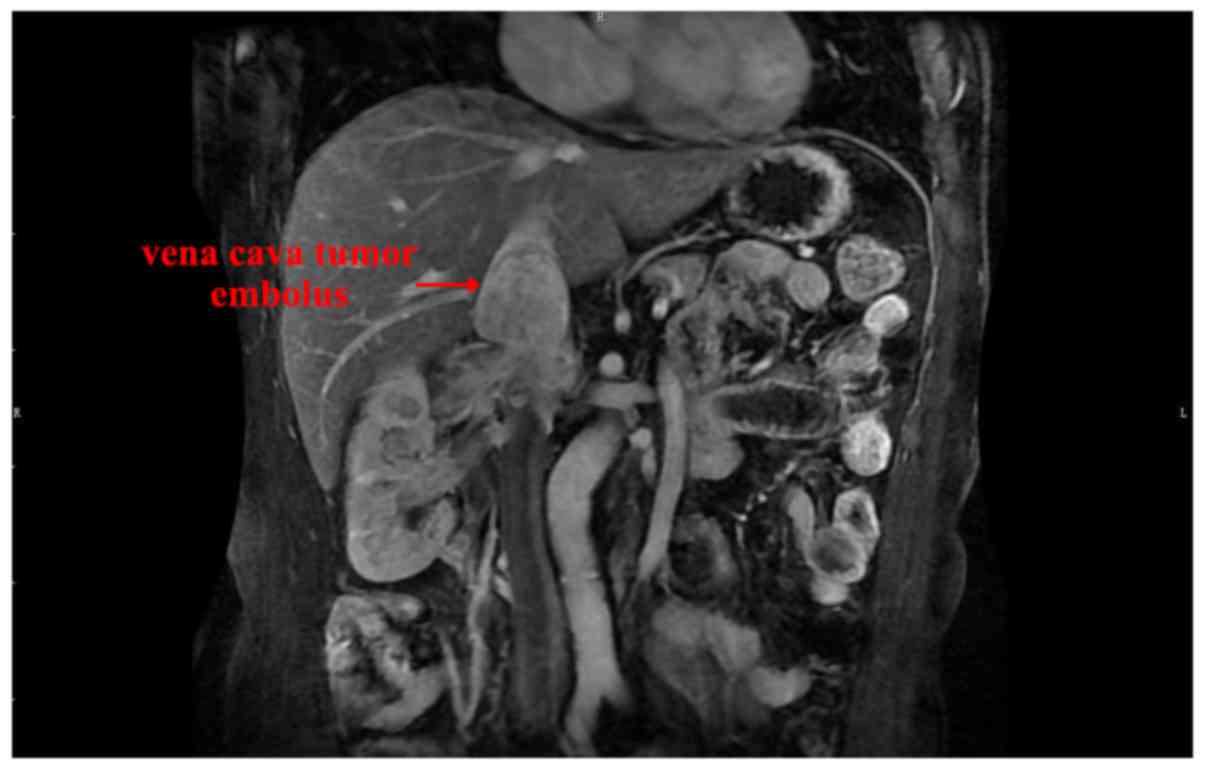
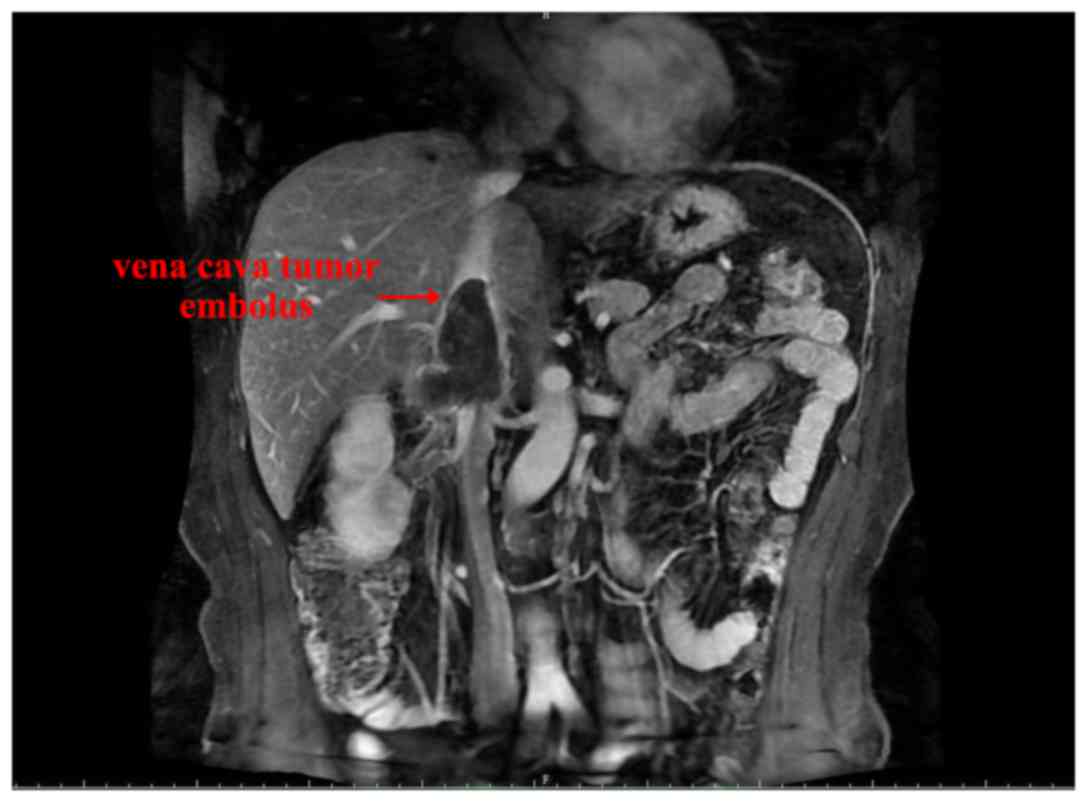
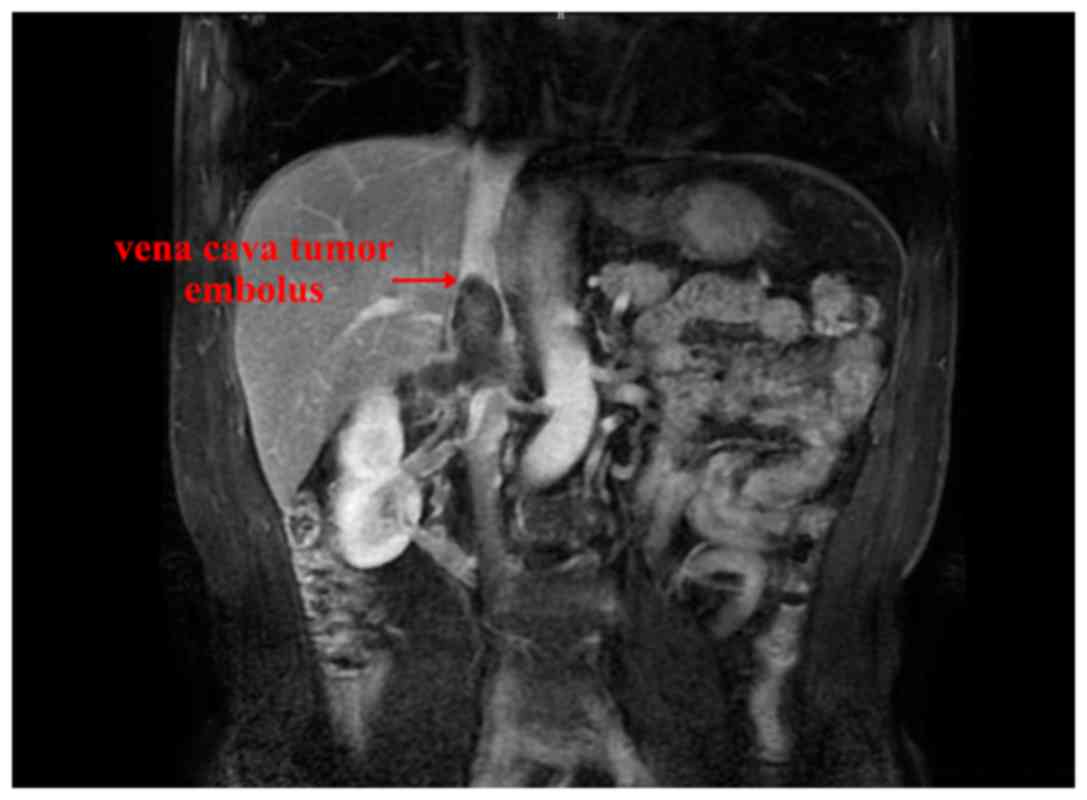
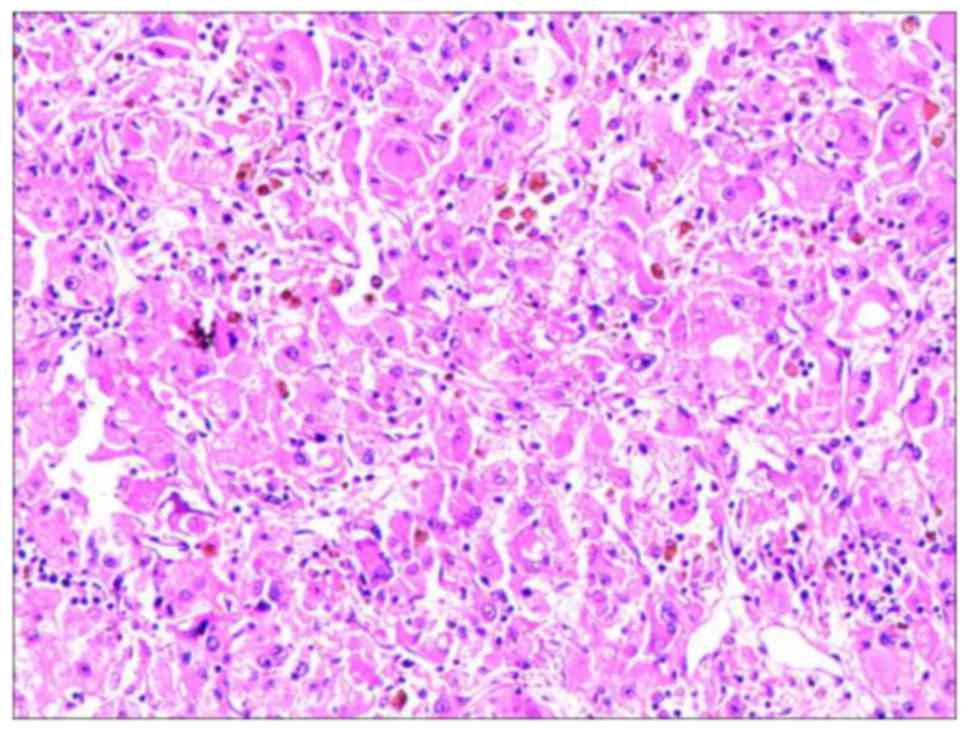
|
1
|
Bhatt JR and Finelli A: Landmarks in the
diagnosis and treatment of renal cell carcinoma. Nat Rev Urol.
11:517–525. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
González J, Gorin MA and Ciancio G:
Long-term survival after radical surgery for renal cell carcinoma
with tumour thrombus. BJU Int. 111:E8–E9. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Novick AC and Campbell SC: Renal
TumorsCampbell's Urology. Walsh PC, Retik AB, Vaughan ED and Wein
AJ: WB Saunders; Philadephia: pp. 2672–2731. 2002
|
|
4
|
Shuch B, Riggs SB, LaRochelle JC,
Kabbinavar FF, Avakian R, Pantuck AJ, Patard JJ and Belldegrun AS:
Neoadjuvant targeted therapy and advanced kidney cancer:
Observations and implications for a new treatment paradigm. BJU
Int. 102:692–696. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Di Silverio F, Sciarra A, Parente U,
