Introduction
Immune cells are associated with carcinogenesis,
tumor growth, invasion and metastasis. Natural killer (NK) cells in
particular serve an important role in immune surveillance, and are
generally accepted as a beneficial cell population for anti-tumor
immunity (1). Several studies have
reported that depletion of NK cells causes increased survival of
circulating tumor cells, resulting in enhanced cancer metastasis
(2–5).
In addition, it has been suggested that a favorable prognosis is
associated with the extent of NK cell infiltration into the tumor
in patients with gastric cancer or colorectal cancer (6,7).
Therefore, inhibition of NK cell activity may promote cancer
metastasis through a decrease in the number of NK cells.
In addition, cluster of differentiation
(CD)4+ and CD8+ T cells, which are specific
for tumor-associated antigens, serve important roles in antitumor
immunity (8,9). CD4+ T cells serve an
important role in generating effective immune responses by
stimulating CD8+ T cell proliferation and establishing
long-lived functional T cell memory (8). It has been reported that CD4+
T cell can enhance CD8+ T cell recruitment and
infiltration into tumors (8).
Similarly, several reports have suggested that the infiltration of
CD8+ T cells is associated with a better prognosis in
colon cancer (10).
Several inflammatory cytokines, including tumor
necrosis factor (TNF)-α and interleukin (IL)-6, serve important
roles in the development and progression of rheumatoid arthritis
(RA) (11,12). Thus, TNF-α inhibitors, including
etanercept and the anti-IL-6 receptor (IL-6R) antibody (Ab)
tocilizumab are efficacious RA treatments (13,14).
Additionally, the novel small-molecule Janus kinase (JAK) inhibitor
tofacitinib, suppresses several cytokine signals, including IL-2,
−4, −6, −7 and −15. Therefore, it is also effective for the
treatment of RA (15,16).
There are concerns about the potential increase in
cancer risk associated with certain RA drug treatments, but these
possibilities remain to be demonstrated. Therefore, in the present
study, the effect of tofacitinib, the anti-mouse IL-6R Ab MR16-1
and etanercept, on the number of NK and T cells and cancer
metastasis was investigated using an experimental lung metastasis
mouse model with a mouse colon cancer cell line.
Materials and methods
Laboratory animals
Female Balb/c mice were obtained from Charles River
Laboratories Japan, Inc. (Yokohama, Japan). The mice were housed
under specific-pathogen-free conditions and were used in
experiments at 6 weeks of age. The mean weight of mice was 20.5 g
(20.3–20.8 g). In total, 32 mice were used for each experiment (a
total of 96 mice were used in the present study). Mice were housed
in cages and received standard mouse chow (CRF1; Oriental Yeast
Co., Ltd., Tokyo, Japan) and water ad libitum. The
environment was maintained between 23 and 24°C with a
time-regulated light period between 8 a.m. and 8 p.m. Experiments
were conducted in accordance with the institutional Ethics
Guidelines of Fukuoka University in Japan (Fukuoka, Japan). The
present study was approved by the Fukuoka University Animal
Experiment Committee (approval no. 1404735).
Cell line
The mouse rectal colon 26 (C26) cancer cell line,
was obtained from the RIKEN BioResource Center (Tsukuba, Japan).
C26 cells were maintained in RPMI-1640 medium supplemented with 10%
fetal bovine serum, 100 U/ml penicillin and 100 µg/ml streptomycin
(all from Thermo Fisher Scientific, Inc., Waltham, MA, USA). C26
cells were incubated at 37°C in air containing 5%
CO2.
Experimental metastasis assay
On day 0, the mice were treated with each agent
(tofacitinib, MR16-1 or etanercept) as described subsequently. C26
cells were suspended in sodium bicarbonate-free RPMI-1640
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). A single injection
of C26 cells (1.0×104 cells/mouse) was injected into
mice via the lateral tail vein on day 3. On day 14, the mice were
sacrificed by removal of blood from caudal vena cava under
isoflurane anesthesia (Wako Pure Chemical Industries, Ltd., Osaka,
Japan). Blood was subsequently collected from the vena cava, and
the spleen and lung were resected. The spleen was dispersed in PBS
and contaminated red blood cells were lysed with lysing solution
(BD Pharm Lyse; BD Biosciences, Franklin Lakes, NJ, USA). The total
number of leukocytes in a splenocyte suspension and a whole blood
sample were counted using an automatic cell counter (Nihon Kohden
Corporation, Tokyo, Japan). Each lung was then weighed and placed
in Bouin's solution (Wako Pure Chemical Industries, Ltd.) for ≥24
h, and the number of surface nodules was then counted using a light
stereo microscope (magnification, ×10; SW-301; Wraymer Inc., Osaka,
Japan).
Treatment of all mice groups
For each experiment with tofacitinib, MR16-1 or
etanercept, the mice were divided into the following four groups
(n=8 per group): No agent + no cancer cell group; no agent + C26
cell injection group; vehicle/control + C26 cell injection group;
and agent + C26 injection group. The vehicle/controls used were
poly (ethylene glycol) 300 (PEG300; Wako Pure Chemical Industries,
Ltd.), rat immunoglobulin G (IgG) and human IgG (both from MP
Biomedicals, LLC, Santa Ana, CA, USA) for tofacitinib, MR16-1 and
etanercept, respectively. The dose of each treatment was determined
as the effective dose reported on a collagen-induced arthritis
model in previous studies (17–19).
Tofacitinib treatment
Tofacitinib (Selleck Chemicals, Houston, TX, USA)
was dissolved in a sterile solution of PEG300, as used previously
(18). Mice in the tofacitinib and
vehicle treatment groups were anesthetized with isoflurane, and
their dorsal surface was shaved 1 day prior to pump insertion. On
day 0, a subcutaneous pocket was created under anesthesia with
isoflurane, and an ALZET Mini-Osmotic Pump (model 2002, release
rate 0.5 µl/h; Durect Co., Cupertino, CA, USA) was then inserted to
deliver tofacitinib at a dosage of 15 mg/kg/day, or PEG300 as a
control, as previously described (18).
