Introduction
Gastric cancer is one of the most common malignant
tumors, and the major cause of cancer-associated mortality
worldwide (1,2). The World Health Organization (WHO) and
International Arctic Research Center indicates that the global
incidence of gastric cancer is 952,000, with 405,000 patients in
China, accounting for 42.6% of the global incidence (3). In China, the majority of patients are
diagnosed in advanced gastric cancer, 10% patients are the early
stage gastric cancer, and the 5-year survival rate is 10–30%
(4). The pathogenesis of gastric
cancer is a complex multi-step process resulting from the
accumulation of multiple genes. As important components of the
extracellular matrix, proteoglycans are involved in the occurrence
and invasiveness of cancer. Small leucine-rich proteoglycans
(SLRPs) not only regulate the extracellular osmotic pressure, but
also affects tissue biomechanics (5,6) and serves
a notable role in tumor growth, proliferation, adhesion, migration
and the regulation of growth factor activity (7,8). As an
important member of SLRP, lumican is thought to modify the fibrous
tissue and induce a tumor-specific ECM (9). Lumican has its own unique structure and
certain special functions that can affect tumor occurrence and
development, processes that remain to be understood (10,11).
To investigate the function of lumican during the
occurrence and development of gastric cancer, lumican expression
was assessed by immunohistochemistry in gastric cancer tissues, and
adjacent normal gastric tissues. The aim of the present study was
to investigate the lumican expression in gastric cancer and its
association with biological behavior and prognosis.
Materials and methods
Materials
The present study was approved by the Ethics
committee of Harbin Medical University Cancer Hospital, Harbin
Medical University (Harbin, China) and informed consent was
obtained from all patients. The retrospective analysis enrolled 146
patients with gastric cancer which were confirmed in accordance
with pathological evidence, and 55 adjacent normal gastric tissue
specimens obtained. All patients were radical correction or
enlarged radical correction in the Department of Gastrointestinal
Surgery, Harbin Medical University Cancer Hospital, Harbin Medical
University (Harbin, China) from June 2010 to December 2010. Of the
146 patients, 110 patients were male and 36 were female, with a
mean age of 57.1 years (range 30–75 years). Selection criteria
included: i) A pathological diagnosis of adenocarcinoma; ii) no
pre-operative radiotherapy or chemotherapy; iii) complete clinical
data and follow-up; iv) no other serious organ diseases; v)
patients had succumbed to the disease as a result of tumor
recurrence or metastasis; vi) all adjacent normal tissues were
sourced >5 cm away from the edge of the cancerous tissue.
Immunohistochemistry
Goat polyclonal lumican antibody (cat. no. AF2846;
R&D Systems, Inc., Minneapolis, MN, USA) diluted to 1:50 were
used as the primary antibody. The SP-9003 Immunohistochemistry kit
was obtained from Origene Technologies, Inc., (Beijing, China). A
polyclonal antibody against the human lumican protein used for
immunohistochemistry was obtained as previously reported (12). Immunohistochemical analysis was
performed using the Histofine Simple Stain PO® Max kit
(Nichirei Corporation, Tokyo, Japan). The present study used
dimethylbenzene to wash the slides three times for 5 min to
deparaffinize, dehydrated slides with sequential concentrations of
ethanol (100, 90, 80 and 70%, for 3 min each). Endogenous
peroxidase activity was blocked by incubation in 0.3% hydrogen
peroxide in methanol for 30 min at 37°C. The tissue sections (3–4
µm-thick) were incubated with the anti-human lumican antibody (cat.
no. AF2846; R&D Systems, Inc.) diluted to 1:100 in PBS
containing 1% bovine serum albumin (Institute of Hematology,
Chinese Academy of Medical Sciences, Beijing, China) for 16 h at
4°C. Bound antibodies were detected using Histofine Simple Stain
PO® Max reagent using diaminobenzidine
tetrahydrochloride as the substrate, with sections counterstained
with 0.0025% Mayer's hematoxylin for 2 min in 37°C. The
immunoreactivity of the lumican protein was scored on the basis of
the intensity of the predominant cytoplasmic staining area using
the following classification system: 0, negative; 1,
weakly-positive; 2, strongly-positive. To examine the associations
between lumican expression levels and the clinicopathological
features or prognostic factors, the tumors were divided into two
groups according to the intensity of staining: Scale 0, negative
group; scales 1 and 2, positive group. All specimens were evaluated
by two investigators blinded to the clinical information of the
patients.
