Introduction
Cancers of the head, neck and esophagus are
associated with the same carcinogens, in particular tobacco and
alcohol (1,2). Smoking and alcohol consumption have been
associated with an increase in the rate of incidence of synchronous
or metachronous cancers in patients with head and neck cancer
(HNC). The independent development of such cancers following
exposure to a common carcinogen is known as the field cancerization
phenomenon (3). In particular,
synchronous or metachronous esophageal cancer has been frequently
detected in patients with HNC (4).
The majority of hypopharyngeal squamous cell
carcinomas (HPSCCs) have a poor prognosis. One reason for this poor
prognosis is that early detection of HPSCC is often difficult since
Lugol chromoendoscopy is used in the esophagus but not at the
laryngopharyngeal site (5). HPSCCs
are therefore often found as advanced disease at the initial
diagnosis (6). However, recent
studies suggested that superficial squamous neoplasms in the
orohypopharynx and esophagus can be diagnosed with a high
diagnostic value using narrow-band imaging (NBI) endoscopy with or
without magnification (6). In
addition, previous Japanese studies suggest that peroral endoscopic
resection is useful for treatment of superficial HPSCC, in which
tumor depth is confined to the subepithelial layer (7,8).
Understanding the molecular events that underlie the
development of HPSCC and its double esophageal squamous cell
carcinoma (DESCC) may aid the development of strategies for the
prevention and therapy of these cancers (1,2). These
findings may also be useful in developing diagnostic tests for
patients with second primary malignancies. It has been reported
that carcinogenesis and its progression in head, neck and
esophageal cancers involves the abnormal expression of several
genes including p53, Fragile Histidine Triad (FHIT) and E-cadherin
(1,9,10).
However, little is known regarding whether these genes are
abnormally expressed in endoscopically resected specimens of
superficial HPSCCs and their DESCC.
p53 is a tumor-suppressor gene, which can be mutated
by tobacco smoke and alcohol consumption (2). Furthermore, Helicobacter
pylori-mediated upregulation of the DNA- and RNA-editing
enzyme, activation-induced cytidine deaminase (AID) (11), was recently reported to result in the
accumulation of nucleotide alterations in the p53 tumor suppressor
gene in gastric cells in vitro (12). The protein encoded by the p53 gene is
essential for growth suppression, apoptosis and DNA repair
(13). Modulation of the FHIT gene,
which is located in the most active human fragile region, FRA3B
(14), and of Fhit protein
expression, has also been linked to ESCC and cigarette smoking.
Thus, Fhit is inactivated at an early stage in the development of
ESCC (15); FHIT gene promoter
methylation has been linked to cigarette smoking (16,17), and
ESCC may be associated with loss of the Fhit protein expression
along with alcohol consumption (15,18). Fhit
protein expression has been reported to be associated with growth
inhibition and the induction of apoptosis (19). Loss of E-cadherin expression has been
reported to correlate with tumor invasiveness, metastasis and
prognosis of head, neck and esophageal cancer (9,20).
E-cadherin is an important cell-to-cell adhesion molecule that is
essential for the development and maintenance of cell polarity and
tissue architecture (21).
The purpose of the present study was to test the
field cancerization hypothesis at the molecular level by
identifying aberrant tumor-related protein expression in
superficial HPSCCs and ESCCs arising in the same patient. To assess
biological properties, the expression of p53, Fhit, E-cadherin and
AID were immunohistochemically examined in endoscopically resected
HPSCCs and their synchronous or metachronous secondary ESCCs.
Patients and methods
Patient and tissue samples
A total of 18 tumor specimens, consisting of 9
HPSCCs and 9 DESCCs, were endoscopically resected from 8 patients
with HPSCC at the University of Tottori (Yonago, Japan) between
January 2010 and December 2014. The 9 DESCCs, including 5
synchronous and 4 metachronous cancers, were obtained from 4
patients with HPSCC. Synchronous or metachronous neoplasms were
defined according to the criteria proposed by Warren and Gates
(22). Thus, a synchronous carcinoma
was defined as a second neoplasm that was diagnosed concurrently or
within 6 months of the primary lesion diagnosis. Prior to or
subsequent to this period, neoplasms were considered metachronous.
