Introduction
Non-small cell lung cancer (NSCLC) accounts for 85%
of all cases of lung cancer and is the leading cause of
cancer-associated mortality worldwide (1). While surgery is the mainstay of
treatment for early-stage and localized NSCLC, combination
chemotherapy is considered the standard of care for patients with
advanced NSCLC (1,2). Combination chemotherapy frequently uses
two drugs, which often includes cisplatin plus one other drug
(3–5).
Cisplatin, also termed cis-diamminedichloroplatinum II (CDDP), is a
platinum-containing compound that has also been used for the
treatment of other human cancers, including head and neck, ovarian,
breast, bladder, and testicular cancers (6–9). To
improve the treatment of late-stage NSCLC, clinical trials of
three-drug combinations have been performed (10). However, the results of these trials
have demonstrated that adding a third drug may add little benefit
as a result of the increased toxicity (11,12).
Therefore, the search for novel drugs that are just as effective
with less toxicity associated remains vigorous.
Ent-11α-hydroxy-15-oxo-kaur-16-en-19-oic acid (5F),
a bioactive compound isolated from the herb Pteris
semipinnata L., has been shown to induce cell apoptosis and
inhibit cell proliferation in various cancer cells, including
thyroid carcinoma, lung cancer, nasopharyngeal carcinoma and
hepatocellular carcinoma cells (13–18). CDDP
and 5F inhibit cancer cell growth by inducing cell apoptosis
(9,14–16). In
view of these findings, it was hypothesized that 5F and CDDP may
have synergistic anticancer activity in human NSCLC cells. The
present study was therefore conducted to examine the effects of 5F
combined with CDDP on cell growth, cell apoptosis, cell cycle
arrest and regulation of gene expression in NCI-H23 cells.
Materials and methods
Drugs
CDDP was purchased from Qilu Pharmaceutical Co.,
Ltd. (Jinan, China). 5F was isolated from Pteris semipinnata
L. as previously described (13), and
the purity was >99%, as analyzed by high-performance liquid
chromatography (19). A stock
solution of CDDP at 1 mg/ml was prepared with PBS (pH 7.4). A stock
solution of 5F at 2 mg/ml was prepared by dissolving 5F in dimethyl
sulfoxide (DMSO).
Cell growth inhibition analysis
Human NSCLC NCI-H23 cells (American Type Culture
Collection; Manassas, VA, USA) were cultured in RPMI-1640 medium
supplemented with 10% fetal bovine serum, 100 U/ml penicillin and
100 µg/ml streptomycin (all from Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) at 37°C under a humidified atmosphere
containing 5% CO2. Cells were detached with 0.25%
trypsin/EDTA (Gibco; Thermo Fisher Scientific, Inc.), washed once
with PBS and re-suspended at a density of 3×104 cells/ml
in RPMI-1640 medium. Cell suspension (100 µl) was seeded onto each
well of 96-well plates and cultured at 37°C overnight. On day 2,
the culture medium was replaced with fresh medium, and cells were
divided into different groups and treated as follows: CDDP group, 5
µg/ml of CDDP (final concentration); 5F group, 40 µg/ml of 5F
(final concentration); combination group, 5 µg/ml of CDDP and 40
µg/ml of 5F (final concentration); and control group, no drug
added. Each group was analyzed in triplicate. Following the
addition of drugs, cells were cultured at 37°C for 24 or 48 h, and
an MTT assay was performed according to the manufacturer's protocol
(Beyotime Institute of Biotechnology, Haimen, China). Briefly, the
culture medium was replaced with 100 µl of fresh culture medium,
and 10 µl MTT (5 mg/ml) was added into each well. Following
incubation for 4 h at 37°C, MTT was removed from the wells and 150
µl DMSO was added, followed by agitating the plate for 10 min.
Subsequently, the absorbance of each well at 540 nm was measured
using a microplate reader (Model 450; Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). The cell proliferation inhibition rate was
calculated as: (Absorbance of control group-absorbance of treatment
group)/absorbance of control group.
Cell apoptosis assay
Cell detachment and wash were performed as
aforementioned. Subsequently, cells were re-suspended at a density
of 1×105 cells/ml in RPMI-1640 medium. Cell suspension
(500 µl) was seeded onto each well of 6-well plates. Following
culture at 37°C for 24 h, cells were divided into four groups and
treated with drugs at 37°C for 48 h as aforementioned. Cell
apoptosis was assessed using an Annexin V-FITC Apoptosis Detection
kit (Beyotime Institute of Biotechnology, Haimen, China) as advised
by the manufacturer. Briefly, cells were detached with PBS/1 mM
EDTA, washed once with PBS and re-suspended in 195 µl Annexin V
binding buffer from the kit, followed by addition of 5 µl Annexin
V-fluorescein isothiocyanate and 10 min, 37°C incubation in the
dark. The fluorescence of the apoptotic cells was then determined
with a flow cytometer (FACSAria III; BD Biosciences, Franklin
Lakes, NJ, USA).
Cell cycle arrest assay
Cells were treated with drugs for 48 h as previously
described. Following treatment, cells were detached and washed once
with PBS. A total of 1×106 cells were fixed in cold 70%
ethanol for 1 h at −20°C. Ethanol was then removed by
centrifugation at 1,000 × g at 4°C for 5 min and cells were washed
once with PBS. Subsequently, 200 µl of propidium iodide (PI; 50
µg/ml in PBS) was added to the cells and incubated at 4°C for 30
min in the dark. The cell cycle distribution was then determined
with a flow cytometer.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
RT-qPCR was performed to determine the relative
messenger RNA (mRNA) levels of β-catenin, glycogen synthase kinase
(GSK)-3β, cyclin D1 and c-Myc in drug-treated and control cells.
