Introduction
Breast cancer is a common malignant tumor in females
and the second most common cause of cancer-associated mortality
(1). In total, 60 to 70% of patients
with metastatic breast cancer exhibit bone metastases (2). Therefore, identifying the mechanisms of
tumor metastasis to bone is critical to therapeutic approach.
Secreted protein acidic and rich in cysteine
(SPARC), also termed osteonectin or basement-membrane protein 40,
is a 34-kDa, calcium-binding glycoprotein that associates with cell
membranes and membrane receptors (3).
Bellahcene and Castronovo (4)
suggested that increased expression of SPARC in malignant breast
tumors may serve a role in the preferential homing of breast cancer
cells to bones. Osteonectin is a factor in bone extracts that
promotes breast and prostate cancer cell invasion to bone in
vitro (5). Additionally, bone
extracts from osteonectin-null mice exhibit reduced chemoattractant
activity for prostate cancer cells (6).
Several studies have revealed that increased SPARC
expression is associated with poor prognosis with respect to breast
cancer: Helleman et al (7)
reported that SPARC expression levels were significantly associated
with a shorter metastasis free survival; Hsiao et al
(8) reported that patients with
positive SPARC expression had 2.34 times higher risk of mortality
compared with those with negative SPARC expression level following
adjusting for factors including positive lymph node,
tumor-node-metastasis tumor stage, estrogen receptor and
progesterone receptor; however, Koblinski et al (9) reported the opposite result. Koblinski
et al (9) reported that
increased endogenous expression of SPARC may inhibit the invasive
activity of breast cancer cells and reduce tumor cell-platelet
aggregation.
To investigate the function of SPARC in breast
cancer bone metastasis, the present study evaluated the predictive
value of SPARC in primary breast cancer and bone metastatic foci.
The effect of endogenous expression of SPARC on the invasion
ability of breast cancer cells and bone metastasis was then
determined. The present study revealed that increased SPARC
expression correlated with a low rate of bone metastasis, and SPARC
may inhibit migration and invasion in vitro. SPARC may also
suppress osteoclast activation in the breast cancer
microenvironment. These results suggest that SPARC serves an
important role in breast cancer bone metastasis and may be a
promising therapeutic target for the treatment of breast cancer
bone metastasis.
Materials and methods
Human tumor cell lines and cell
culture
The breast cancer MDA-MB-231, BT474, MCF-7, SKBR3,
MCF10A, HCC1937, T47D and ZR-75-30 cell lines were purchased from
the American Type Culture Collection (Manassas, VA, USA) and
cultured in Dulbecco's modified Eagle's medium (DMEM; Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA). The SUM1315 cell line
was provided by Dr Stephen Ethier (University of Michigan, Ann
Arbor, MI, USA). SUM1315-bo cells were established and termed as in
our previous study (10). SUM1315-bo
cells are derived from SUM1315 metastatic tumor in implanted bone.
All cells were maintained at 37°C in a humidified chamber
supplemented with 5% CO2.
Patient selection
Formalin-fixed paraffin-embedded tumor samples
(n=50) were collected at the Department of Breast Surgery, Nanjing
Maternity and Child Health Care Hospital Affiliated to Nanjing
Medical University (Nanjing, China) and the Department of Breast
Surgery, The First Affiliated Hospital of Nanjing Medical
University (Nanjing, China) between February 2002 and March 2007
from patients who were surgically treated for clinical stage I–III
breast cancer (aged 34–65 years) (11). The patients of the present study were
followed up until December 2013 to determine whether they were
positive or negative for bone metastasis, with a median follow-up
time of 64 months (range, 50–78 months). Patient characteristics
are listed in Table I. All patients
provided informed consent, and the present study was approved by
the Ethical and Scientific Committee of Nanjing Maternity and Child
Health Care Hospital Affiliated to Nanjing Medical University
(Nanjing, China).
 | Table I.Association between stromal SPARC
expression and the clinicopathological characteristics of patients
with breast cancer. |
Table I.
Association between stromal SPARC
expression and the clinicopathological characteristics of patients
with breast cancer.
| Characteristic | Patients, n | Patients with high
SPARC expression, n (%) | P-value |
|---|
| Age, years |
|
| 0.095 |
| ≤50 | 28 | 9 (32.1) |
|
|
>50 | 22 | 2 (9.0) |
|
| Bone metastasis |
|
| 0.001 |
| Yes | 25 | 2 (8.0) |
|
| No | 25 | 13 (52.0) |
|
| Tumor
sizea |
|
| 0.454 |
| T1 | 20 | 4 (20.0) |
|
| T2 | 22 | 4 (18.1) |
|
| T3 | 8 | 3 (37.5) |
|
| Node
stagea |
|
| 0.087 |
| N0 | 9 | 1 (11.1) |
|
| N1 | 23 | 3 (13.0) |
|
| N2 | 14 | 6 (42.8) |
|
| N3 | 4 | 1 (25.0) |
|
| Histological
grade |
|
| 0.708 |
| 1 | 2 | 0 (0.0) |
|
| 2 | 39 | 9 (23.0) |
|
| 3 | 9 | 2 (22.2) |
|
| ER |
|
| 0.728 |
|
Positive | 17 | 3 (17.6) |
|
|
Negative | 33 | 8 (24.2) |
|
| PR |
|
| 1.000 |
|
Positive | 14 | 3 (21.4) |
|
|
Negative | 36 | 8 (22.2) |
|
| HER2b |
|
| 0.651 |
|
Positive | 10 | 4 (30.0) |
|
|
Negative | 40 | 7 (20.0) |
|
Antibodies and reagents
SPARC antibody (#5420) was purchased from Cell
Signaling Technology, Inc. (Danvers, MA, USA). Goat anti-rabbit
immunoglobulin G (IgG)-horseradish peroxidase secondary antibodies
(BS10003) and antibodies against GAPDH (MB001) were purchased from
Bioworld Technology, Inc. (St. Louis Park, MN, USA).