Patient anonymity was maintained by assigning all specimens a
unique number and excluding all personal information. The present
study was approved by the Institutional Ethics Committee of Tottori
University (no. 314 and 1508A024) and complied with the Declaration
of Helsinki. Written informed consent was obtained from all 8
patients involved in the present study.
Analysis of tobacco and alcohol
involvement
The Brinkman index (BI) was used to calculate the
patients' history of smoking and is defined as the number of
cigarettes per day × number of years of smoking. The drinking index
(DI) was used to calculate the patients' history of drinking and
the accumulated amount of alcohol. DI was defined as the number of
drinks per week × the number of years of drinking (drinks / k × yr)
(18). Taking into account the
different alcohol concentrations, one drink was considered to
correspond to 20 mg of ethanol. Also included were the drinking
status, the type of alcoholic beverage, the age at which drinking
started, and for former drinkers, the age at which drinking
stopped. A characteristic physiological response to drinking
alcohol, termed the alcohol flushing response, was also determined.
Flushing responses are clinically useful as a simple,
cost-effective and non-invasive method for identification of
aldehyde dehydrogenase-2 (ALDH2) deficient patients (23). Flushing responses were determined by
recording of facial flushing, nausea and tachycardia.
Immunohistochemical staining
The following primary antibodies at the indicated
dilutions were used for immunohistochemical staining of 4 µm thick
paraffin-embedded sections: Rabbit polyclonal anti-Fhit antibody
(1:100; clone F130; catalog no. 18163; Immuno-Biological
Laboratories, Gunma, Japan), mouse monoclonal anti-p53 (1:50; clone
DO-7; catalog no. M7001; Dako; Agilent Technologies, Inc., Santa
Clara, CA, USA) or anti-E-cadherin (1:50; clone HECD-1; catalog no.
M106; Takara, Bio Inc., Otsu, Japan) antibody and rat monoclonal
anti-AID antibody (1:100; clone EK2 5G9; catalog no. 4959; Cell
Signaling Technology, Inc., Danvers, MA, USA). Signals were
detected using the avidin-biotin-peroxidase complex technique.
The sections were deparaffinized in xylene and
rehydrated in graded ethanol series for 5 min at room temperature.
Sections were then immersed in citrate buffer (0.01 M, pH 6.0) and
antigens were retrieved by heating in a microwave oven for 20–30
min. Endogenous peroxidase activity was subsequently blocked by
incubation with 3% H2O2. The sections were
then incubated with the primary antibody, or with the same dilution
of negative control [normal serum Immunoglobulin G (IgG)] overnight
at 4°C. Signals were detected using the Vectastain Elite ABC kit
(Vector Laboratories, Burlingame, CA, USA) according to the
manufacturer's protocol as follows. The sections were incubated
with the appropriate secondary antibody either biotinylated
anti-rabbit (catalog no. BA-1000), anti-mouse (catalog no. BA-2000)
or anti-rat IgG (catalog no. BA-4000) and with
avidin-biotin-peroxidase. The secondary antibodies were purchased
from Vector Laboratories (Burlingame, CA, USA), and dilutions of
1:200 were used. Signals were subsequently visualized using
diaminobenzidine tetrahydrochloride as a chromogen, and hematoxylin
as a counterstain. The expression of proteins was evaluated by two
observers, who were blinded to the clinical information with a
light microscope.
Assessment of p53 immunostaining
Two criteria were used to define p53 positive
staining: A distinct nuclear p53 immunoreaction and p53 staining of
>30% of tumor cells.
Assessment of Fhit immunostaining
The intensity of Fhit cytoplasmic staining was used
to grade Fhit expression as reduced, absent or positive, as
previously described (24). The
adjacent normal squamous epithelium and lymphocytes of germinal
centers were used as internal positive controls. Fhit weak-positive
lesions demonstrated positive reactivity at a level that was weak
compared with that in the normal epithelia, and these lesions were
described as reduced.
Assessment of E-cadherin
immunostaining
Cases were categorized as decreased or negative for
E-cadherin expression if they demonstrated definite membrane
staining of E-cadherin in <30% of the tumor cells, or a complete
absence of membrane staining. All other cases were categorized as
normal for E-cadherin expression.