Total RNA of NCI-H23 cells was extracted using TRIzol reagent
(Takara Biotechnology Co., Ltd., Dalian, China) following the
manufacturer's protocol and quantified by photospectrometry
(BioSpectrometer; Eppendorf, Hamburg, Germany). Total RNA (2 µg)
was reversed transcribed using the PrimeScript RT Reagent kit
(Takara Biotechnology Co., Ltd.) according to the manufacturer's
protocol. Complementary DNA (1 µl) was mixed with 5 µl of 2X SYBR
Premix Taq buffer (Takara Biotechnology Co., Ltd.), 200 nM of each
primer (final concentration) and nuclease-free H2O,
which was used to adjust the reaction volume to a final volume of
20 µl. Primers for RT-qPCR were designed according to the published
gene sequences using Oligo software, version 5.0 (Molecular Biology
Insights, Inc., Colorado Springs, CO, USA) and synthesized by
Sangon Biotech Co., Ltd. (Shanghai, China). The sequences of all
primers, which were based on sequences available from GenBank
(https://www.ncbi.nlm.nih.gov/gene/),
and relevant information are listed in Table I. The amplification was performed on
the LightCycler 480 Instrument II (Roche Diagnostics, Basel,
Switzerland) as follows: Stage 1, 95°C for 10 min; and stage 2,
95°C for 10 sec and 60°C for 20 sec. Stage 2 was repeated for 40
cycles. Relative mRNA levels against GAPDH were calculated using
the 2−∆∆Cq method (16).
 | Table I.RT-PCR primer sequences. |
Table I.
RT-PCR primer sequences.
| Genes | GenBank number | Sequences | Product size, bp |
|---|
| β-catenin | X87838.1 | F:
5′-GACAGATCCAAGTCAACGTC-3′ | 257 |
|
|
| R:
5′-CACAAGAGCCTCTATACCAC-3′ |
|
| GSK-3β | NM_002093 | F:
5′-TCCCTCAAATTAAGGCACATC-3′ | 117 |
|
|
| R:
5′-CACGGTCTCCAGTATTAGCATCT-3′ |
|
| c-Myc | E01841.1 | F:
5′-GAACTTACAACACCCGAGCAA-3′ | 205 |
|
|
| R:
5′-GCAGTAGAAATACGGCTGCAC-3′ |
|
| Cyclin D1 | BC023620.2 | F:
5′-TACCCCAATAATCAACTCG-3′ | 245 |
|
|
| R:
5′-GATGCCTAGAACCCCACT-3′ |
|
| GAPDH | NM_002046 | F:
5′-ATGACATCAAGAAGGTGGTG-3′ | 177 |
|
|
| R:
5′-CATACCAGGAAATGAGCTTG-3′ |
|
Western blotting
Total protein was extracted from H23 cells using
radioimmunoprecipitation assay lysis buffer (Thermo Fisher
Scientific, Inc.) and quantified using the Pierce BCA Protein Assay
kit (Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. Total protein (50 µg) was separated by 10%
SDS-PAGE and electrotransferred to a nitrocellulose membrane. The
membrane was blocked in TBS-Tween-20 (TBST) buffer [50 mM Tris-HCl,
150 mM NaCl (pH 7.5) and 0.1% Tween-20] containing 5% skimmed milk
for 1 h at room temperature. Subsequently, the membrane was
incubated with primary antibodies diluted in blocking buffer at 4°C
overnight. The primary antibodies used were as follows: Mouse
anti-human β-catenin monoclonal antibody (dilution 1:1,000, cat.
no. 2698) and rabbit anti-human GSK-3β antibody (dilution 1:1,000,
cat. no. 9315) (both Cell Signaling Technology, Inc., Danvers, MA,
USA). Following incubation with the primary antibodies, the
membrane was washed three times with TBST buffer, followed by
incubation with horseradish peroxidase-conjugated anti-mouse
(dilution 1:5,000, cat. no. L3032-2) or anti-rabbit secondary
antibodies (dilution 1:5,000, cat. no. L3012-2) (both Signalway
Antibody, College Park, MD, USA) at 4°C for 1 h. Subsequently, the
membrane was washed three times with TBST buffer. The specific
bands were visualized with an enhanced chemiluminescence western
blot analysis detection kit (Beyotime Institute of Biotechnology),
and densitometric analysis was performed using Image-Pro Plus 6.0
software (Media Cybernetics, Inc., Rockville, MD, USA) to determine
the relative levels of β-catenin and GSK-3β following normalization
against GAPDH. GAPDH, serving as a loading control, was probed
using a rabbit anti-human GAPDH monoclonal antibody (cat. no. 2118,
dilution 1:3,000; Cell Signaling Technology, Inc.).
