Introduction
Breast cancer is one of the most frequent
malignances and the second most common cause of cancer-associated
mortality in women around the world. The morbidity rate of breast
cancer has gradually increased in recent decades (1). Treatment includes surgical intervention,
radiotherapy (RT), systemic treatment with cytotoxic chemotherapy,
hormone therapy, biological therapy, or a combination of all these
therapies (2). Subsequent to
breast-conserving surgery, the tumor bed represents the region with
the highest probability of recurrence (≤90%) (3,4). It was
determined that the most effective strategy is intraoperative RT
(IORT) (5,6). During IORT, a 10-Gy electron-boost is
delivered within breast-conserving treatment (BCT), which results
in notably low local recurrence rates (7). It is known that post-operative wound
fluids (WFs) collected from breast cancer patients are able to
stimulate in vitro growth of breast cancer cells and are
potent activators of the signal transducer and activator of
transcription factors, due to enriched composition of cytokines and
growth factors related to wound healing. WFs have an important role
in breast cancer cell proliferation, migration and survival
(8,9).
Based on these findings and having the knowledge that IORT
contributes to low recurrence rates (5–7), attempts
were made to determine whether the post-surgery wound fluid after
intraoperative irradiation, may modify the wound microenvironment,
making it less favorable for cancer cell growth and invasion
(8). IORT is the key factor that
affects the wound healing microenvironment, which affects cancer
cell biology by decreasing the growth and invasion potential. A
study by Belletti et al (8)
demonstrated that WFs from patients treated with targeted IORT
significantly modify the proteomic expression profile of molecules
associated with tumor growth and motility. Additionally, the effect
of surgical wounds after intraoperative radiotherapy on the cancer
stem cell (CSC) phenotype was previously investigated in a panel of
human breast cancer cell lines (10).
It was found that WF and WF after IORT treatment affects the
putative stem cell phenotype in breast cancer cell lines.
Additionally, this stimulatory effect was decreased in WF after
IORT treatment.
In breast cancer, a number of microRNAs (miRNAs)
have been identified as tumor suppressors or oncogenes and have
been characterized as critical regulators of tumor initiation,
metastasis and chemoresistance (11).
miRNAs have also emerged as critical regulators of drug resistance
that act by modulating the epithelial-to-mesenchymal transition
(EMT) and cancer-associated immune responses (12). miRNAs are molecules that contribute to
modulating signaling pathways subsequent to radiation exposure and
have emerged as a potential therapeutic target or biomarker in the
radiation response of cancer (13).
However, there is insufficient data on the effect of IORT and the
wound-healing process on miRNA regulation in the breast cancer
cells present in that microenvironment.
Due to evidence that post-surgical WFs after IORT
can modify the proteomic expression profile, and thus the wound
microenvironment, the aim of the present study was to determine
whether post-surgery WFs had an effect on the miRNA expression
level in breast cancer cells, and if those changes are associated
with intraoperative radiation therapy.
Materials and methods
Surgical WF collection
Postoperative WFs were collected from breast cancer
patients that underwent surgical treatment in Greater Poland Cancer
Centre in Poznan, Poland. Wound fluids were collected between
November 2013 and January 2015. Following resection of the tumor
(wide local excision), one group of patients underwent IORT up to a
dose of 10 Gy per tumor bed (Boost) (RT-WF group); the second group
of patients did not receive IORT (WF group). The clinical
characteristics of each group are presented in Table I. The follow-up examination was
scheduled 7 days after surgery at the Greater Poland Cancer Centre
in Poznan, Poland. The patients underwent the ultrasonography and
were assessed for the presence of fluid in the tumor bed. WFs were
collected by means of percutaneous aspiration. Fluids were
centrifuged for 25 min at 300 × g at 4°C, sterile filtered and
stored at −80°C. The study was approved by the Bioethics Committee
of Poznań University of Medical Sciences (Poznań, Poland). Informed
consent was obtained from all patients.
 | Table I.Histopatological classification of
patients. |
Table I.
Histopatological classification of
patients.
|
| Treatment group,
% |
|---|
|
|
|
|---|
| Clinicopathological
features | RT-WF | WF |
|---|
| Total, n | 22.0 | 20.0 |
| Age at diagnosis,
years ± SD | 60.1±9.6 | 55.0±10.2 |
| Histological
type |
|
|
|
Ductal |
59.1 | 65.0 |
|
Lobular |
22.7 | 35.0 |
|
Other |
18.2 |
0.0 |
| ER status |
|
|
|
Positive | 100.0 | 95.0 |
|
Negative |
0.0 |
5.0 |
| HER2 status |
|
|
|
Positive |
0.0 | 10.0 |
|
Negative | 100.0 | 90.0 |
| Molecular
classification |
|
|
| Triple
negative |
0.0 |
5.0 |
|
Non-triple negative | 100.0 | 95.0 |
| Histological
grade |
|
|
| G1 |
40.9 | 25.0 |
| G2 |
31.8 | 55.0 |
| G3 |
27.3 | 20.0 |
Cell culture
Experiments were performed on four breast cancer
cell lines, consisting of MDA-MB-468 [Er/PgR-; human epidermal
growth factor receptor 2 (HER2)/Neu-], BT-549 (Er/PgR-; HER2/Neu-),
MCF-7 (Er/PgR+; HER2/Neu-) and SK-BR-3 (Er/PgR-; HER2/Neu+).
MDA-MB-468 and BT-549 are defined as triple-negative breast cancer
(TNBC). They were chosen due to their distinct molecular profile
(14,15). All cell lines were obtained from the
American Type Culture Collection (Manassas, VA, USA) and maintained
according to the supplier's instructions. Cells were grown at 37°C
in 5% CO2. Cells were seeded on 6-cm Petri dishes and
incubated with standard medium overnight 24 h prior to experiments.