Assessment of AID immunostaining
The internal positive controls comprised lymphocytes
of germinal centers in lymphoid follicles, since they were mostly
activated B cells and all specimens were intensely stained for AID.
Cytoplasm was deemed positive when stained at least to the same
degree as germinal centers.
Statistical analysis
Data were statistically analyzed using the χ2 test
with Yates' correction, the Fisher's test and the Mann-Whitney
U-test. Stat Flex version 6.0 (Artech Co., Ltd, Osaka, Japan) was
used for all statistical computations. P<0.05 was considered to
indicate a statistically significant difference.
Results
Patient background and
clinicopathological features
Backgrounds of the 8 investigated patients with
HPSCC are presented in Table I. The
patients were all Japanese males, ranging in age from 53 to 77
years (mean, 68.7 years; median, 72.5 years). All patients with
HPSCC had a flushing response, along with drinking and smoking
habits. The mean DI and BI value of the 8 HPSCC patients were 1,015
and 937.5, respectively. Clinicopathological characteristics and
immunostaining results of the investigated 9 HPSCCs and 9 DESCCs
from the 8 HPSCC patients are summarized in Table II. Based on a histological
examination, the 9 HPSCCs were classified as 6 carcinoma in
situ (CIS) and 3 subepithelial invasion. The 9 DESCCs were
classified as 6 CIS, 2 carcinoma invading the lamina propria and 1
carcinoma with submucosal invasion. The average maximum diameter of
HPSCCs and DESCCs was 14.0±6.3 and 22.0±13.7 mm, with a range of 3
to 25 mm and 12 to 55 mm, respectively.
 | Table I.Patient background of superficial
hypopharyngeal squamous cell carcinoma cases. |
Table I.
Patient background of superficial
hypopharyngeal squamous cell carcinoma cases.
| Characteristic | Superficial
hypopharyngeal squamous cell carcinoma cases (n=8) |
|---|
| Gender
(male:female) | 8:0 |
| Age, years (mean ±
standard deviation) | 68.7±8.8 |
| Regular alcohol
intake | 8 (100%) |
| Drinking index (mean
± standard deviation) | 1015±531 |
| Alcohol flushing | 8 (100%) |
| Current or previous
smoker | 8 (100%) |
| Brinkman index (mean
± standard deviation) | 937±849 |
| History of esophageal
cancer | 6 (75%) |
 | Table II.Clinicopathological characteristics
and immunostaining results of HPSCCs and their DESCCs. |
Table II.
Clinicopathological characteristics
