Introduction
Oral cavity cancer represents ~2.6 and 1.5% of all
malignancies among males and females, respectively and is a major
global public health problem (1). The
2012 cancer registry report from the Taiwan Health Promotion
Administration of the Ministry of Health and Welfare indicated that
the incidence of oral cavity cancer was 20/100,000 and the annual
mortality rate was 11/100,000, making it the fifth most common
cause of cancer-related mortality in Taiwan. On the basis of
histological data, oral cavity squamous cell carcinoma (OSCC)
accounts for >90% of all oral cavity cancer cases; this
histological subgroup is characterized by increased rates of tumour
cell migration, invasion and metastasis (2).
Marked progress has been made in the treatment of
OSCC. Wide excision of the tumour with neck dissection is the
primary form of treatment with concurrent postoperative
chemoradiotherapy improving outcomes for operable cases of locally
advanced OSCC (3,4). However, despite the availability of
multidisciplinary treatment, the survival rate for oral cavity
cancer over the last decade has remained poor and remains a major
cause of mortality (5,6). Furthermore, radiotherapy may lead to
side effects including trismus and xerostomia (7,8).
Therefore, several studies have attempted to identify prognostic
pathological markers, including human papillomavirus (HPV)
infection, which may help to decrease radiation doses in patients
with favourable prognostic factors for head and neck cancer
(9,10).
Cluster of differentiation (CD) 164, a glycoprotein
and type I integral transmembrane sialomucin that is also known as
endolyn or MGC-24 is encoded by the CD164 gene located on
human chromosome 6q21 (11). Three
isoforms of CD164 have been identified (12–14). CD164
serves important roles in regulating proliferation, adhesion and
differentiation in progenitor and hematopoietic stem cells as well
as negative regulation of haematopoiesis (15). However, few cancer-associated studies
have examined this protein and, to the best of our knowledge, no
studies have considered it in the context of head and neck cancer
(16–22). Therefore, the present study aimed to
investigate the association of CD164 expression with
clinicopathological parameters and prognosis in patients with
OSCC.
Materials and methods
Patients
The present study retrospectively reviewed 70
patients who were diagnosed with malignant OSCC between January
2000 and December 2010 at the Tri-Service General Hospital (Taipei,
Taiwan). This sample only included patients with OSCC who underwent
planned curative primary surgery with or without adjuvant
chemoradiotherapy. Patients with other histological diagnoses
including acinic cell carcinoma, adenoid cystic carcinoma,
verrucous carcinoma, adenocarcinoma, sarcoma and mucoepidermoid
carcinoma were excluded. Patients with metastatic oral cavity
cancer, synchronous oral cancers or a history of malignancy or
treatment at other hospitals were also excluded.
The present study was approved by the institutional
review board of Tri-Service General Hospital (TSGH1-105-05-012) and
the methods were carried out in accordance with the approved
guidelines. Informed written consent was obtained from all
subjects. The 70 eligible patients included 63 males and 7 females
with an age range of between 29 and 72 years (median, 51 years).
Pathological stages were classified in all 70 cases according to
the 2010 staging criteria of the American Joint Committee on Cancer
(AJCC) (23).
Treatment
All patients underwent standard primary surgery
according to their clinical stage (5,6). Wide
excision with supraomohyoid neck dissection was performed for
early-stage cases; wide excision with ipsilateral modified radical
neck, ipsilateral radical neck or bilateral radical neck dissection
was performed for locally advanced cases. The majority of patients
required flap reconstruction due to the large wound that was
created by the surgical procedures.
A total of 21 patients (30%) underwent surgery
alone, with the remaining 49 patients (70%) undergoing
postoperative radiotherapy with or without chemotherapy. The
radiation fields included the tumour bed, the ipsilateral upper
neck for early-stage cases and the ipsilateral whole neck or
bilateral neck for locally advanced cases. The radiation technique
was intensity modulated radiotherapy with prescribed doses of
between 60 and 66 Gy for the tumour bed and upper neck, and between
50 and 54 Gy for the lower neck with a daily fraction size of
between 1.8 and 2.2 Gy.
All 43 cases of stage III–IV disease underwent
postoperative concurrent chemoradiotherapy. The early standard for
chemotherapy was previously cisplatin (80–100 mg/m2 per
day on days 1, 22 and 43) during the radiotherapy (24). However, since 2007, weekly cisplatin
chemotherapy (30–40 mg/m2) has also been considered a
treatment option during radiotherapy (6,25).
Following the concurrent chemoradiotherapy, 3 cycles of monthly
adjuvant chemotherapy were administered to high-risk patients
(cisplatin at 80 mg/m2 on day 1 and fluorouracil at
1,000 mg/m2 on days 1–4 as a 96 h infusion for each
cycle). Overall survival time was defined as the time from the date
of diagnosis to the date of mortality from any cause.