Statistical analysis
The χ2 test was used to analyze the
associations between lumican expression and various prognostic
factors. The survival rate was calculated using the Kaplan-Meier
method, and the significance of the differences in the survival
rate was analyzed by the log-rank test. All statistical analyses
were performing using SPSS software version 17.0 (SPSS, Inc.,
Chicago, IL, USA). α was set at 0.05, and a two-tailed P<0.05
was considered to indicate a statistically significant
difference.
Results
Expression and location of lumican in
human gastric cancer, and adjacent normal gastric tissues
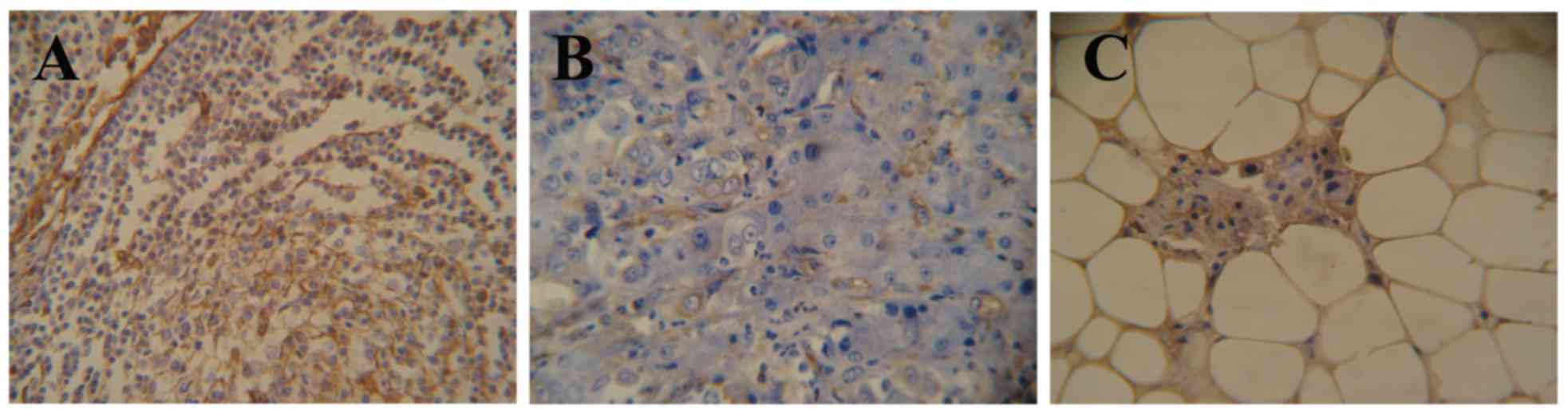
The expression of lumican in the adjacent normal
tissues of 55 patient samples was observed to either be negative or
weakly-positive, with weakly-positive expression only observed in
10.9% (6/55) of the 55 samples (Fig.
