Introduction
Breast cancer is the most common malignancy in women
and a leading cause of cancer-associated mortality globally
(1). Furthermore, the high prevalence
of women diagnosed at an advanced stage of disease, leads to a poor
prognosis (1). Therefore, it is of
crucial importance to identify biomarkers associated with
predicting prognosis and the progression of breast cancer.
MicroRNAs (miRNAs/miRs) are a multifunctional class of small
non-coding RNAs that regulate mRNA expression at the
post-transcriptional level, by inhibiting protein translation or
promoting mRNA degradation (2). An
accumulation of evidence suggests that multiple miRNAs are
frequently aberrantly expressed in and associated with various
processes and phenotypes observed in the initiation and progression
of breast cancer.
Previous studies have demonstrated that miR-24-3p
was upregulated in breast cancer tissues compared with paired
adjacent normal breast tissues (3).
Overexpression of miR-24-3p promoted cell proliferation and
inhibited apoptosis in human breast cancer cells (3). The overexpression of miR-449a observed
in malignant breast tissues, is significantly associated with an
increased incidence of patient relapse, a decreased overall
survival and disease-free survival (4). Elevated miR-449a expression levels
promoted breast cancer cell proliferation, clonogenic survival,
migration, and invasion in vitro, indicating that miR-449a
promotes the progression of breast cancer (4). miR-30e was reported to be downregulated
in plasma and breast cancer tissues, and reduced miR-30e expression
was correlated with the clinical stage of breast cancer (5). Another study reported a marked reduction
in miR-139-5p in breast cancer cells, the effects of which
inhibited cell viability, induced apoptosis, caused cell cycle
arrest in the S phase, and suppressed the migration and invasion of
breast cancer cells (6). Furthermore,
miR-139-5p mediated drug resistance by regulating the expression of
the downstream target gene Notch1 (6). miR-503 expression was markedly
downregulated in breast cancer tissues and cells (7). Overexpression of miR-503 in breast
cancer cell lines reduced cell proliferation through inducing G0/G1
cell cycle arrest (7).
Previous studies have demonstrated that miR-10a
serves contradictory functions in breast cancer. For example,
increased expression of miR-10a in estrogen receptor-positive
tumors was associated with a longer relapse-free time following
tamoxifen treatment (8). Another
previous study revealed that miR-10a was downregulated in breast
tumors from patients with early recurrence, which resulted in an
overall increased proliferative and angiogenic capacity (9). Khan et al (10) demonstrated that the level of miR-10a
expression was significantly decreased in tissues harvested from
patients with breast cancer compared with normal and benign
tissues. However, a study by Chang et al (11) revealed that elevated miR-10a in
relapsed patients was significantly correlated with the hazard
ratio of breast cancer recurrence. Furthermore, a previous study
revealed that increased expression of miR-10a in MCF-7 human breast
adenocarcinoma cells was associated with an inbuilt resistance to
cisplatin (12).
The aim of the present study was to initially
examine the expression of miR-10a in two human breast cancer cell
lines and normal human mammary epithelial cells. Furthermore, the
effects of miR-10a on cell proliferation, migration, and apoptosis
were assayed. In addition, the mechanisms underlying the biological
effects of miR-10a were investigated.
Materials and methods
Cell culture
The human breast cancer cell lines MDA-MB-231 and
MCF-7, and the normal human mammary cell line MCF-10A, were
obtained from the American Type Culture Collection (ATCC; Manassas,
VA, USA). Cells were cultured in Dulbecco's modified Eagle's medium
(DMEM; Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA,
USA), supplemented with 10% fetal bovine serum (FBS; Hyclone; GE
Healthcare Life Sciences, Logan, UT, USA), penicillin (100 U/ml)
and streptomycin (100 µg/ml). Cells were grown at 37°C in a
humidified chamber at 5% CO2 in air.
Cell transfection with miR-10a and
miR-10a inhibitor
miR-10a mimic, miR-10a inhibitor (anti-miR-10a),
miRNA mimic negative control (miR-NC), and miRNA inhibitor negative
control (anti-NC) were designed and synthesized by Shanghai
GenePharma, Ltd. (Shanghai, China). The sequences were as follows:
miR-10a mimic sense, 5′-CAAAUUCGGAUCUACAGGGUAUU-3′ and anti-sense,
5′-UACCCUGUAGAUCCGAAUUUGUG-3′; miR-NC sense,
5′-UUCUCCGAACGUGUCACGUTT-3′ and anti-sense,
5′-UACCCUGUAGAUCCGAAUUUGUG-3′; anti-miR-10a,
5′-CACAAAUUCGGAUCUACAGGGUA-3′; anti-NC,
5′-CAGUACUUUUGUGUAGUACAA-3′. Cells were cultured to ~60–70%
confluency, following which Lipofectamine® 2000 RNAiMAX
reagent (Thermo Fisher Scientific, Inc.) was used to transfect the
MDA-MB-231 and MCF-7 cells with miR-10a mimic, miR-NC, anti-miR-10a
or anti-NC (all 100 nM) for 48 h at 37°C according to the
manufacturer's protocol.
mTOR inhibitor treatment
MCF-7 cells were seeded in 12-well plates at a
density of 1×105 cells/ml and cultured overnight, prior
to pretreatment with an mTOR inhibitor CCI-779 (10 µM or 20 µM,
Selleck Chemicals, Houston, TX, USA) for 12 h, followed by
transfection with anti-miR-10a or anti-NC as aforementioned.