Tissue specimens and
immunohistochemistry
Tumour specimens were soaked in 10% v/v formalin
solution at room temperature for 24 h, and then the specimens were
embedded in paraffin. The paraffin-embedded tumour tissues from the
70 patients prior to chemoradiotherapy treatment were obtained from
the department of pathology, and a tissue microarray slide was
constructed. To construct the tissue microarray, one core of 2 mm
in diameter was taken from a selected area of each
paraffin-embedded tumour tissue. The tissue microarray slide showed
uniform staining as the original paraffin-embedded specimens.
Serial 4 µm sections were excised and stained by a Leica
autostainer XL (Leica Biosystems, Nussloch, Germany) for standard
hematoxylin and eosin staining. Briefly, the procedure of
hematoxylin and eosin staining included xylene 16 min, absolute
alcohol 4 min 30 sec, wash out 1 min 30 sec, hematoxylin 4 min,
wash out 3 min, 0.1% HCL 40 min, wash out 5 min, absolute alcohol 2
min, eosin 30 sec, absolute alcohol 5 min 10 sec and xylene 4 min
20 sec. The histopathological diagnosis of OSCC was confirmed by
two experienced pathologists. On the basis of the histopathological
grading, 17 tumours (24%) exhibited good differentiation, 37
tumours (53%) exhibited moderate differentiation and 16 tumours
(23%) exhibited poor differentiation. The immunohistochemical
staining for CD164 was performed according to the standard protocol
(21). The sections were dried
overnight at 37°C and were deparaffinized using xylene. All
sections were treated using an antigen retrieval solution (Target
Retrieval; Dako; Agilent Technologies, Inc., Santa Clara, CA, USA)
at 95°C for 15 min before incubation overnight at 4°C with a
polyclonal sheep anti-human CD164 antibody (1:100; R&D Systems,
Inc., Minneapolis, MN, USA). A dilution of 1:100 purified rabbit
anti-human CD164 antibody (HPA010636; Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) was set for 60 min at room temperature, and
secondary biotin-linked sheep anti-immunoglobulin antibody (B3275;
Sigma-Aldrich; Merck KGaA) for 30 min at room temperature. The
sections were developed using diaminobenzidine and counterstained
using haematoxylin. These were incubated in horseradish
peroxidase-conjugated streptavidin (Dako; Agilent Technologies,
Inc.) for 2 min at room temperature. All slides were examined using
Olympus BX51 (magnification, ×400) and scored independently by the
two pathologists, who were blinded to the patients' clinical
information.
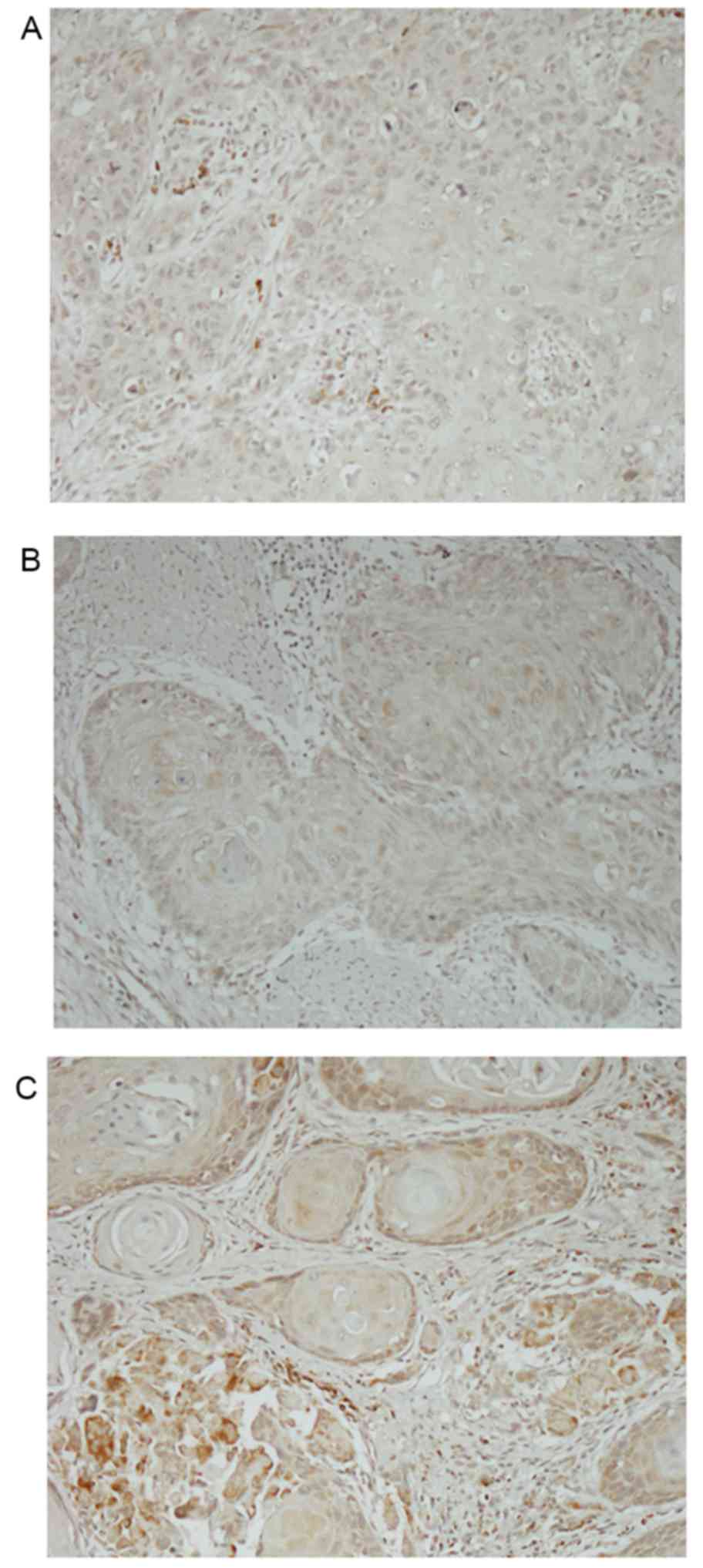
As staining intensity and distribution varied,
cytoplasmic staining was scored using a 4-point scale (0, no
