Introduction
As a cancer imaging technique, diffusion-weighted
magnetic resonance imaging (DW-MRI) has developed into a clinically
valuable tool for the detection and characterization of cancer, and
for monitoring the response to therapy. It is potentially useful
for measuring cellularity and tissue response through assessment of
apparent diffusion coefficient (ADC) values (1–3). This may
be employed to assess the microstructural organization of the cell
density, cell membrane integrity and cell viability, which affect
water diffusion properties in the extracellular space (ECS)
(4). Tumor cell proliferation
increases cellularity, whereas tumor cell apoptosis reduces
cellularity. Tumor cellularity and the shape of the ECS affect
water diffusion; the diffusivity of water molecules is restricted
in microenvironments of high cellularity, as this cellularity
reduces the ratio of the extracellular to intracellular space in a
given area of tissue (4,5). Prior studies have demonstrated that the
tumor ADC inversely correlates with tumor cellularity, and that the
successful treatment of numerous tumor types may be detected by
identifying an early increase in ADC values using DW-MRI (6,7).
Diffusion parameters of the ECS are affected by loss
of cellularity and degradation of the extracellular matrix (ECM).
The ECM and changes in the geometry of the ECS are considered to be
of critical importance in affecting water diffusion and the ADC
values in tumor tissues (8–10). Matrix metalloproteinase 9 (MMP-9) is a
soluble gelatinase B (92 kDa), similar to other MMPs, and a member
of a zinc-containing protease superfamily that efficiently degrades
the protein components of the ECM and basement membranes (BM),
thereby serving a central role in tissue remodeling and degradation
(11–14). There is a large volume of evidence
suggesting that MMP-9 up-regulation is associated with the
progression of cervical squamous cell carcinoma (14). A notable hallmark of cervical cancer
progression is the degradation of the ECM, which allows cancer
cells to invade the surrounding tissue.
Radiation therapy represents a key management
strategy for a number of epithelial tumor types and is an effective
treatment for cervical cancer. However, it has been demonstrated
that ionizing radiation treatment with sub-lethal doses causes the
upregulation of MMP-9 expression and activity, and promotes
MMP-9-mediated ECM degradation, contributing to tumor progression
and invasion (15,16). The mouse U14 cervical carcinoma cell
line provides a useful model to study the association between MMP-9
expression and early changes in ADC values derived from DW-MRI with
tumor image characteristics to predict radiotherapy tumor response
following single higher than conventional-fraction dose
irradiation.
Therefore, the present study examined the early
effects of irradiation on ADC values and MMP-9 expression in U14
allograft tumor tissues following irradiation with a single dose of
20 Gy.
Materials and methods
Tumor cell and tumor allograft
model
The mouse cervical carcinoma U14 strain was
purchased from the Committee on Type Culture Collection of Chinese
Academy of Sciences (Shanghai, China) and preserved under liquid
nitrogen in the Sichuan Cancer Institute (Chengdu, China); these
cells were collected and washed twice with RPMI-1640 medium (Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with
10% fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.), 100
IU/l penicillin and 100 mg/l streptomycin, centrifuged at 140 × g
at 37°C for 10 min and resuspended with RPMI-1640 medium
(2×107) cells/ml. Subsequently, the cell suspension was
incubated in a humidified atmosphere (5% CO2) for 30 min
at 37°C. A total of 26 female BALB/C mice (6 weeks of age; 17–21 g)
were purchased from the Experimental Animal Center of Sichuan
University (SXCKC1172029-09; Chengdu, China). All mice were raised
under specific-pathogen-free conditions and fed with basal diet and
water ad libitum at 26°C in 5% CO2 with a 12-h
light-dark cycles. Finally, all mice were sacrificed by breaking
neck. Single cell suspension (0.1 ml; 1×107/ml in
RPMI-1640 culture medium; Gibco; Thermo Fisher Scientific, Inc.) of
U14 tumor strain resuscitated quickly at 37°C from liquid nitrogen
was inoculated subcutaneously into the right axillary of 2 BALB/c
mice for restoring tumorigenicity. When the tumor volume (TV)
reached 300 mm3, the tumor tissues were removed from
sacrificed mice and prepared into single cell suspension with
RPMI-1640 culture medium. Cell suspension (0.1 ml; 1×107
cells/ml) was reinoculated into the left rear flank of the mice to
establish an allograft model of cervical carcinoma U14. When the
tumor formation rate reached >90%, 24 mice with U14 tumor were
randomly divided into four groups by time of imaging after
irradiation: The control group (without irradiation), 6, 24 and 72
h after the irradiation groups. All experimental procedures were
approved by the Institutional Animal Care and Use Committee of
Sichuan Cancer Institute and conducted in conformity with the
Guiding Principles for Research Involving Animals and Human Beings
(17).