Cell proliferation assay
Confluent MDA-MB-231 and MCF-7 cells transfected
with miR-10a mimic, miR-NC, anti-miR-10a, or anti-NC as
aforementioned were seeded in 96-well plates at a density of
2.5×103 cells/well. After 48 h culture, DMEM culture
medium was replaced with fresh medium supplemented with 100 µl MTT
(Sangon Biotech, Co., Ltd., Shanghai, China), followed by
incubation for an additional 4 h at 37°C. The formazan crystals
were dissolved with 150 µl DMSO for 10 min. The amount of formazan
formed was determined by measuring the absorbance at 550 nm with a
microplate reader (Thermo Fisher Scientific, Inc.).
Cell migration assay
Cell migration activity was detected using a
Transwell assay. Briefly, MDA-MB-231 and MCF-7 cells transfected
with 100 nM miR-10a mimic, miR-NC, anti-miR-10a or anti-NC were
added to the upper chamber of the Transwell assay in 200 µl of
serum-free DMEM without a Matrigel membrane. DMEM culture medium
supplemented with 10% FBS was added to the lower chamber. The
plates were incubated for 48 h at 37°C in 5% CO2, and
non-migrating cells in the upper chambers were removed with a
cotton-tipped swab. Cell migration was determined by counting the
number of migrated cells under a microscope, following fixation and
staining with crystal violet.
Flow cytometry
Apoptosis was determined in MDA-MB-231 and MCF-7
cells using a dual staining method with Annexin V-fluorescein
isothiocyanate (FITC) (BD Biosciences, Franklin Lakes, NJ, USA) and
7-amino-actinomycin (7-AAD; BD Biosciences). Briefly,
1×106 cells were harvested at 48 h by trypsin/EDTA
digestion following transfection with miR-10a mimic or miR-NC
mimic. Annexin V-FITC and 7-AAD were added to the cell suspension
according to the manufacturer's protocol. Cells were analyzed using
a FACSCalibur flow cytometer (Becton Dickinson; BD Biosciences)
using Cell-Quest Pro software (version 5.1; BD Biosciences,
Franklin Lakes, NJ, USA). Apoptosis was assessed by counting the
number of early and late apoptotic cells and determined by relative
apoptotic changes.
Reverse transcription quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from cells using the
RNAisoPlus reagent (Takara, Bio, Inc., Otsu, Japan) according to
the manufacturer's protocol. A total of 1 µg mRNA was reverse
transcribed to cDNA with oligo (dT) primers using TaqMan microRNA
Reverse Transcription kit (Applied Biosystems; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) according to the protocol of
the manufacturer. qPCR analysis was performed using SYBR-Green
(Takara Biotechnology, Co., Ltd, Dalian, China) and the ABI PRISM
7500 Sequence Detection System (Applied Biosystems; Thermo Fisher
Scientific, Inc.). The primer sequences for miR-10a and
Phosphatidylinositol-4,5-Bisphosphate 3-Kinase Catalytic Subunit α
(PIK3CA) were as follows: miR-10a forward
5′-GGATACCCTGTAGATCCGAA-3′ and reverse 5′-CAGTGCGTGTCGTGGAGT-3′;
PIK3CA forward, 5′-GCATACATTCGAAAGACC-3′ and reverse,
5′-CTCAGTTATCTTTTCAG-3′. The expression level of PIK3CA was
calculated using the 2−ΔΔCq method with normalization to
GAPDH (forward, 5′-CTTCACCACCATGGAGAAGGC-3′ and reverse,
5′-GGCATGGACTGTGGTCATGAG-3′) (13).
Western blot analysis
Cells were harvested for total protein extraction
and lysed with 100 µl LRIPA lysis buffer for 30 min on ice. Total
protein concentrations were analyzed using an Easy Protein
Quantitative kit (BCA; Transgen Biotech Co, Ltd, Beijing, China).
Total proteins (30 µg/lane) were separated using SDS-PAGE on a 10%
gel and transferred to nitrocellulose membranes. The membranes were
blocked using 5% non-fat milk for 2 h at room temperature and
incubated with the following antibodies overnight at 4°C:
Anti-PIK3CA (1:5,000; cat. no. ab40776; Abcam, Cambridge, MA, USA)
anti-p-Akt (1:5,000; cat. no. ab81283; Abcam), anti-p-mTOR
(1:1,000; cat. no. ab1093; Abcam), anti-p-ribosomal protein S6
kinase β-1 (p-p70S6K; 1:500; cat. no. ab5231; Abcam), anti-B-cell
lymphoma 2 (Bcl-2; 1:1,000; cat. no. 15071; Cell Signaling
Technology, Inc., Danvers, MA, USA), anti-BCL-2 associated-X,
apoptosis regulator (Bax; 1:1,000; cat. no. 2772; Cell Signaling
Technology, Inc.), anti-cleaved caspase-3 (1:1,000; cat. no. 9661;
Cell Signaling Technology, Inc.), and anti-cytochrome-C (Cyt-C;
1:1,000; cat. no. 4280; Cell Signaling Technology, Inc.). Following
washing with TBST, the membranes were incubated with goat
anti-rabbit IgG horseradish peroxidase-conjugated secondary
antibodies (1:5,000; cat. no. sc-2004; Santa Cruz Biotechnology,
Santa Cruz, CA, USA) for 1 h at room temperature and proteins were
visualized using an enhanced chemiluminescence reagent (EMD
Millipore, Billerica, MA, USA). Data were quantified by using Image
J software (version 1.48u; National Institutes of Health, Bethesda,
MD, USA).
Statistical analysis
Data were expressed as the mean ± standard
deviation. The statistical analyses were performed using one-way
analysis of variance followed by Least Significant Difference test
or unpaired Student's t test with SPSS software (version 13.0;
SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to indicate
a statistically significant difference.