staining; 1+, light staining at high magnification; 2+,
intermediate staining; 3+, dark staining of linear membrane at low
magnification; Fig. 1). Additionally,
the percentage of stained cells was estimated for each intensity.
The percentage of CD164-stained cells for each intensity was
multiplied by the corresponding intensity score to obtain an
immunostaining score (H-score) that ranged between 0 and 300
(26).
Statistical analysis
SPSS version 20.0 (IBM Corp., Armonk, NY, USA) was
used for all analyses. Pearson's χ2 test and Fisher's
exact test were used to evaluate the association of CD164
expression with the patients' clinicopathological characteristics.
Cumulative survival rate was evaluated using Kaplan-Meier estimator
analysis and the log-rank test. All the independent factors were
further tested using Cox's regression for multivariate comparison.
P<0.05 was considered to indicate a statistically significant
difference for all known confounding factors, although P<0.025
was used to evaluate the significance of CD164 expression as its
clinical relevance was unclear.
Results
A total of 9 patients (13%) had stage I disease, 18
patients (26%) had stage II disease, 16 patients (23%) had stage
III disease and 27 patients (38%) had stage IV disease. None of the
patients exhibited distant metastases at their presentation. The
tongue was the most commonly affected site [30 patients (43%)]
followed by the buccal mucosa [29 patients (41%)], gingiva [7
patients (10%)], tonsils (2 patients), palate (1 patient) and lip
(1 patient). At the last follow-up, 34 patients (49%) had succumbed
and 36 patients (51%) remained alive. The median follow-up for the
surviving patients was 46 months (range, 4–120 months). Among all
patients, the 5-year locoregional control and overall survival
rates were 48.0 and 54.4%, respectively.
CD164 was primarily detected in the cytoplasm and
cell membrane of the cancer cells and in the lymphocytes
surrounding the tumours. A total of 17 patients (24%) exhibited a
CD164 staining intensity of 3+, compared with 42 patients (60%) for
2+ and 11 patients (16%) for 1+. The median H-score was 106.5
(range, 23–243) and the samples were arbitrarily classed as having
low CD164 expression (H-score <120) or high CD164 expression
(H-score ≥120). Table I indicates
that the H-score was not significantly associated with known
prognostic factors including sex (P=0.515), age (P=0.324), AJCC
stage (P=0.27), tumour location (P=0.241), histopathological grade
(P=0.972) or surgical margin (P=0.143).
 | Table I.Associations between H-score and
patient characteristics. |
Table I.
Associations between H-score and
patient characteristics.