Irradiation
Radiation was delivered using the Cobalt-60
teletherapy unit (GWGP80; Nuclear Power Institute of China, Leshan,
China) with a dose rate of 0.87 Gy/min. Dosimetry was confirmed
using an ionization chamber and LiF thermoluminescent dosimeters.
For irradiation, when the TV reached 300–500 mm3, the
tumor-bearing mice were anesthetized with 0.3% sodium pentobarbital
(10 ml/kg) administered intraperitoneally, and placed under a
radiation field so only the left rear flank bearing the tumor was
irradiated with a single dose of 20 Gy (source skin distance=80 cm,
d=0.5 cm, a=6 cm).
MRI protocol
Female BALB/C mice with unilateral subcutaneous U14
cervical carcinoma in the left rear flank underwent a baseline MRI
scan using a Philips 3.0T system (Achieva/Intera; Philips
Healthcare, Amsterdam, The Netherlands) equipped with a small
animal receiver coil (CG-MUC18-H300-AP; product no., 5000002301,
serial no., 001001; Shanghai Chenguang Medical Technologies Co.,
Ltd., Shanghai, China). The MRI protocols included the T1-weighted
and T2-weighted spin-echo sequences with two b-factors (0 and 800
sec/mm2) in the axial direction. The scan parameters
were as follows: For T2-weighted spin echo sequence, repetition
time (TR)/echo time (TE): 4,000/66 msec; matrix: 136×134;
bandwidth: 156 Hz; field of view: 50 mm; slice thickness: 2 mm;
intersection gap: 0.2 mm. For T1-weighted spin echo sequence, T1WI
3D-FFE-mice TR/TE: 9.214/4.604 msec; matrix: 80×96; bandwidth:
434.5 Hz; field of view: 60 mm; slice thickness: 1 mm; intersection
gap: 0 mm.
TV assessment and ADC calculation
The tumor borders were segmented manually on the
images obtained with the smaller b factor, based on the signal
intensity between the region of interest (ROI) and background by
two independent investigators. TV was measured with the formula
V=πab2/6, where ‘a’ is the greatest length and ‘b’ is
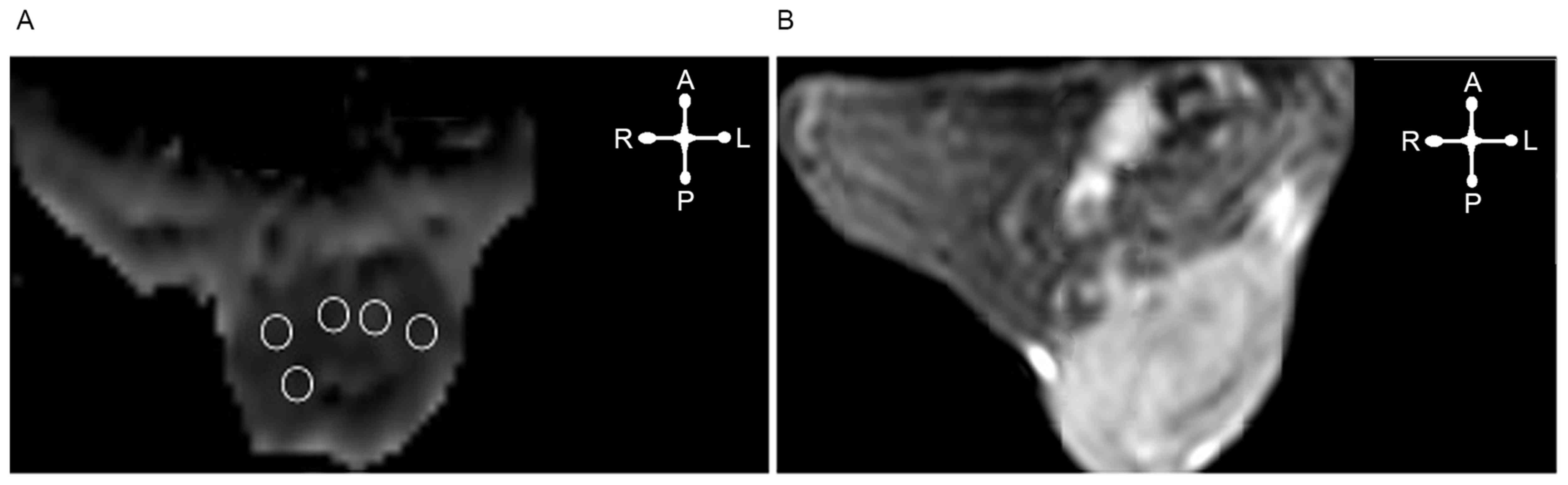
the perpendicular width. For ADC calculation, ≤3 slices of the ADC
map depicting the largest tumor diameter were selected, depending
on the volume of the tumor. In each slice an ROI was delineated
according to the tumor geometry. An ADC value from five sections of
the ROI on the same axial section levels of the same lesion was
calculated (Fig. 1). The ADC image
was obtained by subtracting two sequences of DWI (b=0, b=800