Results
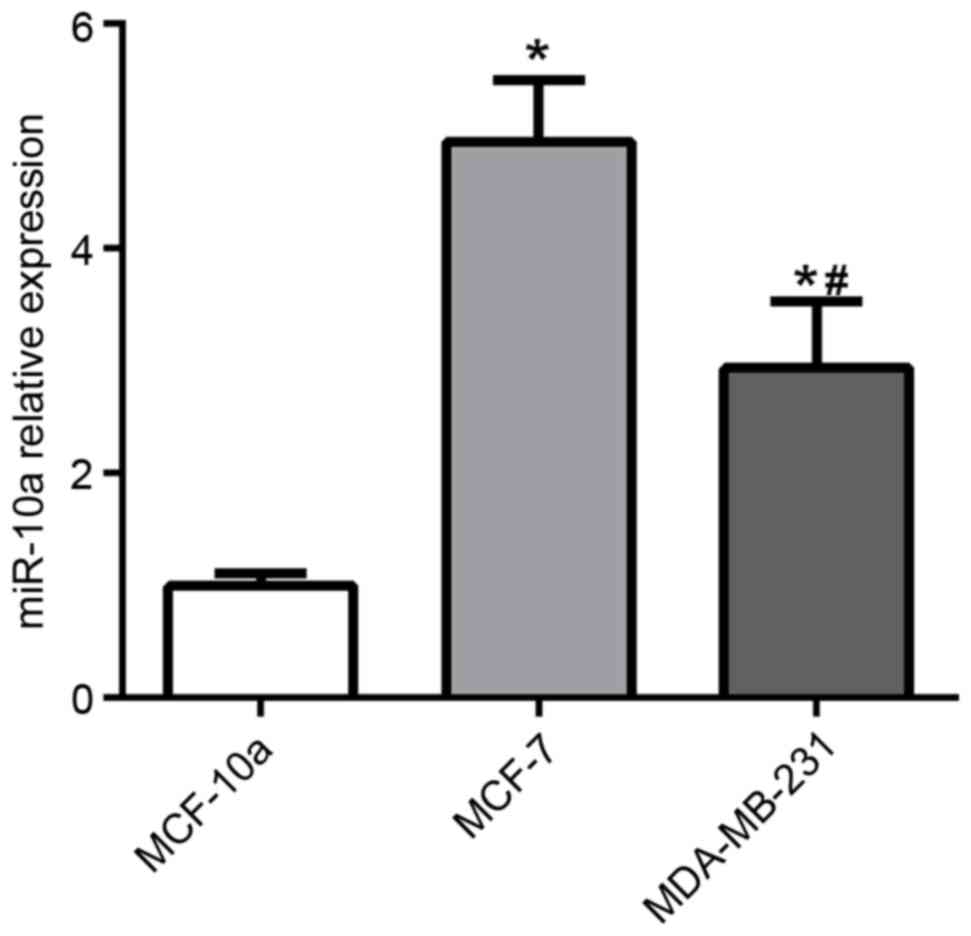
miR-10a is downregulated in high
aggressive breast cancer cell line
To determine the function of miR-10a in breast
cancer, miR-10a expression was examined in MCF-10A human mammary
cells, MCF-7, and MDA-MB-231 breast cancer cell lines. The
expression of miR-10a was significantly increased in MCF-7 and
MDA-MB-231 cells compared with MCF-10A cells, as presented in
Fig. 1. Furthermore, the level of
miR-10a expression was significantly lower in MDA-MB-231 cells
compared with MCF-7 cells.
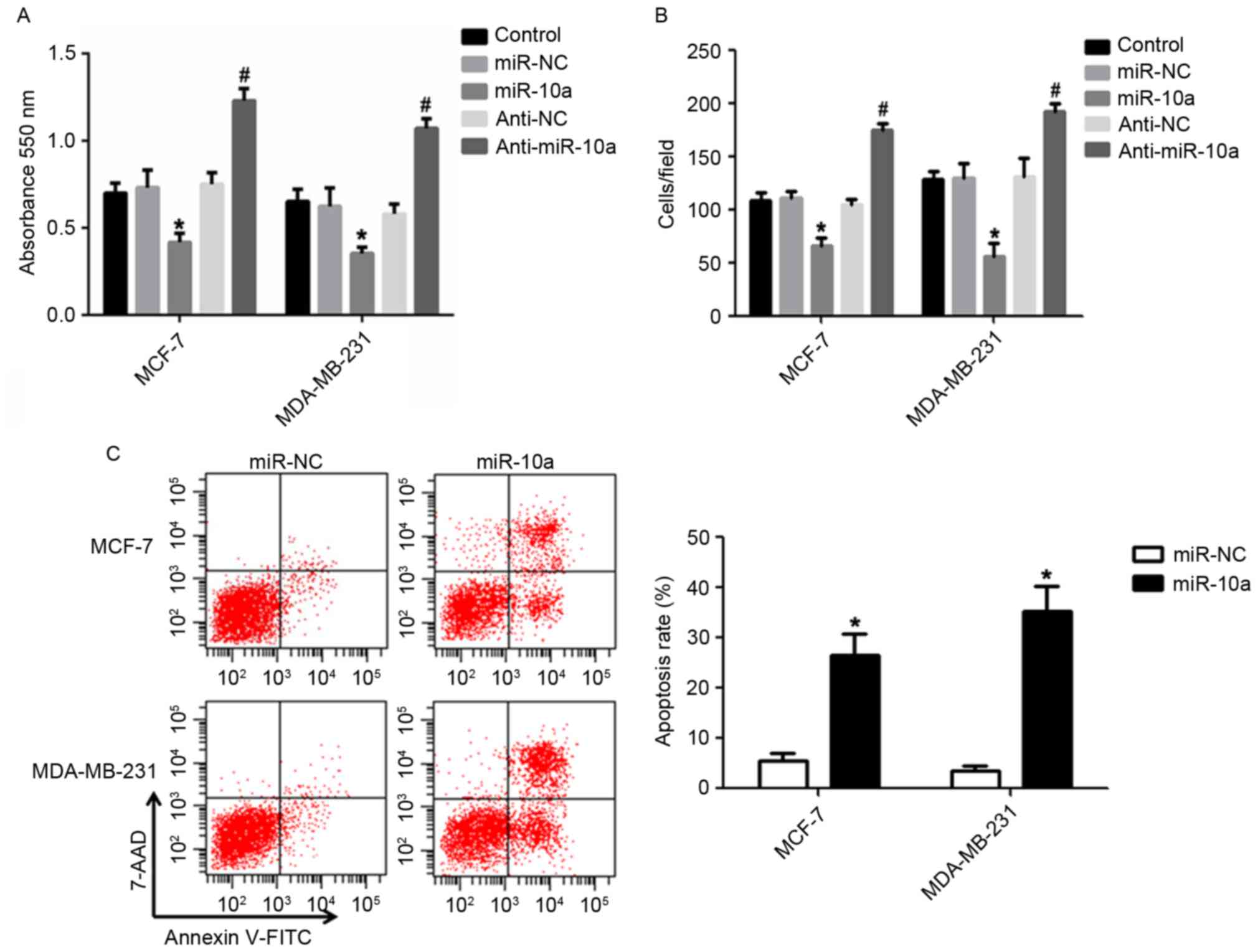
miR-10a inhibits breast cancer cell
proliferation and migration, and induces cell apoptosis
The results from the present study revealed that
miR-10a was significantly downregulated in MDA-MB-231 cells
compared with MCF-7 cells (Fig. 1),
which may indicate that miR-10a functions as a tumor suppressor.