Plasmids and viral production
Wild-type SPARC open reading frame (ORF) was
isolated from human SUM1315 breast cancer cells complementary DNA
using polymerase chain reaction (PCR). The primers used were as
follows: Forward, 5′-GGAAGAAACTGTGGCAGAGG-3′ and reverse,
5′-ATTGCTGCACACCTTCTCAA-3′. The ORF was 1983 bp in length. The
fragment was cloned into pGFP-LV5 via a NotI/NsiI
site. The recombinant lentiviral pGFP-LV5-SPARC expression plasmid
was packaged into a mature lentivirus using 293T cells (American
Type Culture Collection), and the supernatant (4°C, 1,000 × g, 15
min) containing the virus was harvested, concentrated and titrated.
Transfection with pGFP-LV5 empty vector was used as a control.
SUM1315 cells were subsequently infected using the recombinant
lentiviral vector. Flow cytometry was used to select the green
fluorescent protein-cells. Western blot analysis was performed to
detect the SPARC expression level (10).
Transfection
The oligonucleotides were transfected into SUM1315
and SUM1315-bo cells with Lipofectamine 2000 reagent (Invitrogen;
Thermo Fisher Scientific, Inc.) at 80–90% confluence according to
the manufacturer's protocol. All oligonucleotides, small
interfering RNAs (siRNAs) of SPARC, GGAAGAAACUGUGGCAGAGGUGACU
(si-S1); CAAGAACGUCCUGGUCACCCUGUA U (si-S2);
GCGGGUGAAGAAGAUCCAUGAGAAU (si-S3), were obtained from Invitrogen
(Thermo Fisher Scientific, Inc.). The oligonucleotides were
chemically modified (20-O-Methyl) oligos. They were revealed to
exert long-term effects (2 weeks) and high gene knockout
efficiency. Transfection efficiency was analyzed using a
fluorescence microscope 24 h subsequent to transfection and defined
as the number of cells capable of exhibiting fluorescence divided
by the number of untransfected controls.
Reverse transcription-quantitative
(RT-q)PCR analysis
Total RNA was extracted using TRIzol total RNA
isolation reagent (Invitrogen; Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol. Specific primers from
Invitrogen; Thermo Fisher Scientific, Inc., (Shanghai, China) were
used for transcript detection: Actin forward,
5′-CTCCATCCTGGCCTCGCTTGT-3′ and reverse,
5′-GCTGTCACCTTCACCGTTCC-3′; SPARC forward,
5′-GGAAGAAACTGTGGCAGAGG-3′ and reverse, 5′-ATTGCTGCACACCTTCTCAA-3′.
qPCR (5X PrimeScript buffer 2 µl, PrimeScript RT Enzyme Mix 1 0.5
µl, Oligo Dt Prime 0.5 µl, Random 6 mers 0.5 µl, total RNA and
RNase Free dH2O up to 10 µl; Takara Bio, Inc., Otsu,
Japan) was performed with SYBR Green I (Takara Bio, Inc.). The
progression consisted of 40 cycles (95°C for 15 sec and 60°C for 1
min) subsequent to an initial denaturation step (95°C for 10 min).
The mean number of three independent analyses for each gene and
sample was calculated and normalized to the endogenous GAPDH
(12) reference control gene actin
using the 2−ΔΔCq method (13).
Western blot analysis
The cells were harvested in radioimmunoprecipitation
assay lysis buffer (Beyotime Institute of Biotechnology, Shanghai,
China). Samples were incubated for 1 h on ice with agitation and
centrifuged at 4°C 12,000 × g for 20 min. A total of 10 g protein
samples (based on concentration) were subject to electrophoresis on
12% SDS-PAGE, transferred to a polyvinylidene difluoride membrane,
blocked in 5% nonfat milk in TBS-Tween-20 and hybridized with
antibodies against SPARC (dilution, 1:1,000) and GAPDH (dilution,
1:5,000). GAPDH was used as a loading control. Signals were
determined subsequent to incubation with horseradish
peroxidase-conjugated anti-rabbit IgG secondary antibody (dilution,
1:1,000) using enhanced chemiluminescence. Protein expression
levels were evaluated by densitometric analysis (Quantity One
software version 4.62; Bio-Rad Laboratories, Inc., Hercules, CA,
USA).