MR16-1 treatment
As tocilizumab is an anti-human IL-6R Ab, it does
not cross-react with murine IL-6R (20). Therefore, in the present study MR16-1
[obtained from hybridoma, established and gifted by Chugai
Pharmaceutical Co., Ltd., Tokyo, Japan (20)], a specific rat anti-mouse IL-6R Ab,
was used instead of tocilizumab. An intraperitoneal (i.p.) dose of
10 mg/ml MR16-1 in PBS or rat IgG (cat. no. 55951; MP Biomedicals,
LLC) of 8 mg/mouse was injected once a week.
Etanercept treatment
Etanercept is a human TNF receptor-Fc fusion protein
that inhibits TNF-α function of humans and mice (17). Etanercept was purchased from Pfizer,
Inc. (Tokyo, Japan). Etanercept or human IgG (cat. no. 55908; MP
Biomedicals, LCC) (1 mg/mouse, 3 times a week) was injected i.p. in
mice.
Flow cytometric analysis
Splenocyte suspension was incubated with the
Fc-receptor-blocking antibodies anti-CD16 and anti-CD32 (BD
Biosciences) and then stained for 30 min with fluorescent
antibodies (Table I) at 4°C. Blood
sample was incubated with the Fc-receptor-blocking antibodies
anti-CD16 and anti-CD32 (BD Biosciences) and stained with
fluorescent antibodies (Table I) for
30 min at room temperature. Red blood cells were then lysed with
lysing solution (BD Pharm Lyse; BD Biosciences). Following
antibodies (all from BD Biosciences): Fluorescein isothiocyanate
(FITC)-conjugated anti-CD3, phycoerythrin (PE)-conjugated
anti-natural killer cell p46-related protein (NKp46),
allophycocyanin (APC)-cyanine (Cy)7-conjugated anti-CD11b,
APC-conjugated anti-CD27, PE-Cy7-conjugated
anti-granulocyte-differentiation antigen-1 (Gr1)/Ly6 G and 6c for
analysis of NK cell populations; and FITC-conjugated anti-CD3,
APC-conjugated anti-CD4, PE-conjugated anti-CD8 and
PE-Cy7-conjugated anti-CD19 were used for analysis of lymphocyte
populations. Manufacturer-recommended isotype controls were used
for each antibody. Antibodies used for FACS in the present study
are summarized in Table I. The
frequency of labeled cells was visualized using FACSCanto™II (BD
Bioscience). In flow cytometric analysis of splenocyte and blood, T
cells were gated as the CD3+ cells, and NK cells were
gated as the CD3− NKp46+ Gr1−
cells.
 | Table I.Antibodies used. |
Table I.
Antibodies used.
| A, Antibodies used
for FACS |
|---|
|
|---|
| Fluorescent
antibody for FACS | Cat. no. | Volume, µl |
|---|
| FITC anti-mouse
CD3 | 561798 | 1 |
| PE anti-mouse CD335
(NKp46) | 560757 | 1 |
| APC-Cy7 rat
anti-mouse CD11b | 557657 | 1 |
| APC hamster
anti-mouse CD27 | 560691 | 1 |
| PE-Cy7 rat
anti-mouse Ly6g and | 552985 | 1 |
| Ly6c (Gr1) |
|
|
| APC rat anti-mouse
CD4 | 553051 | 1 |
| PE anti-mouse
CD8a | 553032 | 1 |
| PE-Cy7 rat
anti-mouse CD19 | 552854 | 1 |
|
| B, Isotype
controls |
|
| Isotype
control | Cat. no. | Volume, µl |
|
| FITC rat
IgG2bκ | 556923 | 1 |
| PE rat IgG2aκ | 553930 | 1 |
| APC-Cy7 rat
IgG2bκ | 552773 | 1 |
| APC hamster
IgG1κ | 553974 | 1 |
| PE-Cy7 rat
IgG2bκ | 552849 | 1 |
| APC rat IgG2aκ | 553932 | 1 |
| PE rat IgG2aκ | 353930 | 1 |
| PE-Cy7 rat
IgG2aκ | 552784 | 1 |
Statistical analysis
All data are presented as the mean ± standard error
of the mean. Statistical analysis was performed using one-way
analysis of variance with Dunnett's test as a post hoc comparison.
P<0.05 was considered to indicate a statistically significant
difference. All data were analyzed using SPSS software (version
22.0; IBM Corp., Armonk, NY, USA).
Results
Tofacitinib treatment
The tofacitinib-treated group had significantly
reduced numbers of NK cells in the blood and spleen compared with
those in all other groups (P<0.001; Fig. 1A). Compared with those in the
vehicle-treated group, the number of NK cells in blood and spleen
samples in the tofacitinib-treated group was decreased by 90 and
88%, respectively (Fig. 1A).