Subsequently, culture medium was changed to fresh medium containing
10% RT-WF or 10% WF. The cells were incubated in standard culture
conditions (37°C in an atmosphere containing 5% CO2) for
4 days. Control cells (untreated cells cultured in the same
conditions) were harvested in standard medium under the same
conditions.
RNA isolation
Total RNA from 20 samples for each cell line (10
samples per group; RT-WF or WF) was extracted with TRIzol reagent
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany), according to the
manufacturer's instructions. Cells were lysed using TRIzol reagent.
For the miRNA normalization process, 3.5 µl of miRNeasy
Serum/Plasma Spike-In Control working solution (1.6×108
copies/µl; Qiagen, Hilden, Germany) was added to each sample. The
RNA eluted in diethylpyrocarbonate-treated H2O was
stored at −80°C until further analyses.
Reverse transcription (RT)
The RT reactions were performed using the Taq-Man
Reverse Transcription kit and miRNA-specific stem-loop primers:
Human (hsa-) mir-21-5p (assay ID, 000397), hsa-mir-155-5p (assay
ID, 002623), hsa-mir-221 (assay ID, 000524), cel-miR-39 (reference
gene; assay ID, 000200; Applied Biosystems; Thermo Fisher
Scientific, Inc., Waltham, MA, USA). Each reaction consisted of 10
ng miRNA, 10X RT buffer, 20 U/µl RNase inhibitor, 5X TaqMan miRNA
RT primer, 100 mmol/l dNTP and 50 U/µl MultiScribe Reverse
Transcriptase. The RT reaction was performed using a
Veriti® 96-Well Fast Thermal Cycler (Applied Biosystems;
Thermo Fisher Scientific, Inc.) at 16°C for 30 min, 42°C for 30 min
and 85°C for 5 min.
Quantitative polymerase chain reaction
(qPCR)
qPCR analysis of hsa-miR-21, hsa-miR-155,
hsa-miR-221 and miRNeasy Serum/Plasma Spike-In Control
(1.6×108 copies/µl) as a reference, was performed using
a miR-specific TaqMan MicroRNA Assay kit (Applied Biosystems;
Thermo Fisher Scientific, Inc.). Analyzed miRNA primers and
reference miRNA primers used were as aforementioned for reverse
transcription (miRNA-specific stem-loop primers; Applied
Biosystems; Thermo Fisher Scientific, Inc.). The amplification
protocol was as follows: 95°C for 10 min, followed by 50 cycles of
95°C for 15 sec, 60°C for 1 min, and 4°C for 30 sec. The qPCR
reactions were performed in triplicate according to the
manufacturer's instructions using LightCycler480 (Roche
Diagnostics, Basel, Switzerland). The fold-differences in miRNA
expression between the samples were calculated using the
comparative Cq (2−ΔΔCq) method (16).
Statistical analysis
Quantitative differential expression of miRNAs
between patients was calculated as 2−ΔΔCq. For the
statistical analysis the GraphPad Prism program (version 6;
GraphPad Software, Inc., La Jolla, CA, USA) was used. Data were