Transwell migration and invasion
assays
The in vitro invasion studies were performed
using a BD BioCoat Matrigel invasion assay system (BD Biosciences,
Franklin Lakes, NJ, USA). Cells were seeded at
5×104/well on 8-µm pore Transwell inserts (Corning Inc.,
Corning, NY, USA). The lower chamber was filled with DMEM
containing 10% fetal bovine serum (Gibco; Thermo Fisher Scientific,
Inc.). Subsequent to incubation for 24 h, the number of cells in
the lower chamber of the filter membranes was determined. All in
vitro experiments were performed in triplicate, and all trials
produced similar results. For Transwell migration assays, the cells
were plated at 1×105/well in the upper chamber on an
8-µm membrane (BD Biosciences) precoated with 100 µg/ml fibronectin
and 2.5% bovine serum albumin (both from Gibco; Thermo Fisher
Scientific, Inc.). Cells were incubated for the indicated lengths
of time (8 h) under standard culture conditions. Tumor cells
remaining on the upper surface of the membrane were removed and
cells that had migrated to the underside were fixed and stained
with 0.1% crystal violet for 30 min, rinsed in PBS and subjected to
microscopic inspection (Omega Bio-Tek, Inc., Norcross, GA, USA). A
total of five images of the preset fields per insert were captured.
Subsequent to staining and the capturing of images, the cells that
had migrated to the underside were eluted using 33% acetic acid,
and the optical density values of absorbance in the positively
stained cells were measured using a microplate reader (Omega
Bio-Tek, Inc.) at 570 nm.
Preparation of conditioned medium
(CM)
The SUM1315 cells were grown until sub-confluence,
and subsequently starved in serum-free DMEM for 24 h. The CM was
collected, centrifuged (4°C, 1,000 × g, 15 min), concentrated and
aliquoted, and stored at −20°C until use.
Osteoblast differentiation and
osteoclastogenesis assay in vitro
Human mesenchymal stem cells (HMSCs) were purchased
from ScienCell (Carlsbad, CA, USA), plated in cell culture flasks
and expanded in mesenchymal stem cell growth medium (MSCM;
ScienCell) at 37°C in a 5% CO2 atmosphere for 7–10 days.
Subsequent to having grown to an adequate density (70–80%
convergence), the adherent cells were trypsinized and seeded at a
density of 5,000 cells/cm2 on a 24-well plastic plate.
After 24 h, MSCM was removed, and osteogenic induction medium
(ScienCell) was added to induce osteoblast differentiation (day 0).
On the seventh day, the osteoblast cells were plated at
1×106 cells per well in 24-well plates, at a 1:1 ratio
of basal culture medium/filtered CM (harvested from 24 h incubation
of confluent tumor cells). The medium was replaced every 2 days.
Tartrate-resistant acid phosphatase (TRAP) staining was performed
on day 7 using a Leukocyte Acid Phosphatase kit from Sigma-Aldrich;
Merck KGaA (Darmstadt, Germany) (14). TRAP-positive multinucleated cells were
considered mature osteoclasts and were included in the number of
osteoclasts per well.
Immunohistochemistry (IHC)
Paraffin-embedded tissue sections were
deparaffinized, rehydrated, rinsed, immersed in 10 mM sodium
citrate, microwaved for 20 min and cooled for 20 min. For
immunocytohistochemistry, the slides were fixed in PBS (pH 7.4)
solution containing 4% paraformaldehyde for 10 min and rinsed.
Subsequent to incubation in methanol containing 3% hydrogen
peroxide for 10 min to block endogenous peroxidase activity, the
slides were microwaved in 0.01 mmol/l sodium citrate (pH 6.0) for
antigen retrieval, incubated with rabbit polyclonal antibody
against SPARC (1:1,000; Cell Signaling Technology, Inc.) for 2 h at
37°C, incubated with horseradish peroxidase-labeled rabbit
anti-goat secondary antibody for 1 h at 37°C, incubated with
3,3′-diaminobenzidine solution for 10 min and counterstained with
hematoxylin.
Evaluation of IHC results
Immunohistochemical staining results were
interpreted by two experienced pathologists, and the mean staining
density was determined using Image-Pro Plus 6.0 (Media Cybernetics,
Inc., Rockville, MD, USA). SPARC expression was evaluated under a
light microscope at magnification, ×400. For each specimen, five
images of representative areas were acquired, and a total of 1,000
to 2,000 tumor cells were counted. For human samples, IHC scoring
was performed using a modified histoscore, which included a
semi-quantitative assessment of the fraction of positive cells and
the intensity of staining. The extent of the staining, defined as
the relative area of positive staining within the tumor cells
relative to the entire tissue area, was scored on a scale of 0–4 as
follows: 0, 0–10%; 1, 11–25%; 2, 26–50%; 3, 51–75%; and 4, >75%.