Therefore, the effects of miR-10a on the proliferation, migration
and apoptosis of breast cancer cells via the
upregulation/downregulation of miR-10a were determined. The results
presented in Fig. 2A revealed that a
slower rate of cell proliferation was observed in cells transfected
with an miR-10a mimic compared with miR-NC mimic in breast cancer
cell lines. However, transfection of anti-miR-10a resulted in the
opposite effect. Furthermore, the miR-10a mimic significantly
dampened the migration of MCF-7 and MDA-MB-231 cells, and
anti-miR-10a enhanced cell migration compared with the control
(Fig. 2B). In addition, transfection
with the miR-10a mimic significantly enhanced the apoptosis of the
breast cancer cell lines compared with the control (Fig. 2C). The data presented indicated that
miR-10a is efficient at suppressing the proliferation and migration
and facilitating the apoptosis of breast cancer cells.
miR-10a regulates the PI3K/Akt/mTOR
pathway
Previous studies have demonstrated that the
PI3K/Akt/mTOR pathway is an important target in breast cancer
(14). To evaluate whether the
inhibitory effects of miR-10a on the proliferation and migration of
breast cancer cells and the enhanced apoptotic effects are
regulated via the PI3K/Akt/mTOR pathway, the expression of p-Akt,
p-mTOR, and p-p70S6K was determined in breast cancer cells.
Transfection with the miR-10a mimic significantly suppressed the
levels of p-Akt, p-mTOR, and p-p70S6K in MDA-MB-231 cells (Fig. 3A and B). In addition, anti-miR-10a
markedly increased the expression of p-Akt, p-mTOR, and p-p70S6K in