sec/mm2). The ADC value in each ROI was calculated
independently by two experienced investigators using the following
formula: ADC=[ln(S1/sec2)]/(b2-b1), where ‘S’ represents
the signal strength at different b values (b=0, b=800
sec/mm2) in a specific ROI (18).
Immunohistochemistry for MMP-9 expression. For
immunohistochemistry, 4-µm thick sections were cut from the
paraffin-embedded U14 cervical tumor biopsy samples. These sections
were mounted on amino-propyl-ethoxy-silane coated glass slides.
Slides were deparaffinized in xylene and rehydrated with ethanol,
and antigen retrieval was performed using the autoclave oven
technique (125°C, 103 KPa, 8 min). Endogenous peroxidase was
blocked by incubation with 0.3% hydrogen peroxidase at 37°C for 30
min. The primary antibody (4–5 µg/ml) was incubated with the
samples overnight at 4°C (catalog no., BA2202; rabbit anti-mouse;
dilution, 1:200; Wuhan Boster Biological Technology, Ltd., Wuhan,
China). Following three washes with PBS, the specimens were
incubated with a goat anti-rabbit horseradish peroxidase
immunoglobulin G (ZSBio; OriGene Technologies, Inc., Beijing,
China; 5 µg/ml) for 30 min at 37°C. Staining was visualized using
3′3-diaminobenzidine tetrahydrochloride (0.05%) for 12 min at 37°C
and counterstaining was performed with HE for 3 min. PBS without
the primary antibody served as the negative control. Two
independent pathologists using a 1–4+ semi-quantitative scale
scored MMP-9 immunostaining (19).
Statistical analysis
GraphPad Prism (GraphPad Software, version 5.02,
Inc., La Jolla, CA, USA) was used for statistical analysis. Data
are expressed as the mean ± standard deviation. Inter-observer
agreement was assessed with Cohen's Kappa: κ≤0.40, poor agreement;
κ=0.41–0.75, good agreement; κ≥0.76, excellent agreement. The
correlation between the change in mean ADC and the TV was
calculated using Pearson's correlation coefficient, and the
correlation between the change in mean ADC and MMP-9 expression was
calculated using Spearman's correlation coefficient. Two-tailed
P<0.05 values we considered to indicate statistically
significant differences.
Results
Inter-observer agreement
There was an excellent inter-observer agreement
between the two readers, with a κ coefficient of 0.91 for the
assessment of TV, 0.87 for ADC values and 0.79 for H-scoring.
