Introduction
In recent years, the incidence of rectal cancer has
risen. In addition, about 60% of rectal cancer cases are in
advanced stages at diagnosis. Neoadjuvant chemoradiotherapy is the
standard treatment for advanced rectal cancer, and
chemoradiotherapy can effectively reduce the tumor size, increase
the rates of successful resection and sphincter preservation, and
reduce the local recurrence rate (1,2). However,
complications of concurrent chemoradiotherapy, such as fecal
incontinence, urinary incontinence, and sexual dysfunction, cannot
be ignored (3–5).
Individualized therapy is the current trend in the
diagnosis and treatment of rectal cancer (6). It is proposed that T3 patients with
early microinvasion do not require neoadjuvant chemotherapy
(7,8).
Many studies have found that the extent of mesorectal invasion
(EMI) is a significant independent prognostic factor for T3 rectal
cancer (9–12). The MERCURY study showed that rectal
cancer patients with an EMI ≤5 mm (pT3a) had a better prognosis
than those with an EMI >5 mm (pT3b) (13). When the EMI was <5 mm, total
mesorectal excision was sufficient to ensure complete tumor
resection, and thus, the complications of chemoradiotherapy could
be avoided. Thus, determination of substages within the T3 stage
based on the EMI is very important for predicting prognosis and
treatment planning. However, the currently used pre-operative TNM
staging based on radiographic data is insufficient to meet the
clinical needs. More prognostic information is needed, such as the
EMI, circumferential resection margin (CRM), and so on.
Currently, the EMI assessed by magnetic resonance
imaging (MRI) is typically used for substaging of T3 rectal cancer,
and the accuracy of this approach has been confirmed by multiple
studies (13,14). Endorectal ultrasound (ERUS) has
advantages in TN staging over MRI, such as higher accuracy, lower
cost, and easier operation (15).
However, the value of ERUS in assessing the EMI has not been widely
recognized (16–18).
The present study aimed to assess the consistency
between ultrasound and pathological measurements of EMI as well as
to assess interobserver agreement for EMI measurements. In
addition, the accuracy of ERUS and MRI in T3 and substaging of T3