The sum of the staining-intensity and staining-extent scores was
used as the final staining score for SPARC (0–7). For the
statistical analysis, a final staining score of 0–5 was considered
indicative of low expression, and scores of 6–7 were considered
indicative of high expression. The immunostained slides were
evaluated by two board-certified pathologists at two separate
institutions (Nanjing Maternity and Child Health Care Hospital
Affiliated to Nanjing Medical University and The First Affiliated
Hospital of Nanjing Medical University, Nanjing, China). The
pathologists independently examined the entire tissue section and
were blinded to the clinical data. Reading agreement was found to
be 96% concordant between the two pathologists. Non-concordant
cases were resolved by a third pathologist who blindly scored those
cases, and the two out of three rule was used for the determination
of final scores.
Statistical analyses
The data are presented as the mean ± standard
deviation. A Student's t-test (two-tailed) was used to determine
the statistical significance of the differences between the groups.
P<0.05 was considered to indicate a statistically significant
difference. The intensity of SPARC expression in human breast
cancer samples was analyzed using the χ2 test.
Statistical analysis was performed using SPSS version 19.0 software
(IBM SPSS, Armonk, NY, USA).
Results
Association between stromal SPARC
expression and breast cancer bone metastasis
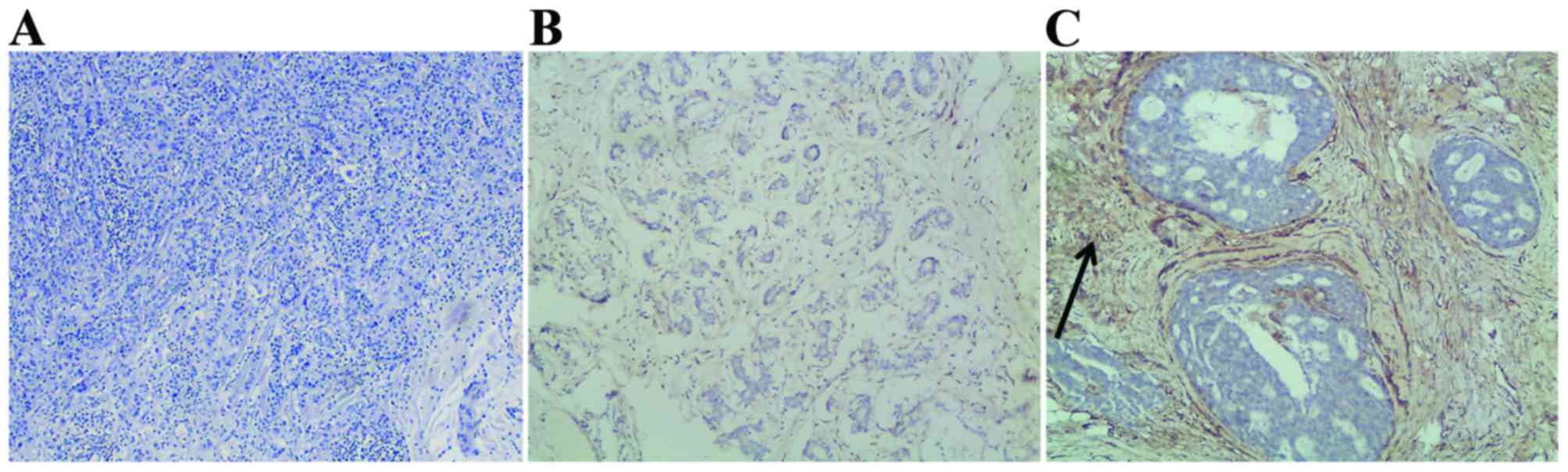
Stromal SPARC expression was evaluated in 50
patients with breast cancer using IHC. Representative images of
SPARC staining are shown in Fig. 1.
High stromal SPARC expression was associated with a decreased risk
of bone metastasis in patients with breast cancer. Myoepithelial
cells exhibited significantly strong stromal SPARC expression in 8%
(2/25) and 52% (13/25) of the patients, respectively (Table I). The association between stromal
SPARC expression and breast cancer bone metastasis was analyzed.
High levels of stromal SPARC expression were closely associated
with decreased levels of breast to bone metastasis (P=0.001).