and immunostaining results of HPSCCs and their DESCCs.
| Patient no. | Age, years | Gender | Tumor |
Metachronous/synchronous | Diameter, mm | Depth | p53 | Fhit | E-ca | AID |
|---|
| 1 | 73 | Male | HPSCC |
| 20 | SE | AE | AE | NE | AE |
|
|
|
| HPSCC | Metachronous | 10 | EP | AE | AE | AE | AE |
| 2 | 75 | Male | HPSCC |
| 15 | EP | AE | AE | AE | AE |
|
|
|
| DESCC | Metachronous | 14 | EP | AE | AE | NE | NE |
|
|
|
| DESCC | Metachronous | 12 | EP | AE | AE | NE | NE |
|
|
|
| DESCC | Metachronous | 15 | EP | AE | AE | AE | AE |
| 3 | 75 | Male | HPSCC |
| 10 | EP | AE | AE | AE | NE |
|
|
|
| DESCC | Synchronous | 55 | SM | AE | AE | AE | NE |
| 4 | 72 | Male | HPSCC |
| 14 | SE | AE | NE | NE | NE |
| 5 | 60 | Male | HPSCC |
| 17 | EP | AE | AE | NE | AE |
| 6 | 53 | Male | HPSCC |
| 25 | EP | AE | AE | NE | AE |
| 7 | 77 | Male | HPSCC |
| 3 | SE | AE | AE | NE | NE |
|
|
|
| DESCC | Synchronous | 30 |
LPM | AE | AE | AE | AE |
|
|
|
| DESCC | Synchronous | 15 | EP | AE | NE | NE | AE |
|
|
|
| DESCC | Synchronous | 20 | EP | AE | AE | NE | AE |
| 8 | 62 | Male | HPSCC |
| 12 | EP | NE | AE | AE | AE |
|
|
|
| DESCC | Synchronous | 13 | EP | NE | AE | AE | NE |
|
|
|
| DESCC | Metachronous | 24 |
LPM | AE | AE | NE | NE |
Immunohistochemical analysis of p53,
Fhit, E-cadherin and AID in HPSCC and DESCC
Table II presents the
expression of p53, Fhit, E-cadherin and AID in the endoscopically
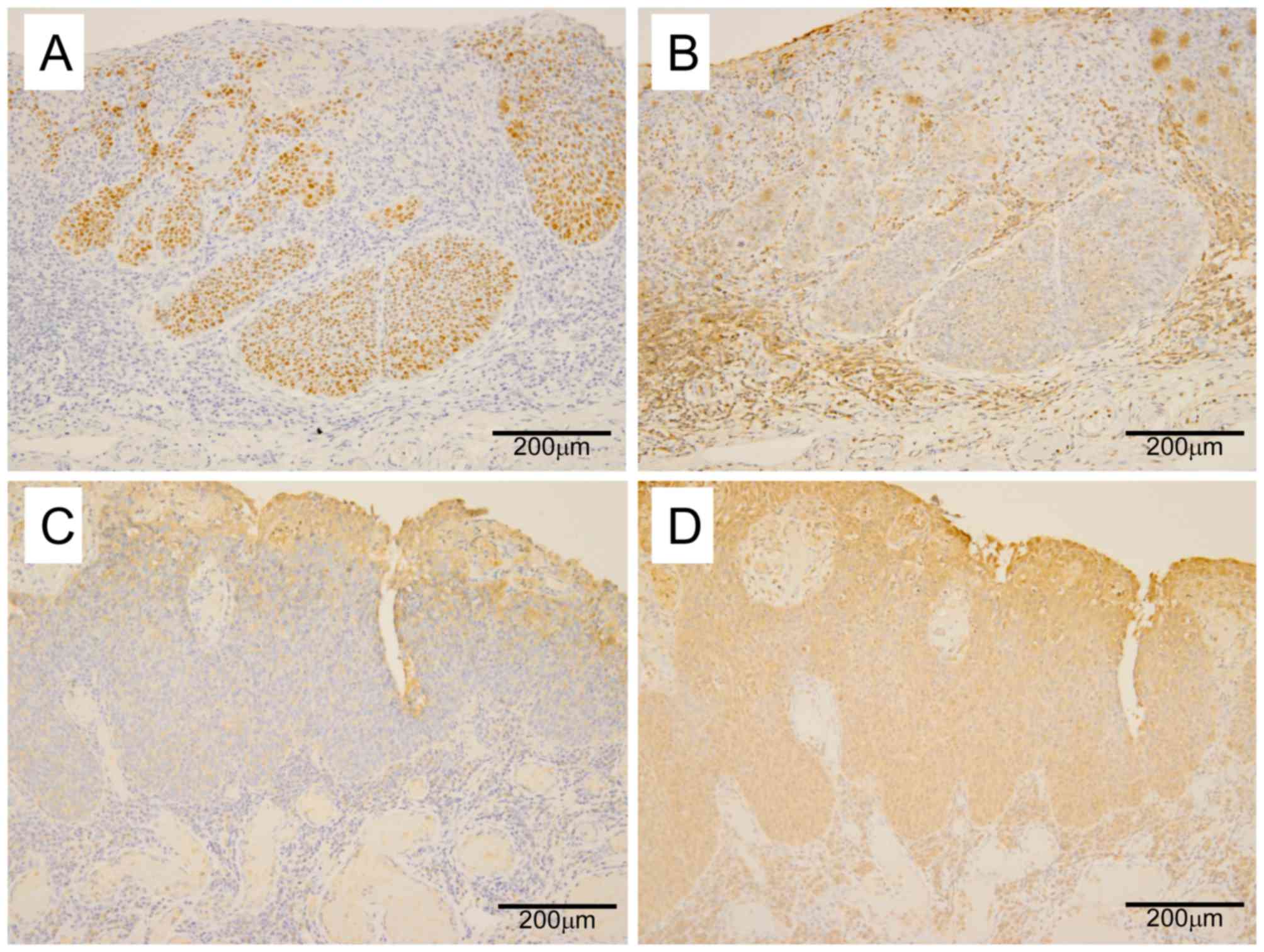
resected HPSCCs and their DESCCs. Representative
immunohistochemical stainings are shown in Fig. 1. Immuno-histochemical staining
detected overexpression of p53 and loss of Fhit expression in 8
(89%) and 8 (89%) of the 9 HPSCCs, and in 8 (89%) and 8 (89%) of
the 9 DESCCs, respectively, showing a high frequency of such
expression. Aberrant E-cadherin and AID expression was identified
in 4 (44%) and 6 (67%) of the 9 HPSCCs and in 4 (44%) and 4 (44%)
of the 9 DESCCs, respectively (Table
III). Aberrant expression of p53 and Fhit was identified in 7
tumors out of 9 HPSCCs and in 7 tumors out of their DESCCs
(Table IV). In addition, p53
expression was not significantly associated with AID expression
(P=0.86; Table V). In univariate
analyses, no significant correlation was identified between the
expression levels of these proteins and various clinicopathological
parameters, including age, sex, size of tumor and location.