| Characteristic | H-score <120, n
(%) | H-score ≥120, n
(%) | P-value |
|---|
| All cases | 42 (60) | 28 (40) |
|
| Sex |
|
|
|
| Male | 37 (88) | 26 (93) | 0.515 |
|
Female | 5 (12) | 2 (7) |
|
| Age, years |
|
|
|
|
<51 | 28 (67) | 15 (54) | 0.324 |
| ≥51 | 14 (33) | 13 (46) |
|
| AJCC stage |
|
|
|
| I–II | 31 (38) | 12 (39) | 0.270 |
|
III–IV | 50 (62) | 21 (42) |
|
| Tumour location |
|
|
|
|
Buccal-gingival | 24 (57) | 12 (43) | 0.241 |
|
Others | 18 (43) | 14 (57) |
|
| Histopathological
grade |
|
|
|
| 1 | 10 (24) | 7
(25) | 0.972 |
| 2 | 22 (52) | 15 (54) |
|
| 3 | 10 (24) | 6
(21) |
|
| Surgical margin |
|
|
|
|
Negative | 36 (86) | 27 (96) | 0.143 |
|
Positive | 6
(14) | 1 (4) |
|
Univariate analyses revealed that no factors were
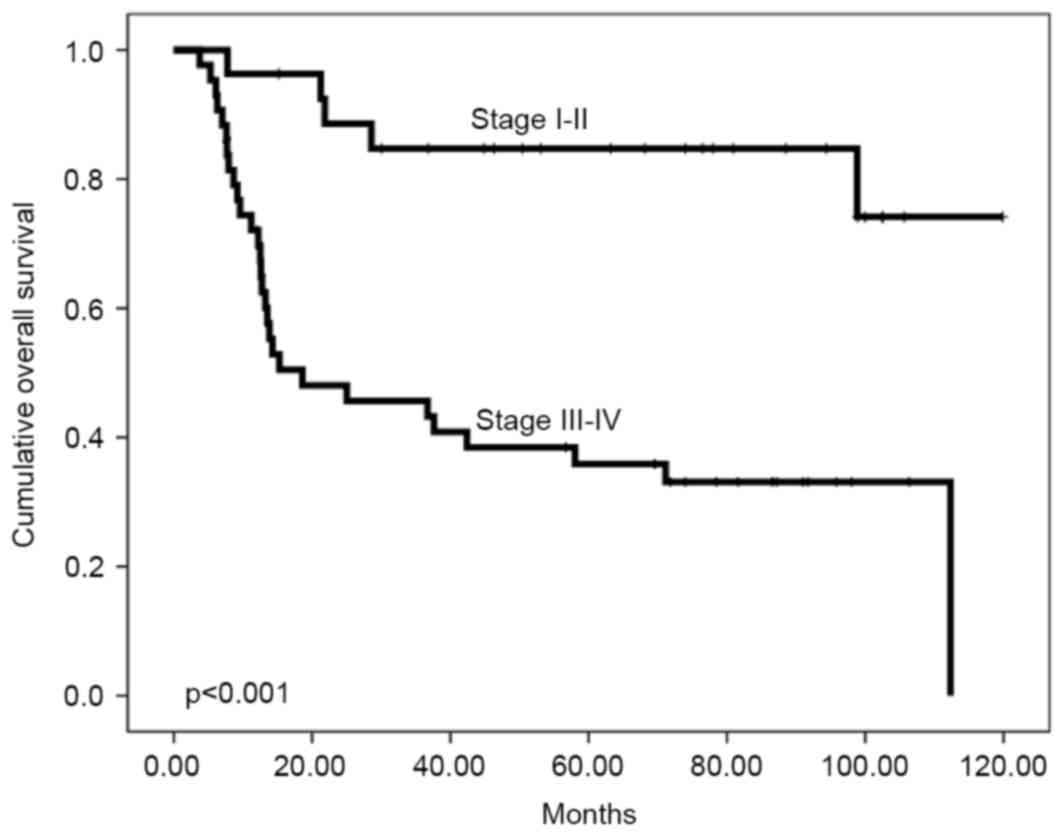
significantly associated with locoregional control (Table II). However, poor overall survival