Statistical analysis
All data are expressed as the mean ± standard
deviation. Statistical analysis of the differences between multiple
groups was performed by one-way analysis of variance using the SPSS
13.0 software (SPSS, Inc., Chicago, IL, USA). Additional two-group
comparisons were performed using the least-significant difference
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Synergistic anti-proliferative effects
of 5F and CDDP
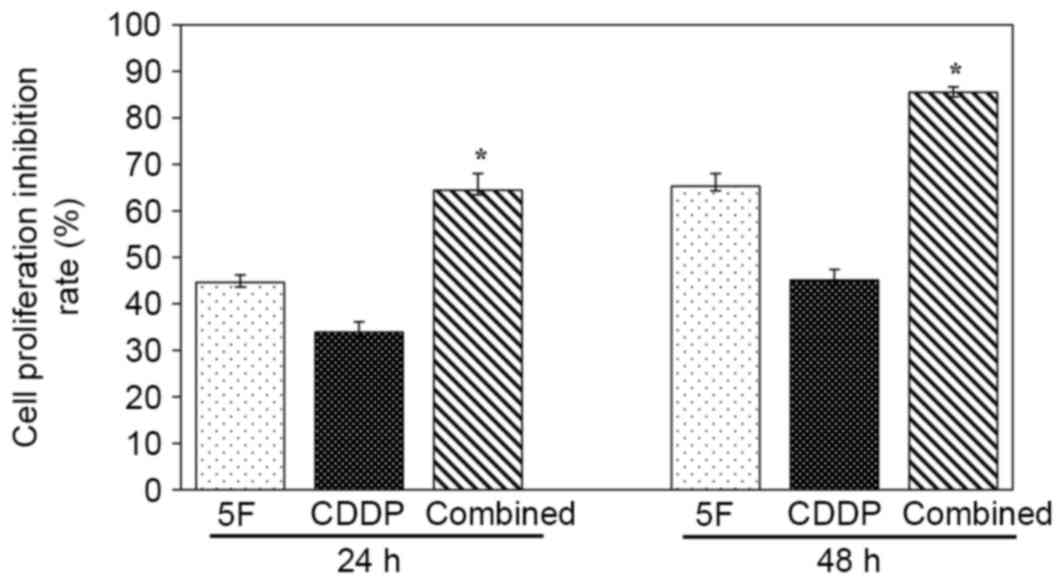
At 24 h after drug treatment, the cell proliferation
inhibition rate for the combined group was 64.5±3.6%, which was
significantly higher than that of the 5F (44.6±1.6%; P<0.05;
n=4) and CDDP (33.9±2.2%; P<0.05; n=4) groups (Fig. 1). When cells were treated for 48 h,
the inhibition rates for the combined, 5F and CDDP groups were
85.5±1.2, 65.3±2.7 and 45.1±2.3%, respectively (P<0.05 compared
with the combined group; n=4) (Fig.
1).
Synergistic pro-apoptotic effects of
5F and CDDP
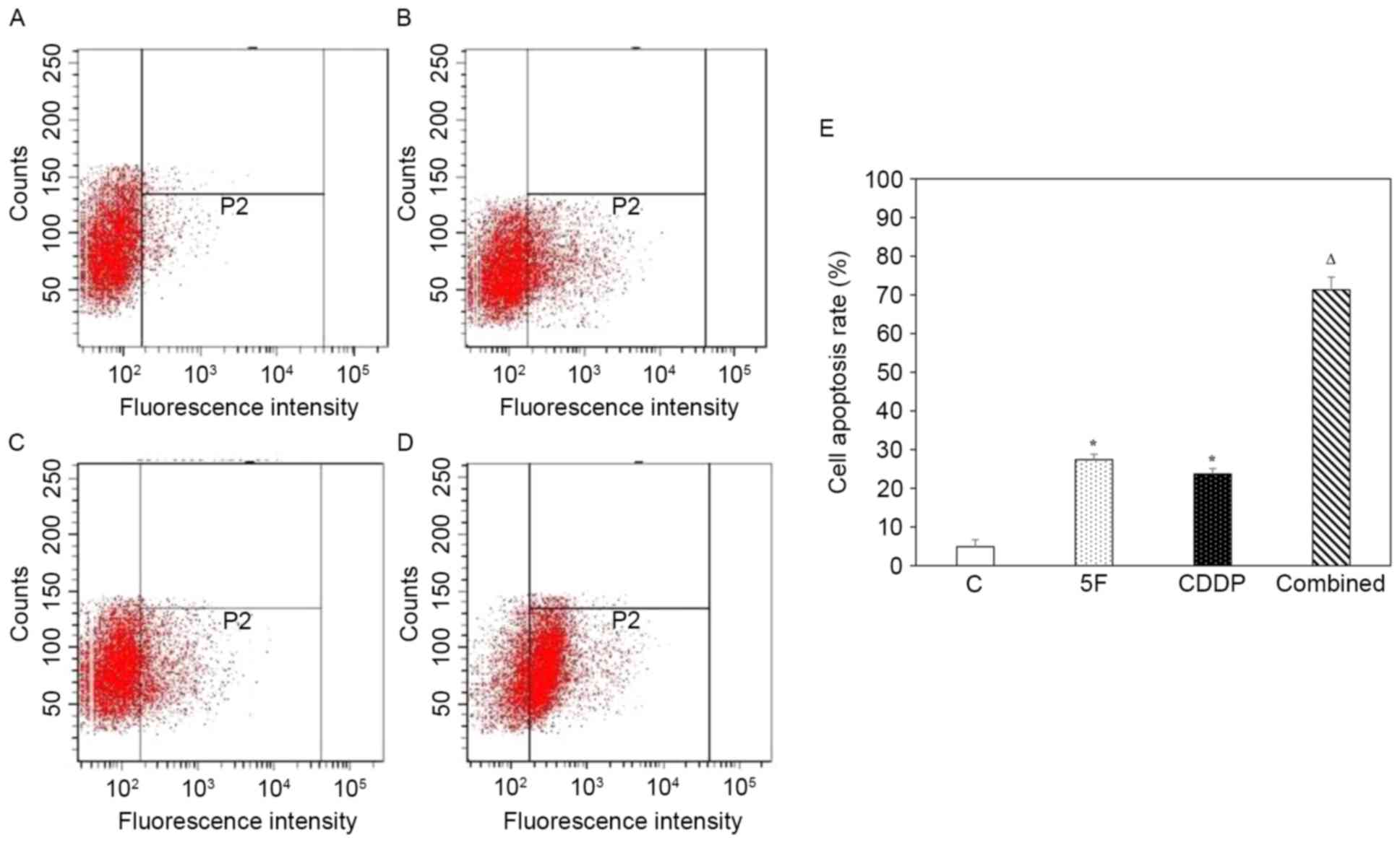
Representative histograms of cell apoptotic analysis
by flow cytometry for the control, 5F, CDDP and combined groups are
shown in Fig. 2A-D, respectively. The
apoptotic rates for the control, 5F, CDDP and combined groups were
4.9±1.8, 27.4±1.4, 23.7±1.4 and 71.3±3.3%, respectively. Treatment
with 5F or CDDP alone markedly increased cell apoptosis compared
with that of no-drug treatment (P<0.05). Statistical analysis
revealed that the combined group had a significantly higher
apoptotic rate than the 5F (P<0.001; n=6) and CDDP (P<0.001;
n=6) groups (Fig. 2E).