examined using one-way analysis of variance with Tukey's post hoc
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Evaluation of miRNA expression in
breast cancer cell lines
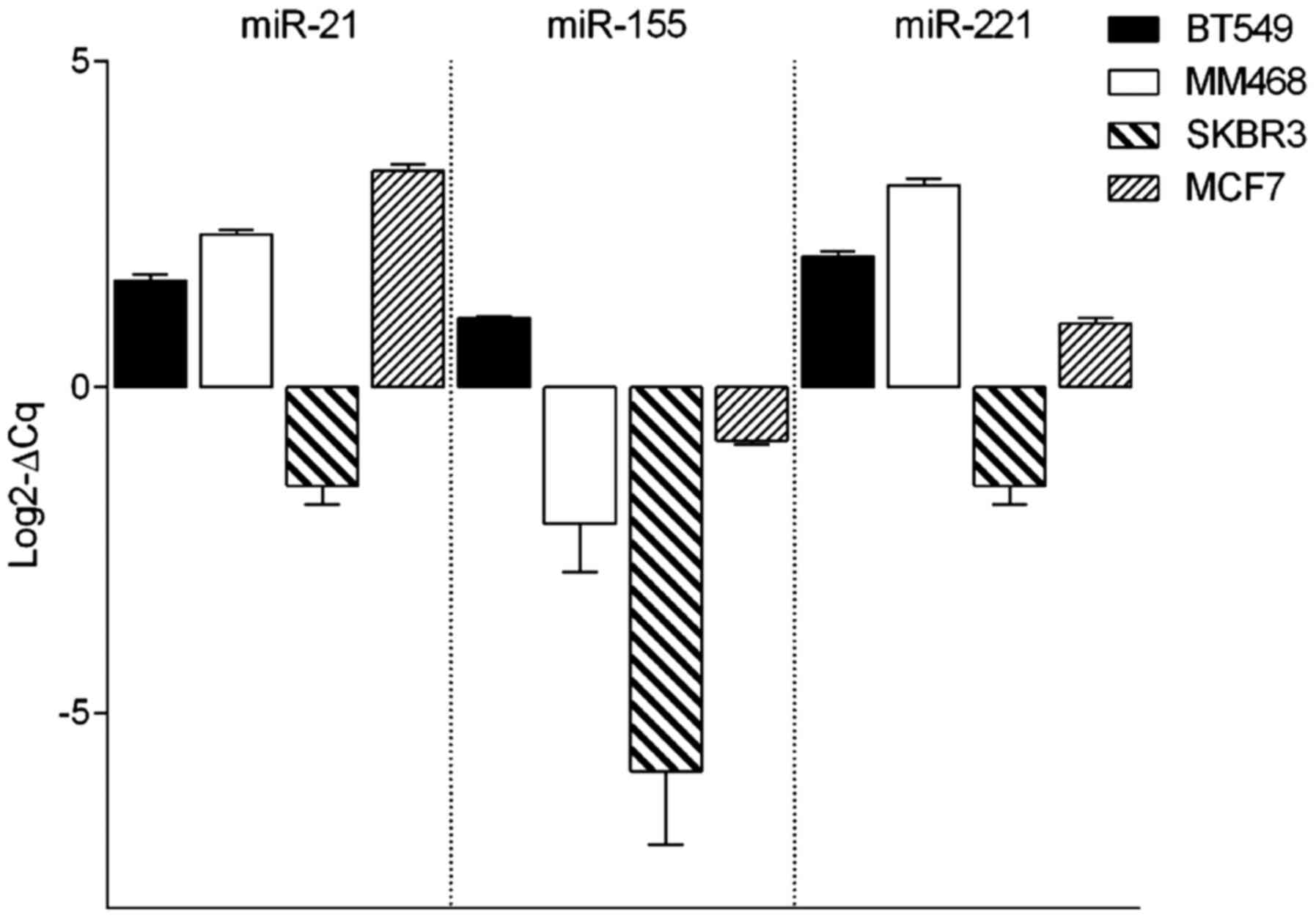
The expression level of three different miRNAs,
consisting of miR-21, miR-155 and miR-221, was assessed by RT-qPCR
analysis in cell lines from 4 subtypes of breast cancer, consisting
of the basal/mesenchymal BT-549, basal/epithelial MDA-MB-468,
HER2-overexpressed SB-BR-3 and luminal MCF7 cell line (Fig. 1). The expression profiles of miR-21
and miR-221 were similar in all analyzed cell lines. BT-549,
MDA-MB-468 and MCF7 cells showed similar expression levels of
miR-21 and miR-221. Similar patterns were also observed in SK-BR-3
cell line; however, the expression of those miRNAs was much lower.
miR-155 represented the lowest expression profile among all
analyzed cell lines. The expression of all analyzed miRNAs was
lowest in the SK-BR3 cell line.
Expression of miR-21, miR-155 and
miR-221 is affected by WFs in different subtypes of breast cancer
cells
The present study confirmed that all the miRNAs were
differentially expressed in breast cancer cells (Fig. 1). To establish the effect of WFs on
the expression of the 3 analyzed miRNAs, cells were incubated with
10% of WF or RT-WF and harvested after 4 days. The levels of miRNA
expression were evaluated using the TaqMan MicroRNA Assay.
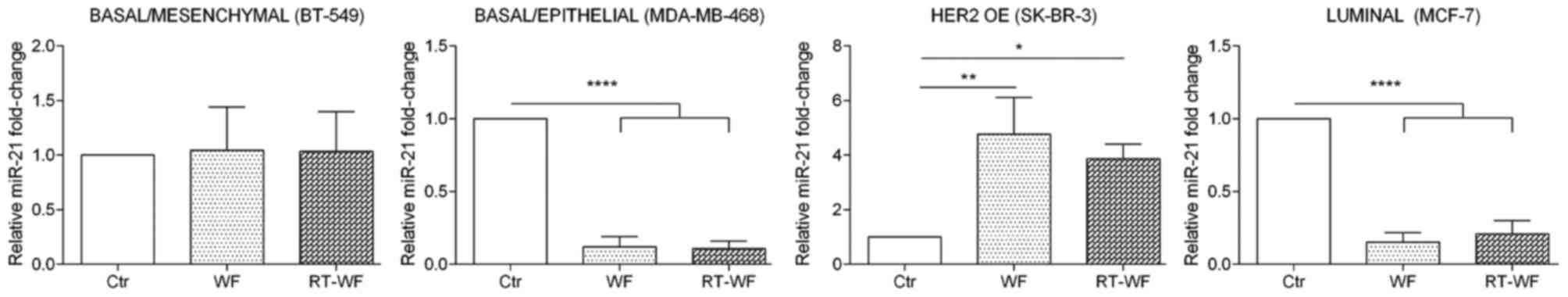
miR-21 expression
Breast cancer cells from 4 subtypes of breast cancer
demonstrated an altered expression level of miR-21 after 4-day
stimulation with 10% WF or RT-WF, and the results varied markedly
between each cell line (Fig. 2). The
basal/mesenchymal BT-549 cell line did not exhibit any changes in
the expression of miR-21 following incubation with WF or RT-WF (WF,
1.04±0.26; RT-WF, 1.04±0.24). Notably, the basal/epithelial
MDA-MB-468 and luminal MCF7 cell lines exhibited a similar
expression profile of miR-21. Following incubation with WF and
RT-WF, the expression of analyzed miRNA was significantly decreased
(P<0.0001). No changes were observed between the WF and RT-WF
groups in the MDA-MB-468 cell line (WF, 0.12±0.05; RT-WF,
0.11±0.03). By contrast, the RT-WF group exhibited slightly
increased expression of miR-21 compared with the WF group in the
MCF7 cell line; however, those results were not statistically
significant (WF, 0.15±0.04; RT-WF, 0.21±0.07). In contrast to the
aforementioned cell line, the HER-positive SK-BR-3 cell line
reacted differently to WF and RT-WF. Both WFs significantly
increased miR-21 expression (WF, P=0.005; RT-WF, P=0.02); however,
the stimulation with WF was markedly higher (WF, 4.47±0.9; RT-WF,
3.86±0.4).