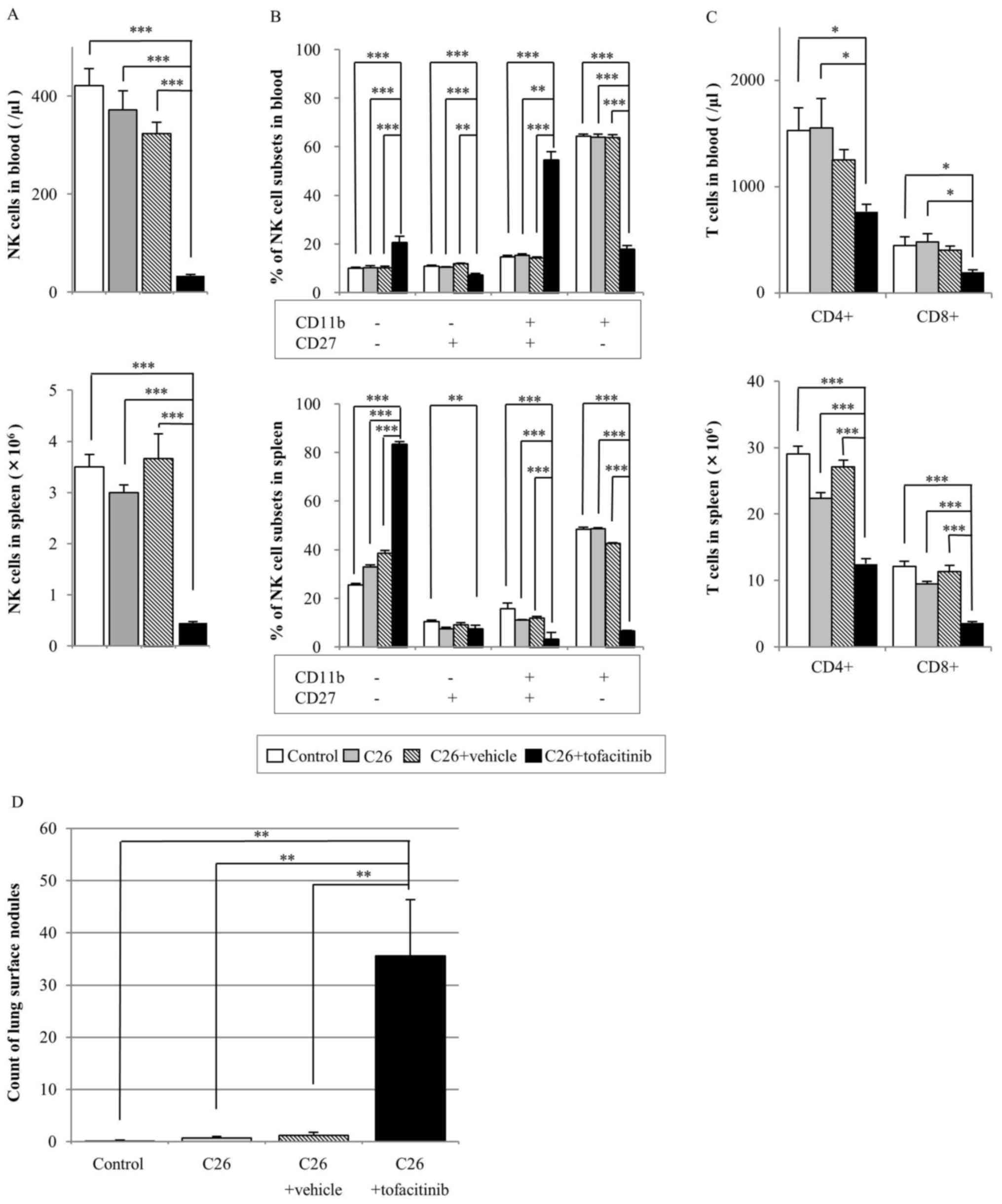 | Figure 1.Effect of tofacitinib treatment on NK
cells, T cells and metastatic nodules in a mouse tumor model.
Balb/c mice were injected with or without C26 colon cancer cells,
and with or without tofacitinib or a vehicle [poly (ethylene
glycol) 300] control as indicated. The following parameters were
assayed after 14 days: (A) NK cell count in the blood (top) and
spleen (bottom); (B) percentage of NK cell subsets, which were
defined based on CD27 and CD11b expression, in the blood (top) and
spleen (bottom); (C) CD3+CD4+ and
CD3+CD8+ cell counts in the blood (top) and
spleen (bottom); and (D) number of metastatic lung surface nodules.
Data are presented as the mean ± standard error of the mean
(control group n=7, C26 group n=7, C26+vehicle n=5, C26+tofacitinib
n=7). *P<0.05, **P<0.01, ***P<0.001. CD, cluster of
differentiation; NK, natural killer. |
In addition, the effect of tofacitinib treatment on
the percentage of NK cell subsets defined by CD11b and CD27 surface
expression was assayed to analyze NK cell activity (Fig. 1B). The percentage of
CD11b+CD27− NK cells in the blood and spleen
samples of the tofacitinib-treated group was significantly
decreased compared with that in the other three groups. By
contrast, the percentage of CD11b−CD27− NK
cell subsets was significantly increased in the tofacitinib-treated
group compared with that in the other groups for blood and spleen
analyses.
The number of CD4+ and CD8+ T
cells in the blood samples of the tofacitinib-treated group was
significantly decreased compared with that in the control and C26
cell-injected groups (Fig. 1C). No
significant differences were identified in the number of
CD4+ (P=0.381) or CD8+ (P=0.189) T cells in
the blood samples between the tofacitinib-treated and
vehicle-treated groups.
In the spleen of the tofacitinib-treated group, the
number of CD4+ and CD8+ T cells was
significantly decreased compared with that in all the other groups
(Fig. 1C). The number of
CD4+ and CD8+ T cells in the spleens of the
tofacitinib-treated group was 39 and 51% lower, respectively
compared with that in the vehicle-treated group.
In the experimental lung metastasis assay, no
significant difference was observed in the lung weight among all
groups (data not shown). The number of lung surface nodules was
significantly increased in the tofacitinib-treated mice compared
with that in the other three groups (vehicle-treated, 1.20±0.58;
tofacitinib-treated, 35.6±10.81; all P<0.01; Fig. 1D).
The following mice were excluded from this analysis:
One mouse in the vehicle-treated group died prior to being injected
with C26 cells due to trouble at surgery; two mice in the
vehicle-treated group failed to receive the C26 injection due to
mistake of tail vein injection; and one mouse in the tofacitinib
group had problem at drug administration (failure of skin
anastomosis).