Synergistic cell cycle arrest effects
of 5F and CDDP
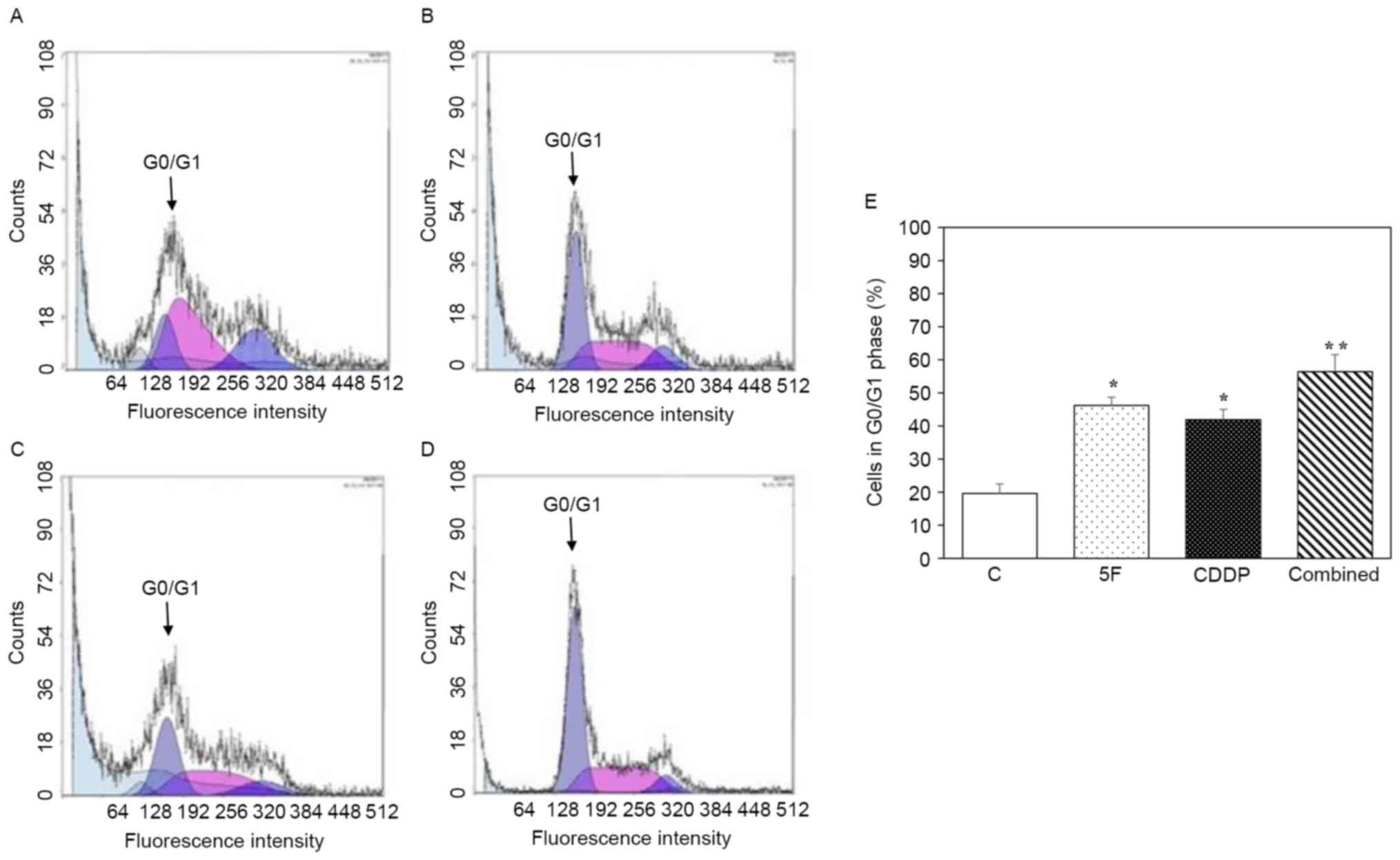
PI staining and flow cytometric analysis were
employed to determine cell numbers at each phase of the cell cycle
following drug treatment. Fig. 3A-D
shows representative histograms of cytometric analysis of the cell
cycle for the control, 5F, CDDP and combined groups, respectively.
As demonstrated in Fig. 3E, treatment
with 5F or CDDP alone caused cell cycle arrest at the G0/G1 phase.