 | Table III.Summary of analysis of p53, Fhit,
E-cadherin and AID protein expression in superficial HPSCCs and
their DESCCs. |
Table III.
Summary of analysis of p53, Fhit,
E-cadherin and AID protein expression in superficial HPSCCs and
their DESCCs.
| Type | p53 (%) | Fhit (%) | E-cadherin (%) | AID (%) |
|---|
| HPSCC (n=9) | 8 (89) | 8 (89) | 4 (44) | 6 (67) |
| DESCC (n=9) | 8 (89) | 8 (89) | 4 (44) | 4 (44) |
 | Table IV.Association between p53 and Fhit
expression in superficial HPSCCs and DESCCs. |
Table IV.
Association between p53 and Fhit
expression in superficial HPSCCs and DESCCs.
| Protein
expression | HPSCC n=9 | DESCC n=9 |
|---|
| p53(−)/Fhit(+) | 0 | 0 |
| p53(−)/Fhit(−) | 1 | 1 |
| p53(+)/Fhit(+) | 1 | 1 |
| p53(+)/Fhit(−) | 7 | 7 |
 | Table V.Association between p53 and AID
expression in superficial HPSCCs and DESCCs. |
Table V.
Association between p53 and AID
expression in superficial HPSCCs and DESCCs.
|
| p53 |
|---|
|
|
|
|---|
| AID | Positive | Negative | Total |
|---|
| Positive | 9 | 1 | 10a |
| Negative | 7 | 1 |
8a |
| Total | 16 | 2 | 18 |
Discussion
It is well established that the multiple primary SCC
and the widespread epithelial oncogenic alterations that develop in
the head and neck region and the esophagus can be attributed to the
field cancerization phenomenon (1,2). NBI
combined with magnifying endoscopy has recently been shown to be a
useful method for the identification of superficial SCC in the head
and neck region as well as in the esophagus (6). In addition, peroral endoscopic resection
of superficial HPSCC has also been suggested as a practicable and
useful treatment for such tumors (7,8). The
present study investigated clinicopathological and biological
features of endoscopically resected primary HPSCCs and their
DESCCs, which were detected by NBI endoscopy with magnification
and/or Lugol chromoendoscopy. To assess biological properties, the
expression of p53, Fhit, E-cadherin and AID was
immunohistochemically examined. A high frequency of aberrant p53
and Fhit expression was observed in HPSCCs and DESCCs. Furthermore,
the status of p53 and Fhit expression in HPSCCs and their DESCCs
demonstrated a close similarity, indicating field carcinogenesis
had occurred.
Lifestyle factors including heavy smoking and
excessive consumption of alcohol have been associated with an
increased risk of HNC and esophageal cancer (2). It has been demonstrated for East Asian
drinkers that there is a strong association between inactive ALDH2
and the risk of HNC and esophageal cancer (23,25). The
presence of inactive ALDH2 can be predicted based on facial
flushing in Japanese men aged 40 years or older. In the present
study, all patients were males with facial flushing who had
drinking and smoking habits.