MR16-1 treatment
The blood NK cell numbers in the MR16-1-treated
group were significantly decreased compared with those in the
control group (Fig. 2A). In the
spleen, no significant differences were identified between groups.
The percentage of CD11b+CD27− NK cells in the
blood and spleen was highest in all NK cell subsets in all groups,
and the percentages of this subset in the MR16-1-treated group were
not different among the other groups (Fig. 2B).
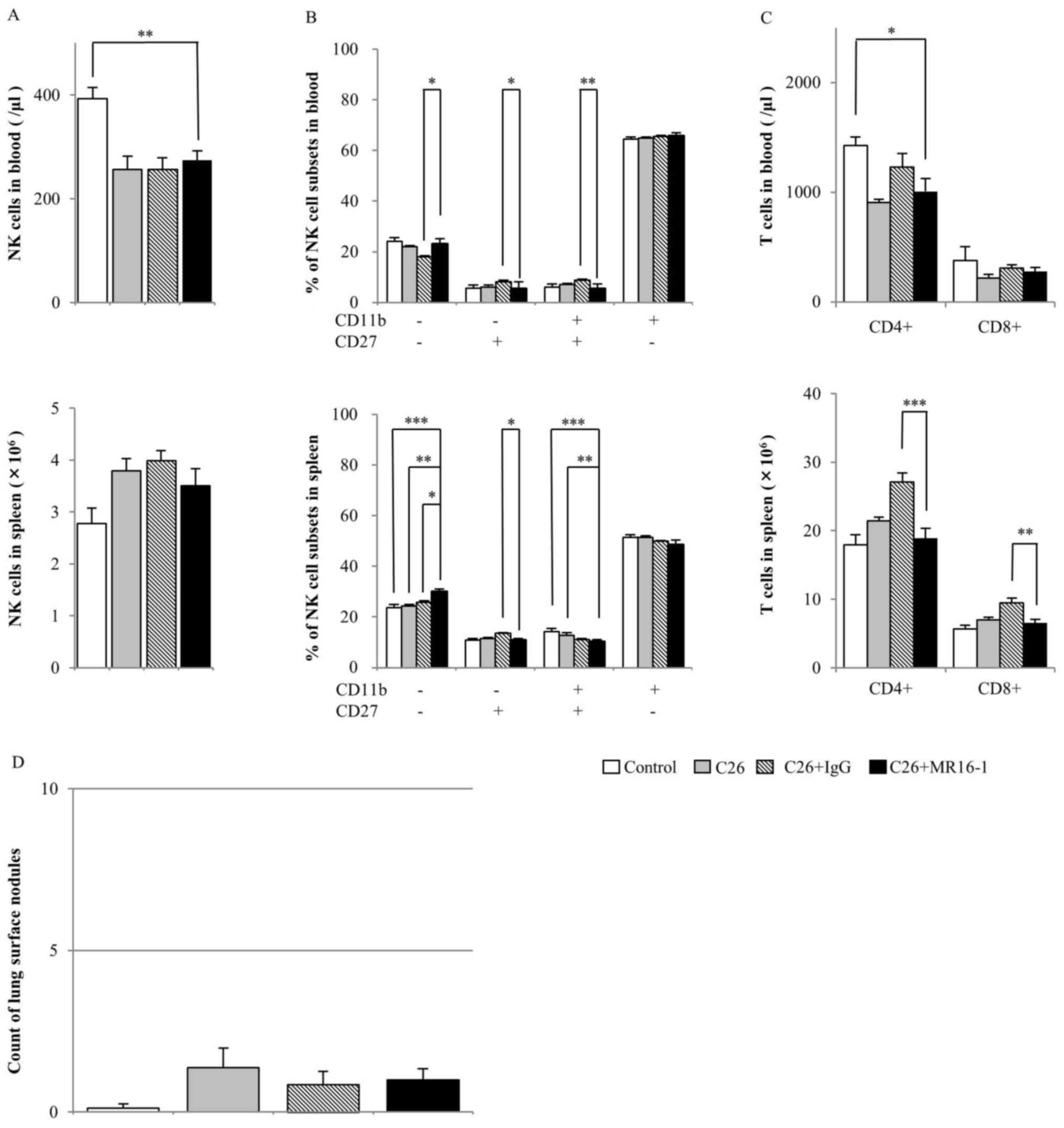 | Figure 2.Effect of MR16-1 treatment on NK
cells, T cells and metastatic nodules in a mouse tumor model.
Balb/c mice were injected with or without C26 colon cancer cells,
and with or without MR16-1 or a rat IgG control as indicated. The
following parameters were assayed following 14 days: (A) NK cell
count in the blood (top) and spleen (bottom); (B) percentage of NK
cell subsets defined based on CD27 and CD11b expression in the
blood (top) and spleen (bottom); (C) CD3+CD4+
and CD3+CD8+ cell counts in the blood (top)
and spleen (bottom); and (D) number of metastatic lung surface
nodules. Data are presented as the mean ± standard error of the
mean (control group n=8, C26 group n=8, C26+IgG group n=7,
C26+MR16-1 n=8). *P<0.05, **P<0.01, ***P<0.001. CD,
cluster of differentiation; NK, natural killer; IgG, Immunoglobulin
G. |
The CD4+ and CD8+ T cell
numbers in the blood of the MR16-1-treated group were not different
from those of any other groups (Fig.
2C). The CD4+ and CD8+ T cell numbers in
blood exhibited similar results, although the CD4+ T
cell number was decreased in the MR16-1-treated group compared with
that in the control group (Fig. 2C).
In the splenocyte of the MR16-1-treated group, the CD4+
and CD8+ T cell number was significantly decreased
compared with that in the rat IgG-treated group, but not with that
in the control or C26-injected groups (Fig. 2C).