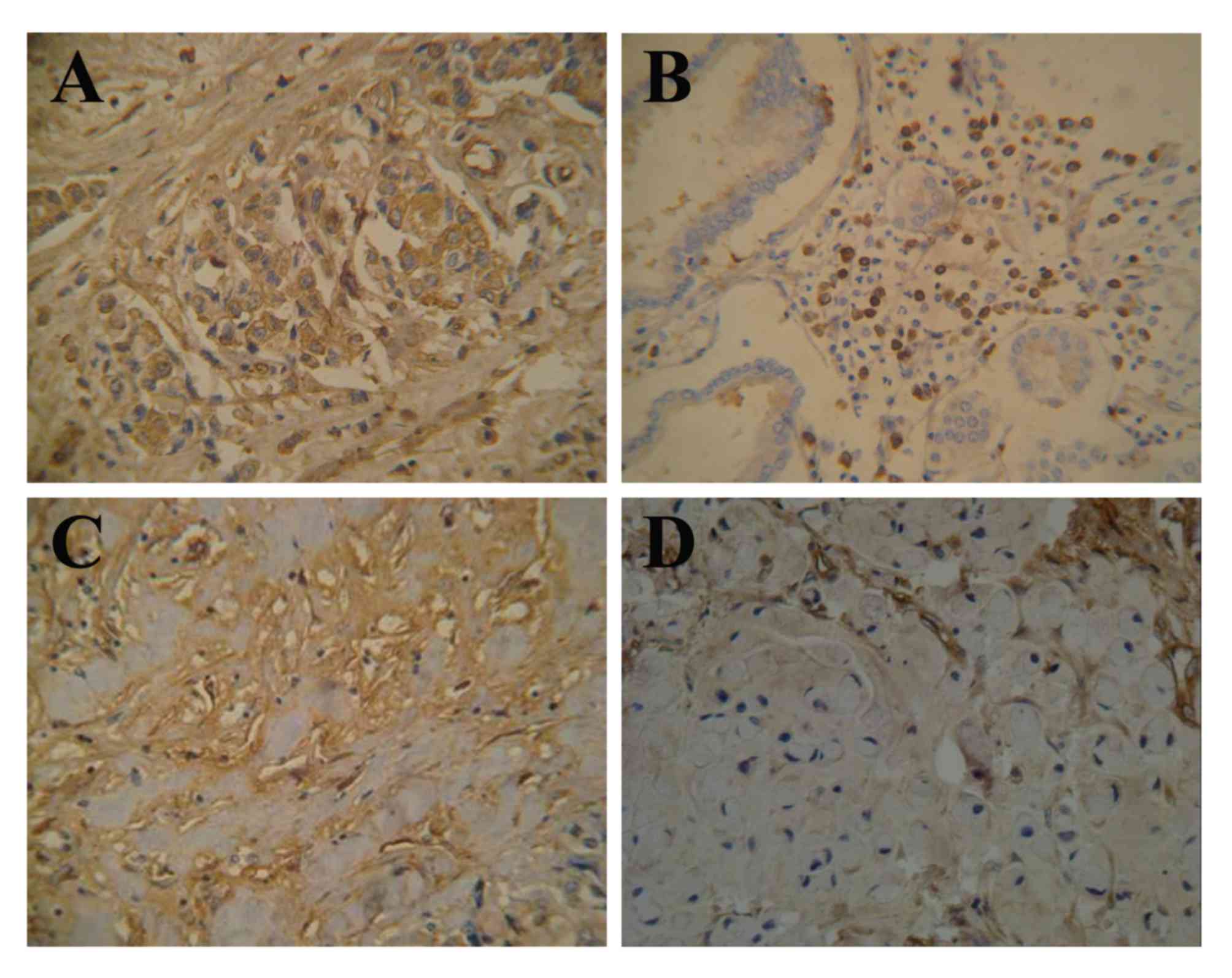
1A). In the present study, lumican expression was localized to
the cytoplasm (58.2%, 85/146) and membrane (8.2%, 12/146) of the
gastric cancer cells (Fig. 1B-D). Of
the 146 gastric cancer tissue samples, lumican expression was
observed in 66.4% (97/146), of which 34.9% (51/146) were
weakly-positive and 31.5% (46/146) were strongly-positive. The rate
of positive expression in organ metastatic and lymphatic metastatic
tissues was 77.8% (28/36) and 76.5% (65/85), respectively (Fig. 2).