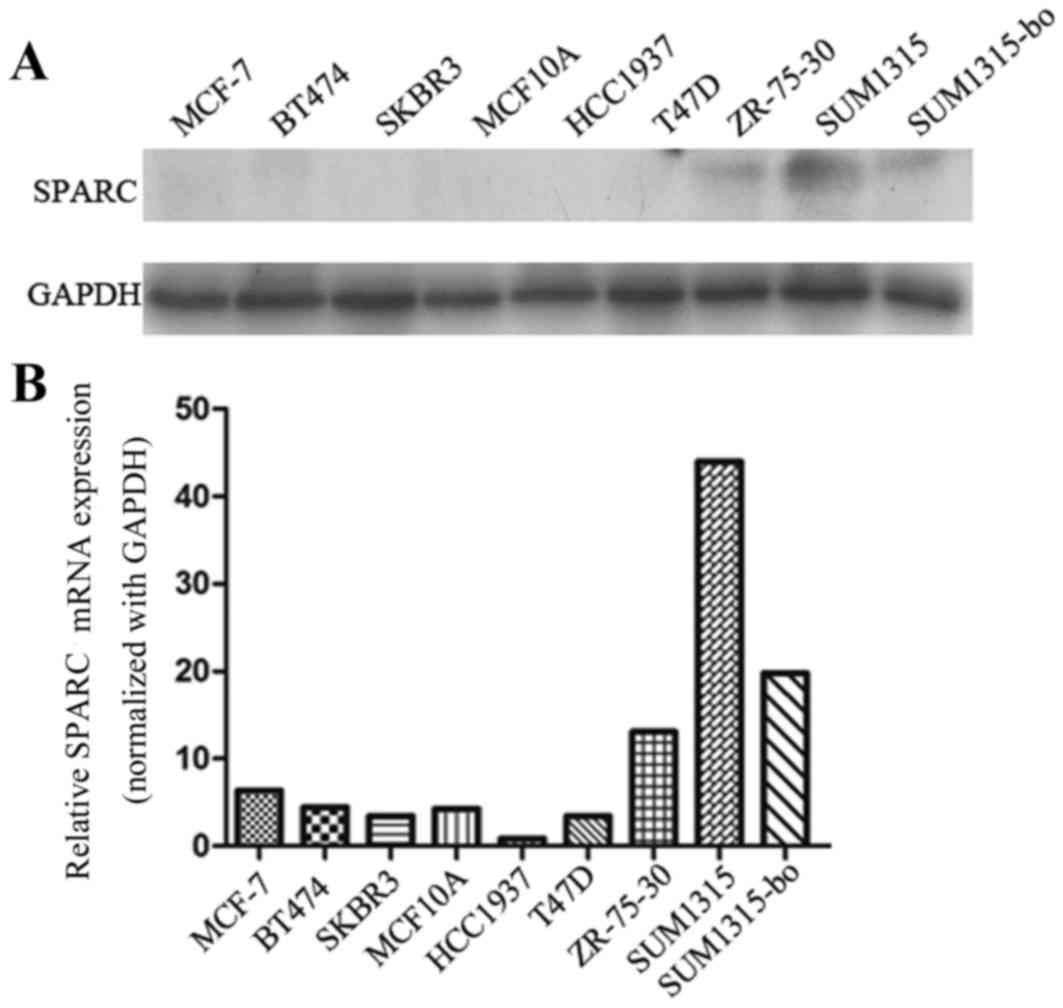
SPARC expression in a panel of breast
cancer cells
The SUM1315-bo and SUM1315 cells exhibited increased
SPARC protein levels compared with the MCF-7, BT474, SKBR3, MCF10A,
T47D, ZR-75-30 and HCC1937 cells. The present study revealed that
SUM1315-bo cells exhibited reduced SPARC expression compared with
SUM1315 cells (Fig. 2).
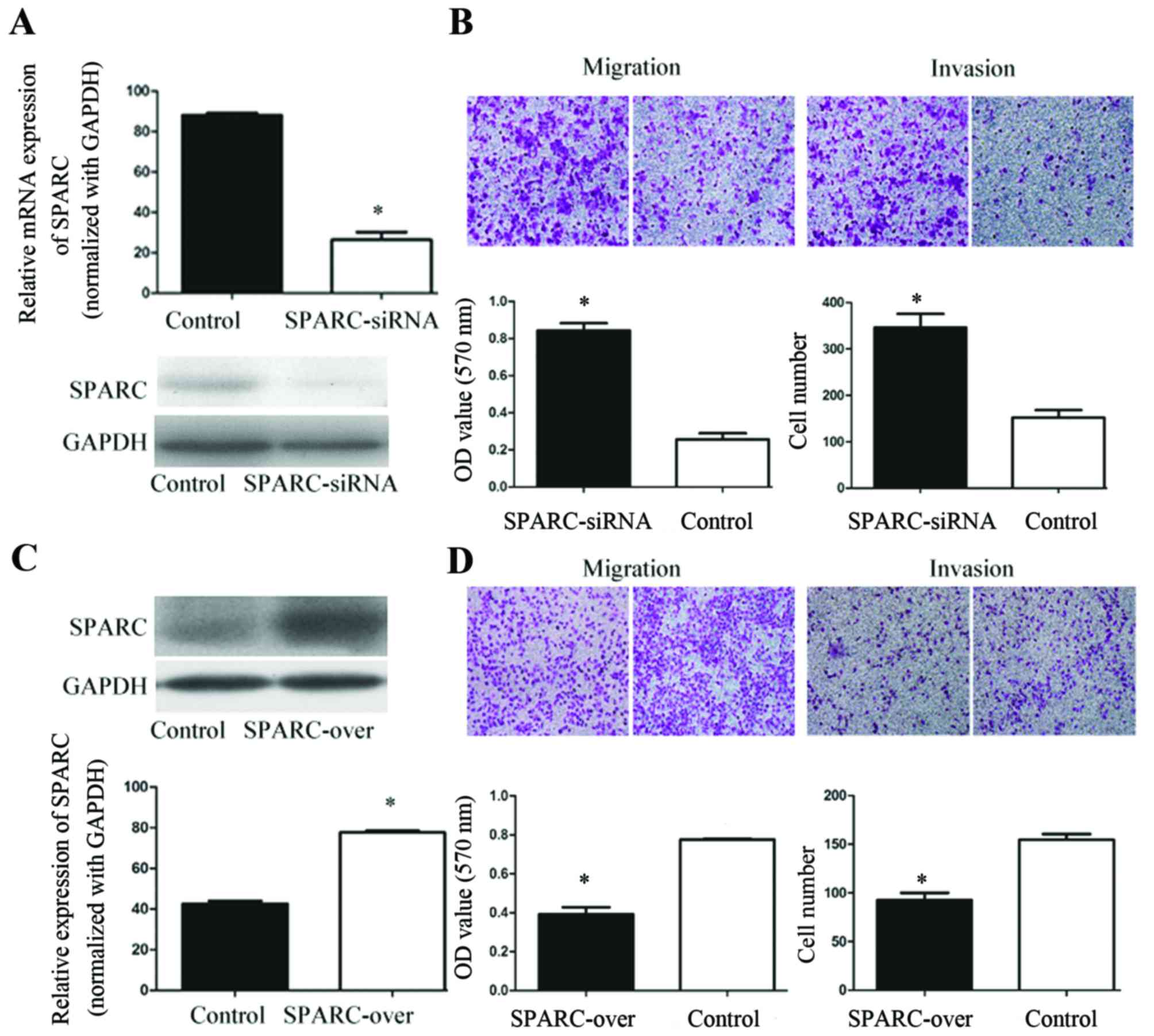
Inhibition of endogenous SPARC and
migration and invasion of SUM1315 cells
The role of SPARC in SUM1315 cell migration and
invasion was investigated by knocking down SPARC using siRNA. The
present study used SUM1315 cells as they exhibit a high migratory
potential and express endogenous SPARC at high levels. A total of
three siRNA-coding oligos against human SPARC were designed and
compared. The most effective SPARC siRNA construct exhibited a
target sequence of GCGGGUGAAGAAGAUCCAUGAGAAU (si-S3). This sequence
was verified at the mRNA and protein levels (Fig. 3A). Notably, SPARC silencing was
associated with significantly increased invasion and migration in
SUM1315 cells (Fig. 3B).
Overexpression of SPARC and invasion
and migration of SUM1315-bo cells
The present study transfected SUM1315-bo cells with
the constructed expression vector pGFP-LV5-SPARC to determine
whether SPARC overexpression decreased the level of cell migration
and invasion. Using western blot analysis, it was revealed that
SPARC levels increased 2.12-fold subsequent to transfection
(Fig. 3C). The effects of SPARC on
the migratory and invasive behavior of SUM1315-bo cells were
analyzed. The results identified a 2.13-fold decrease in cell
motility and a 2.32-fold decrease in cell invasiveness subsequent
to transfection of the constructed expression vector pGFP-LV5-SPARC
(Fig. 3D). These results indicated
that SPARC overexpression inhibited migration and invasion of
SUM1315-bo cells in vitro.
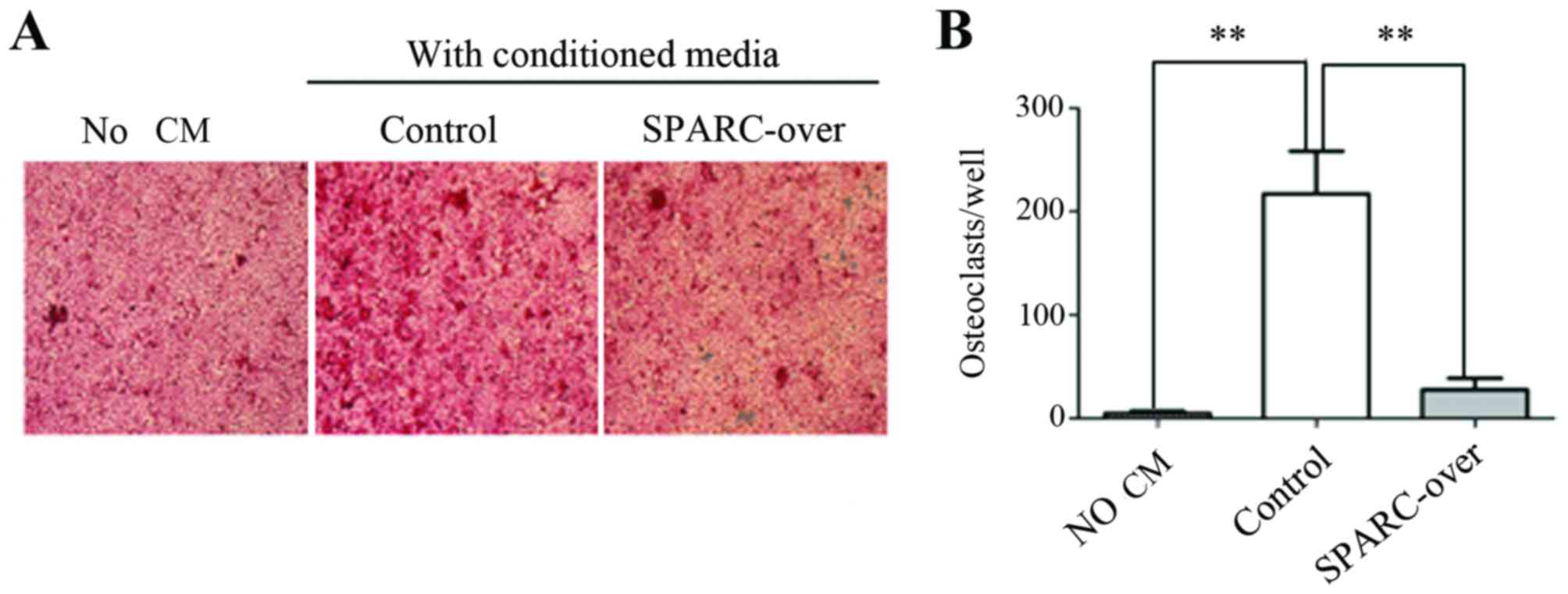
SPARC inhibits tumor-stimulated
osteoclast activation in SUM1315-bo cancer cells
The present study investigated the role of SPARC in