rectal cancer were compared, so as to demonstrating the role of
ERUS in the treatment of T3 rectal cancer.
Patients and methods
Patients
We retrospectively analyzed the clinical data of
rectal cancer patients admitted to Peking Union Medical College
Hospital between January 2014 and July 2015. The clinical
pathological and imaging data were retrieved. This study was
approved by the Peking Union Medical College Hospital Ethics
Committee.
The inclusion criteria included: i) histologically
(biopsy) confirmed rectal carcinoma; ii) did not receive
neoadjuvant chemoradiotherapy before surgery; iii) achieved radical
resection in accordance with the principles of total mesorectal
excision (TME), as the removal en bloc of the tumor together with
its mesorectum; iv) both ERUS and MRI were performed before
surgery, v) complete ERUS with dynamic imaging for 120s
continuously; vi) tumor located <10 cm above the anal verge, and
vii) tumor not causing intestinal stenosis.
ERUS imaging and interpretation
Ultrasound was performed on a Hitachi Vision Preirus
ultrasound machine, equipped with a EUP-R54AW-33 endorectal probe,
with the frequency of 5–10 MHz and a scanning angle of 360°. Two
doctors conducted TN staging and EMI measurements independently.
Dynamic US images were retrieved from the imaging workstation. The
Hildebrandt uTN classification was used for ERUS staging of tumors
(19). The best images on which the
EMI was measured were selected according to the following criteria:
i) probe was located at the center of the intestine; and ii)
maximum diameter of tumor invasion was shown. There is currently no
accepted method for the measurement of the EMI by ERUS, and with
reference to MRI measurement methods (20), the following ERUS measurement methods
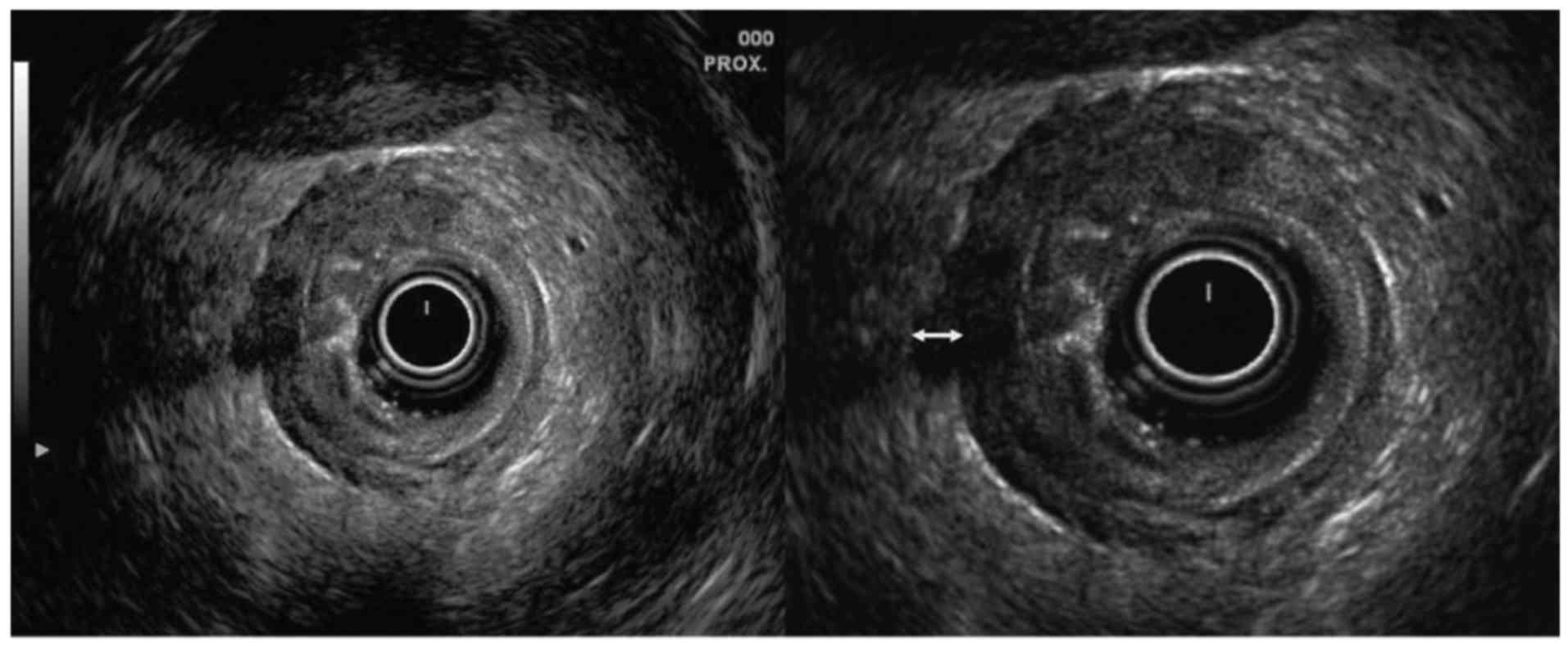
were created. If the muscularis propria was completely
identifiable, the maximum distance from the deepest part of tumor
invasion to the outer border of the muscularis propria was measured
(Fig. 1); if the muscularis propria
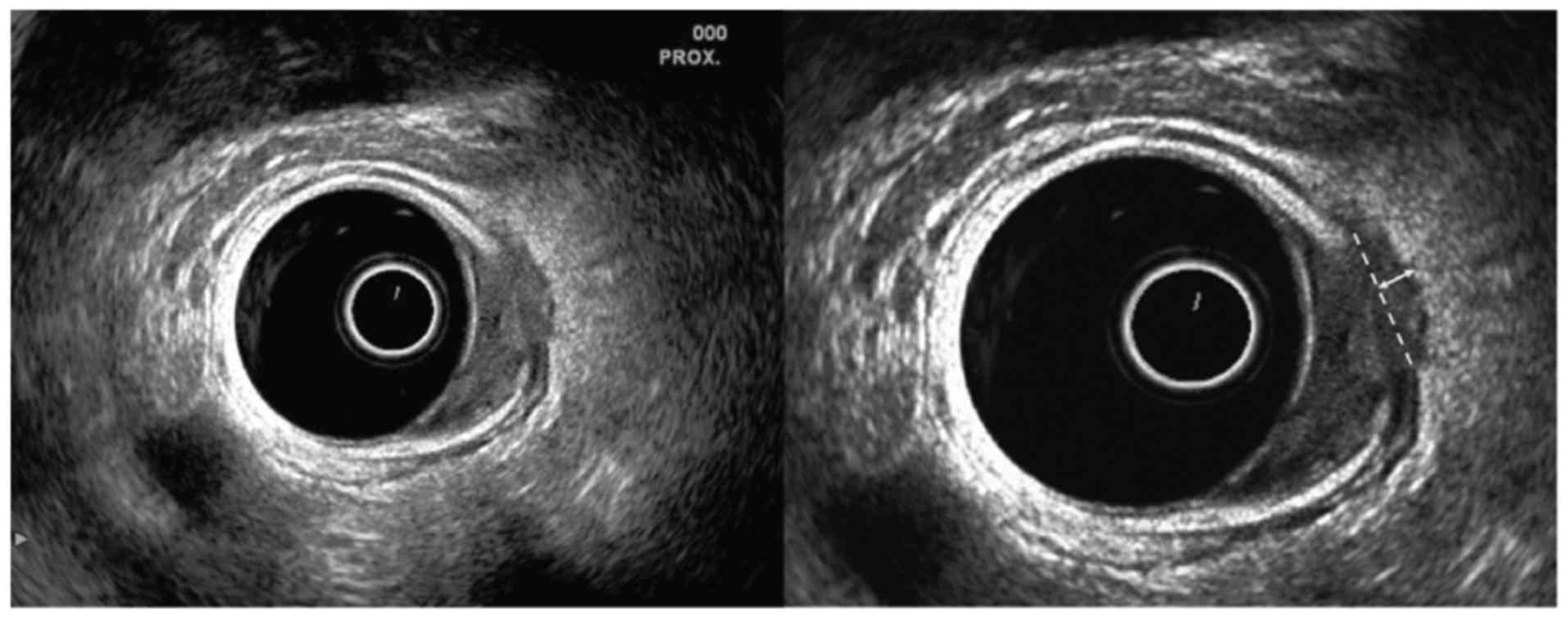
was not entirely identifiable, the maximum distance from the
deepest part of tumor invasion to an imaginary line connecting two
break points of the muscularis propria was measured (Fig. 2).