When cells were incubated with 5F combined with CDDP, the
percentage of cells at the G0/G1 phase was 56.4±5.2%, which was
significantly higher than that of the 5F (46.2±2.5%; P<0.05;
n=6) and CDDP (41.9±3.1%; P<0.05; n=6) groups.
Synergistic effects of 5F and CDDP on
the regulation of β-catenin, GSK-3β, c-Myc and cyclin D1
Fig. 4 shows the
RT-qPCR data (n=6 for each gene). The results revealed that
treatment with 5F or CDDP alone resulted in reduced mRNA expression
of β-catenin, c-Myc and cyclin D1, but increased mRNA expression of
GSK-3β. When compared with that of the 5F or CDDP group, the
combined group had a significantly lower level of β-catenin, c-Myc
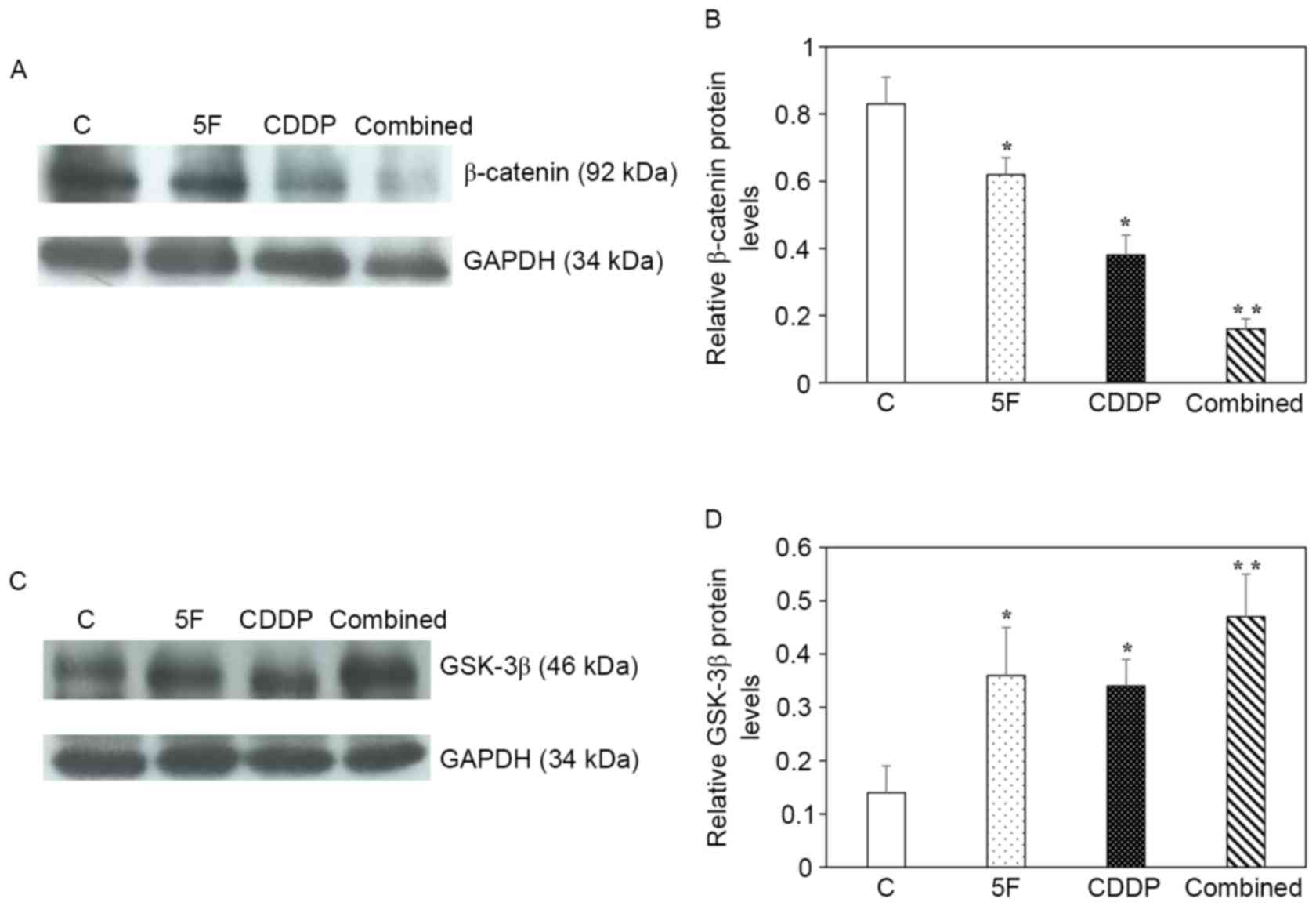
and cyclin D1, but a markedly higher level of GSK-3β (Fig. 4A-D). Western blot analysis of
β-catenin and GSK-3β was performed (Fig.
5A and B, respectively), and statistical analysis of β-catenin
and GSK-3β protein levels (Fig. 5C and
D, respectively) confirmed the mRNA data for β-catenin and
GSK-3β.