In the experimental lung metastasis model, no
significant difference was observed in lung weight (data not shown)
or in the number of lung surface nodules between the MR16-1-treated
group and any other groups (Fig. 2D).
For one mouse in the rat IgG group, the spleen cells could not be
analyzed due to technical failure (missing the sample).
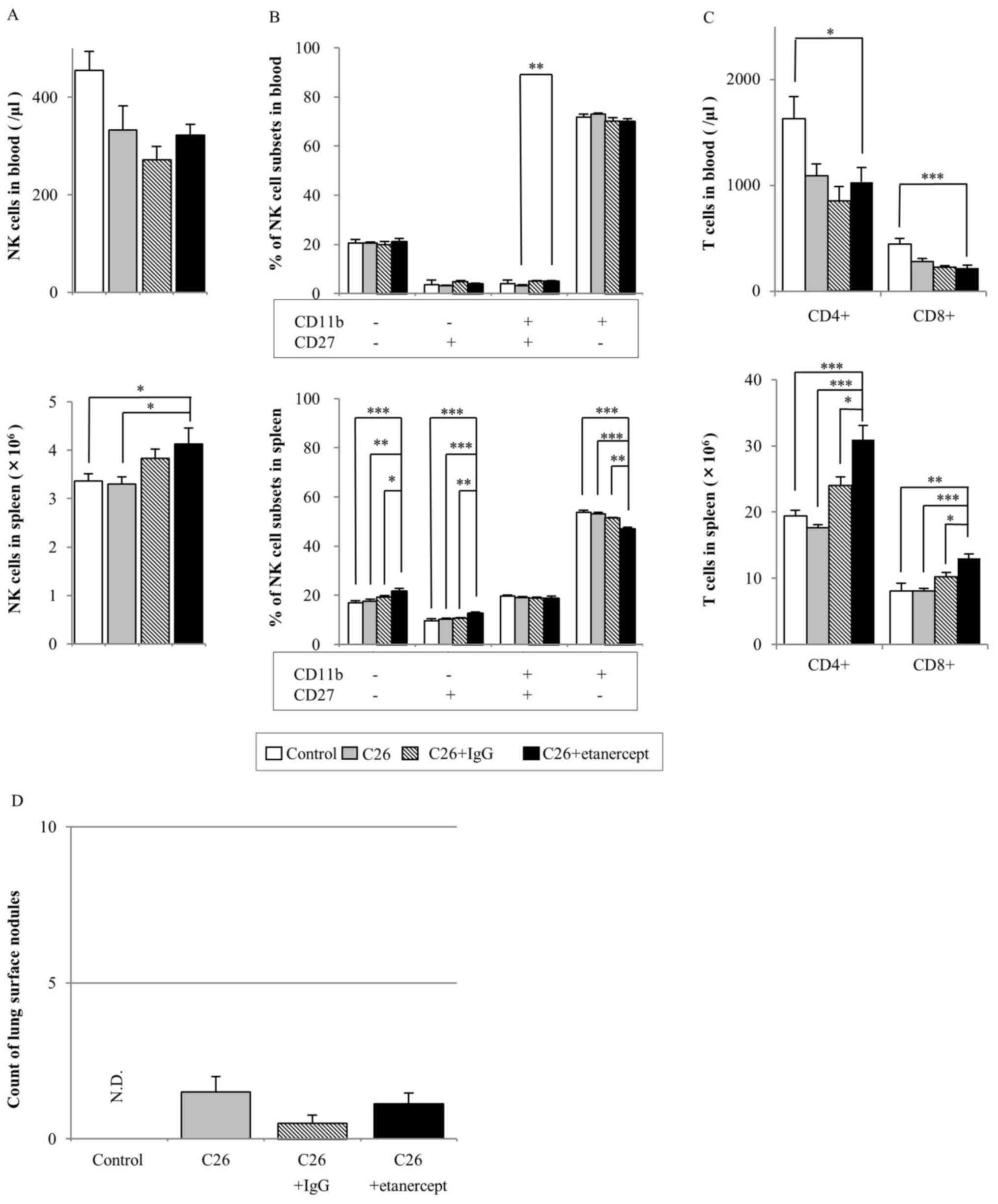
Etanercept treatment
The NK cell numbers in the blood of the
etanercept-treated group did not differ from those of any of the
other groups. The NK cell number in the spleens of the
etanercept-treated group was not different from that in the human
IgG-treated group, but was significantly increased compared with
that in the control and C26-injected group (Fig. 3A). The percentage of
CD11b+CD27− NK cells in blood and spleen was
the highest of all the NK cell subsets in all the groups (Fig. 3B). However, in the spleen, the
percentage of CD11b+CD27− NK cells of the
etanercept-treated group was significantly decreased compared with
that of the other groups.
CD4+ and CD8+ T cell numbers
in the blood of the etanercept-treated group were significantly
decreased compared with those of the C26 only-injected group
(Fig. 3C). However, the
CD4+ and CD8+ T cell numbers in the spleen
were significantly increased in the etanercept-treated group
compared with those in all other groups.
In the experimental lung metastasis assay, no
significant difference was identified in the number of lung surface
nodules between the etanercept-treated group and any other groups
(Fig. 3D). Representative images of
the lungs of mice treated with tofacitinib, MR16-1 and etanercept
are shown in Fig. 4.
Discussion
In the present study, the effect of three cytokine
signal inhibitors, tofacitinib, MR16-1 and etanercept, on NK cells,
T cells and cancer metastasis was investigated. Only tofacitinib
significantly enhanced cancer metastasis as determined by the
number of lung surface nodules, with a significant decrease in NK
cells in the mouse model.
Several previous reports have suggested that
tofacitinib reduces NK cell counts in vivo (21,22).