Magnetic resonance imaging and
interpretation
One experienced radiologist who had no previous
information concerning the clinical or pathologic findings,
interpreted whole MRI scans on the PACS viewer regarding T and N
stages. The extent of mesorectal invasion was measured blindly to
the ERUS findings in this study, with special focus on the thin
slice axial T2 weighted MRI images. The EMI in millimeters was
measured in the same manner as the ERUS measurement.
T3 substaging of rectal cancer by ERUS
and MRI
In T3 substaging, there is currently no cut-off
point for the EMI measured by ERUS. Based on a previous study
(13), 5 mm was set as the cut-off
point, and T3 stage was divided into u, rT3a stage (u, rEMI ≤5 mm)
and u, rT3b stage (u, rEMI >5 mm).
Pathological measurement and
staging
An experienced pathologist was in charge of the
pathological measurement and staging. For all the specimens, the
intestine was opened along the opposite side of the mesentery.
Specimens were fixed for at least 12 h in 10% formalin. Then the
deepest point of tumor infiltration was selected, and one or more
sections were made and subdivided into appropriately sized pieces.
Specimens were embedded in paraffin and stained with
hematoxylin-eosin, and then the pEMI was measured. The pathologist
measured the distance between the outer border of the identifiable
muscularis propria and the outermost border of the tumor. When the
muscularis propria could not be identified, an imaginary line
connecting two break points of the muscularis propria was used. A
5-mm cut-off value was used for categorizing pT3 stage specimens
into pT3a and pT3b.
The pathological staging results were considered as
the gold standard, and the sensitivity, specificity, and accuracy
of ERUS and MRI for T3 rectal cancer and substaging of T3 rectal
cancer were assessed.
Statistical analysis
The MedCalc11.4.2.0 statistical package was used for
statistical analysis. Data are expressed as mean ± standard
deviation. The TN staging and subT3 staging by ERUS and MRI were
compared with the pathological findings, considered as the
criterion standard. The consistency between the measurement of EMI
by ERUS and MRI and pathological measurement were evaluated using
the Bland-Altman analysis. The intraclass correlation coefficient
(ICC) was calculated by a two-factor random effects model to assess
the consistency of measurements between two ultrasound doctors. An
ICC >0.80 indicates good consistency, 0.61–0.80 medium
consistency, 0.41–0.60 moderate consistency, 0.21–0.40 poor
consistency, and ≤0.20 no consistency. The accuracy of ERUS and MRI
in substaging of T3 tumors were compared by using the χ2
test. P-values less than 0.05 were considered significant.
Results
Basic characteristics
According to the inclusion criteria, 61 patients
with different stages of rectal cancer were enrolled in the study,
including 35 males and 26 females with a mean age of 54. 36±10.93
years (range, 30–82 years). Radical resection in accordance with
the principles of TME was achieved for all patients. Laparoscopic
resection was performed in 46 cases, and open surgery was performed
in 15 cases. Postoperative chemotherapy was performed in seven
cases. Patient characteristics are summarized in Table I.
 | Table I.General characteristic of the
lesions. |
Table I.
General characteristic of the
lesions.
| Characteristic of the
lesions | n (%) |
|---|
| Distance from the
anal margin |
|
| Middle
segment (5–10 cm) | 40 (66) |
| Lower
segment (<5 cm) | 21 (34) |
| Depth of tumor
invasion (pT stage) |
|
| pT1 | 8 (13) |
| pT2 | 10 (16) |
| pT3 | 43 (71) |
| pN stage |
|
| pN0 | 24 (39) |
| pN+ | 37 (61) |
| Circumferential
margin (CRM) |
|
| CRM
(−) | 44 (96) |
| CRM
(+) | 2 (4) |
| Postoperative
chemotherapy |
|
| Not
performed | 54 (89) |
|
Performed | 7 (11) |
Accuracy of MRI and ERUS in TN
staging
The MRI correctly staged the depth of rectal wall
invasion in 49 cases, for an overall accuracy rate of 80.3%
(49/61). MRI understaged the depth in two cases and overstaged it
in ten. Two pT3 tumors were understaged as rT2. Six pT1 tumors were
overstaged as rT2 tumors and four pT2 tumors were overstaged as rT3
(Table I). MRI correctly predicted
the N stage in 48 cases, for an overall accuracy rate of 78.7%
(48/61). MRI overstaged the N stage in three cases and understaged
it in 10.
The accuracies of ERUS in T stage by the two
ultrasound doctors were 85.2% (52/61) and 81.9% (50/61),
respectively. The first doctor overstaged six cases and understaged
three cases. The second doctor overstaged six cases and understaged
five cases. They mainly overstaged pT2 tumors as uT3 tumors. The
two doctors correctly predicted the N stage in 46 cases and 43
cases, respectively. The first doctor overstaged four cases and
understaged 11 cases. The second doctor overstaged five cases and
understaged 13 cases. The TN stage by MRI and ERUS are summarized
in Tables II and III.
 | Table II.The T stage by ERUS and MRI. |
Table II.