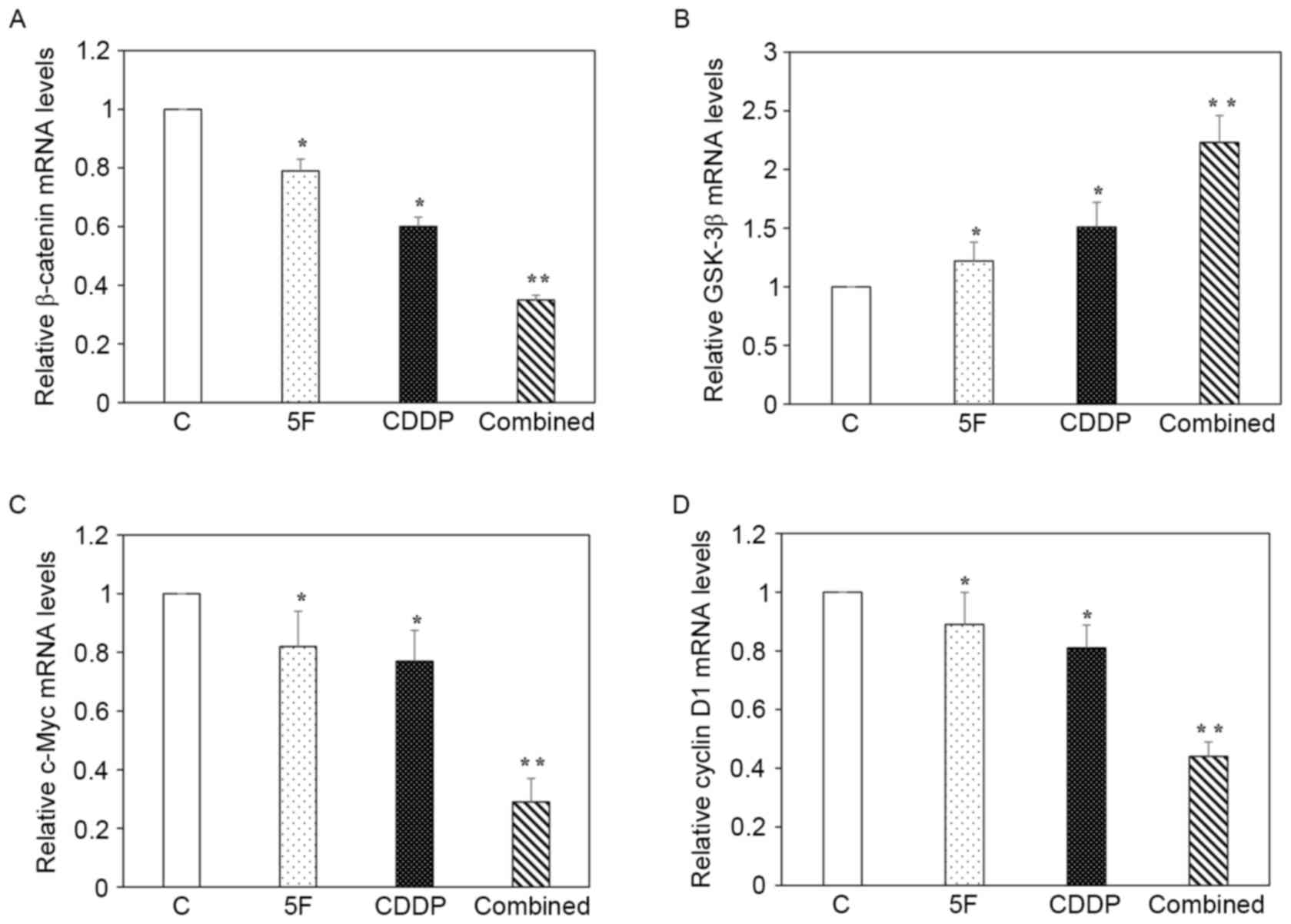 | Figure 4.Effects of 5F and CDDP on regulating
the mRNA expression of β-catenin, GSK-3β, c-Myc and cyclin D1.
Relative mRNA levels of (A) β-catenin, (B) GSK-3β, (C) c-Myc and
(D) cyclin D1. The combined group had significantly lower mRNA
levels of β-catenin, c-Myc and cyclin D1, but markedly higher mRNA
levels of GSK-3β, when compared with those of the 5F or CDDP
groups. *P<0.05 compared with control; **P<0.05 compared with
5F or CDDP groups. 5F, ent-11α-hydroxy-15-oxo-kaur-16-en-19-oic
acid; CDDP, cisplatin; C, control; GSK-3β, glycogen synthase
kinase-3β; mRNA, messenger RNA. |
Discussion
CDDP-based combination chemotherapy has been shown
to be effective in improving survival and quality of life in
patients with advanced NSCLC (4,5). Recently,
the identification of abnormal molecular pathways in a number of
NSCLC cases has led to the development of targeted therapies for a
subset of patients (20,21). However, >50% of patients with
advanced NSCLC are usually treated with combination chemotherapy
(20). In the past two decades,
several anticancer drugs, including gemcitabine, vinorelbine,
paclitaxel, docetaxel, pemetrexed and vinblastine, have been
developed and combined with CDDP to form a doublet therapy regimen
(22). Over the last decade, the
5-year survival rate of patients with advanced NSCLC has only
marginally improved with combination therapy (20). To improve the treatment of advanced
NSCLC, clinical trials using three-drug combinations have been
performed (10). However, the results
show that adding a third drug does not add much benefit due to
increased toxicity (11,12). Therefore, the search for novel drugs
that are just as effective but with less toxicity remains vigorous
(2,22).
5F has been shown to induce apoptosis and inhibit
cell proliferation in various cancer cells (14–18). One
outstanding property of 5F is its minimal side effects (14), making it a promising anticancer agent.
In a preliminary experiment, the half-maximal inhibitory
concentration (IC50) for CDDP and 5F was determined in
H23 cells (data not shown). In the present study, a final
concentration of 5F of 40 µg/ml and of CDDP of 5 µg/ml, which was
close to their IC50, was selected to treat H23 cells. It
was observed that 5F and CDDP synergistically induced apoptosis and
inhibited cell growth, arrested cell cycles in the G0/G1 phase, and
regulated the expression of β-catenin, GSK-3β, c-Myc and cyclin D1
genes.