MCF-7 cells (Fig. 3A and C).
Additionally, the effects of pretreatment with an mTOR inhibitor
CCI-779 (0, 10 or 20 µM) for 24 h prior to transfection with
anti-miR-10a in MCF-7 cells, decreased the effect of anti-miR-10a
on the migration of MCF-7 cells in a dose-dependent manner
(Fig. 3D). These data indicated that
miR-10a functions via the PI3K/Akt/mTOR signaling pathway in breast
cancer cells.
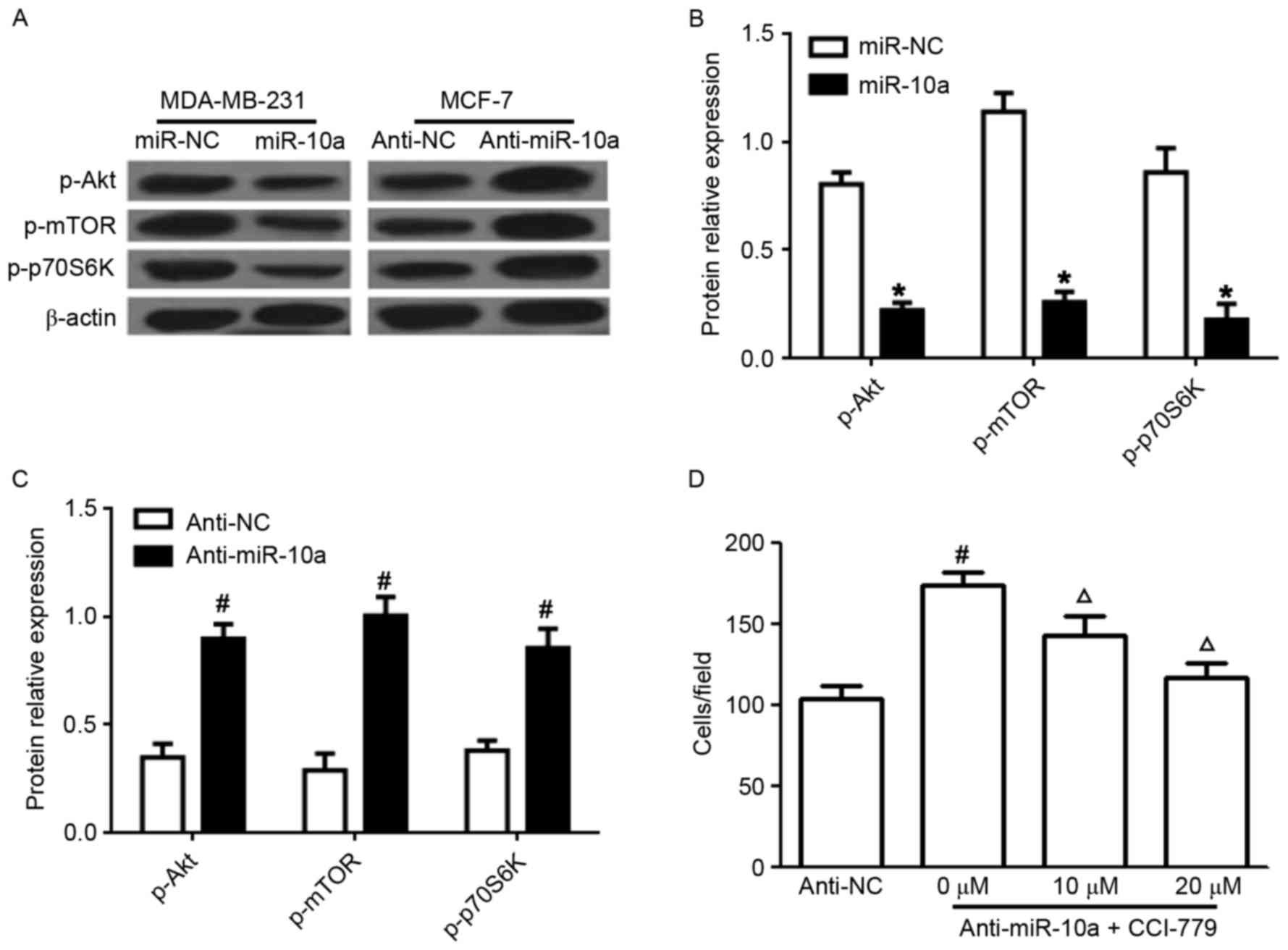 | Figure 3.Effects of miR-10a on PI3K/Akt/mTOR
signaling. (A) Western blot analysis for the protein expression of
p-Akt, p-mTOR, p-p70S6K in MDA-MB-231 and MCF-7 cells transfected
with miR-10a or anti-miR-10a. Statistical analysis of p-AKT, mTOR
and p-P-70S6K expression in cells transfected with (B) miR-10a
mimic and miR-NC and (C) anti-miR-10a and anti-NC. (D) Cell
migration of MCF-7 cells pretreated with an mTOR inhibitor CCI-779
(0, 10 or 20 µM) for 24 h followed by transfection with
anti-miR-10a, determined by Transwell analysis. Each experiment is
representative of three independent repeats. Each value represents
the mean ± standard deviation. *P<0.05 vs. miR-NC,
#P<0.05 vs. anti-NC, ΔP<0.05 vs.
anti-miR-10a. miR-10a, microRNA-10a; P13K,
phosphatidylinositol-3-kinase; Akt, protein kinase B; mTOR,
mammalian target of rapamycin; p70S6K, ribosomal protein S6 kinase
β-1; p-, phosphorylated. |
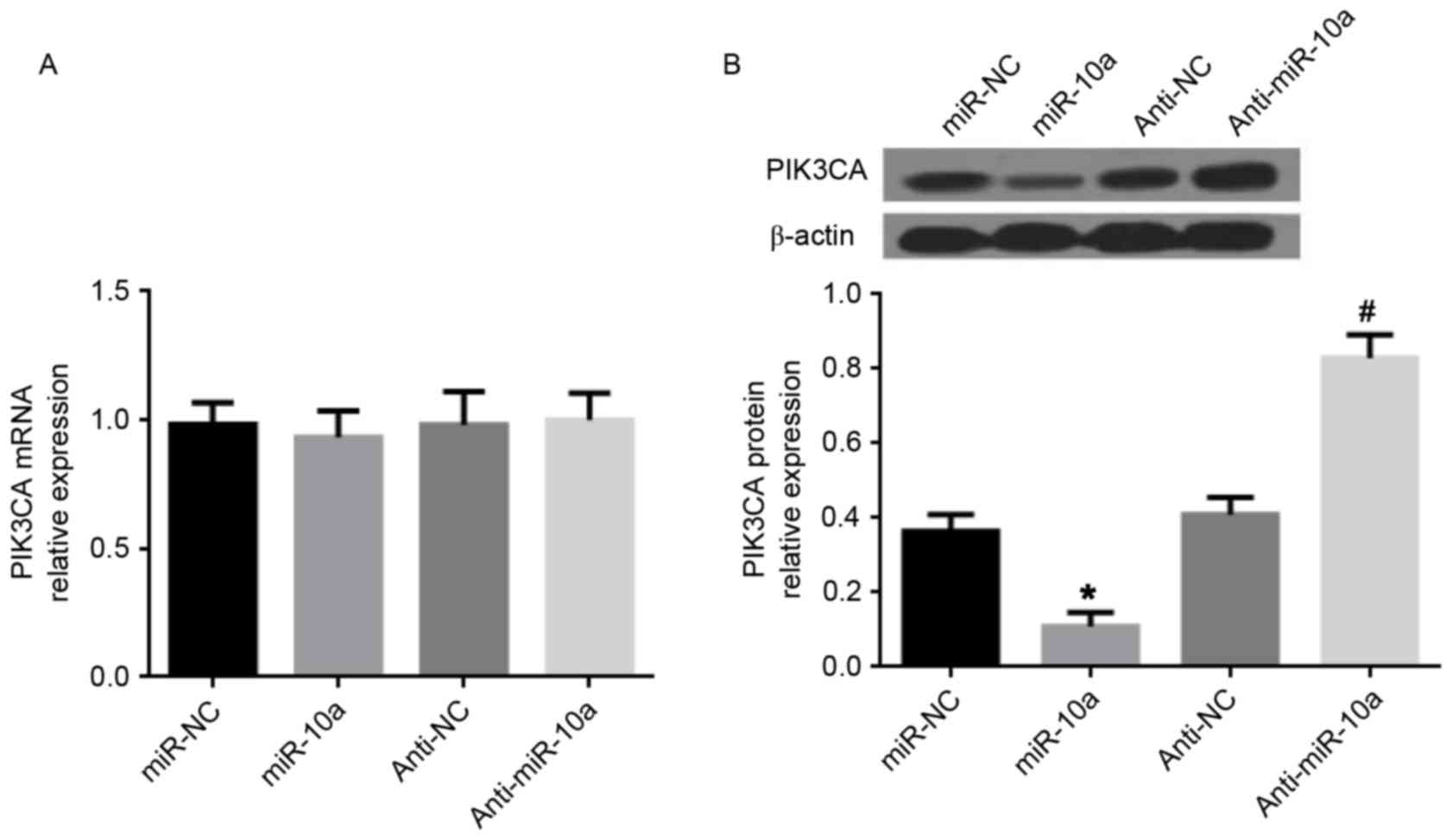
miR-10a decreases PIK3CA expression in
MDA-MB-231 cells
It has previously been demonstrated that
PIK3CA, the central component of the PI3K pathway, is a
direct target gene of miR-10a (15).