rate was associated with advanced AJCC stage, buccogingival tumour
location and low CD164 expression. The 5-year overall survival
rates were 84.7% for patients with stage I–II disease and 35.9% for
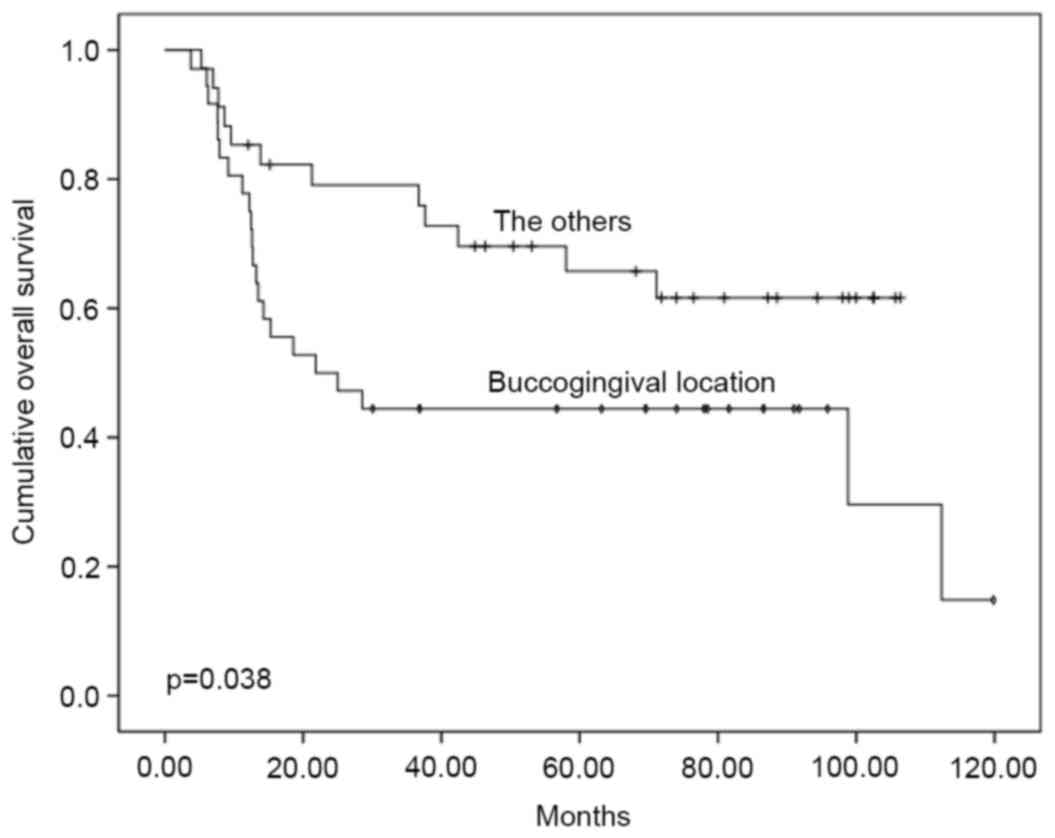
patients with stage III–IV disease (P<0.001; Fig. 2). Buccogingival tumour location was
associated with a significantly decreased 5-year overall survival
rate (44.4%) compared with the other sites (65.7%) (P=0.038;
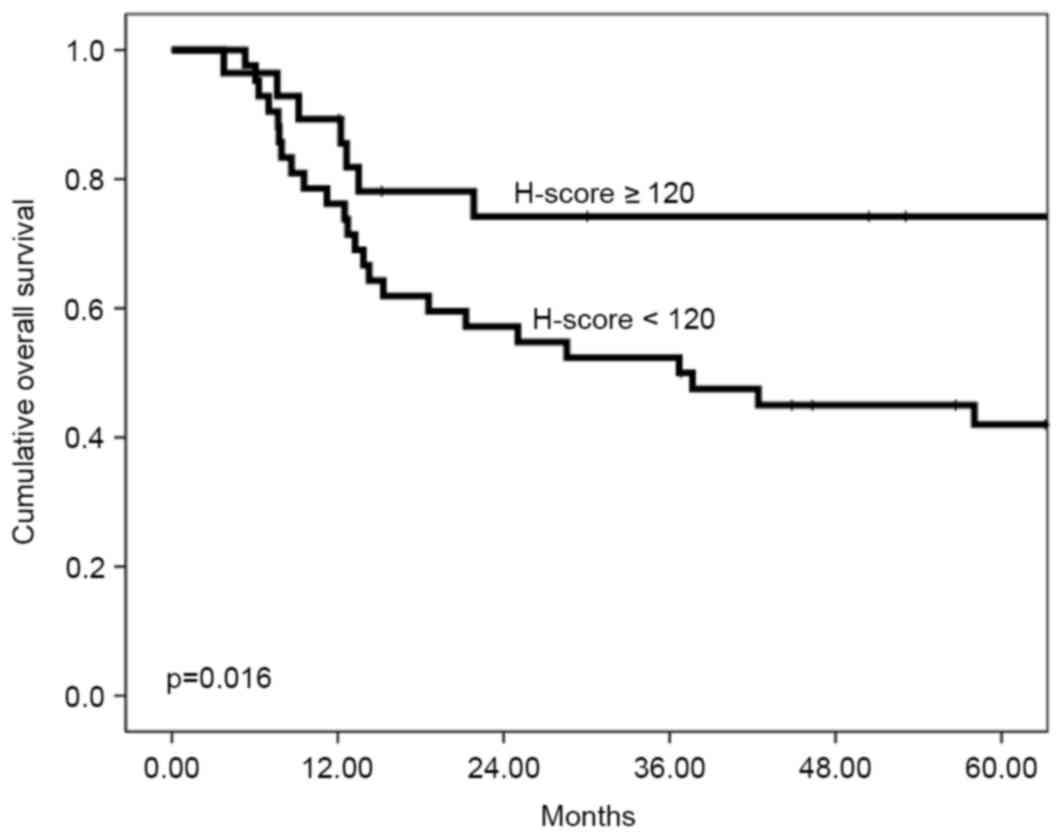
Fig. 3). The 5-year overall survival
rate was 42.0% for a low H-score compared with 74.2% for a high
H-score (P=0.016, Fig. 4). All the
independent factors were incorporated into the multivariate
analyses which revealed that poor survival rate was only associated
with AJCC stage III–IV disease (P=0.001) and a low H-score
(P=0.040).
 | Table II.Patient characteristics and
prognostic factors identified using univariate analysis. |
Table II.