Liu et al (15)
observed that treatment of thyroid carcinoma cells with 5F led to
the translocation of B-cell lymphoma-2-associated X protein into
mitochondria, and the release of cytochrome c and
apoptosis-inducing factor from mitochondria into the cytosol,
indicating that the cell death induced by 5F was through a
mitochondrial-mediated pathway. It is known that the anticancer
activity of CDDP is associated with its ability to interact with
the purine bases of DNA, causing DNA damage and subsequently
inducing apoptosis in cancer cells (9,23).
Therefore, 5F and CDDP likely exert synergistic anticancer effects
in H23 cells through targeting different pathways, although the
mechanisms behind these interactions were not elucidated in the
present study.
The Wnt signaling pathway was first identified due
to its role in carcinogenesis (24).
Activation of the canonical or Wnt/β-catenin-dependent signaling
pathway in numerous cases promotes cell growth. It has been
reported that the Wnt signaling pathway is activated in ~50% of
human NSCLC cell lines and primary tumors, and downregulation of
activated Wnt signaling inhibits NSCLC proliferation and induces a
more differentiated phenotype (21).
Wnt ligands initiate pathway activation by binding to the Frizzled
receptor on the cell membrane. Subsequently, the signal is
transduced to the cytoplasmic phosphoprotein Dishevelled (Dsh)
(25,26). Once Dsh is activated, it leads to the
accumulation and stabilization of β-catenin. Subsequently,
β-catenin is translocated into nuclei, where it modulates target
gene expression, including upregulation of cyclin D1 and c-Myc, to
stimulate cell proliferation (27,28).
GSK-3β negatively regulates the Wnt signaling pathway by
destabilizing β-catenin and inhibiting β-catenin accumulation
(28). Overexpression of cyclin D1
and c-Myc has been revealed to be associated with cancer onset and
progression (29–32). The present study revealed that 5F and
CDDP concurrently downregulated β-catenin but upregulated GSK-3β in
H23 cells, leading to reduced expression of cyclin D1 and c-Myc,
which may represent one of the mechanisms for the synergistic
anticancer activity of 5F and CDDP. In addition, as cyclin D1 is
required for progression through the G1 phase of the cell cycle,
reduced cyclin D1 levels may also be responsible for G0/G1 cell
cycle arrest resulting from 5F and CDDP treatment.
Considering that 5F exerts anticancer activity with
minimal side effects, future studies should investigate whether
combination of 5F and CDDP will have the same or greater anticancer
activity but with less side effects compared with that of CDDP plus
one other drug, and whether addition of 5F as a third drug to
CDDP-based two-drug combinations will enhance the anticancer
effects without increasing toxicity. To address these questions,
in vivo studies using animal models of NSCLC are
required.
Acknowledgements
The present study was supported by the Foundation
for Distinguished Young Talents in Higher Education (grant no.
LYM10083) and the Science and Technology Planning Project (grant
no. 2011B031800343) of Guangdong Province, China.
References
|
1
|
Boolell V, Alamgeer M, Watkins DN and
Ganju V: The evolution of therapies in non-small cell lung cancer.
Cancers (Basel). 7:1815–1846. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ramalingam S and Belani C: Systemic
chemotherapy for advanced non-small cell lung cancer: Recent
advances and future directions. Oncologist. 13 Suppl 1:S5–S13.
2008. View Article : Google Scholar
|
|
3
|
Schiller JH, Harrington D, Belani CP,
Langer C, Sandler A, Krook J, Zhu J and Johnson DH: Eastern
Cooperative Oncology Group: Comparison of four chemotherapy
regimens for advanced non-small-cell lung cancer. N Engl J Med.
346:92–98. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cullen MH, Billingham LJ, Woodroffe CM,
Chetiyawardana AD, Gower NH, Joshi R, Ferry DR, Rudd RM, Spiro SG,
Cook JE, et al: Mitomycin, ifosfamide, and cisplatin in
unresectable non-small-cell lung cancer: Effects on survival and
quality of life. J Clin Oncol. 17:3188–3194. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Billingham LJ and Cullen MH: The benefits
of chemotherapy in patient subgroups with unresectable
non-small-cell lung cancer. Ann Oncol. 12:1671–1675. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Creagan ET, Woods JE, Schutt AJ and
O'Fallon JR: Cyclophosphamide, adriamycin, and
cis-diamminedichloroplatinum (II) in the treatment of advanced
nonsquamous cell head and neck cancer. Cancer. 52:2007–2010. 1983.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Colombo N, Sessa C, Landoni F, Sartori E,
Pecorelli S and Mangioni C: Cisplatin, vinblastine, and bleomycin
combination chemotherapy in metastatic granulosa cell tumor of the
ovary. Obstet Gynecol. 67:265–268. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Decatris MP, Sundar S and O'Byrne KJ:
Platinum-based chemotherapy in metastatic breast cancer: Current
status. Cancer Treat Rev. 30:53–81. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dasari S and Tchounwou PB: Cisplatin in
cancer therapy: Molecular mechanisms of action. Eur J Pharmacol.