The T stage by ERUS and MRI.
|
|
| Pathological
stage |
|
|
|---|
|
|
|
|
|
|
|---|
| Doctors | Imaging stage | pT1 (n=8) | pT2 (n=10) | pT3 (n=43) | Accuracy (%) |
|---|
| Ultrasound doctor
1 | uT1 | 7 | 0 | 0 | 85.2 (52/61) |
|
| uT2 | 1 | 5 | 3 |
|
|
| uT3 | 0 | 5 | 40 |
|
| Ultrasound doctor
2 | uT1 | 6 | 0 | 0 | 82.0 (50/61) |
|
| uT2 | 2 | 6 | 5 |
|
|
| uT3 | 0 | 4 | 38 |
|
| Radiologist | rT1 | 2 | 0 | 0 | 80.3 (49/61) |
|
| rT2 | 6 | 6 | 2 |
|
|
| rT3 | 0 | 4 | 41 |
|
 | Table III.The N stage by ERUS and MRI. |
Table III.
The N stage by ERUS and MRI.
|
|
| Pathological
stage |
|
|---|
|
|
|
|
|
|---|
| Doctor | Imaging stage | pN1 (n=8) | pN0 (n=10) | Accuracy (%) |
|---|
| Ultrasound doctor
1 | uN1 | 26 | 4 | 75.4 (46/61) |
|
| uN0 | 11 | 20 |
|
| Ultrasound doctor
2 | uN1 | 24 | 5 | 70.5 (43/61) |
|
| uN0 | 13 | 19 |
|
| Radiologist | rN1 | 27 | 3 | 78.7 (48/61) |
|
| rN0 | 10 | 21 |
|
Reliability of EMI measurements by
ERUS and interobserver agreement
Two doctors measured the EMI on ERUS, and the
average uEMI values were 4.8±2.7 mm (0.8–10.2 mm) and 4.6±2.3 mm
(1.2–11 mm) for doctors 1 and 2, respectively. The average pEMI
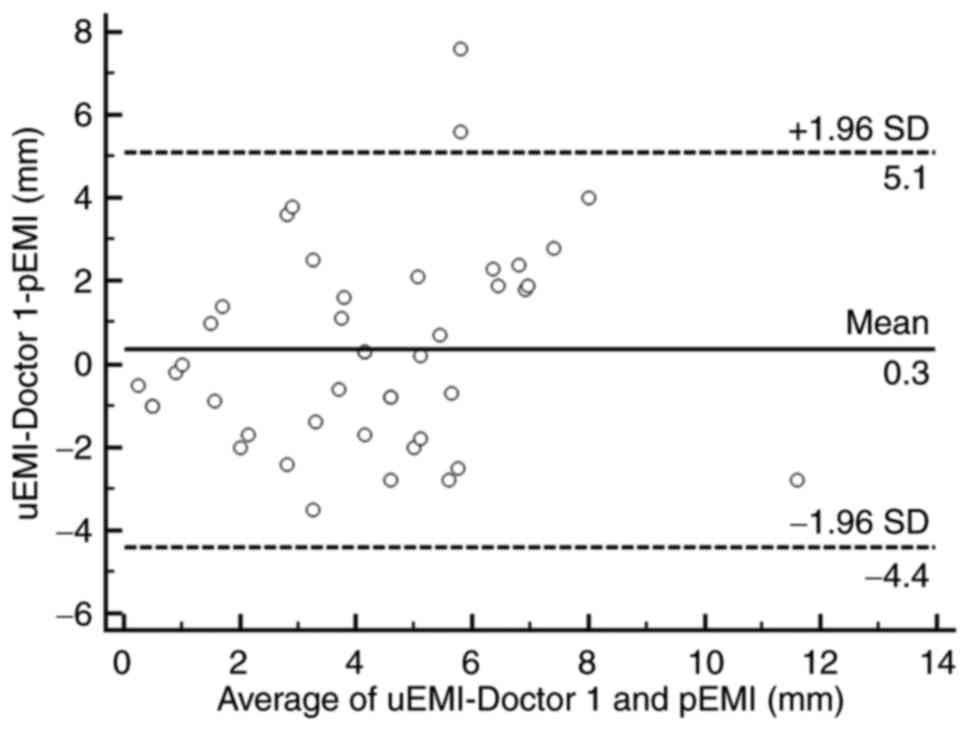
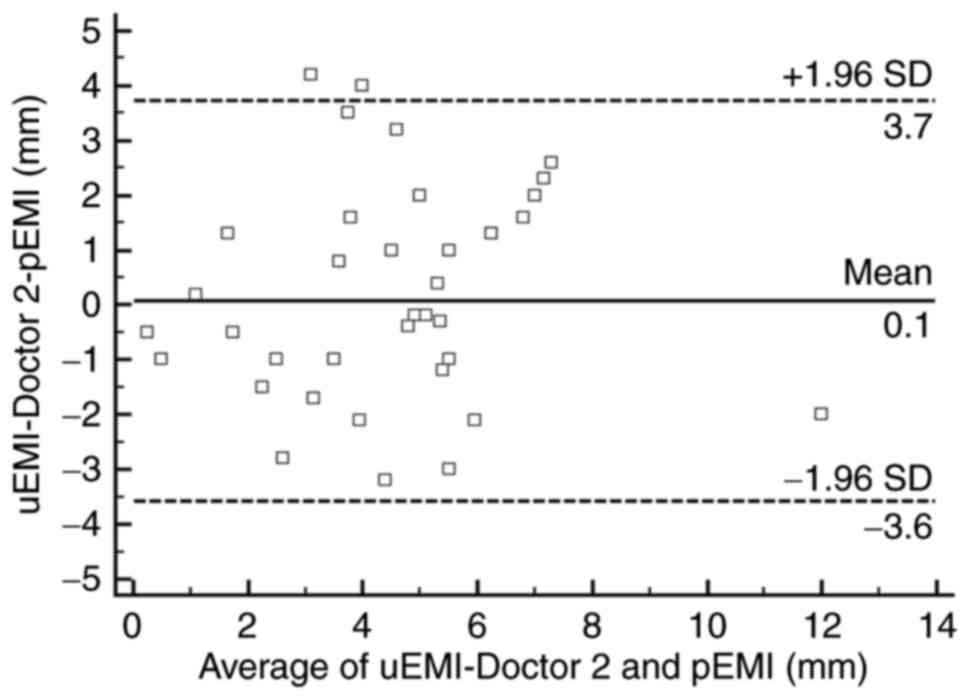
value was 4.1±2.4 mm (0.5–12.5 mm). As shown in the Bland-Altman
scatter plots (Figs. 3 and 4), most of the plotted points were located
within the range of mean ± 1.96 SD, showing that the ERUS
measurements and pathology measurements were in good agreement. The
arithmetic mean difference between the ERUS measurement and
pathology measurement was 0.3 mm (95% confidence interval [CI],
−0.397–1.094 mm) for doctor 1 and 0.1 mm (95% CI, −0.493–0.651 mm)
for doctor 2. The 95% CIs of the limits of agreement were 5.1 mm
(95% CI, 3.815–6.384 mm) to −4.4 mm (95% CI, −5.686 to −3.117 mm)
for doctor 1 and 37 mm (95% CI, 2.739–4.710 mm) to −3.5 mm (95% CI,
−4.552 to −2.581 mm) for doctor 2.