tumor-stimulated osteoclast activation. HMSC-derived osteoblasts
were treated with CM from the control and SPARC-overexpression
sublines of SUM1315-bo cells in an in vitro
osteoclastogenesis assay, and TRAP-positive multinucleated mature
osteoclasts were scored (Fig. 4).
HMSC-derived osteoblasts were cultured with CM from tumor cells and
exhibited numerous TRAP-positive cells. The CM of the SUM1315-bo
cell line induced extensive osteoclast differentiation. However,
the osteoclast-activating ability of the CM was significantly
decreased when SPARC expression was overexpressed.
Discussion
The present study aimed to elucidate the function of
SPARC in breast cancer bone metastasis. The overexpression of SPARC
stroma expression was revealed to be associated with a decreased
risk of bone metastasis in patients with breast cancer.
SPARC, also termed osteonectin, is a 32-kDa secreted
glycoprotein that interacts with extracellular matrix (ECM)
proteins to promote the disassociation of cells from the matrix,
thereby promoting cell motility. SPARC serves an important role in
wound healing, embryonic development and tumorigenesis (15). In addition to interacting with ECM
components, SPARC interacts with growth factors, including vascular
endothelial growth factor and fibroblast growth factor.
Increased SPARC expression has been identified in
multiple types of tumor and is associated with poor prognosis
(12,16,17). In
breast carcinoma, SPARC has been identified as a member of a
cluster of genes associated with increased invasive capacity
(18). In addition, the mRNA levels
of SPARC inversely correlate with estrogen receptor status,
indicating that SPARC expression is associated with more aggressive
types of breast cancer (12). The
role of SPARC in tumorigenesis is complex as it is expressed in
epithelial and stromal compartments. Notably, epithelial SPARC
expression does not confer a poorer prognosis in lung and
pancreatic cancer, whereas stromal SPARC expression has been
associated with poor clinical outcomes independent of common