Alterations in the p53 and FHIT genes and their
expression have been reported in a variety of epithelial tumors and
premalignant lesions including in HNC and esophageal cancer
(1,2).
In the present study, a high frequency of aberrant p53 and Fhit
expression in HPSCCs and in their DESCCs was observed.
Additionally, the pattern of p53 and Fhit expression in the DESCCs
closely paralleled that in their HPSCCs. These findings may be
compatible with the exposure of the patients to a common
carcinogen, such as smoking and drinking habits, from the viewpoint
of field cancerization. The frequency of aberrant p53 and Fhit
expression was increased compared with what has been observed in
previous reports (1,2,26,27). The present data were obtained from
endoscopically resected, early carcinoma. Therefore, the staging of
the present cases differed from those of previous reports. However,
the present data are consistent with previous reports that p53
protein overexpression is closely linked with the multiplicity of
esophageal cancer and with the development of multiple primary
cancers (28,29). Therefore, the p53 and Fhit alterations
observed in patients clearly associated with field cancerization
may frequently occur in the early stages of head, neck and
esophageal carcinogenesis. The biological nature of HPSCC based on
the p53 and Fhit status may share strong similarities with that of
their double ESC. Based on the above data the present study
considered that patients at high risk of having DESCCs may by
usefully screened by p53 and Fhit expression and that the results
of such screening may have value for directing the clinical
management of patients with HNCs.
Complete loss or reduced expression of E-cadherin
was previously detected in patients with invasive HNC (60% of
patients) and invasive ESCC (42.3% of patients) (9,30). In
addition, loss of E-cadherin expression in HNC and ESCC is
associated with tumor invasiveness, metastasis and prognosis
(9,20). Although the present study analyzed
early stage tumors, it demonstrated a similar frequency of
E-cadherin loss/reduced expression compared with several published
studies (9,30). Aberrant AID expression induces p53
gene mutation in Helicobacter pylori infected gastric
epithelial cells and is associated with the development of
precancerous lesions and the progression of malignant tumors
(12). We previously detected
aberrant AID expression in 33–45% of early esophageal squamous
neoplasia (27). This aberrant AID
expression rate was similar to that detected in the present study.
In our previous study, we proposed that p53 expression was
independent of aberrant AID expression in the early stage of
esophageal carcinogenesis (27).
Similarly, the present study identified no association between
aberrant AID expression and p53 overexpression in the superficial
HPSCCs and their DESCC samples. The molecular mechanism underlying
AID upregulation in hypopharyngeal and esophageal tissues is
unclear. Previous studies have indicated that AID expression does
perform an important role in the early stages of oral
carcinogenesis and that AID expression in oral squamous cells is
induced in response to inflammatory cytokine stimulation (31,32).
Additionally, the frequencies of aberrant E-cadherin and AID
expression were similar in HPSCCs and DESCCs, although their
expression did not correlate with each other. These results may
also be due to the field effect of tobacco and alcohol.
In conclusion, the frequency and pattern of aberrant
p53, Fhit, E-cadherin, and AID expression in the examined
superficial HPSCCs and their DESCCs was consistent with the field
theory of cancerization. Furthermore, it is proposed that p53 and
Fhit expression may be candidate biomarkers for predicting the
development of DESCC in patients with HPSCC. A knowledge of the
aberrant expression of these genes could also provide a basis for
improving the strategies used for the early endoscopic detection
and treatment of early stage HPSCC and ESCC.
References
|
1
|
Perez-Ordoñez B, Beauchemin M and Jordan
RC: Molecular biology of squamous cell carcinoma of the head and
neck. J Clin Pathol. 59:445–453. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Toh Y, Oki E, Ohgaki K, Sakamoto Y, Ito S,
Egashira A, Saeki H, Kakeji Y, Morita M, Sakaguchi Y, et al:
Alcohol drinking, cigarette smoking, and development of squamous
cell carcinoma of the esophagus: Molecular mechanisms of
carcinogenesis. Inc J Clin Oncol. 15:135–144. 2010. View Article : Google Scholar
|
|
3
|
Slaughter DP, Southwick HW and Smejkal W:
Field cancerization in oral stratified squamous epithelium;
clinical implications of multicentric origin. Cancer. 6:963–968.