Patient characteristics and
prognostic factors identified using univariate analysis.
|
|
| 5-year locoregional
control rate | 5-year overall
survival rate |
|---|
|
|
|
|
|
|---|
| Characteristic | n (%) | % | P-value | % | P-value |
|---|
| Sex |
|
| 0.544 |
| 0.871 |
|
Male | 63 (90) | 46.8 |
| 54.2 |
|
|
Female | 7
(10) | 57.1 |
| 57.1 |
|
| Age, years |
|
| 0.553 |
| 0.702 |
|
<50 | 30 (43) | 56.2 |
| 59.8 |
|
|
≥50 | 40 (57) | 42.4 |
| 50.1 |
|
| AJCC staging |
|
| 0.327 |
| <0.001 |
|
I–II | 27 (39) | 61.7 |
| 84.7 |
|
|
III–IV | 43 (61) | 39.3 |
| 35.9 |
|
| Tumour
location |
|
| 0.604 |
| 0.038 |
|
Buccal-gingival | 36 (51) | 52.8 |
| 44.4 |
|
|
Others | 34 (49) | 44.3 |
| 65.7 |
|
| Histological
grade |
|
| 0.975 |
| 0.265 |
| 1 | 17 (24) | 47.1 |
| 70.6 |
|
| 2 | 37 (53) | 47.6 |
| 47.0 |
|
| 3 | 16 (23) | 53.0 |
| 54.5 |
|
| Surgical
margins |
|
| 0.507 |
| 0.170 |
|
Negative | 63 (90) | 26.8 |
| 55.9 |
|
|
Positive | 7
(10) | 49.8 |
| 38.1 |
|
| H-score |
|
| 0.203 |
| 0.016 |
|
<120 | 42 (60) | 41.8 |
| 42.0 |
|
|
≥120 | 28 (40) | 57.1 |
| 74.2 |
|
Discussion
To the best of our knowledge, the present study is
the first to demonstrate that a low CD164 H-score was associated
with poor overall survival rate in patients with OSCC. Therefore,
CD164 expression may be a useful marker for predicting prognosis in
these patients as it is independent of other known
clinicopathological parameters including AJCC stage and
histopathological grade. Previous studies have attempted to
identify parameters that may facilitate decreases in dose- and
treatment-associated side effects in head and neck cancer (9,10).
Matsui et al (22) evaluated 92 patients with advanced
colorectal carcinoma and analysed the association of CD164
expression with metastatic potential, demonstrating that lower
CD164 expression in colon carcinoma was associated with a trend
towards invasion into the lymphatic vessels. McGuckin et al
(27) identified that CD164 and CD34
exhibit marked co-localization patterns in cells that express the
two antigens, suggesting a functional link between the two
sialomucins; it was concluded that CD164 and CD34 act as negative
regulators of cell proliferation in the transplantation area.
Jorgensen-Tye et al (28) also
demonstrated that CD164 was a negative regulator of haematopoiesis.
Therefore, the results cited above may support the hypothesis that
CD164 protects against cell proliferation.
However, other studies have demonstrated that CD164
serves a distinct role in other solid and haematological
malignancies. For example, Tang et al (17) examined specimens from human
colorectal, breast and ovarian cancer cell lines and revealed that
decreased CD164 expression in a human colon cancer cell line
significantly inhibited cell proliferation, mobility and
metastasis. Thus, it was concluded that CD164 may be a useful
target for diagnosing and treating colon cancer. Havens et
al (18) analysed the role of
CD164 in prostate cancer cell lines and identified that blocking
CD164 impaired the ability of prostate cancer cells to adhere to
bone marrow endothelial cells and invade extracellular matrices.
They also stained human tissue microarrays for CD164 and observed a
positive association with prostate-specific antigen levels which
led to the conclusion that CD164 participates in the localization
of prostate cancer cells to the bone marrow and is involved in
tumour metastasis. Huang et al (21) evaluated the role of CD164 in ovarian
surface epithelial cells from 97 cases and identified that high
CD164 expression was significantly associated with high-grade
ovarian tumours, advanced-stage disease and tumour metastasis.
Thus, they suggested that increased CD164 expression is involved in
ovarian cancer progression through the stromal cell-derived factor
1a/C-X-C chemokine receptor type 4 axis, which promotes
tumourigenicity. Wysocka et al (20) evaluated 6 patients with Sézary
syndrome and 3 healthy donors, and identified that CD164 could be
used to diagnose and monitor cases of the disease. This potential
diagnostic role was also observed by Guenova et al (19) who investigated CD164 expression in
malignant T-cells from 8 patients with Sézary syndrome and revealed
that CD164 expression on CD4+ lymphocytes was
significantly increased compared with healthy controls. The role of
CD164 is ambiguous and remains unclear in the aforementioned
studies; the results of the present study support the hypothesis
that CD164 inhibits cell proliferation (17–22,27,28).