The interobserver agreement for uEMI measurements
between the two doctors was good (ICC=0.9344; 95% CI,
0.8789–0.9645).
Reliability of EMI measurements by
MRI
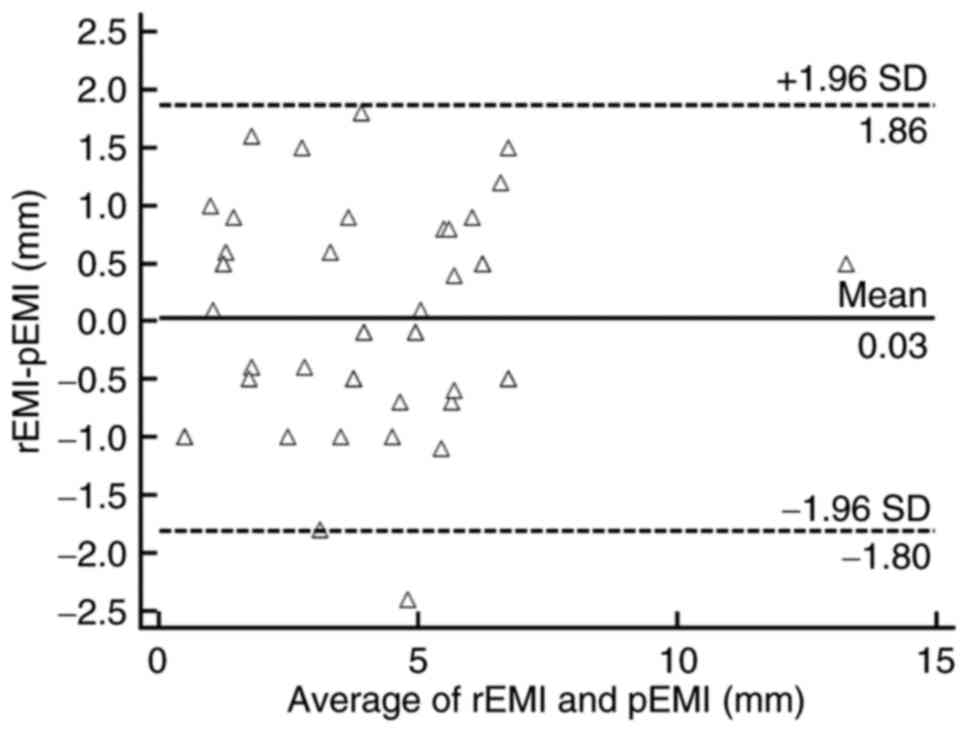
The Bland-Altman plot showed that most plotted
points were located within the mean ±1.96 SD, which indicates good
agreement (Fig. 5). The average value
of rEMI was 4.3±2.4 mm. The arithmetic mean difference between the
MRI measurement and pathology measurement was 0.03 mm (95% CI,
−0.397–1.094 mm). The 95% CIs of the limits of agreement were 1.9
mm (95% CI, 1.365–2.356 mm) to −1.8 mm (95% CI, −2.301 to −1.309
mm).
Efficacy of ERUS and MRI for T3 and T3
substaging of rectal cancer
The diagnostic accuracy, sensitivity, and
specificity for the two individual doctors for the diagnosis of T3
rectal cancer were 86.9% (53/61), 93.0% (40/43), and 72.2% (13/18)
compared with 85.2% (52/61), 88.4 (38/43), and 77.8% (14/18),
respectively. The diagnostic accuracy, sensitivity, and specificity
of MRI for the diagnosis of T3 rectal cancer were 90.2% (55/61),
95.3% (41/43), and 77.8% (14/18), respectively.