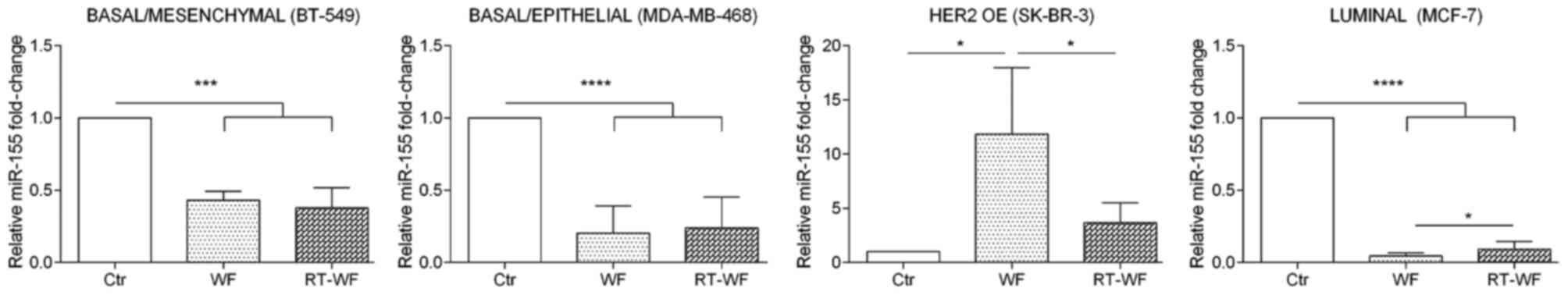
miR-155 expression
As shown in Fig. 1,
miR-155 exhibits the lowest expression in breast cell lines in
comparison with the two other analyzed miRNAs. In the BT-549 cell
line, expression of miR-155 was significantly decreased (P=0.0003)
after incubation with WF and RT-WF in comparison to the control.
However, differences between cells exposed to WF or RT-WF were not
observed (WF, 0.43±0.05; RT-WF, 0.38±0.08; Fig. 3). Similar results were also identified
for the MDA-MB-468 cell line (P<0.0001). In contrast to the
BT-549 cell line, in MDA-MB-468 cells a slight increase in miR-155
expression was observed following incubation with RT-WF (compared
with WF); however, this was not statistically significant (WF,
0.22±0.1; RT-WF, 0.24±0.15). By contrast, the HER2-overexpressing
SK-BR-3 breast cancer cell line exhibits the most pronounced
differences in miR-155 expression. WF causes the highest
upregulation of miR-155 (11.84±5.2; P=0.012) in comparison to the
control and RT-WF groups. Increased expression of miR-155 was also
observed following exposure to RT-WF, but at a significantly lower
level (3.67±1.3; P=0.024). Additionally, the MCF7 cell line
exhibited significantly decreased miR-155 expression (P<0.0001)
and this decrease was more noticeable in the WF group compared with
the RT-WF group (P=0.012; WF, 0.05±0.01; RT-WF, 0.09±0.03).
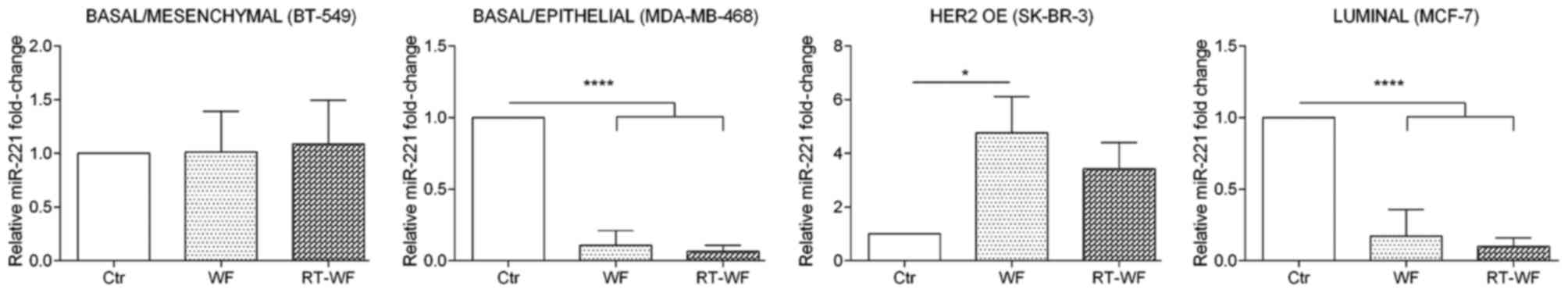
miR-221 expression
miR-221 expression shows a similar expression
profile to miR-21 expression subsequent to WF and RT-WF
supplementation of culture medium (Fig.
4). In the BT-549 cell line, no changes in miRNA expression
were observed subsequent to WF or RT-WF stimulation (WF, 1.02±0.27;
RT-WF, 1.09±0.3). Incubation of MDA-MB-468 and MCF7 cells with WF
and RT-WF resulted in a prominent decrease in miR-221 expression
(P<0.0001). For MDA-MB-468 cells, no statistical changes were
observed between the WF and RT-WF groups (WF, 0.11±0.06; RT-WF,
0.07±0.03); however, non-significant decreased expression of
miR-221 in the RT-WF group was observed. In contrast to other cell
lines, in SK-BR-3 cells the miR-221 expression was increased
following incubation with both WFs; however, statistical
significance was observed only in WF-treated cells (P=0.0015; WF,
4.76±0.9; RT-WF, 3.42±0.7).
Discussion
Surgery itself can activate numerous inflammatory
responses that are known to modify the growth kinetics of breast
cancer micro-metastasis, suggesting that it can be also a factor in
local recurrence or metastasis development (17–19).