Expression of lumican and its
association with pathological features
According to the WHO 2010 pathological grouping
standard, gastric cancer was divided into adenocarcinoma (tubular
adenocarcinoma and papillary adenocarcinoma), mucinous
adenocarcinoma, signet-ring cell carcinoma, mixed carcinoma and
others (13). Lumican expression,
tissue histology and other clinicopathological parameters were
presented in Table I. Tumors were
located in proximal (14), middle
(17), distal (99), and diffuse
(16) areas of stomach. A total of 57
tumors exhibited a diameter <5 cm, 12 with a diameter >10 cm,
and 99 with a diameter of 5–10 cm. Of these patients, 36 cases
exhibited organ metastasis, and 110 did not. A total of 19 tumors
had invaded the mucosa, 26 in the submucosa, 65 in the muscularis
and 34 in the serosa of the stomach. Borrmann type was categorized
into five groups: Borrmann 0, Borrmann I, Borrmann II, Borrmann III
and Borrmann IV (14). 17 cases were
Borrmann 0, 12 cases were Borrmann I, 21 cases were Borrmann II, 93
cases were Borrmann III, 3 cases were Borrmann IV. A total of 85
cases exhibited lymphatic metastasis, and 61 did not.
Pathologically, 102 patients exhibited adenocarcinoma and 44
patients exhibited mucinous carcinoma/signet-ring cell carcinoma.
Lumican expression was determined to be significantly associated
with organ metastasis, lymphatic metastasis and histological type
(P<0.05), but not with the tumor location, size, invasion depth
or the Borrmann type (P>0.05).
 | Table I.Association between patient
characteristics and lumican expression. |
Table I.
Association between patient
characteristics and lumican expression.
|
|
| Lumican, n |
|
|
|---|
|
|
|
|
|
|
|---|
| Parameter | All | Negative | Weak | Strong | χ2
value | P-value |
|---|
| Age, years |
|
|
|
| 4.290 | 0.296 |
|
<40 | 11 | 7 | 2 | 2 |
|
|
|
40–60 | 73 | 23 | 27 | 23 |
|
|
|
>60 | 62 | 19 | 22 | 21 |
|
|
| Sex |
|
|
|
| 5.240 | 0.073 |
| Male | 110 | 33 | 37 | 40 |
|
|
|
Female | 36 | 16 | 14 | 6 |
|
|
| Tumor location |
|
|
|
| 6.963 | 0.324 |
|
Proximal | 14 | 6 | 3 | 5 |
|
|