clinicopathological parameters (16,19). The
mechanism by which stromal SPARC expression confers a poor
prognosis is not known. A potential mechanism by which SPARC
promotes tumorigenesis is through the stimulation of angiogenesis.
SPARC was initially identified as a protein secreted by endothelial
cells in vitro (20).
Increased SPARC expression was detected in newly formed vessels in
malignant melanoma xenografts and during neovascularization of
aortic stenosis (21,22). SPARC has also been demonstrated to
mediate several stages of the epithelial-to-mesenchymal transition,
and its expression is a feature of metaplastic breast carcinoma
(23).
Both gain- and loss-of-function studies have
revealed that SPARC inhibits breast cancer cell migration and
invasion. Furthermore, SPARC inhibits the osteoclast-activating
ability of the CM in SUM1315 cancer cells. Therefore, chemotherapy
agents targeting SPARC may be suitable to reverse the potential of
breast cancer cells for bone metastasis.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81172501).
References
|
1
|
DeSantis C, Siegel R, Bandi P and Jemal A:
Breast cancer statistics, 2011. CA Cancer J Clin. 61:409–418. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Solomayer EF, Diel IJ, Meyberg GC, Gollan
C and Bastert G: Metastatic breast cancer: Clinical course,
prognosis and therapy related to the first site of metastasis.
Breast Cancer Res Treat. 59:271–278. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yan Q and Sage EH: SPARC, a matricellular
glycoprotein with important biological functions. J Histochem
Cytochem. 47:1495–1506. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bellahcène A and Castronovo V: Increased
expression of osteonectin and osteopontin, two bone matrix
proteins, in human breast cancer. Am J Pathol. 146:95–100.
1995.PubMed/NCBI
|
|
5
|
Jacob K, Webber M, Benayahu D and Kleinman
HK: Osteonectin promotes prostate cancer cell migration and
invasion: A possible mechanism for metastasis to bone. Cancer Res.