It was shown that not only surgery, but also the
post-surgery WF drainage collected from breast cancer patients can
have a prominent role in breast cancer cell proliferation, motility
and survival (8,20,21). Thus,
it is possible that modification of the local microenvironment
caused by surgery may alter the growth kinetics of cancer cells,
supporting the ‘seed and soil’ hypothesis first proposed by Sir
Stephen Paget to explain the pathogenesis of cancer metastasis
(22,23). Additionally, it has been proposed that
radiotherapy may affect not only breast cancer cell survival, but
may also change the cell phenotype, physical interactions,
signaling between cells and thus the entire tumor microenvironment
(24,25).
miRNAs have revealed functions in cancer biology and
processes in the local microenvironment. However, only a few
studies have described the effect of radiation on the miRNA
expression profile and little is known about the underlying
molecular mechanism (26–28). In the present study, with knowledge of
the role of miRNAs in breast cancer development and different
expression patterns between the breast cancer cell lines, and based
on previous studies investigating the potential effect of
postoperative WF on the tumor microenvironment (8,9,29), it was decided to explore whether
stimulation of breast cancer cells by WF can affect the expression
of distinct miRNAs in cell culture.
Previous studies have revealed that miRNAs also act
as potential agents for predicting radiation responses or
modulating the tumor radiation response of lymphoblastic cell
lines, endothelial cells and lung cancer cells (26,27,30).
Additionally, the dysregulation of miR-21 and miR-155 expression is
associated with tumor progression (31–33).
miR-21 is known as a common inflammation-inducible miRNA that
targets phosphatase and tensin homolog, nuclear factor I B and
pro-inflammatory programmed cell death protein 4 (32,34,35). In
breast cancer cells, miR-21 contributes to radiation resistance by
compromising cell cycle progression (radiation-induced G2/M arrest)
(36). miR-21 is also one of the most
consistently upregulated onco-miRs in a wide range of cancers,
including breast, lung or colon cancer (31). Anastasov et al (36) observed that the changes in miR-21
expression depend on the cells being radiosensitive. In the
aforementioned study using two breast cancer cell lines, the
authors demonstrated that miR-21 expression was significantly
increased in radiation-resistant cells, but remained unchanged in
the radiosensitive cell line (36).
This data supports the hypothesis that regulation of miR-21
expression is not associated with oncogenesis, but rather acts as
radioresistant miRNA when it is transiently overexpressed
subsequent to radiation treatment (37). The present study showed that miR-21
was highly expressed in 3 of 4 analyzed breast cancer cells,
consisting of the basal/mesenchymal BT-549, basal/epithelial
MDA-MB-468 and luminal MCF7 cell lines. Significantly decreased
expression of miR-21 was observed in the HER2-positive SK-BR3 cell
line, which represents the HER2-positive histological type of
breast cancer. HER2 abnormality has a high prevalence (~22%) in
breast cancer, where overexpression of HER2 is associated with an
increased histological tumor grade, increased cell proliferation,
cell motility, tumor invasiveness, metastases and angiogenesis,
decreased apoptosis, and a poor overall prognosis (38,39).
While comparing the responses of different subtypes
of breast cancer to WFs obtained from patients that underwent
surgery alone or surgery followed by IORT, a different profile of
miR-21 was observed. The present study found that WFs have no
effect on the BT-549 cell line. In MDA-MB-468 and MCF7 cells, a
prominent decrease in miR-21 expression was observed. By contrast,
SK-BR3 cells had increased expression of miR-21 following
stimulation with WF and RT-WF. Based on the present data, it was
hypothesized that both WFs, and particularly WF can increase the
malignancy of SK-BR-3. Thus, the present results confirm the
findings of previous studies, indicating that local recurrence
after surgery is particularly common in tumors characterized by
HER2 overexpression, where miR-21 is over-expressed (40,41).
Additionally, it was previously reported that WFs contain growth
factors that induce proliferation of HER2-positive breast cancer
(29).
miR-155 is also one of the most consistently
upregulated onco-miRs in a wide range of cancers and it is
associated with tumor progression (31,32). The
clinical data indicate that miR-155 has a crucial role in tumor
development, tumor diagnosis and prognosis. It was also suggested
that this miRNA promotes tumor growth, invasion and metastasis and
thus, acts as an onco-miR in human cancer (42). Notably, the present results clearly
indicate that the WF and RT-WF groups decreased miR-155 expression
in BT-549, MDA-MB-468 and MCF7 cell lines. The SK-BR-3 cell line
showed a different profile of miRNA expression in the response to
WF and RT-WF. The level of miR-155 expression was 12 times higher
in the WF group and 4 times higher in the RT-WF group compared with
untreated cells. The present findings may contribute to the
clinical response of the patients. According to Chen et al
(43), decreased miR-155 expression
by antisense targeting could increase the sensitivity of breast
cancer cells to irradiation. Since the RT-WF group shows a
decreased expression level of miR-155 compared with WF, it may be
hypothesized that radiation therapy, given to the two groups of
patients following surgery, may have exhibited an improved outcome
in patients who had already received IORT.
These results can be highly informative, but do not
provide unambiguous data to allow a simple conclusion of how
surgery followed by IORT alters the tumor microenvironment.
Additionally, it is important to indicate that increased miR-155
expression in breast cancer has been shown to be significantly
associated with increased tumor grade, advanced tumor stage and
lymph node metastasis, suggesting its potential as a clinical
prognostic value (44). Shibuya et
al (45) showed that increased
miR-155 expression may be an effective biomarker for the prediction
of poor prognosis. The present study found that in the case of
SK-BR-3 stimulation by postoperative WF after IORT, the expression
of miR-155 was significantly downregulated compared to the WF
group. This finding may indicate that radiotherapy on the tumor bed
immediately after surgery (IORT) may modulate the properties of
post-operative WF and consequently change the miRNA expression.