The present study has several limitations that
warrant consideration. First, the sample size was small and a
larger cohort study is required to validate the results. Secondly,
having used a retrospective design, it is impossible to accurately
consider all potential confounding factors (e.g., smoking status,
alcohol consumption and paan consumption). Thirdly, H-scores
<120 were arbitrarily defined as low based on a median score of
106.5, and a larger cohort is required to determine a more accurate
and sensitive threshold value. Fourthly, the present study did not
consider the cellular and molecular basis of the association of
CD164 expression with patient survival rate, although this issue is
currently being investigated by the present authors. Fifthly, as a
consequence of betel chewing, the majority of OSCC occurs on the
tongue, buccal mucosa and gingiva in Taiwan (5,6). However,
the three most common sites worldwide for OSCC are the tongue,
floor of the mouth and retromolar trigone (29). Owing to this disparity, it remains
unknown whether the results of the present study are applicable to
other countries or patients with OSCC who do not chew betel.
In conclusion, the results of the present study
revealed that CD164 overexpression in patients with OSCC was
associated with favourable overall survival rates. Therefore, in
addition to the known prognostic factors, CD164 may be another
clinically useful parameter.
Acknowledgements
The authors would like to thank the Cancer Registry
Group at the Tri-Service General Hospital for providing the
clinical data. The present study was supported by the Tri-Service
General Hospital (grant no. TSGH-C106-041).
References
|
1
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lin CS, Lin YC, Adebayo BO, Wu A, Chen JH,
Peng YJ, Cheng MF, Lee WH, Hsiao M, Chao TY and Yeh CT: Silencing
JARID1B suppresses oncogenicity, stemness and increases radiation
sensitivity in human oral carcinoma. Cancer Lett. 368:36–45. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cooper JS, Pajak TF, Forastiere AA, Jacobs
J, Campbell BH, Saxman SB, Kish JA, Kim HE, Cmelak AJ, Rotman M, et
al: Postoperative concurrent radiotherapy and chemotherapy for
high-risk squamous-cell carcinoma of the head and neck. N Engl J
Med. 350:1937–1944. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Blanchard P, Baujat B, Holostenco V,
Bourredjem A, Baey C, Bourhis J and Pignon JP; and MACH-CH
Collaborative group, . Meta-analysis of chemotherapy in head and
neck cancer (MACH-NC): A comprehensive analysis by tumour site.
Radiother Oncol. 100:33–40. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lin CS, Jen YM, Cheng MF, Lin YS, Su WF,
Hwang JM, Chang LP, Chao HL, Liu DW, Lin HY and Shum WY: Squamous
cell carcinoma of the buccal mucosa: An aggressive cancer requiring
multimodality treatment. Head Neck. 28:150–157. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lin CS, Jen YM, Kao WY, Ho CL, Dai MS,
Shih CL, Cheng JC, Chang PY, Huang WY and Su YF: Improved outcomes
in buccal squamous cell carcinoma. Head Neck. 35:65–71. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Machtay M, Moughan J, Trotti A, Garden AS,
Weber RS, Cooper JS, Forastiere A and Ang KK: Factors associated
with severe late toxicity after concurrent chemoradiation for
locally advanced head and neck cancer: An RTOG analysis. J Clin
Oncol. 26:3582–3589. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bjordal K, Hammerlid E, Ahlner-Elmqvist M,
de Graeff A, Boysen M, Evensen JF, Biörklund A, de Leeuw JR, Fayers
PM, Jannert M, et al: Quality of life in head and neck cancer
patients: Validation of the european organization for research and
treatment of cancer quality of life questionnaire-H&N35. J Clin
Oncol. 17:1008–1019. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Westra WH, Taube JM, Poeta ML, Begum S,
Sidransky D and Koch WM: Inverse relationship between human
papillomavirus-16 infection and disruptive p53 gene mutations in
squamous cell carcinoma of the head and neck. Clin Cancer Res.
14:366–369. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chera BS, Amdur RJ, Tepper J, Qaqish B,
Green R, Aumer SL, Hayes N, Weiss J, Grilley-Olson J, Zanation A,
et al: Phase 2 trial of De-intensified chemoradiation therapy for
favorable-risk human papillomavirus-associated oropharyngeal
squamous cell carcinoma. Int J Radiat Oncol Biol Phys. 93:976–985.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Watt SM, Buhring HJ, Rappold I, Chan JY,
Lee-Prudhoe J, Jones T, Zannettino AC, Simmons PJ, Doyonnas R,
Sheer D and Butler LH: CD164, a novel sialomucin on CD34(+) and
erythroid subsets, is located on human chromosome 6q21. Blood.
92:849–866. 1998.PubMed/NCBI
|
|
12
|
Doyonnas R, Chan Yi-Hsin J, Butler LH,
Rappold I, Lee-Prudhoe JE, Zannettino AC, Simmons PJ, Bühring HJ,
Levesque JP and Watt SM: CD164 monoclonal antibodies that block
hemopoietic progenitor cell adhesion and proliferation interact
with the first mucin domain of the CD164 receptor. J Immunol.