Therefore, PIK3CA expression was investigated following
transfection of the miR-10a mimic or anti-miR-10a in MDA-MB-231
cells. The data presented in Fig. 4
demonstrated that the miR-10a mimic did not affect PIK3CA
gene expression, but significantly reduced PIK3CA at the protein
level. Conversely, anti-miR-10a increased PIK3CA at the protein
level, whereas no effect was observed on PIK3CA gene
expression. Thus, it is suggested that miR-10a inhibits PIK3CA
expression at the posttranscriptional level to suppress
PI3K/Akt/mTOR signaling in breast cancer cells.
miR-10a induces the mitochondrial
apoptotic pathway
The PI3K/Akt/mTOR pathway is associated with the
mitochondrial mediated apoptosis in tumor cells including human
breast cancer cells (16,17). To further investigate the intrinsic
pathway of apoptosis, the protein expression of Cyt C, Bcl-2, Bax,
and cleaved caspase-3 was determined in MCF-7 cells following
transfection of miR-10a mimic. Transfection with the miR-10a mimic
significantly increased the expression of Cyt C and cleaved
caspase-3 (Fig. 5A and B).
Furthermore, the ratio of Bax/Bcl-2 was significantly increased
following overexpression of the miR-10a, compared with miR-NC mimic
(Fig. 5C).
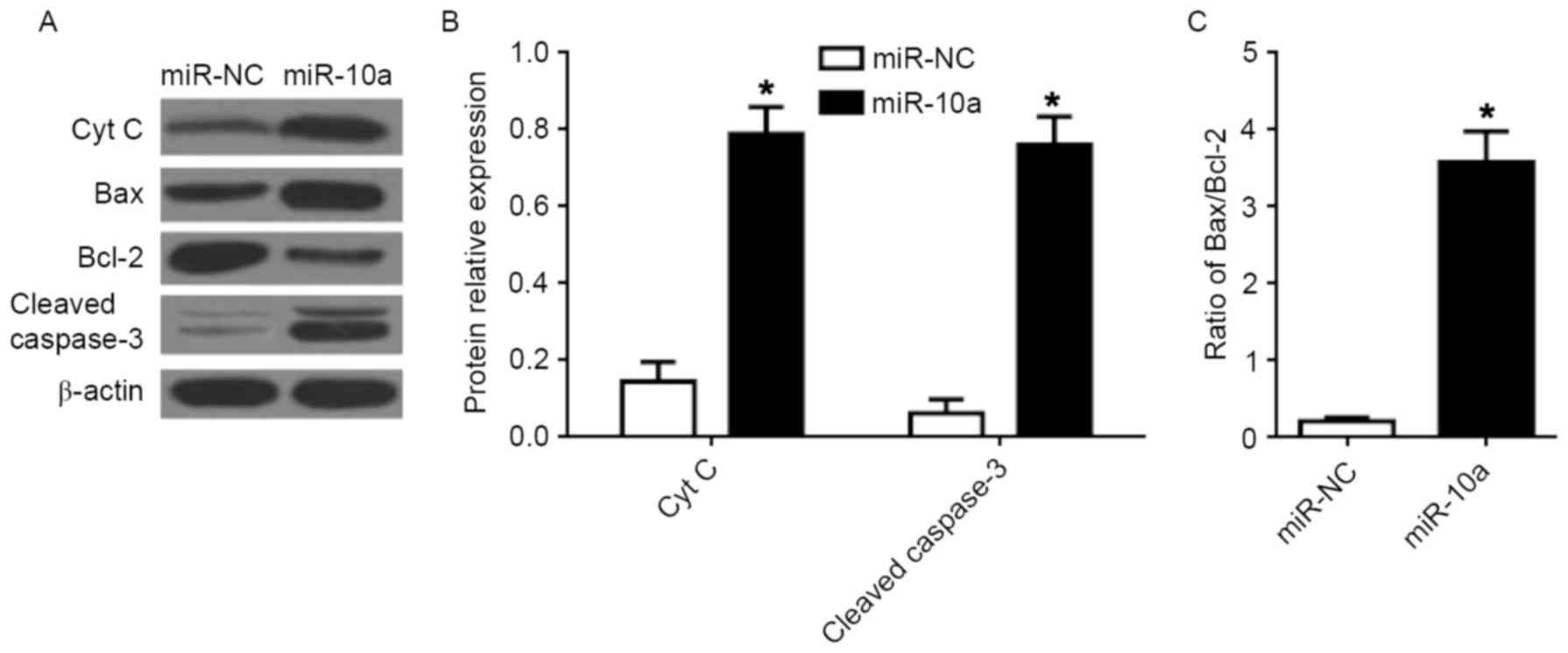 | Figure 5.Effects of miR-10a on the
mitochondrial apoptotic pathway in breast cancer cells. (A) Western
blot analysis of Cyt C, Bax, Bcl-2, and cleaved caspase-3 protein
expression in MCF-7 cells. Quantification of the protein levels of
(B) Cyt C and cleaved caspase-3, and (C) the ratio of Bax/Bcl-2.