According to Jiang et al (46)
overexpression of miR-155 increases the proliferation of breast
cancer cell, while downregulation (using anti-miR-155) reduces the
cell growth. Downregulation of miR-155, which the present study
observed in RT-WF group, may then reduce the proliferation of tumor
cells and finally the local recurrence of cancer. Opposite results
were observed in the MCF7 cell line. The level of miR-155
expression was slightly increased in the RT-WF group compared to WF
group. However, as shown by Gasparini et al (47), overexpression of miR-155 in the MCF7
cell line decreased the efficiency of homologous recombination and
thus enhanced sensitivity to IR both in vivo and in
vitro. This statement stays on the contrary with aforementioned
study by Chen et al (48).
Thus, additional data are required to better understand the key
aspect on miR-155 impact on response to ionizing radiation
treatment.
miR-221 has been found to be overexpressed in
numerous human tumors (49–52). While analyzing breast cancer cells,
Roscigno et al (53) showed
that miR-221 is expressed at increased levels within the CSC
population. It is also responsible for promoting tumorigenesis in
triple-negative breast cancer cells by promoting EMT in those
cells. Thus, patients with increased miR-221 levels show worse
overall survival (54). The findings
presented in the present study indicate that miR-221 expression is
affected by WFs in two basal/epithelial HER2-positive and luminal
subtypes of breast cancer. MDA-MB-468 and MCF7 cells show decreased
expression of miR-221 subsequent to WF/RT-WF addition, with a
slightly increased expression in the WF group; again, SK-BR-3 shows
a different expression profile. In SK-BR-3 cells, WF statistically
increases miR-221 expression (5-fold), while RT-WF only slightly
stimulates its expression, without any statistical significance. As
miR-221 is upregulated in the population of CSCs, we wondered
whether this effect is also dependent on enriched population of
CSCs. As shown in a previous study, the SK-BR-3 cell line is
enriched in CSC phenotype measured by both ALDH activity and
CD44/CD24−/low population after stimulation with both
fluids (10). Furthermore, MCF7,
MDA-MB-468 and BT-549 also represented elevated levels of CSC
population after WF stimulation. However, as the present results
indicate, the level of miR-221 expression was decreased in those
cells. Therefore, the present study speculates, that the
correlation between miR-221, CSC phenotype and WF is related to the
subtype of breast cancer cell line. Additionally, it was also
demonstrated that miR-221 together with miR-222 directly targets
estrogen receptor alpha and overexpression of these miRNAs in
breast cancer aid in the progression of the more aggressive
basal-like breast cancer (55). Thus,
it was hypothesized that WF may have a role in the progression of
HER2-positive breast cancer, and that this effect can be partially
abrogated by intraoperative radiotherapy. However, due to the
preliminary nature of the current study, additional research is
required to reach definitive conclusions.
Overall, the present study demonstrated that
post-surgical WFs affect the expression level of selected miRNAs
associated with tumor development and progression. The
understanding of the crosstalk mechanism between surgery and
recurrence and metastases may be particularly relevant for
identifying effective treatments for breast cancer.
Acknowledgements
The present study was supported by the National
Science Center, Kraków, Poland (grant no.
UMO-2013/09/N/NZ4/02844).
References
|
1
|
Westlake S and Cooper N: Cancer incidence
and mortality: Trends in the United Kingdom and constituent
countries, 1993 to 2004. Health Stat Q. 33–46. 2008.PubMed/NCBI
|
|
2
|
Carlson RW, Allred DC, Anderson BO,
Burstein HJ, Edge SB, Farrar WB, Forero A, Giordano SH, Goldstein
LJ, Gradishar WJ, et al: Metastatic breast cancer, version 1.2012:
Featured updates to the NCCN guidelines. J Natl Compr Canc Netw.
10:821–829. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Demicheli R, Ardoino I, Boracchi P,
Coradini D, Agresti R, Ferraris C, Gennaro M, Hrushesky WJ and
Biganzoli E: Recurrence and mortality according to estrogen
receptor status for breast cancer patients undergoing conservative
surgery. Ipsilateral breast tumour recurrence dynamics provides
clues for tumour biology within the residual breast. BMC Cancer.
10:6562010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Faverly DR, Hendriks JH and Holland R:
Breast carcinomas of limited extent: Frequency,
radiologic-pathologic characteristics, and surgical margin
requirements. Cancer. 91:647–659. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bitterman A, Kessner R, Goldman I, Shiloni
E and Steiner M: Intraoperative radiotherapy for breast cancer. Isr
Med Assoc J. 14:256–259. 2012.PubMed/NCBI
|
|
6
|
Murawa P, Murawa D, Adamczyk B and Polom
K: Breast cancer: Actual methods of treatment and future trends.
Rep Pract Oncol Radiother. 19:165–172. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sedlmayer F, Reitsamer R, Fussl C, Ziegler
I, Zehentmayr F, Deutschmann H, Kopp P and Fastner G: Boost IORT in
breast cancer: Body of evidence. Int J Breast Cancer.
2014:4725162014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Belletti B, Vaidya JS, D'Andrea S,
Entschladen F, Roncadin M, Lovat F, Berton S, Perin T, Candiani E,
Reccanello S, et al: Targeted intraoperative radiotherapy impairs
the stimulation of breast cancer cell proliferation and invasion
caused by surgical wounding. Clin Cancer Res. 14:1325–1332. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Segatto I, Berton S, Sonego M, Massarut S,
Perin T, Piccoli E, Colombatti A, Vecchione A, Baldassarre G and
Belletti B: Surgery-induced wound response promotes stem-like and
tumor-initiating features of breast cancer cells, via STAT3
signaling. Oncotarget. 5:6267–6279. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zaleska K, Suchorska WM, Przybyła A and
Murawa D: Effect of surgical wound fluids after intraoperative
electron radiotherapy on the cancer stem cell phenotype in a panel
of human breast cancer cell lines. Oncol Lett. 12:3707–3714.