740:364–378. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Okamoto I, Miyazaki M, Morinaga R, Kaneda
H, Ueda S, Hasegawa Y, Satoh T, Kawada A, Fukuoka M, Fukino K, et
al: Phase I clinical and pharmacokinetic study of sorafenib in
combination with carboplatin and paclitaxel in patients with
advanced non-small cell lung cancer. Invest New Drugs. 28:844–853.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Han JY, Nam BH, Kim HY, Yoon SJ, Kim HT
and Lee JS: A randomized phase II study of irinotecan plus
cisplatin versus irinotecan plus capecitabine with or without
isosorbide-5-mononitrate in advanced non-small-cell lung cancer.
Ann Oncol. 23:2925–2930. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Paz-Ares L, Mezger J, Ciuleanu TE, Fischer
JR, von Pawel J, Provencio M, Kazarnowicz A, Losonczy G, de Castro
G Jr, Szczesna A, et al: Necitumumab plus pemetrexed and cisplatin
as first-line therapy in patients with stage IV non-squamous
non-small-cell lung cancer (INSPIRE): An open-label, randomised,
controlled phase 3 study. Lancet Oncol. 16:328–337. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li MY, Leung J, Kong AW, Liang NC, Wu K,
Hsin MK, Deng YF, Gong X, Lv Y, Mok TS, et al: Anticancer efficacy
of 5F in NNK-induced lung cancer development of A/J mice and human
lung cancer cells. J Mol Med (Berl). 88:1265–1276. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li MY, Liang NC and Chen GG:
Ent-11alpha-hydroxy-15-oxo-kaur-16-en-19-oic-acid induces apoptosis
of human malignant cancer cells. Curr Drug Targets. 13:1730–1737.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu ZM, Chen GG, Vlantis AC, Liang NC,
Deng YF and van Hasselt CA: Cell death induced by
ent-11alpha-hydroxy-15-oxo-kaur-16-en-19-oic-acid in anaplastic
thyroid carcinoma cells is via a mitochondrial-mediated pathway.
Apoptosis. 10:1345–1356. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wu K, Liu Y, Lv Y, Cui L, Li W, Chen J,
Liang NC and Li L: Ent-11α-hydroxy-15-oxo-kaur-16-en-19-oic-acid
induces apoptosis and cell cycle arrest in CNE-2Z nasopharyngeal
carcinoma cells. Oncol Rep. 29:2101–2108. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ye H, Wu Q, Guo M, Wu K, Lv Y, Yu F, Liu
Y, Gao X, Zhu Y, Cui L, et al: Growth inhibition effects of
ent-11α-hydroxy-15-oxo-kaur-16-en-19-oic-acid on colorectal
carcinoma cells and colon carcinoma-bearing mice. Mol Med Rep.
13:3525–3532. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen GG, Leung J, Liang NC, Li L, Wu K,
Chan UP, Leung BC, Li M, Du J, Deng YF, et al:
Ent-11α-hydroxy-15-oxo-kaur-16-en-19-oic-acid inhibits
hepatocellular carcinoma in vitro and in vivo via stabilizing IkBα.
Invest New Drugs. 30:2210–2218. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lu Y, Wu K, Liang N and Chen GG: LC method
for quantification of ent-11α-Hydroxy-15-oxo-kaur-16-en-19-oic acid
in rabbit plasma: Validation and application to a pharmacokinetic
study. Chromatographia. 70:15992009. View Article : Google Scholar
|
|
20
|
Alamgeer M, Ganju V and Watkins DN: Novel
therapeutic targets in non-small cell lung cancer. Curr Opin
Pharmacol. 13:394–401. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Akiri G, Cherian MM, Vijayakumar S, Liu G,
Bafico A and Aaronson SA: Wnt pathway aberrations including
autocrine Wnt activation occur at high frequency in human
non-small-cell lung carcinoma. Oncogene. 28:2163–2172. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
de Castria TB, da Silva EM, Gois AF and
Riera R: Cisplatin versus carboplatin in combination with
third-generation drugs for advanced non-small cell lung cancer.
Cochrane Database Syst Rev: CD009256. 2013.doi:
10.1002/14651858.CD009256.pub2. View Article : Google Scholar
|
|
23
|
Byun JM, Jeong DH, Lee DS, Kim JR, Park
SG, Kang MS, Kim YN, Lee KB, Sung MS and Kim KT: Tetraarsenic oxide
and cisplatin induce apoptotic synergism in cervical cancer. Oncol
Rep. 29:1540–1546. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nusse R and Varmus HE: Wnt genes. Cell.
69:1073–1087. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gao C and Chen YG: Dishevelled: The hub of
Wnt signaling. Cell Signal. 22:717–727. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wong HC, Bourdelas A, Krauss A, Lee HJ,
Shao Y, Wu D, Mlodzik M, Shi DL and Zheng J: Direct binding of the
PDZ domain of Dishevelled to a conserved internal sequence in the
C-terminal region of Frizzled. Mol Cell. 12:1251–1260. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gordon MD and Nusse R: Wnt signaling:
Multiple pathways, multiple receptors, and multiple transcription
factors. J Biol Chem. 281:22429–22433. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
MacDonald BT, Tamai K and He X:
Wnt/beta-catenin signaling: Components, mechanisms, and diseases.
Dev Cell. 17:9–26. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Alao JP: The regulation of cyclin D1
degradation: Roles in cancer development and the potential for
therapeutic invention. Mol Cancer. 6:242007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Diehl JA: Cycling to cancer with cyclin
D1. Cancer Biol Ther. 1:226–231. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Meyer N and Penn LZ: Reflecting on 25
years with MYC. Nat Rev Cancer. 8:976–990. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nesbit CE, Tersak JM and Prochownik EV:
MYC oncogenes and human neoplastic disease. Oncogene. 18:3004–3016.
1999. View Article : Google Scholar : PubMed/NCBI
|