Each value represents the mean ± standard deviation, *P<0.05 vs.
miR-NC. miR, microRNA; Bax, BCL-2 associated X, apoptosis
regulator; Bcl-2, B-cell lymphoma 2; Cyt C, cytochrome C; NC,
negative control. |
Discussion
The present study aimed to investigate the
involvement of miR-10a in the pathogenesis of breast cancer.
Previous studies have demonstrated that the expression of miR-10a
is contradictory in breast cancer (10,11). In
the present study, the expression of miR-10a was initially detected
in MCF-7 and MDA-MB-231 breast cancer cells and MCF-10A normal
mammary cells. The data presented demonstrated that the expression
of miR-10a was increased in breast cancer cell lines. Notably, the
level of miR-10a expression was lower in the more aggressive breast
cancer cell line MDA-MB-231 compared with the less aggressive MCF-7
cells (Fig. 1). The loss of miR-10a
expression in gastric cancer highlighted a potential tumor
suppressor function for miR-10a (18). Thus, we speculated that miR-10a may
function as a tumor suppressor in breast cancer, and miR-10a
expression levels may be considered as a predictor for the
progression of breast cancer.
To evaluate the exact function of miR-10a in breast
cancer, the induced upregulation and downregulation of miR-10a
expression were studies in breast cancer cells. Transfection of
miR-10a markedly inhibited proliferation and migration, whilst
promoting the apoptosis of breast cancer cells. Conversely,
transfection with anti-miR-10a induced the opposite effects on cell
proliferation and migration (Fig. 2).
The PI3K/Akt/mTOR signaling pathway serves a key regulatory
function in cell survival, proliferation, migration, metabolism,
and apoptosis, and its aberrant activation has been observed in
multiple types of cancer, including breast cancer (16). p70S6K is the downstream effector of
mTOR. The results of the present study demonstrated that p-Akt,
p-mTOR, and p-p70S6K expression was significantly decreased
following overexpression of miR-10a in MDA-MB-231 cells. The role
of miR-10a on PI3K/Akt/mTOR signalling subsequent to downregulation
of miR-10a in MCF-7 cells was also confirmed, and the results
indicated that the levels of p-Akt, p-mTOR, and p-p70S6K were
enhanced by anti-miR-10a expression in MCF-7 cells. In addition, an
mTOR inhibitor, CCI-779, reversed the effect of anti-miR-10a on the
migration of MCF-7 cells in a dose-dependent manner (Fig. 3). The results from the present study
indicated that miR-10a suppresses proliferation and migration in,
yet promotes the apoptosis of breast cancer cells via impeding
PI3K/Akt/mTOR signaling.
PIK3CA is a fundamental molecule of the
PI3K/Akt/mTOR pathway. Enhanced activation of PIK3CA has been
observed in a high proportion of several types of human tumors, and
is associated with mammary tumorigenesis and angiogenesis (19). In addition, PIK3CA is relevant in
terms of resistance to endocrine or anti-HER2 therapies (20,21).
Previous studies have demonstrated that miR-10a directly suppresses
the expression of PIK3CA by targeting the 3y-untranslated region of
the gene in airway smooth muscle cells to control cell
proliferation (15). To confirm
whether miR-10a functions through targeting PIK3CA, the gene and
protein expression of PIK3CA following transfection with miR-10a
mimic or anti-miR-10a in MDA-MB-231 cells was determined. The
results of the present study revealed that miR-10a markedly
decreased the protein levels of PIK3CA, however no detectable
changes were observed at the mRNA level, compared with the control
mimic. Notably, the protein expression of PIK3CA was upregulated in
response to anti-miR-10a, but no changes were observed at the mRNA
level (Fig. 4). The results from the
present study indicated that miR-10a targets PIK3CA expression to
inhibit the PI3K/Akt/mTOR pathway in breast cancer cells.
To further investigate the mechanism of miR-10a on
the apoptosis of breast cancer cells, the activity of the
mitochondrial apoptotic pathway in MCF-7 cells. Mitochondria are
known to play a central role in cell apoptosis. The mitochondrial
mediated apoptotic pathway is upstream of caspase activation and is
regulated by the Bcl-2 family of proteins. Any imbalance in the
expression of Bcl-2 and Bax protein will disrupt the outer membrane
of mitochondria (22). Previous
studies have demonstrated that the increase of Bax/Bcl-2 modulates
mitochondrial permeability, releasing Cyt C from mitochondria
(23). In the present study,
transfection with the miR-10a mimic significantly increased the
expression of Cyt C and cleaved caspase-3, and enhanced the
Bax/Bcl-2 ratio. Taken together, the results of the present study
indicated that miR-10 regulates breast cancer cell apoptosis via
the mitochondrial apoptotic pathway.
In conclusion, to the best of our knowledge, the
present study is the first to report that miR-10a is downregulated
in an aggressive breast cancer cell line, which indicates that
miR-10a expression may be a potential predictor of the development
of breast cancer. Furthermore, miR-10a suppresses proliferation and
migration in, and promotes apoptosis of, breast cancer cells
through targeting the PI3K/Akt/mTOR pathway and promoting the
mitochondrial mediated apoptotic pathway; which indicates that
miR-10a functions as a suppressor in the progression of breast
cancer.