Andrea A, Von Heland M, Panebianco V and Passariello R: Neoadjuvant
therapy with sorafenib in advanced renal cell carcinoma with vena
cava extension submitted to radical nephrectomy. Urol Int.
80:451–453. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Karakiewicz PI, Suardi N, Jeldres C, Audet
P, Ghosn P, Patard JJ and Perrotte P: Neoadjuvant sutent induction
therapy may effectively down-stage renal cell carcinoma atrial
thrombi. Eur Urol. 53:845–848. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Peters I, Winkler M, Jüttner B, Teebken
OE, Herrmann TR, von Klot C, Kramer M, Reichelt A, Abbas M, Kuczyk
MA and Merseburger AS: Neoadjuvant targeted therapy in a primary
metastasized renal cell cancer patient leads to down-staging of
inferior vena cava thrombus (IVC) enabling a cardiopulmonary
bypass-free tumor nephrectomy: A case report. World J Urol.
32:245–248. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Horn T, Thalgott MK, Maurer T, Hauner K,
Schulz S, Fingerle A, Retz M, Gschwend JE and Kübler HR:
Presurgical treatment with sunitinib for renal cell carcinoma with
a level III/IV vena cava tumour thrombus. Anticancer Res.
32:1729–1735. 2012.PubMed/NCBI
|
|
9
|
Amin C, Wallen E, Pruthi RS, Calvo BF,
Godley PA and Rathmell WK: Preoperative tyrosine kinase inhibition
as an adjunct to debulking nephrectomy. Urology. 72:864–868. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kondo T, Hashimoto Y, Kobayashi H, Iizuka
J, Nishikawa T, Nakano M and Tanabe K: Presurgical targeted therapy
with tyrosine kinase inhibitors for advanced renal cell carcinoma:
Clinical results and histopathological therapeutic effects. Jpn J
Clin Oncol. 40:1173–1179. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cowey CL, Amin C, Pruthi RS, Wallen EM,
Nielsen ME, Grigson G, Watkins C, Nance KV, Crane J, Jalkut M, et
al: Neoadjuvant clinical trial with sorafenib for patients with
stage II or higher renal cell carcinoma. J Clin Oncol.