59:4453–4457. 1999.PubMed/NCBI
|
|
6
|
De S, Chen J, Narizhneva NV, Heston W,
Brainard J, Sage EH and Byzova TV: Molecular pathway for cancer
metastasis to bone. J Biol Chem. 278:39044–39050. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Helleman J, Jansen MP, Ruigrok-Ritstier K,
van Staveren IL, Look MP, Meijer-van Gelder ME, Sieuwerts AM, Klijn
JG, Sleijfer S, Foekens JA and Berns EM: Association of an
extracellular matrix gene cluster with breast cancer prognosis and
endocrine therapy response. Clin Cancer Res. 14:5555–5564. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hsiao YH, Lien HC, Hwa HL, Kuo WH, Chang
KJ and Hsieh FJ: SPARC (osteonectin) in breast tumors of different
histologic types and its role in the outcome of invasive ductal
carcinoma. Breast J. 16:305–308. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Koblinski JE, Kaplan-Singer BR, VanOsdol
SJ, Wu M, Engbring JA, Wang S, Goldsmith CM, Piper JT, Vostal JG,
Harms JF, et al: Endogenous osteonectin/SPARC/BM-40 expression
inhibits MDA-MB-231 breast cancer cell metastasis. Cancer Res.
65:7370–7377. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ni X, Xia T, Zhao Y, Zhou W, Wu N, Liu X,
Ding Q, Zha X, Sha J and Wang S: Downregulation of miR-106b induced
breast cancer cell invasion and motility in association with
overexpression of matrix metalloproteinase 2. Cancer Sci.
105:18–25. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
van Nijnatten TJA, Moossdorff M, de Munck
L, Goorts B, Vane MLG, Keymeulen KBMI, Beets-Tan RGH, Lobbes MBI
and Smidt ML: TNM classification and the need for revision of pN3a
breast cancer. Eur J Cancer. 79:23–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Watkins G, Douglas-Jones A, Bryce R,
Mansel RE and Jiang WG: Increased levels of SPARC (osteonectin) in
human breast cancer tissues and its association with clinical
outcomes. Prostaglandins Leukot Essent Fatty Acids. 72:267–272.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Marshall MS: Ras target proteins in
eukaryotic cells. FASEB J. 9:1311–1318. 1995.PubMed/NCBI
|
|
15
|
Funk SE and Sage EH: The Ca2(+)-binding
glycoprotein SPARC modulates cell cycle progression in bovine
aortic endothelial cells. Proc Natl Acad Sci USA. 88:2648–2652.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Infante JR, Matsubayashi H, Sato N,
Tonascia J, Klein AP, Riall TA, Yeo C, Iacobuzio-Donahue C and
Goggins M: Peritumoral fibroblast SPARC expression and patient
outcome with resectable pancreatic adenocarcinoma. J Clin Oncol.
25:319–325. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yamanaka M, Kanda K, Li NC, Fukumori T,
Oka N, Kanayama HO and Kagawa S: Analysis of the gene expression of
SPARC and its prognostic value for bladder cancer. J Urol.
166:2495–2499. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Iacobuzio-Donahue CA, Argani P, Hempen PM,
Jones J and Kern SE: The desmoplastic response to infiltrating
breast carcinoma: Gene expression at the site of primary invasion
and implications for comparisons between tumor types. Cancer Res.
62:5351–5357. 2002.PubMed/NCBI
|
|
19
|
Koukourakis MI, Giatromanolaki A, Brekken
RA, Sivridis E, Gatter KC, Harris AL and Sage EH: Enhanced
expression of SPARC/osteonectin in the tumor-associated stroma of
non-small cell lung cancer is correlated with markers of
hypoxia/acidity and with poor prognosis of patients. Cancer Res.
63:5376–5380. 2003.PubMed/NCBI
|
|
20
|
Sage H, Johnson C and Bornstein P:
Characterization of a novel serum albumin-binding glycoprotein
secreted by endothelial cells in culture. J Biol Chem.
259:3993–4007. 1984.PubMed/NCBI
|
|
21
|
Prada F, Benedetti LG, Bravo AI, Alvarez
MJ, Carbone C and Podhajcer OL: SPARC endogenous level, rather than
fibroblast-produced SPARC or stroma reorganization induced by
SPARC, is responsible for melanoma cell growth. J Invest Dermatol.
127:2618–2628. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Charest A, Pépin A, Shetty R, Côté C,
Voisine P, Dagenais F, Pibarot P and Mathieu P: Distribution of
SPARC during neovascularisation of degenerative aortic stenosis.
Heart. 92:1844–1849. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lien HC, Hsiao YH, Lin YS, Yao YT, Juan
HF, Kuo WH, Hung MC, Chang KJ and Hsieh FJ: Molecular signatures of
metaplastic carcinoma of the breast by large-scale transcriptional
profiling: Identification of genes potentially related to
epithelial-mesenchymal transition. Oncogene. 26:7859–7871. 2007.
View Article : Google Scholar : PubMed/NCBI
|