165:840–851. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kurosawa N, Kanemitsu Y, Matsui T, Shimada
K, Ishihama H and Muramatsu T: Genomic analysis of a murine
cell-surface sialomucin, MGC-24/CD164. Eur J Biochem. 265:466–472.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Watt SM, Butler LH, Tavian M, Bühring HJ,
Rappold I, Simmons PJ, Zannettino AC, Buck D, Fuchs A, Doyonnas R,
et al: Functionally defined CD164 epitopes are expressed on CD34(+)
cells throughout ontogeny but display distinct distribution
patterns in adult hematopoietic and nonhematopoietic tissues.
Blood. 95:3113–3124. 2000.PubMed/NCBI
|
|
15
|
Zannettino AC, Bühring HJ, Niutta S, Watt
SM, Benton MA and Simmons PJ: The sialomucin CD164 (MGC-24v) is an
adhesive glycoprotein expressed by human hematopoietic progenitors
and bone marrow stromal cells that serves as a potent negative
regulator of hematopoiesis. Blood. 92:2613–2628. 1998.PubMed/NCBI
|
|
16
|
Shi JA, Lu DL, Huang X and Tan W: miR-219
inhibits the proliferation, migration and invasion of
medulloblastoma cells by targeting CD164. Int J Mol Med.
34:237–243. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tang J, Zhang L, She X, Zhou G, Yu F,
Xiang J and Li G: Inhibiting CD164 expression in colon cancer cell
line HCT116 leads to reduced cancer cell proliferation, mobility
and metastasis in vitro and in vivo. Cancer Invest. 30:380–389.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Havens AM, Jung Y, Sun YX, Wang J, Shah
RB, Bühring HJ, Pienta KJ and Taichman RS: The role of sialomucin
CD164 (MGC-24v or endolyn) in prostate cancer metastasis. BMC
Cancer. 6:1952006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Guenova E, Ignatova D, Chang YT, Contassot
E, Mehra T, Saulite I, Navarini AA, Mitev V, Dummer R and Kazakov
DV: Expression of CD164 on malignant T cells in Sézary syndrome.
Acta Derm Venereol. 96:464–467. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wysocka M, Kossenkov AV, Benoit BM, Troxel
AB, Singer E, Schaffer A, Kim B, Dentchev T, Nagata S, Ise T, et
al: CD164 and FCRL3 are highly expressed on CD4+CD26- T cells in
Sézary syndrome patients. J Invest Dermatol. 134:229–236. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Huang AF, Chen MW, Huang SM, Kao CL, Lai
HC and Chan JY: CD164 regulates the tumorigenesis of ovarian
surface epithelial cells through the SDF-1α/CXCR4 axis. Mol Cancer.
12:1152013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Matsui T, Kurosawa N, Hibi K, Akiyama S,
Kasai Y, Sakamoto J, Ito K, Nakao A and Muramatsu T: The ratio of
splicing variants of MGC-24/CD164, a sialomucin, correlates with
the metastatic potential of colorectal carcinomas. J Biochem.
127:1103–1107. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Edge SB and Compton CC: The american joint
committee on cancer: The 7th edition of the AJCC cancer staging
manual and the future of TNM. Ann Surg Oncol. 17:1471–1474. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Marcial VA, Pajak TF, Mohiuddin M, Cooper
JS, al Sarraf M, Mowry PA, Curran W, Crissman J, Rodríguez M and
Vélez-García E: Concomitant cisplatin chemotherapy and radiotherapy
in advanced mucosal squamous cell carcinoma of the head and neck.
Long-term results of the radiation therapy oncology group study
81–17. Cancer. 66:1861–1868. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
van den Bent MJ, van Putten WL, Hilkens
PH, de Wit R and van der Burg ME: Retreatment with dose-dense
weekly cisplatin after previous cisplatin chemotherapy is not
complicated by significant neuro-toxicity. Eur J Cancer.
38:387–391. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mazieres J, Brugger W, Cappuzzo F, Middel
P, Frosch A, Bara I, Klingelschmitt G and Klughammer B: Evaluation
of EGFR protein expression by immunohistochemistry using H-score
and the magnification rule: Re-analysis of the SATURN study. Lung
Cancer. 82:231–237. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
McGuckin CP, Forraz N, Baradez MO,
Lojo-Rial C, Wertheim D, Whiting K, Watt SM and Pettengell R:
Colocalization analysis of sialomucins CD34 and CD164. Stem Cells.
21:162–170. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jorgensen-Tye B, Levesque JP, Royle L,
Doyonnas R, Chan JY, Dwek RA, Rudd PM, Harvey DJ, Simmons PJ and
Watt SM: Epitope recognition of antibodies that define the
sialomucin, endolyn (CD164), a negative regulator of
haematopoiesis. Tissue Antigens. 65:220–239. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen AY and Myers JN: Cancer of the oral
cavity. Dis Mon. 47:275–361. 2001. View Article : Google Scholar : PubMed/NCBI
|