2016.PubMed/NCBI
|
|
11
|
Kong W, He L, Coppola M, Guo J, Esposito
NN, Coppola D and Cheng JQ: MicroRNA-155 regulates cell survival,
growth and chemosensitivity by targeting FOXO3a in breast cancer. J
Biol Chem. 285:17869–17879. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
De Mattos-Arruda L, Bottai G, Nuciforo PG,
Di Tommaso L, Giovannetti E, Peg V, Losurdo A, Pérez-Garcia J,
Masci G, Corsi F, et al: MicroRNA-21 links
epithelial-to-mesenchymal transition and inflammatory signals to
confer resistance to neoadjuvant trastuzumab and chemotherapy in
HER2-positive breast cancer patients. Oncotarget. 6:37269–37280.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Leung CM, Chen TW, Li SC, Ho MR, Hu LY,
Liu WS, Wu TT, Hsu PC, Chang HT and Tsai KW: MicroRNA expression
profiles in human breast cancer cells after multifraction and
single-dose radiation treatment. Oncol Rep. 31:2147–2156. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Prat A and Perou CM: Deconstructing the
molecular portraits of breast cancer. Mol Oncol. 5:5–23. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Neve RM, Chin K, Fridlyand J, Yeh J,
Baehner FL, Fevr T, Clark L, Bayani N, Coppe JP, Tong F, et al: A
collection of breast cancer cell lines for the study of
functionally distinct cancer subtypes. Cancer Cell. 10:515–527.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Demicheli R, Valagussa P and Bonadonna G:
Does surgery modify growth kinetics of breast cancer
micrometastases? Br J Cancer. 85:490–492. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fisher B, Gunduz N, Coyle J, Rudock C and
Saffer E: Presence of a growth-stimulating factor in serum
following primary tumor removal in mice. Cancer Res. 49:1996–2001.
1989.PubMed/NCBI
|
|
19
|
Holmgren L, O'Reilly MS and Folkman J:
Dormancy of micrometastases: Balanced proliferation and apoptosis
in the presence of angiogenesis suppression. Nat Med. 1:149–153.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Segatto I, Berton S, Sonego M, Massarut S,
Fabris L, Armenia J, Mileto M, Colombatti A, Vecchione A,
Baldassarre G and Belletti B: p70S6 kinase mediates breast cancer
cell survival in response to surgical wound fluid stimulation. Mol
Oncol. 8:766–780. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Segatto I, Berton S, Sonego M, Massarut S,
D'Andrea S, Perin T, Fabris L, Armenia J, Rampioni G, Lovisa S, et
al: Inhibition of breast cancer local relapse by targeting p70S6
kinase activity. J Mol Cell Biol. 5:428–431. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fidler IJ: The organ microenvironment and
cancer metastasis. Differentiation. 70:498–505. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Paget S: The distribution of secondary
growths in cancer of the breast. 1889. Lancet. 133:571–573. 1889.
View Article : Google Scholar
|
|
24
|
Barcellos-Hoff MH, Park C and Wright EG:
Radiation and the microenvironment-tumorigenesis and therapy. Nat
Rev Cancer. 5:867–875. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Vaidya JS, Tobias JS, Baum M, Keshtgar M,
Joseph D, Wenz F, Houghton J, Saunders C, Corica T, D'Souza D, et
al: Intraoperative radiotherapy for breast cancer. Lancet Oncol.
5:165–173. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chaudhry MA: Real-time PCR analysis of
micro-RNA expression in ionizing radiation-treated cells. Cancer
Biother Radiopharm. 24:49–56. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Weidhaas JB, Babar I, Nallur SM, Trang P,
Roush S, Boehm M, Gillespie E and Slack FJ: MicroRNAs as potential
agents to alter resistance to cytotoxic anticancer therapy. Cancer
Res. 67:11111–11116. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Maes OC, An J, Sarojini H, Wu H and Wang
E: Changes in MicroRNA expression patterns in human fibroblasts
after low-LET radiation. J Cell Biochem. 105:824–34. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tagliabue E, Agresti R, Carcangiu ML,
Ghirelli C, Morelli D, Campiglio M, Martel M, Giovanazzi R, Greco
M, Balsari A and Ménard S: Role of HER2 in wound-induced breast
carcinoma proliferation. Lancet. 362:527–533. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kraemer A, Anastasov N, Angermeier M,
Winkler K, Atkinson MJ and Moertl S: MicroRNA-mediated processes
are essential for the cellular radiation response. Radiation Res.
176:575–586. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yang H, Kong W, He L, Zhao JJ, O'Donnell
JD, Wang J, Wenham RM, Coppola D, Kruk PA, Nicosia SV and Cheng JQ:
MicroRNA expression profiling in human ovarian cancer: miR-214
induces cell survival and cisplatin resistance by targeting PTEN.
Cancer Res. 68:425–433. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhu S, Wu H, Wu F, Nie D, Sheng S and Mo
YY: MicroRNA-21 targets tumor suppressor genes in invasion and
metastasis. Cell Res. 18:350–359. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang JG, Wang JJ, Zhao F, Liu Q, Jiang K
and Yang GH: MicroRNA-21 (miR-21) represses tumor suppressor PTEN
and promotes growth and invasion in non-small cell lung cancer
(NSCLC). Clin Chim Acta. 411:846–852. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li X, Xin S, He Z, Che X, Wang J, Xiao X,
Chen J and Song X: MicroRNA-21 (miR-21) post-transcriptionally
downregulates tumor suppressor PDCD4 and promotes cell
transformation, proliferation, and metastasis in renal cell
carcinoma. Cell Physiol Biochem. 33:1631–1642. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Fujita S, Ito T, Mizutani T, Minoguchi S,
Yamamichi N, Sakurai K and Iba H: miR-21 gene expression triggered
by AP-1 is sustained through a double-negative feedback mechanism.