Clinically, tofacitinib does not significantly decrease NK cell
counts in patients with RA (23).
However, the Food and Drug Administration has reported that NK
numbers exhibit a dose-dependent decrease following tofacitinib
treatment (24). It was therefore
suggested that tofacitinib reduces NK cells depending on the status
of the patient. Additionally, it was reported that infiltration of
CD8+ T cells into the tumor was associated with an
improved prognosis, and that the depletion of CD8+ T
cells reduces anti-tumor immunity and enhances growth and
metastasis in a mouse lung metastasis model (10,25,26). It is
therefore assumed that NK and CD8+ T cell reduction
following tofacitinib treatment can promote cancer metastasis.
Tofacitinib is a JAK inhibitor that suppresses inflammatory
signaling downstream of γc-chain cytokines, IL-2, −4, −7
and −15 (22). IL-15 has an important
role in the life and death of NK and CD8+ T cells
(27,28). It is considered that IL-15 inhibition
following tofacitinib treatment is the main mechanism underlying
the significant reduction observed in NK and CD8+ T cell
numbers.
Regarding the effect of tofacitinib on NK cell
numbers and NK subsets in the present study, the results suggest
that tofacitinib reduces total NK cell numbers and the percentage
of the CD11b+CD27− NK cell subset. It has
been proposed that CD11b−CD27−,
CD11b−CD27+,
CD11b+CD27+ and
CD11b+CD27− NK subsets are present in
proportion to maturation of murine and human NK cells (29,30).
CD11b+CD27− NK cells are considered to be
effector cells, expressing a high level of CD107a and producing
interferon (IFN)-γ and cytotoxic granules, including granzyme B and
perforin (31). It was suggested that
perforin and IFN-γ in particular, produced by NK cells, have an
important role in tumor surveillance (32,33).
Therefore, it is considered that the
CD11b+CD27− subset has the most important
role for immunosurveillance of cancer. Thus, in the current study,
it was considered that the reduction of CD8+ and NK cell
counts, and the inhibition of NK cell maturation following
tofacitinib treatment promotes lung metastasis due to the
activities described above.
Cancer metastasis and NK cell count was not
significantly affected by MR16-1 treatment in the present study.
IL-6 is an inflammatory cytokine that serves multiple roles,
including developmental differentiation, proliferation, survival
and anti-apoptosis of various cells (34). These same signaling pathways serve to
maintain cell progression towards neoplastic growth, protecting
cells from apoptotic death (35).
With regards to NK cell activity, a previous study reported that
human NK cells exposed to IL-6 exhibited reduced perforin and
granzyme-B expression, which was recovered in the presence of the
anti-human IL-6R Ab tocilizumab (36). In that study, no significant
differences in NK cell expression of CD69 or CD107a were observed
between IL-6 transgenic, and wild-type mice. However, perforin and
granzyme expression in NK cells was reduced in IL-6 transgenic mice
compared with that in wild-type mice (36). Therefore, it may be assumed that NK
cell activity is inhibited by IL-6; however, in the present study,
the IL-6R Ab did not affect NK cell numbers or maturation, and did
not promote cancer metastasis in the lung metastasis mouse
model.
Etanercept is a recombinant human TNF
receptor-Fragment crystallizable (R-Fc) fusion protein that
inhibits TNF-α activity (37). Due to
the immunosuppressive properties of this TNF-α inhibitor, it has
been suggested that TNF-α inhibitor therapy may increase the risk
of malignancy (38,39). However, a consensus has not been
reached on whether this TNF-α inhibitor enhances carcinogenesis,
tumor growth and metastasis in patients with cancer. The present
study revealed no enhancement of lung metastasis in
etanercept-treated mice. Etanercept has been reported to reduce the
number and size of tumors in a spontaneous colon cancer mouse model
associated with chronic colitis (40). Furthermore, blockade of TNF-α has been
reported to inhibit lung metastasis in a mouse model (41,42).
Concerning the effect of etanercept on NK cells, etanercept was
reported to inhibit the production of transforming growth factor
(TGF)-β1, which subsequently led to the inhibition of NK cells and
cytotoxic activity (42). In an
experimental lung metastasis mouse model, etanercept inhibited
TGF-β1 production, which induced IL-13, restored CD8+
cell cytolytic activity and reduced lung metastasis (42). In the present study, there was a
significant decrease in the percentage of
CD11b+CD27− NK cells in the spleen following
treatment with etanercept compared with that in other groups.
Accompanied by the decrease in the
CD11b+CD27− ratio, the ratio of
CD11b−CD27− and
CD11b−CD27+ was increased; however, the ratio
of CD11b+CD27+ to total NK cells was
unchanged. However, the total NK cell count in the
etanercept-treated group was significantly increased compared with
that in the untreated control and C26-treated groups. Furthermore,
no statistically significant differences were identified in the
total count of CD11b+CD27− NK cells in the
spleen compared with those in other groups. The effect of
etanercept may depend on the TNF-α status of the experimental
model; for example, whether the model exhibits enhanced TNF-α
expression or not. It was assumed that lung metastasis was not
significantly enhanced following etanercept treatment in the
present study, as etanercept exhibited little effect on NK cells.
This finding does not conflict with previous studies reporting that
TNF blockade inhibits carcinogenesis and cancer metastasis
(41,42).
The present study has certain limitations. Firstly,
the study used an experimental mouse model. Thus, the dose or
administration method of each drug was referred from other previous
experimental animal reports, and the clinical use of these drugs in
humans may differ from the lung metastasis model used. In
particular, tofacitinib is orally administered in humans, and
therefore, it is unclear whether an increase in cancer metastasis
would occur in patients with cancer following tofacitinib treatment
as it did in the mice. Therefore, validation of the results of the
current study in patients is warranted. Secondly, the present study
used a normal mouse-bearing cancer cell line but not a RA mouse
model. Thus, further studies are required to address these
limitations.