We used 5 mm as the cut-off value for the EMI for
substaging of T3 rectal cancer. The accuracies of substaging T3
rectal cancer made by the two doctors were 79.1% (37/43) and 67.4%
(31/43). The accuracy of MRI in substaging T3 rectal cancer was
86.0% (37/43). Though it was obviously much higher than those of
ERUS, there was no significantly statistical difference among them
(P=0.394>0.05; P=0.112>0.05). The detailed diagnostic
accuracy, sensitivity, and specificity values are summarized in
Table IV.
 | Table IV.The accuracy, sensitivity, and
specificity of ERUS EMI measurements for T3 substaging. |
Table IV.
The accuracy, sensitivity, and
specificity of ERUS EMI measurements for T3 substaging.
|
|
| Pathological
stage |
|
|
|
|---|
|
|
|
|
|
|
|
|---|
| Doctor | Imaging stage | pT3b (n=15) | pT3a (n=28) | Accuracy (%) | Sensitivity
(%) | Specificity
(%) |
|---|
| Ultrasound doctor
1 | uT3b | 10 | 4 | 79.1 (34/43) | 66.7 (10/15) | 85.7 (24/28) |
|
| uT3a | 5 | 24 |
|
|
|
| Ultrasound doctor
2 | uT3b | 9 | 5 | 67.4 (31/43) | 60 (9/15) | 82.1 (23/28) |
|
| uT3a | 6 | 23 |
|
|
|
| Radiologist | rT3b | 12 | 3 | 86.0 (37/43) | 80 (12/15) | 89.3 (25/28) |
|
| rT3a | 3 | 25 |
|
|
|
Discussion
This is the first study to compare EMI measurements
determined by ERUS and pathology measurements. Currently, the value
of ERUS in measuring the EMI has not been widely recognized. ERUS
has been used to measure the EMI and for substaging of T3 rectal
cancer (16), but the reliability of
ultrasound measurements of the EMI has not been evaluated. This
study verified the reliability of ERUS for measuring the EMI. The
Bland-Altman scatter plot showed good consistency between
ultrasound and pathology measurements. Meanwhile, the good
agreement between uEMI measurements made by two ultrasound doctors
also suggests the promising clinical potential of this method.
Among the imaging methods used for staging, MRI is
now the preferred technique for EMI assessment, and its reliability
has been demonstrated (21). In the
present study, it was demostrated that MRI was a reliable method
for the measurement of EMI, and the accuracy of T3 and T3
substaging was also high. ERUS was more accurate than MRI for the
evaluation of local invasion, especially for T1 and T2 tumors, our
study also demonstrated that ERUS was superior in diagnosis T1
tumor. In recent years, there has been a shift away from ERUS for
the staging of rectal cancer. This can be partially explained by
factors linked to ERUS such as the reported low accuracy in the
staging of advance rectal cancer in some recent studies and the
operator dependency. In addition, MRI can be used to assess the
circumferential resection margin and EMI which are the most
important prognostic factors with regard to TN stage.
However, MRI has some shortcomings, such as high
cost and long exam duration. Moreover, there are many MRI
contraindications; for example, patients with a pacemaker,
intrauterine device, or claustrophobia are not suitable for MRI
(21). Compared to MRI, ERUS has the
following advantages: Low cost, fewer contraindications, and easy
operation. Although ERUS is less accurate in substaging of T3 tumor
than MRI, ERUS can overcome the shortcomings of MRI in preoperative
stage of T3 rectal cancer. For complementary to each other, MRI and
ERUS should be used together so as to selecting the best
therapeutical approach.
Maximal tumor thickness (MTT) also has been used for
substaging. Esclapez et al divided the T3 stage into uT3a
(uMTT ≤19 mm) and uT3b (uMTT >19 mm) (22). They believed that because the
muscularis propria was replaced by tumor, the ERUS would be
unlikely to clearly show the muscularis propria. Thus, the
measurement of the EMI would suffer from errors. However, our
previous study (23) demonstrated
that the vast majority of patients have an uMTT >19 mm, and
therefore, if we use 19 mm as the cut-off point, many patients with
a high risk of local recurrence might be missed, resulting in
insufficient treatment. In the present study, the average errors of
the uEMI were 0.3 and 0.1 mm, which are acceptable. In addition,
when most of the tumor protrudes into the intestine lumen, MTT is
not a good indicator of tumor aggressiveness. By contrast, the EMI
can more directly reflect the tumor aggressiveness as well as the
prognosis, and thus, the EMI is a preferred indicator for
substaging of T3 rectal cancer.
Compared with the EMI measured by pathology, the
uEMI showed some deviations. Possible reasons for these deviations
includes: 1) it was difficult to choose the same section for both
the ultrasound measurement and pathology measurement; 2) the
muscularis propria may have been completely replaced by the tumor
over a wide area, which could make measuring the EMI more difficult
(21); 3) peritumoral inflammation
and real transmural tumor extension cannot easily, reliably, or
precisely distinguished on ultrasound, which commonly results in
overestimation of the uEMI (24–26); and
4) bulky tumors can lie outside the focal length of the transducer
(27).
There is no consensus on a cut-off value for the EMI
for substaging T3 stage rectal cancer. Harewood et al
divided T3 stage rectal cancer into minimally invasive T3 (EMI ≤2
mm) and advanced T3 (EMI >2 mm) substages (17). Rafaelsen et al divided T3 stage
rectal cancer into four substages according to UICC standards: T3a
(EMI: 0–1 mm), T3b (EMI: 2–5 mm), T3c (EMI: 5–15 mm), and T3d (EMI
>15 mm). In their system T3a and T3b are considered early T3
stage rectal cancer, and T3c and T3d are considered advanced T3
rectal cancer (16). In this study,
the uEMI in most cases was larger than 2 mm, and we also found that
when the EMI was less than 2 mm, it was difficult to distinguish
minor tumor infiltration from paraneoplastic fibrosis and
inflammation. In this study, with 5 mm as the cut-off value for
classifying the uEMI measurements, substaging of T3 rectal cancer
showed good consistency with the pathology results.