References
|
1
|
Lauby-Secretan B, Scoccianti C, Loomis D,
Benbrahim-Tallaa L, Bouvard V, Bianchini F and Straif K:
International Agency for Research on Cancer Handbook Working Group:
Breast-cancer screening-viewpoint of the IARC Working Group. N Engl
J Med. 372:2353–2358. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lu K, Wang J, Song Y, Zhao S, Liu H, Tang
D, Pan B, Zhao H and Zhang Q: miRNA-24-3p promotes cell
proliferation and inhibits apoptosis in human breast cancer by
targeting p27Kip1. Oncol Rep. 34:995–1002. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shi W, Bruce J, Lee M, Yue S, Rowe M,
Pintilie M, Kogo R, Bissey PA, Fyles A, Yip KW and Liu FF: MiR-449a
promotes breast cancer progression by targeting CRIP2. Oncotarget.
7:18906–18918. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lin Z, Li JW, Wang Y, Chen T, Ren N, Yang
L, Xu W, He H, Jiang Y, Chen X, et al: Abnormal miRNA-30e
Expression is Associated with Breast Cancer Progression. Clin Lab.
62:121–128. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang HD, Sun DW, Mao L, Zhang J, Jiang
LH, Li J, Wu Y, Ji H, Chen W, Wang J, et al: MiR-139-5p inhibits
the biological function of breast cancer cells by targeting Notch1
and mediates chemosensitivity to docetaxel. Biochem Biophys Res
Commun. 465:702–713. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Long J, Ou C, Xia H, Zhu Y and Liu D:
MiR-503 inhibited cell proliferation of human breast cancer cells
by suppressing CCND1 expression. Tumour Biol. 36:8697–8702. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hoppe R, Achinger-Kawecka J, Winter S,
Fritz P, Lo WY, Schroth W and Brauch H: Increased expression of
miR-126 and miR-10a predict prolonged relapse-free time of primary
oestrogen receptor-positive breast cancer following tamoxifen
treatment. Eur J Cancer. 49:3598–3608. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pérez-Rivas LG, Jerez JM, Carmona R, de
Luque V, Vicioso L, Claros MG, Viguera E, Pajares B, Sánchez A,
Ribelles N, et al: A microRNA signature associated with early
recurrence in breast cancer. PLoS One. 9:e918842014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Khan S, Wall D, Curran C, Newell J, Kerin
MJ and Dwyer RM: MicroRNA-10a is reduced in breast cancer and
regulated in part through retinoic acid. BMC Cancer. 15:3452015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chang CH, Fan TC, Yu JC, Liao GS, Lin YC,
Shih AC, Li WH and Yu AL: The prognostic significance of RUNX2 and
miR-10a/10b and their inter-relationship in breast cancer. J Transl
Med. 12:2572014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pogribny IP, Filkowski JN, Tryndyak VP,
Golubov A, Shpyleva SI and Kovalchuk O: Alterations of microRNAs
and their targets are associated with acquired resistance of MCF-7
breast cancer cells to cisplatin. Int J Cancer. 127:1785–1794.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Guo Y, Chang H, Li J, Xu XY, Shen L, Yu ZB
and Liu WC: Thymosin alpha 1 suppresses proliferation and induces
apoptosis in breast cancer cells through PTEN-mediated inhibition
of PI3K/Akt/mTOR signaling pathway. Apoptosis. 20:1109–1121. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hu R, Pan W, Fedulov AV, Jester W, Jones
MR, Weiss ST, Panettieri RA Jr, Tantisira K and Lu Q: MicroRNA-10a
controls airway smooth muscle cell proliferation via direct
targeting of the PI3Kinase pathway. FASEB J. 28:2347–2357. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kamal A, Nayak Lakshma V, Nagesh N,
Vishnuvardhan MV and Reddy Subba NV: Benzo [b]furan derivatives
induces apoptosis by targeting the PI3K/Akt/mTOR signaling pathway
in human breast cancer cells. Bioorg Chem. 66:124–131. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wani ZA, Guru SK, Rao AV, Sharma S,
Mahajan G, Behl A, Kumar A, Sharma PR, Kamal A, Bhushan S and
Mondhe DM: A novel quinazolinone chalcone derivative induces
mitochondrial dependent apoptosis and inhibits PI3K/Akt/mTOR
signaling pathway in human colon cancer HCT-116 cells. Food Chem
Toxicol. 87:1–11. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jia H, Zhang Z, Zou D, Wang B, Yan Y, Luo
M, Dong L, Yin H, Gong B, Li Z, et al: MicroRNA-10a is
down-regulated by DNA methylation and functions as a tumor
suppressor in gastric cancer cells. PLoS One. 9:e880572014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Renner O, Blanco-Aparicio C, Grassow M,
Canamero M, Leal JF and Carnero A: Activation of
phosphatidylinositol 3-kinase by membrane localization of p110alpha
predisposes mammary glands to neoplastic transformation. Cancer
Res. 68:9643–9653. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Egeland NG, Lunde S, Jonsdottir K, Lende
TH, Cronin-Fenton D, Gilje B, Janssen EA and Søiland H: The role of
MicroRNAs as predictors of response to tamoxifen treatment in
breast cancer patients. Int J Mol Sci. 16:24243–24275. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang SX, Polley E and Lipkowitz S: New
insights on PI3K/AKT pathway alterations and clinical outcomes in
breast cancer. Cancer Treat Rev. 45:87–96. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Suen DF, Norris KL and Youle RJ:
Mitochondrial dynamics and apoptosis. Genes Dev. 22:1577–1590.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Breckenridge DG and Xue D: Regulation of
mitochondrial membrane permeabilization by BCL-2 family proteins
and caspases. Curr Opin Cell Biol. 16:647–652. 2004. View Article : Google Scholar : PubMed/NCBI
|