Out of the three cytokine signal inhibitors
evaluated in the present study, only tofacitinib significantly
enhanced lung metastasis with inhibition of the proliferation and
differentiation of NK cells in the lung metastasis mouse model.
These data suggest that agents that reduce NK cell numbers have the
potential to promote cancer metastasis. Monitoring of the NK cell
number in patients with RA treated with cytokine signal inhibitors
may be important in reducing the risk of cancer.
Acknowledgements
English language editing of the manuscript was
received from Elsevier Language Editing Services (Elsevier, San
Diego, CA, USA). The research grant and the anti-mouse IL-6R Ab
were provided by Chugai Pharmaceutical Co., Ltd. (grant awarded to
Dr Shinsuke Takeno; Department of Surgery, Miyazaki University
Faculty of Medicine, Miyazaki, Japan).
Glossary
Abbreviations
Abbreviations:
|
C26
|
colon 26
|
|
IL-6R Ab
|
IL-6 receptor antibody
|
|
NK
|
natural killer
|
|
RA
|
rheumatoid arthritis
|
References
|
1
|
Whiteside TL and Herberman RB: The role of
natural killer cells in immune surveillance of cancer. Curr Opin
Immunol. 7:704–710. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hanna N and Burton RC: Definitive evidence
that natural killer (NK) cells inhibit experimental tumor
metastases in vivo. J Immunol. 127:1754–1758. 1981.PubMed/NCBI
|
|
3
|
Kelly SA, Gschmeissner S, East N and
Balkwill FR: Enhancement of metastatic potential by
gamma-interferon. Cancer Res. 51:4020–4027. 1991.PubMed/NCBI
|
|
4
|
Mailloux AW, Clark AM and Young MR: NK
depletion results in increased CCL22 secretion and Treg levels in
Lewis lung carcinoma via the accumulation of CCL22-secreting
CD11b+CD11c+ cells. Int J Cancer. 127:2598–2611. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yano S, Nishioka Y, Izumi K, Tsuruo T,
Tanaka T, Miyasaka M and Sone S: Novel metastasis model of human
lung cancer in SCID mice depleted of NK cells. Int J Cancer.
67:211–217. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Coca S, Perez-Piqueras J, Martinez D,
Colmenarejo A, Saez MA, Vallejo C, Martos JA and Moreno M: The
prognostic significance of intratumoral natural killer cells in
patients with colorectal carcinoma. Cancer. 79:2320–2328. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ishigami S, Natsugoe S, Tokuda K, Nakajo
A, Che X, Iwashige H, Aridome K, Hokita S and Aikou T: Prognostic
value of intratumoral natural killer cells in gastric carcinoma.
Cancer. 88:577–583. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bos R and Sherman LA: CD4+ T-cell help in
the tumor milieu is required for recruitment and cytolytic function
of CD8+ T lymphocytes. Cancer Res. 70:8368–8377. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Schild HJ, Kyewski B, von Hoegen P and
Schirrmacher V: CD4+ helper T cells are required for
resistance to a highly metastatic murine tumor. Eur J Immunol.
17:1863–1866. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Funada Y, Noguchi T, Kikuchi R, Takeno S,
Uchida Y and Gabbert HE: Prognostic significance of CD8+
T cell and macrophage peritumoral infiltration in colorectal
cancer. Oncol Rep. 10:309–313. 2003.PubMed/NCBI
|
|
11
|
Waldburger JM and Firestein GS: Garden of
therapeutic delights: New targets in rheumatic diseases. Arthritis
Res Ther. 11:2062009. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Siebert S, Tsoukas A, Robertson J and
McInnes I: Cytokines as therapeutic targets in rheumatoid arthritis
and other inflammatory diseases. Pharmacol Rev. 67:280–309. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Emery P, Breedveld F, van der Heijde D,
Ferraccioli G, Dougados M, Robertson D, Pedersen R, Koenig AS and
Freundlich B; Combination of Methotrexate and Etanercept in Early
Rheumatoid Arthritis Trial Group, : Two-year clinical and
radiographic results with combination etanercept-methotrexate
therapy versus monotherapy in early rheumatoid arthritis: A
two-year, double-blind, randomized study. Arthritis Rheum.
62:674–682. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Genovese MC, Rubbert-Roth A, Smolen JS,
Kremer J, Khraishi M, Gómez-Reino J, Sebba A, Pilson R, Williams S
and Van Vollenhoven R: Longterm safety and efficacy of tocilizumab
in patients with rheumatoid arthritis: A cumulative analysis of up
to 4.6 years of exposure. J Rheumatol. 40:768–780. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lundquist LM, Cole SW and Sikes ML:
Efficacy and safety of tofacitinib for treatment of rheumatoid
arthritis. World J Orthop. 5:504–511. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
van Vollenhoven RF, Fleischmann R, Cohen
S, Lee EB, García Meijide JA, Wagner S, Forejtova S, Zwillich SH,
Gruben D, Koncz T, et al: Tofacitinib or adalimumab versus placebo
in rheumatoid arthritis. N Engl J Med. 367:508–519. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fujimoto M, Serada S, Mihara M, Uchiyama
Y, Yoshida H, Koike N, Ohsugi Y, Nishikawa T, Ripley B, Kimura A,
et al: Interleukin-6 blockade suppresses autoimmune arthritis in
mice by the inhibition of inflammatory Th17 responses. Arthritis
Rheum. 58:3710–3719. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Milici AJ, Kudlacz EM, Audoly L, Zwillich
S and Changelian P: Cartilage preservation by inhibition of Janus
kinase 3 in two rodent models of rheumatoid arthritis. Arthritis
Res Ther. 10:R142008. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Takagi N, Mihara M, Moriya Y, Nishimoto N,
Yoshizaki K, Kishimoto T, Takeda Y and Ohsugi Y: Blockage of
interleukin-6 receptor ameliorates joint disease in murine
collagen-induced arthritis. Arthritis Rheum. 41:2117–2121. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Okazaki M, Yamada Y, Nishimoto N,
Yoshizaki K and Mihara M: Characterization of anti-mouse
interleukin-6 receptor antibody. Immunol Lett. 84:231–240. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Conklyn M, Andresen C, Changelian P and
Kudlacz E: The JAK3 inhibitor CP-690550 selectively reduces NK and
CD8+ cell numbers in cynomolgus monkey blood following
chronic oral dosing. J Leukoc Biol. 76:1248–1255. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kudlacz E, Perry B, Sawyer P, Conklyn M,
McCurdy S, Brissette W, Flanagan And M and Changelian P: The novel
JAK-3 inhibitor CP-690550 is a potent immunosuppressive agent in
various murine models. Am J Transplant. 4:51–57. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sonomoto K, Yamaoka K, Kubo S, Hirata S,
Fukuyo S, Maeshima K, Suzuki K, Saito K and Tanaka Y: Effects of
tofacitinib on lymphocytes in rheumatoid arthritis: Relation to
efficacy and infectious adverse events. Rheumatology (Oxford).