| Mid | 17 | 3 | 10 | 4 |
|
|
|
Distal | 99 | 35 | 34 | 30 |
|
|
|
Diffuse | 16 | 5 | 4 | 7 |
|
|
| Diameter, cm |
|
|
|
| 7.857 | 0.097 |
|
<5 | 57 | 16 | 27 | 14 |
|
|
|
5–10 | 77 | 29 | 19 | 29 |
|
|
|
>10 | 12 | 4 | 5 | 3 |
|
|
| Organ
metastasis |
|
|
|
| 7.717 | 0.021a |
| No | 110 | 41 | 41 | 28 |
|
|
|
Yes | 36 | 8 | 10 | 18 |
|
|
| Depth |
|
|
|
| 3.791 | 0.705 |
|
Mucosa | 19 | 5 | 10 | 4 |
|
|
|
Submucosa | 28 | 11 | 8 | 9 |
|
|
|
Muscularis | 65 | 21 | 23 | 21 |
|
|
|
Serosa | 34 | 12 | 10 | 12 |
|
|
| Borrmann type |
|
|
|
| 11.290 | 0.186 |
| 0 | 17 | 4 | 8 | 5 |
|
|
| 1 | 12 | 3 | 8 | 1 |
|
|
| 2 | 21 | 6 | 7 | 8 |
|
|
| 3 | 93 | 35 | 26 | 32 |
|
|
| 4 | 3 | 1 | 2 | 0 |
|
|
| Lymphatic
metastasis |
|
|
|
| 13.736 | 0.001a |
| No | 61 | 29 | 22 | 10 |
|
|
|
Yes | 85 | 20 | 29 | 36 |
|
|
| Histological
type |
|
|
|
|
7.696 | 0.021a |
|
Adenocarcinoma | 102 | 27 | 40 | 35 |
|
|
|
Mucinous/signet-ring cell
carcinoma | 44 | 22 | 11 | 11 |
|
|
Lumican expression in gastric cancer
and its association with long-term survival rate
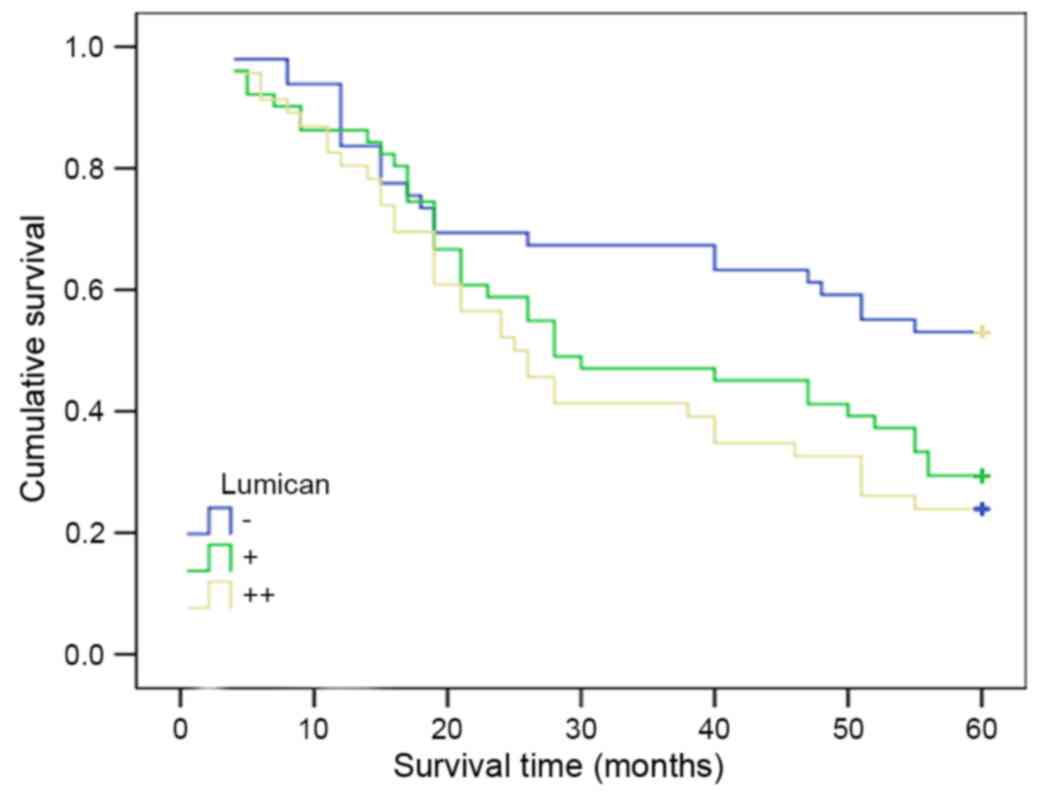
For the 146 gastric cancer patient samples, in which
lumican expression was negative (49 cases), weakly-positive (51
cases) and strongly-positive (46 cases), the median survival time
were 46.3, 39.6 and 24.3 months, respectively. Kaplan-Meier
survival analysis revealed that the difference was statistically
significant amongst the three groups (χ2=8.492; P=0.014;
Fig. 3). There was a negative
association between lumican expression and the long-term survival
of patients with gastric cancer.
Discussion
The lumican gene is 338 amino acids long and
contains a recognized 18-residue signal peptide of 38 kDa (15–17), which
is located at the 12q21.3-q22 area of the 12th chromosome (18,19).
Lumican, as an important member of SLRP, possesses four functional
regions: Region I, contains a signal peptide; region II,
cysteine-rich; region III, a leucine-rich region that contains 10
leucine-rich repeats; region IV, a cysteine-rich region that
combines with an N-terminal cysteine into a disulfide domain
(20–23).