J Mol Biol. 378:492–504. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Anastasov N, Höfig I, Vasconcellos IG,
Rappl K, Braselmann H, Ludyga N, Auer G, Aubele M and Atkinson MJ:
Radiation resistance due to high expression of miR-21 and G2/M
checkpoint arrest in breast cancer cells. Radiat Oncol. 7:2062012.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Iliopoulos D, Jaeger SA, Hirsch HA, Bulyk
ML and Struhl K: STAT3 activation of miR-21 and miR-181b-1 via PTEN
and CYLD are part of the epigenetic switch linking inflammation to
cancer. Mol Cell. 39:493–506. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ross JS, Slodkowska EA, Symmans WF,
Pusztai L, Ravdin PM and Hortobagyi GN: The HER-2 receptor and
breast cancer: Ten years of targeted anti-HER-2 therapy and
personalized medicine. Oncologist. 14:320–368. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Slamon DJ, Godolphin W, Jones LA, Holt JA,
Wong SG, Keith DE, Levin WJ, Stuart SG, Udove J, Ullrich A, et al:
Studies of the HER-2/neu proto-oncogene in human breast and ovarian
cancer. Science. 244:707–712. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lee JA, Lee HY, Lee ES, Kim I and Bae JW:
Prognostic implications of microRNA-21 overexpression in invasive
ductal carcinomas of the breast. J Breast Cancer. 14:269–275. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Gong C, Yao Y, Wang Y, Liu B, Wu W, Chen
J, Su F, Yao H and Song E: Up-regulation of miR-21 mediates
resistance to trastuzumab therapy for breast cancer. J Biol Chem.
286:19127–19137. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhang CM, Zhao J and Deng HY: MiR-155
promotes proliferation of human breast cancer MCF-7 cells through
targeting tumor protein 53-induced nuclear protein 1. J Biomed Sci.
20:792013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chen X, Chen Y, Wang Y, Yan M, Zhang J,
Liu Q, Yang H and Li J: Role of miR-155 in myasthenia gravis and
effect of dexamethasone on miR-155. Zhong Nan Da Xue Xue Bao Yi Xue
Ban. 37:777–782. 2012.(In Chinese). PubMed/NCBI
|
|
44
|
Cortez MA, Welsh JW and Calin GA:
Circulating microRNAs as noninvasive biomarkers in breast cancer.
Recent Results Cancer Res. 195:151–161. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Shibuya H, Iinuma H, Shimada R, Horiuchi A
and Watanabe T: Clinicopathological and prognostic value of
microRNA-21 and microRNA-155 in colorectal cancer. Oncology.
79:313–320. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Jiang S, Zhang HW, Lu MH, He XH, Li Y, Gu
H, Liu MF and Wang ED: MicroRNA-155 functions as an OncomiR in
breast cancer by targeting the suppressor of cytokine signaling 1
gene. Cancer Res. 70:3119–3127. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Gasparini P, Lovat F, Fassan M, Casadei L,
Cascione L, Jacob NK, Carasi S, Palmieri D, Costinean S, Shapiro
CL, et al: Protective role of miR-155 in breast cancer through
RAD51 targeting impairs homologous recombination after irradiation.
Proc Natl Acad Sci USA. 111:4536–4541. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Chen J, Wang BC and Tang JH: Clinical
significance of microRNA-155 expression in human breast cancer. J
Surg Oncol. 106:260–266. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Garofalo M, Quintavalle C, Romano G, Croce
CM and Condorelli G: miR221/222 in cancer: Their role in tumor
progression and response to therapy. Curr Mol Med. 12:27–33. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Pineau P, Volinia S, McJunkin K, Marchio
A, Battiston C, Terris B, Mazzaferro V, Lowe SW, Croce CM and
Dejean A: miR-221 overexpression contributes to liver
tumorigenesis. Proc Natl Acad Sci USA. 107:264–269. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Garofalo M, Quintavalle C, Di Leva G,
Zanca C, Romano G, Taccioli C, Liu CG, Croce CM and Condorelli G:
MicroRNA signatures of TRAIL resistance in human non-small cell
lung cancer. Oncogene. 27:3845–3855. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Pallante P, Visone R, Ferracin M, Ferraro
A, Berlingieri MT, Troncone G, Chiappetta G, Liu CG, Santoro M,
Negrini M, et al: MicroRNA deregulation in human thyroid papillary
carcinomas. Endocr Relat Cancer. 13:497–508. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Roscigno G, Quintavalle C, Donnarumma E,
Puoti I, Diaz-Lagares A, Iaboni M, Fiore D, Russo V, Todaro M,
Romano G, et al: MiR-221 promotes stemness of breast cancer cells
by targeting DNMT3b. Oncotarget. 7:580–592. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Ke J, Zhao Z, Hong SH, Bai S, He Z, Malik
F, Xu J, Zhou L, Chen W, Martin-Trevino R, et al: Role of
microRNA221 in regulating normal mammary epithelial hierarchy and
breast cancer stem-like cells. Oncotarget. 6:3709–3721. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Zhao JJ, Lin J, Yang H, Kong W, He L, Ma
X, Coppola D and Cheng JQ: MicroRNA-221/222 negatively regulates
estrogen receptor alpha and is associated with tamoxifen resistance
in breast cancer. J Biol Chem. 283:31079–31086. 2008. View Article : Google Scholar : PubMed/NCBI
|