1953. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ina H, Shibuya H, Ohashi I and Kitagawa M:
The frequency of a concomitant early esophageal cancer in male
patients with oral and oropharyngeal cancer. Screening results
using Lugol dye endoscopy. Cancer. 73:2038–2041. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dubuc J, Legoux J..Winnock M, Seyrig
J..Barbier J..Barrioz T, Laugier R, Boulay G, Grasset D, Sautereau
D, et al: Endoscopic screening for esophageal squamous cell
carcinoma in high-risk patients: A prospective study conducted in
62 French endoscopy centers. Endoscopy. 57:690–695. 2006.
View Article : Google Scholar
|
|
6
|
Goda K, Dobashi A and Tajiri H:
Perspectives on narrow-band imaging endoscopy for superficial
squamous neoplasms of the orohypopharynx and esophagus. Dig Endosc.
26 Suppl 1:S1–S11. 2014. View Article : Google Scholar
|
|
7
|
Shimizu Y, Yamamoto J, Kato M, Yoshida T,
Hirota J, Ono Y, Nakagawa M, Nakagawa S, Oridate N and Asaka M:
Endoscopic submucosal dissection for treatment of early stage
hypopharyngeal carcinoma. Gastrointest Endosc. 64:255–262. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hanaoka N, Ishihara R, Takeuchi Y, Suzuki
M, Otozai S, Kida K, Yoshii T, Fujii T, Yoshino K, Sugawa T, et al:
Endoscopic submucosal dissection as minimally invasive treatment
for superficial pharyngeal cancer: A phase II study (with video).
Gastrointest Endosc. 82:1002–1008. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mattijssen V, Peters HM, Schalkwijk L,
Manni JJ, van't Hof-Grootenboer B, de Mulder PH and Ruiter DJ:
E-cadherin expression in head and neck squamous-cell carcinoma is
associated with clinical outcome. Int J Cancer. 55:580–585. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xu XC: Risk factors and gene expression in
esophageal cancer. Methods Mol Biol. 471:335–360. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Muramatsu M, Kinoshita K, Fagarasan S,
Yamada S, Shinkai Y and Honjo T: Class switch recombination and
hypermutation require activation-induced cytidine deaminase (AID),
a potential RNA editing enzyme. Cell. 102:553–563. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Matsumoto Y, Marusawa H, Kinoshita K, Endo
Y, Kou T, Morisawa T, Azuma T, Okazaki IM, Honjo T and Chiba T:
Helicobacter pylori infection triggers aberrant expression of
activation-induced cytidine deaminase in gastric epithelium. Nat
Med. 13:470–476. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hollestein M, Sidransky D, Vogelstein B
and Harris CC: p53 mutations in human cancers. Science. 253:49–53.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ohta M, Inoue H, Cotticelli MG, Kastury K,
Baffa R, Palazzo J, Siprashvili Z, Mori M, McCue P, Druck T, et al:
The FHIT gene, spanning the chromosome 3p14.2 fragile site and
renal carcinoma-associated t(3;8) breakpoint, is abnormal in
digestive tract cancer. Cell. 84:587–597. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mori M, Mimori K, Shiraishi T, Alder H,
Inoue H, Tanaka Y, Sugimachi K, Huebner K and Croce CM: Altered
expression of Fhit in carcinoma precarcinomatous lesions of the
esophagus. Cancer Res. 60:1177–1182. 2000.PubMed/NCBI
|
|
16
|
Lee EJ, Lee BB, Kim JW, Shim YM, Hoseok I,
Han J, Cho EY, Park J and Kim DH: Aberrant methylation of Fragile
Histidine Triad gene is associated with poor prognosis in early
stage esophageal squamous cell carcinoma. Eur J Cancer. 42:972–980.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Soma T, Kaganoi J, Kawabe A, Kondo K,
Imamura M and Shimada Y: Nicotine induces the fragile histidine
triad methylation in human esophageal squamous epithelial cells.