53:914–918. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
U.S. Food and Drug Administration, .
Advisory Committee meeting. Tofacitinib for treatment of rheumatoid
arthritis (NDA 203214). Pfizer Inc.; 2012
|
|
25
|
Ando T, Ito H, Arioka Y, Ogiso H and
Seishima M: Combination therapy with α-galactosylceramide and a
Toll-like receptor agonist exerts an augmented suppressive effect
on lung tumor metastasis in a mouse model. Oncol Rep. 33:826–832.
2015.PubMed/NCBI
|
|
26
|
Mlecnik B, Tosolini M, Kirilovsky A,
Berger A, Bindea G, Meatchi T, Bruneval P, Trajanoski Z, Fridman
WH, Pagès F and Galon J: Histopathologic-based prognostic factors
of colorectal cancers are associated with the state of the local
immune reaction. J Clin Oncol. 29:610–618. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lodolce JP, Boone DL, Chai S, Swain RE,
Dassopoulos T, Trettin S and Ma A: IL-15 receptor maintains
lymphoid homeostasis by supporting lymphocyte homing and
proliferation. Immunity. 9:669–676. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Waldmann TA: The biology of IL-15:
Implications for cancer therapy and the treatment of autoimmune
disorders. J Investig Dermatol Symp Proc. 16:pp. S28–S30. 2013;
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chiossone L, Chaix J, Fuseri N, Roth C,
Vivier E and Walzer T: Maturation of mouse NK cells is a 4-stage
developmental program. Blood. 113:5488–5496. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fu B, Wang F, Sun R, Ling B, Tian Z and
Wei H: CD11b and CD27 reflect distinct population and functional
specialization in human natural killer cells. Immunology.
133:350–359. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Clinthorne JF, Beli E, Duriancik DM and
Gardner EM: NK cell maturation and function in C57BL/6 mice are
altered by caloric restriction. J Immunol. 190:712–722. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Smyth MJ, Thia KY, Cretney E, Kelly JM,
Snook MB, Forbes CA and Scalzo AA: Perforin is a major contributor
to NK cell control of tumor metastasis. J Immunol. 162:6658–6662.
1999.PubMed/NCBI
|
|
33
|
Street SE, Cretney E and Smyth MJ:
Perforin and interferon-gamma activities independently control
tumor initiation, growth and metastasis. Blood. 97:192–197. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hunter CA and Jones SA: IL-6 as a keystone
cytokine in health and disease. Nat Immunol. 16:448–457. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hodge DR, Hurt EM and Farrar WL: The role
of IL-6 and STAT3 in inflammation and cancer. Eur J Cancer.
41:2502–2512. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Cifaldi L, Prencipe G, Caiello I,
Bracaglia C, Locatelli F, De Benedetti F and Strippoli R:
Inhibition of natural killer cell cytotoxicity by interleukin-6:
Implications for the pathogenesis of macrophage activation
syndrome. Arthritis Rheumatol. 67:3037–3046. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Feldmann M: Development of anti-TNF
therapy for rheumatoid arthritis. Nat Rev Immunol. 2:364–371. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Brown SL, Greene MH, Gershon SK, Edwards
ET and Braun MM: Tumor necrosis factor antagonist therapy and
lymphoma development: Twenty-six cases reported to the Food and
Drug Administration. Arthritis Rheum. 46:3151–3158. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Diak P, Siegel J, La Grenade L, Choi L,
Lemery S and McMahon A: Tumor necrosis factor alpha blockers and
malignancy in children: Forty-eight cases reported to the Food and
drug administration. Arthritis Rheum. 62:2517–2524. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Popivanova BK, Kitamura K, Wu Y, Kondo T,
Kagaya T, Kaneko S, Oshima M, Fujii C and Mukaida N: Blocking
TNF-alpha in mice reduces colorectal carcinogenesis associated with
chronic colitis. J Clin Invest. 118:560–570. 2008.PubMed/NCBI
|
|
41
|
Choo MK, Sakurai H, Koizumi K and Saiki I:
TAK1-mediated stress signaling pathways are essential for
TNF-alpha-promoted pulmonary metastasis of murine colon cancer
cells. Int J Cancer. 118:2758–2764. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Fichtner-Feigl S, Terabe M, Kitani A,
Young CA, Fuss I, Geissler EK, Schlitt HJ, Berzofsky JA and Strober
W: Restoration of tumor immunosurveillance via targeting of
interleukin-13 receptor-alpha 2. Cancer Res. 68:3467–3475. 2008.
View Article : Google Scholar : PubMed/NCBI
|