To identify an effective strategy for the treatment
of T3 rectal cancer, we need to accurately evaluate the prognostic
factors preoperatively and assess the risk of recurrence, before a
specific, individual treatment plan can be formulated. Another
important factor in determining the prognosis of T3 rectal cancer
is the CMR. Residual tumor in CRM was found to be related to a high
risk of local recurrence, and thus, patients with positive CRM need
to be treated with neoadjuvant chemoradiotherapy (28). ERUS was once considered to offer
limited resolution and visualization ability, and it could not
clearly show the mesorectal fascia. Thus, it has not been a
conventional method used for pre-operative assessment of CRM. Phang
first reported that ERUS can be used for the diagnosis of CRM
(29), and then another study
reported the diagnostic accuracy of ERUS for CRM is 83.7% (30). In addition, our previous studies
demonstrated an accuracy of 98.1% and a negative predictive value
of 100% for ERUS (23). ERUS can not
only be applied to TN staging, but also can provide information on
the EMI, CRM, and location of the tumor. This information is
helpful for predicting the prognosis and planning treatment. For
example, when the EMI is greater than 5 mm, the lymph node status
is N2 stage, more than 4 lymph nodes are involved, CRM is positive,
and lesions are in the lower section of the rectum, the risks for
local recurrence and distant metastasis are extremely high, and
preoperative neoadjuvant therapy should be selected.
The present study has some limitations. First, this
was a retrospective study, and therefore, the data analyses are
prone to bias. Second, in the present study the frequency of the
probe was not fixed, which may have some impact on the diagnostic
accuracy. The resolution of a 5.0 MHz endorectal transducer is
high, which may be approximately the same or higher as the
resolution for thin slice axial T2 weighted MRI. Further study will
be need to demonstrate this.
ERUS is a valuable tool for measurement of the EMI,
and a cut-off point of 5 mm can be used for substaging of T3 rectal
cancer. ERUS and MRI can be used together which will be quite
helpful for selecting appropriate treatments for rectal cancer
patients.
References
|
1
|
Berardi R, Maccaroni E, Onofri A, Morgese
F, Torniai M, Tiberi M, Ferrini C and Cascinu S: Locally advanced
rectal cancer: The importance of a multidisciplinary approach.
World J Gastroenterol. 20:17279–17287. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Marks J, Nassif G, Schoonyoung H, DeNittis
A, Zeger E, Mohiuddin M and Marks G: Sphincter-sparing surgery for
adenocarcinoma of the distal 3 cm of the true rectum: Results after
neoadjuvant therapy and minimally invasive radical surgery or local
excision. Surg Endosc. 27:4469–4477. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Loos M, Quentmeier P, Schuster T, Nitsche
U, Gertler R, Keerl A, Kocher T, Friess H and Rosenberg R: Effect
of preoperative radio(chemo)therapy on long-term functional outcome
in rectal cancer patients: A systematic review and meta-analysis.
Ann Surg Oncol. 20:1816–1828. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bruheim K, Guren MG, Dahl AA, Skovlund E,
Balteskard L, Carlsen E, Fosså SD and Tveit KM: Sexual function in
males after radiotherapy for rectal cancer. Int J Radiat Oncol Biol
Phys. 76:1012–1017. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bruheim K, Guren MG, Skovlund E, Hjermstad
MJ, Dahl O, Frykholm G, Carlsen E and Tveit KM: Late side effects
and quality of life after radiotherapy for rectal cancer. Int J
Radiat Oncol Biol Phys. 76:1005–1011. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wilson PM, Ladner RD and Lenz HJ:
Exploring alternative individualized treatment strategies in
colorectal cancer. Clin Colorectal Cancer. 7 Suppl 1:S28–S36. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cecil TD, Sexton R, Moran BJ and Heald RJ:
Total mesorectal excision results in low local recurrence rates in
lymph node-positive rectal cancer. Dis Colon Rectum. 47:1145–1150.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Frasson M, Garcia-Granero E, Roda D,
Flor-Lorente B, Roselló S, Esclapez P, Faus C, Navarro S, Campos S
and Cervantes A: Preoperative chemoradiation may not always be
needed for patients with T3 and T2N+ rectal cancer. Cancer.
117:3118–3125. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cawthorn SJ, Parums DV, Gibbs NM, A'Hern
RP, Caffarey SM, Broughton CI and Marks CG: Extent of mesorectal
spread and involvement of lateral resection margin as prognostic
factors after surgery for rectal cancer. Lancet. 335:1055–1059.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Willett CG, Badizadegan K, Ancukiewicz M
and Shellito PC: Prognostic factors in stage T3N0 rectal cancer: Do
all patients require postoperative pelvic irradiation and
chemotherapy? Dis Colon Rectum. 42:167–173. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Steel MC, Woods R, Mackay JM and Chen F:
Extent of mesorectal invasion is a prognostic indicator in T3
rectal carcinoma. ANZ J Surg. 72:483–487. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Miyoshi M, Ueno H, Hashiguchi Y, Mochizuki
H and Talbot IC: Extent of mesorectal tumor invasion as a
prognostic factor after curative surgery for T3 rectal cancer
patients. Ann Surg. 243:492–498. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Merkel S, Mansmann U, Siassi M,
Papadopoulos T, Hohenberger W and Hermanek P: The prognostic
inhomogeneity in pT3 rectal carcinomas. Int J Colorectal Dis.