Numerous regulatory factors affect the abnormal
expression of lumican, with various tumors having differing degrees
of lumican expression (24–26). A non-sulfuric or poorly-sulfuric
polylactosamine chain has been observed to be attached to lumican
in certain pancreatic cancer cell lines (27). Lumican in cancer cells has a
polylactosamine chain; these glycosylated lumican proteins promote
the proliferation of cancer cells (28). In breast cancer, lumican expression in
cancer cells and acrotenic fibroblasts has been observed to be high
(29). Lumican expression is closely
associated with the degree of tumor cell differentiation, cancer
estrogen receptor levels and patient age (30). In the present study, it was observed
that, in adjacent normal gastric tissues, the expression of lumican
was negative or weakly positive and the majority of positive
expression was focal or diffuse, localized in the cytoplasm or at
the membrane. In the gastric glands, which are formed primarily of
mature cells, the expression of lumican protein was usually
negative, and suggesting that the expression of lumican was weak or
negative in differentiated non-proliferating cells.
In the 146 gastric cancer samples analyzed in the
present study, the majority expressed lumican (66.4%, 97/146), with
that expression concentrated in the cytoplasm or at the membrane.
The differential expression of lumican may be associated with
damage to normal interstitial tissue and the reconstruction of the
tumor stroma in the process of invasion. In colorectal cancer
tissues, lumican was diffusely localized in the cytoplasm of the
cancer cells and its expression was detected in 99 of the 158 cases
(62.7%) of a previous study (31).
During the development of the tumor, lumican promotes the
rebuilding of tumor stroma by participating in the biochemical
synthesis and degradation of the extracellular matrix (32).
The expression of lumican in patients with organ and
lymphatic metastatic tissues was 77.8 (28/36) and 76.5% (65/85),
respectively. The expression of lumican in patients without organ
metastatic and lymphatic metastatic tissues was 62.7 (69/110) and
52.5% (32/61), respectively. It was observed that the expression of
lumican in the patients with gastric cancer with organ or lymphatic
metastasis were higher compared with those without lymphatic or
organ metastasis (77.8 vs. 62.7; 76.5 vs. 52.5%). These data
indicate that lumican expression may be associated with the
invasion and metastasis of gastric cancer. As lumican has more
non-sulfated or poorly-sulfated polylactosamine side-chains than
highly-sulfated keratan sulfate side chains, positive expression in
colorectal and pancreatic cancer exhibits more invasive and
metastatic characteristics (33,34).
Lumican expression was stronger in adenocarcinoma, compared with in
mucinous adenocarcinoma or signet-ring cell carcinoma. No
incidences of strongly-positive lumican expression were identified
in mucinous adenocarcinoma or signet-ring cell carcinoma.
Co-adjustment cannot be achieved between lumican and fibroblasts
via transforming growth factor-β and mothers against
decapentaplegic homolog protein signaling, due to a lack of tumor
stromal tissues (35,36).
Statistical analysis of patient survival indicated
that there is a significant negative association between high
lumican expression levels and the long-term survival rate. The
stronger the expression of lumican, the poorer the prognosis of the
patient. The results of the present study suggested that lumican
could be used as a sensitive, independent molecular target to judge
the prognosis of patients with gastric cancer, but its expression
and regulatory mechanism necessitates further investigation.
In summary, there was a significant association
between the expression of lumican and the invasive potential of
gastric cancer; thus, lumican could serve as an independent
prognostic factor.
Acknowledgements
The present study was supported by grants from
Clinical Research Foundation of Wu Jieping Medical Foundation
(grant no. 320.6750.13105) and the Educational Institutions of
Heilongjiang Province (grant no. 12541458).