Int J Cancer. 119:1023–1027. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Morita M, Oyama T, Nakata S, Ono K, Sugaya
M, Uramoto H, Yoshimatsu T, Hanagiri T, Sugio K and Yasumoto K:
Expression of FHIT in esophageal epithelium and carcinoma:
Reference to drinking, smoking and multicentric carcinogenesis.
Anticancer Res. 26:2243–2248. 2006.PubMed/NCBI
|
|
19
|
Ishii H, Dumon KR, Vecchione A, Trapasso
F, Mimori K, Alder H, Mori M, Sozzi G, Baffa R, Huebner K and Croce
CM: Effect of adenoviral transduction of the fragile histidine
triad gene into esophageal cancer cells. Cancer Res. 61:1578–1584.
2001.PubMed/NCBI
|
|
20
|
Tamura S, Shiozaki H, Miyata M, Kadowaki
T, Inoue M, Matsui S, Iwazawa T, Takayama T, Takeichi M and Monden
M: Decreased E-cadherin expression is associated with haematogenous
recurrence and poor prognosis in patients with squamous cell
carcinoma of the oesophagus. Br J Surg. 83:1608–1614. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hirohashi S: Inactivation of the
E-cadherin-mediated cell adhesion system in human cancers. Am J
Pathol. 153:333–339. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Warren S and Gates O: Multiple primary,
malignant tumors: A survey of the literature and statistical study.
Am J Cancer. 16:1358–1414. 1932.
|
|
23
|
Brooks PJ, Enoch MA, Goldman D, Li TK and
Yokoyama A: The alcohol flushing response: An unrecognized risk
factor for esophageal cancer from alcohol consumption. PLoS Med.
6:e502009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Saldivar JC, Shibata H and Huebner K:
Pathology and biology associated with the fragile FHIT gene and
gene product. J Cell Biochem. 109:858–865. 2010.PubMed/NCBI
|
|
25
|
Yokoyama A, Mizukami T and Yokoyama T:
Genetic polymorphisms of alcohol dehydrogense-1B and aldehyde
dehydrogenase-2, alcohol flushing, mean corpuscular volume, and
aerodigestive tract neoplasia in Japanese drinkers. Adv Exp Med
Biol. 815:265–279. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sauter ER, Cleveland D, Trock B, Ridge JA
and Klein-Szanto AJ: p53 is overexpressed in fifty percent of
pre-invasive lesions of head and neck epithelium. Carcinogenesis.
15:2269–2274. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hayashi A, Yashima K, Takeda Y, Sasaki S,
Kawaguchi K, Harada K, Murawaki Y and Ito H: Fhit, E-cadherin, p53,
and activation-induced cytidine deaminase expression in
endoscopically resected early stage esophageal squamous neoplasia.
J Gastroenterol Hepatol. 27:1752–1758. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kohmura T, Hasegawa Y, Ogawa T, Matsuura
H, Takahashi M, Yanagita N and Nakashima T: Cyclin D1 and p53
overexpression predicts multiple primary malignant neoplasms of the
hypopharynx and esophagus. Arch Otolaryngol Head Neck Surg.
125:1351–1354. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kato H, Yoshikawa M, Miyazaki T, Nakajima
M, Fukai Y, Tajima K, Masuda N, Tsukada K, Fukuda T, Nakajima T and
Kuwano H: Expression of p53 protein related to smoking and
alcoholic beverage drinking habits in patients with esophageal
cancers. Cancer Lett. 167:65–72. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chung Y, Lam AK, Luk JM, Law S, Chan KW,
Lee PY and Wong J: Altered E-cadherin expression and p120 catenin
localization in esophageal squamous cell carcinoma. Ann Surg Oncol.
14:3260–3267. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Miyazaki Y, Fujinami M, Inoue H, Kikuchi
K, Ide F and Kusama K: Expression of activation-induced cytidine
deaminase in oral epithelial dysplasia and oral squamous cell
carcinoma. J Oral Sci. 55:293–299. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nakanishi Y, Kondo S, Wakisaka N, Tsuji A,
Endo K, Murono S, Ito M, Kitamura K, Muramatsu M and Yoshizaki T:
Role of activation-induced cytidine deaminase in the development of
oral squamous cell carcinoma. PLoS One. 8:e620662013. View Article : Google Scholar : PubMed/NCBI
|