28:1502–1507. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rini BI, Garcia J, Elson P, Wood L, Shah
S, Stephenson A, Salem M, Gong M, Fergany A, Rabets J, et al: The
effect of sunitinib on primary renal cell carcinoma and
facilitation of subsequent surgery. J Urol. 187:1548–1554. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cost NG, Delacroix SE Jr, Sleeper JP,
Smith PJ, Youssef RF, Chapin BF, Karam JA, Culp S, Abel EJ,
Brugarolas J, et al: The impact of targeted molecular therapies on
the level of renal cell carcinoma vena caval tumor thrombus. Eur
Urol. 59:912–918. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Therasse P, Arbuck SG, Eisenhauer EA,
Wanders J, Kaplan RS, Rubinstein L, Verweij J, van Glabbeke M, van
Oosterom AT, Christian MC, et al: New guidelines to evaluate the
response to treatment in solid tumors. J Natl Cancer Inst.
92:205–216. 2000. View Article : Google Scholar
|
|
15
|
National Cancer Institute, . Cancer
Therapy Evaluation Program, Common Terminology Criteria for Adverse
Events v3.0 (CTCAE). http://ctep.cancer.gov
|
|
16
|
Kiernan JA: Histological and Histochemical
Methods: Theory and Practice. 4th. Scion; Bloxham, UK: 2008
|
|
17
|
Bex A, Kroon BK and de Bruijn R: Is there
a role for neoadjuvant targeted therapy to downsize primary tumors
for organ sparing strategies in renal cell carcinoma? Int J Surg
Oncol. 2012:2504792012.PubMed/NCBI
|
|
18
|
Kroon BK, de Bruijn R, Prevoo W, Horenblas
S, Powles T and Bex A: Probability of downsizing primary tumors of
renal cell carcinoma by targeted therapies is related to size at
presentation. Urology. 81:111–115. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Vogelzang NJ, Samlowski W and Weissman A:
Long-term response in primary renal cancer to sequential
antiangiogenic therapy. J Clin Oncol. 27:e106–e107. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hutson TE, Bukowski RM, Cowey CL, Figlin
R, Escudier B and Sternberg CN: Sequential use of targeted agents
in the treatment of renal cell carcinoma. Crit Rev Oncol Hematol.
77:48–62. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Escudier B, Eisen T, Porta C, Patard JJ,
Khoo V, Algaba F, Mulders P and Kataja V; ESMO Guidelines Working
Group, : Renal cell carcinoma: ESMO clinical practice guidelines
for diagnosis, treatment and follow-up. Ann Oncol. 23 Suppl
7:vii65–vii71. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Escudier B, Pluzanska A, Koralewski P,
Ravaud A, Bracarda S, Szczylik C, Chevreau C, Filipek M, Melichar
B, Bajetta E, et al: Bevacizumab plus interferon alfa2a for
treatment of metastatic renal cell carcinoma: A randomised,
double-blind phase III trial. Lancet. 370:2103–2111. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Escudier R, Eisen T, Stadler WM, Szczylik
C, Oudard S, Siebels M, Negrier S, Chevreau C, Solska E, Desai AA,
et al: Sorafenib in advanced clear-cell renal-cell carcinoma. N
Engl J Med. 356:125–134. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sternberg CN, Davis ID, Mardiak J,
Szczylik C, Lee E, Wagstaff J, Barrios CH, Salman P, Gladkov OA,
Kavina A, et al: Pazopanib in locally advanced or metastatis renal
cell carcinoma: Results of a randomized phase III trial. J Clin
Oncol. 28:1061–1068. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Thomas AA, Rini BI, Stephenson AJ, Garcia
JA, Fergany A, Krishnamurthi V, Novick AC, Gill IS, Klein EA, Zhou
M, et al: Surgical resection of renal cell carcinoma after targeted
therapy. J Urol. 182:881–886. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chapin BF, Delacroix SE Jr, Culp SH,
Gonzalez GM Nogueras, Tannir NM, Jonasch E, Tamboli P and Wood CG:
Safety of presurgical targeted therapy in the setting of metastatic
renal cell carcinoma. Eur Urol. 60:964–971. 2011. View Article : Google Scholar : PubMed/NCBI
|