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen L, Zuo Y, Zhu L, Zhang Y, Li S, Ma F,
Han Y, Song H and Xue Y: Peripheral venous blood
neutrophil-to-lymphocyte ratio predicts survival in patients with
advanced gastric cancer treated with neoadjuvant chemotherapy. Onco
Targets Ther. 10:2569–2580. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen S and Birk DE: The regulatory roles
of small leucine-rich proteoglycans in extracellular assembly. FEBS
J. 280:2120–2137. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Moreth K, Iozzo RV and Schaefer L: Small
leucine-rich proteoglycans orchestrate receptor crosstalk during
inflammation. Cell Cycle. 11:2084–2091. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Baghy K, Dezso K, László V, Fullár A,
Péterfia B, Paku S, Nagy P, Schaff Z, Iozzo RV and Kovalszky I:
Ablation of the decorin gene enhances experimental hepatic fibrosis
and impairs hepatic healing in mice. Lab Invest. 91:439–451. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Schaefer L and Iozzo RV: Small
leucine-rich proteoglycans, at the crossroad of cancer growth and
inflammation. Curr Opin Genet Dev. 22:56–57. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
de Wit M, Carvalho B, Delis-van Diemen PM,
van Alphen C, Beliën JAM, Meijer GA and Fijneman RJA: Lumican and
versican protein expression are associated with colorectal
adenoma-to-carcinoma progression. PLoS One. 12:e01747682017.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kalamajski S and Oldberg A: The role of
small leucine-rich proteoglycans in collagen fibrillogenesis.
Matrix Biol. 29:248–p253. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nikitovic D, Papoutsidakis A, Karamanos NK
and Tzanakakis GN: Lumican affects tumor cell functions, tumor-ECM
interactions, angiogenesis and inflammatory response. Matrix Biol.
35:206–214. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Appunni S, Anand V, Khandelwal M, Seth A,
Mathur S and Sharma A: Altered expression of small leucine-rich
proteoglycans (Decorin, Biglycan and Lumican): Plausible diagnostic
marker in urothelial carcinoma of bladder. Tumour Biol.
39:10104283176991122017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xiao LL and Xiao XY: Histological and
molecular classification of gastric cancer and personalized
therapy. WCJD. 23:4141–4149. 2015. View Article : Google Scholar
|
|
14
|
Liu JW, Liu HL and Zhang T: Research
progress of molecular type of gastric cancer. Chin J Clin.
8:4444–4448. 2014.
|
|
15
|
Guggenheim JA, Zayats T, Hammond C and
Young TL: Lumican and muscarinic acetylcholine receptor 1 gene
polymorphisms associated with high myopia. Eye (Lond).
24:1411–1412. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Matsuda Y, Yamamoto T, Kudo M, Kawahara K,
Kawamoto M, Nakajima Y, Koizumi K, Nakazawa N, Ishiwata T and Naito
Z: Expression and roles of lumican in lung adenocarcinoma and
squamous cell carcinoma. Int J Oncol. 33:1177–1185. 2008.PubMed/NCBI
|
|
17
|
Hayashi Y, Call MK, Chikama T, Liu H,
Carlson EC, Sun Y, Pearlman E, Funderburgh JL, Babcock G, Liu CY,
et al: Lumican is required for neutrophil extravasation following
corneal injury and wound healing. J Cell Sci. 123:2987–2995. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kalamajski S and Oldberg A: Homologous
sequence in lumican and fibromodulin leucine-rich repeat 5–7
competes for collagen binding. J Biol Chem. 284:534–539. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nikitovic D, Katonis P, Tsatsakis A,
Karamanos NK and Tzanakakis GN: Lumican, a small leucine-rich
proteoglycan. IUBMB Life. 60:818–823. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Seomun Y and Joo CK: Lumican induces human
corneal epithelial cell migration and integrin expression via ERK
1/2 signaling. Biochem Biophys Res Commun. 372:221–225. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nikitovic D, Berdiaki A, Zafiropoulos A,
Katonis P, Tsatsakis A, Karamanos NK and Tzanakakis GN: Lumican
expression is positively correlated with the differentiation and
negatively with the growth of human osteosarcoma cells. FEBS J.