16:298–304. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
MERCURY Study Group: Extramural depth of
tumor invasion at thin-section MR in patients with rectal cancer:
Results of the MERCURY study. Radiology. 243:132–139. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Marone P, de Bellis M, D'Angelo V, Delrio
P, Passananti V, Di Girolamo E, Rossi GB, Rega D, Tracey MC and
Tempesta AM: Role of endoscopic ultrasonography in the
loco-regional staging of patients with rectal cancer. World J
Gastrointest Endosc. 7:688–701. 2015.PubMed/NCBI
|
|
16
|
Rafaelsen SR, Vagn-Hansen C, Sørensen T,
Pløen J and Jakobsen A: Transrectal ultrasound and magnetic
resonance imaging measurement of extramural tumor spread in rectal
cancer. World J Gastroenterol. 18:5021–5026. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Harewood GC, Kumar KS, Clain JE, Levy MJ
and Nelson H: Clinical implications of quantification of mesorectal
tumor invasion by endoscopic ultrasound: All T3 rectal cancers are
not equal. J Gastroenterol Hepatol. 19:750–755. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Muñoz E, Granero-Castro P, Frasson M,
Escartin J, Esclapez P, Campos S, Flor-Lorente B and Garcia-Granero
E: Modified Wong's classification improves the accuracy of rectal
cancer staging by endorectal ultrasound and MRI. Dis Colon Rectum.
56:1332–1338. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hildebrandt U and Feifel G: Preoperative
staging of rectal cancer by intrarectal ultrasound. Dis Colon
Rectum. 28:42–46. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cho SH, Kim SH, Bae JH, Jang YJ, Kim HJ,
Lee D and Park JS: Society of North America (RSNA): Prognostic
stratification by extramural depth of tumor invasion of primary
rectal cancer based on the Radiological Society of North America
proposal. AJR Am J Roentgenol. 202:1238–1244. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim YW, Cha SW, Pyo J, Kim NK, Min BS, Kim
MJ and Kim H: Factors related to preoperative assessment of the
circumferential resection margin and the extent of mesorectal
invasion by magnetic resonance imaging in rectal cancer: A
prospective comparison study. World J Surg. 33:1952–1960. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Esclapez P, Garcia-Granero E, Flor B,
Garcia-Botello S, Cervantes A, Navarro S and Lledó S: Prognostic
heterogeneity of endosonographic T3 rectal cancer. Dis Colon
Rectum. 52:685–691. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhong G, Xiao Y, Zhang J, Dai Q, Li J and
Jiang Y: Value of endorectal ultasound in predicting the
circumferential resection margin and maximum tumor thickness of T3
rectal cancer. Zhonghua Wei Chang Wai Ke Za Zhi. 18:252–256.
2015.(In Chinese). PubMed/NCBI
|
|
24
|
Siddiqui AA, Fayiga Y and Huerta S: The
role of endoscopic ultrasound in the evaluation of rectal cancer.
Int Semin Surg Oncol. 3:362006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Assenat E, Thézenas S, Samalin E, Bibeau
F, Portales F, Azria D, Quenet F, Rouanet P, Aubert Saint B and
Senesse P: The value of endoscopic rectal ultrasound in predicting
the lateral clearance and outcome in patients with lower-third
rectal adenocarcinoma. Endoscopy. 39:309–313. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bipat S, Glas AS, Slors FJ, Zwinderman AH,
Bossuyt PM and Stoker J: Rectal cancer: Local staging and
assessment of lymph node involvement with endoluminal US, CT and MR
imaging-a meta-analysis. Radiology. 232:773–783. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fernández-Esparrach G, Ayuso-Colella JR,
Sendino O, Pagés M, Cuatrecasas M, Pellisé M, Maurel J,
Ayuso-Colella C, González-Suárez B, Llach J, et al: EUS and
magnetic resonance imaging in the staging of rectal cancer: A
prospective and comparative study. Gastrointest Endosc. 74:347–354.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Burton S, Brown G, Daniels IR, Norman AR,
Mason B and Cunningham D: Royal Marsden Hospital, Colorectal Cancer
Network: MRI directed multidisciplinary team preoperative treatment
strategy: The way to eliminate positive circumferential margins? Br
J Cancer. 94:351–357. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Phang PT, Gollub MJ, Loh BD, Nash GM,
Temple LK, Paty PB, Guillem JG and Weiser MR: Accuracy of
endorectal ultrasound for measurement of the closest predicted
radial mesorectal margin for rectal cancer. Dis Colon Rectum.
55:59–64. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Granero-Castro P, Munoz E, Frasson M,
Garcia-Granero A, Esclapez P, Campos S, Flor-Lorente B and
Garcia-Granero E: Evaluation of mesorectal fascia in mid and low
anterior rectal cancer using endorectal ultrasound is feasible and
reliable: A comparison with MRI findings. Dis Colon Rectum.
57:709–714. 2014. View Article : Google Scholar : PubMed/NCBI
|