275:350–361. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chakravarti S, Stallings RL, SundarRaj N,
Cornuet PK and Hassell JR: Primary structure of human Lumican
(keratan sulfate proteoglycan) and localization of the gene (LUM)
to chromosome 12q21.3-q22. Genomics. 27:481–488. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kafienah W, Cheung FL, Sims T, Martin I,
Miot S, Von Ruhland C, Roughley PJ and Hollander AP: Lumican
inhibits collagen deposition in tissue engineered cartilage. Matrix
Biol. 27:526–534. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sharma B, Ramus MD, Kirkwood CT, Sperry
EE, Chu PH, Kao WW and Albig AR: lumican exhibits anti-angiogenic
activity in a context specific manner. Cancer Microenviron.
6:263–271. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kashyap MK, Marimuthu A, Peri S, Kumar GS,
Jacob HK, Prasad TS, Mahmood R, Kumar KV, Kumar MV, Meltzer SJ, et
al: Overexpression of periostin and lumican in esophageal squamous
cell carcinoma. Cancers. 2:133–142. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Colak D, Chishti MA, Al-Bakheet AB,
Al-Qahtani A, Shoukri MM, Goyns MH, Ozand PT, Quackenbush J, Park
BH and Kaya N: Integrative and comparative genomics analysis of
early hepatocellular carcinoma differentiated from liver
regeneration in young and old. Mol Cancer. 9:1462010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li X, Truty MA, Kang Y, Chopin-Laly X,
Zhang R, Roife D, Chatterjee D, Lin E, Thomas RM, Wang H, et al:
Extracellular lumican inhibits pancreatic cancer cell growth and is
associated with prolonged survival after surgery. Clin Cancer Res.
20:6529–6540. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li X, Roife D, Kang Y, Dai B, Pratt M and
Fleming JB: Extracellular lumican augments cytotoxicity of
chemotherapy in pancreatic ductal adenocarcinoma cells via
autophagy inhibition. Oncogene. 35:4881–4890. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Eshchenko TY, Rykova VI, Chernakov AE,
Sidorov SV and Grigorieva EV: Expression of different proteoglycans
in human breast tumors. Biochemistry (Moscow). 72:1016–1020. 2007.
View Article : Google Scholar
|
|
30
|
Kang Y, Roife D, Lee Y, Lv H, Suzuki R,
Ling J, Perez Rios MV, Li X, Dai B, Pratt M, et al: Transforming
Growth Factor-β limits secretion of lumican by activated stellate
cells within primary pancreatic adenocarcinoma tumors. Clin Cancer
Res. 22:4934–4946. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Seya T, Tanaka N, Shinji S, Yokoi K,
Koizumi M, Teranishi N, Yamashita K, Tajiri T, Ishiwata T and Naito
Z: Lumican expression in advanced colorectal cancer with nodal
metastasis correlates with poor prognosis. Oncol Rep. 16:1225–1230.
2006.PubMed/NCBI
|
|
32
|
Radwanska A, Litwin M, Nowak D, Baczynska
D, Wegrowski Y, Maquart FX and Malicka-Blaszkiewicz M:
Overexpression of lumican affects the migration of human colon
cancer cells through up-regulation of gelsolin and filamentous
actin reorganization. Exp Cell Res. 318:2312–2323. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yamamoto T, Matsuda Y, Kawahara K,
Ishiwata T and Naito Z: Secreted 70 kDa lumican stimulates growth
and inhibits invasion of human pancreatic cancer. Cancer Lett.
320:31–39. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
de Wit M, Belt EJ, Delis-van Diemen PM,
Carvalho B, Coupé VM, Stockmann HB, Bril H, Beliën JA, Fijneman RJ
and Meijer GA: Lumican and versican are associated with good
outcome in stage II and III colon cancer. Ann Surg Oncol. 20 Suppl
3:S348–S359. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Nikitovic D, Chalkiadaki G, Berdiaki A,
Aggelidakis J, Katonis P, Karamanos NK and Tzanakakis GN: Lumican
regulates osteosarcoma cell adhesion by modulating TGFβ2 activity.
Int J Biochem Cell Biol. 43:928–935. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Naito Z: Role of the small leucine-rich
proteoglycan (SLRP) family in pathological lesions and cancer cell
growth. J Nippon Med Sch. 72:137–145. 2005. View Article : Google Scholar : PubMed/NCBI
|