DW-MRI and ADC values
DW-MRI was used to detect the response of cervical
carcinoma U14 allograft tumors to irradiation. ADC maps and
high-resolution axial T1WI and T2WI from tumors prior to
irradiation and at various time points post-irradiation are
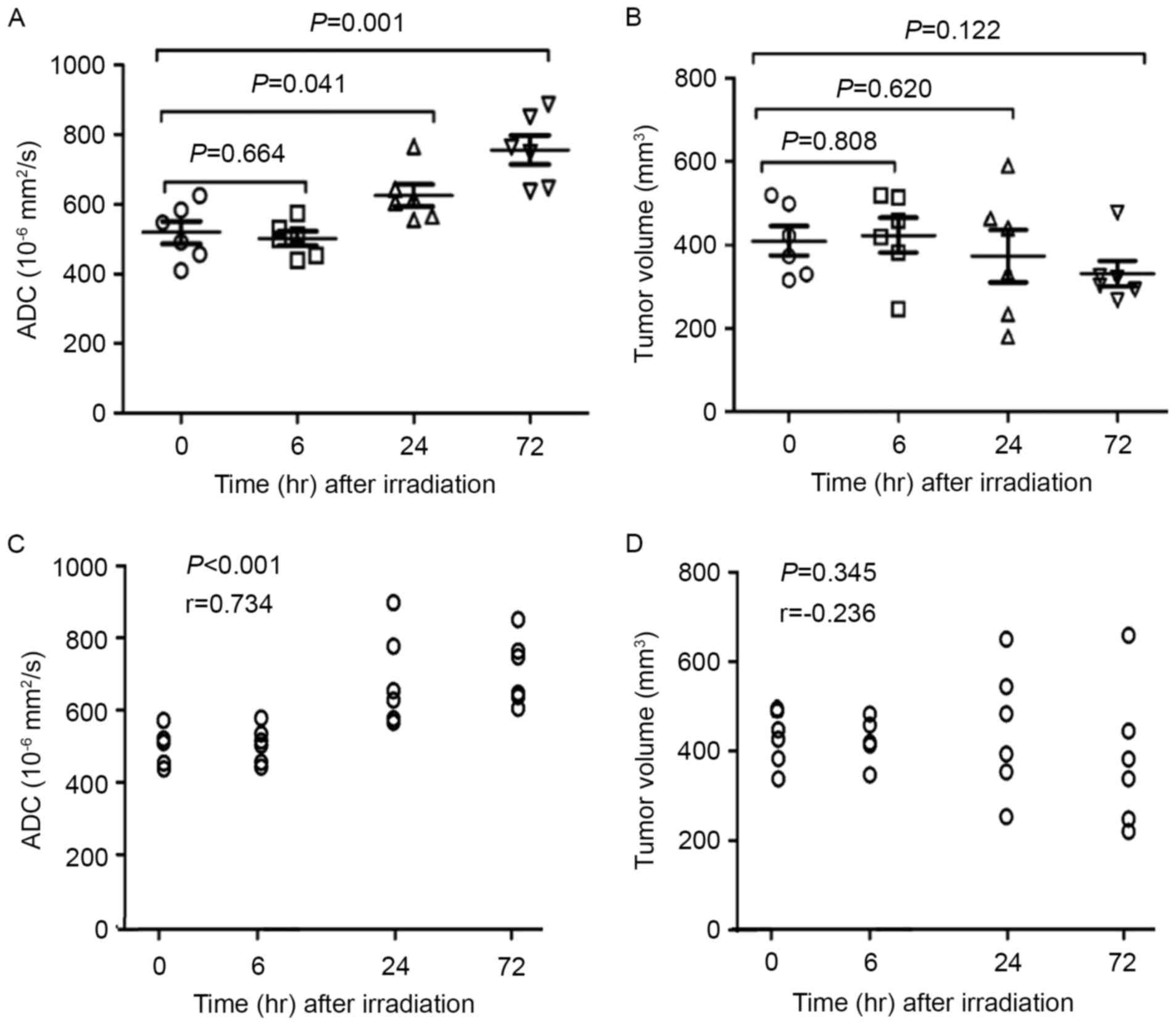
presented in Fig. 2. A significant
and time-dependent increase of ADC value was observed in irradiated
vs. non-irradiated tumors 72 h following irradiation (P=0.001).
Non-irradiated tumors were typically homogeneously hyperintense on
the T2WI and DWI images with a low mean ADC value
(0.501±0.052×10−3 mm2/sec; Fig. 2A-D); irradiated tumors were
hyperintense on T2WI and hypointense on T1WI, and the mean ADC
values of the irradiated tumors at 6, 24 and 72 h subsequent to
irradiation were 0.518±0.081×10−3,
0.625±0.076×10−3 and 0.756±0.102×10−3
mm2/sec, respectively (Fig.
2E-H for 6 h; 2I-L for 24 h; 2M-P for 72 h). Fig. 3 presents a summary of the correlations
between ADC values and the post-irradiation time and TV for all
tumors. ADC values of solid portions within the irradiated tumors
suggested a notable correlation with post-irradiation time
(r=0.734; P<0.0001), but TV did not exhibit a correlation with
the post-irradiation time (r=−0.236; P=0.345).
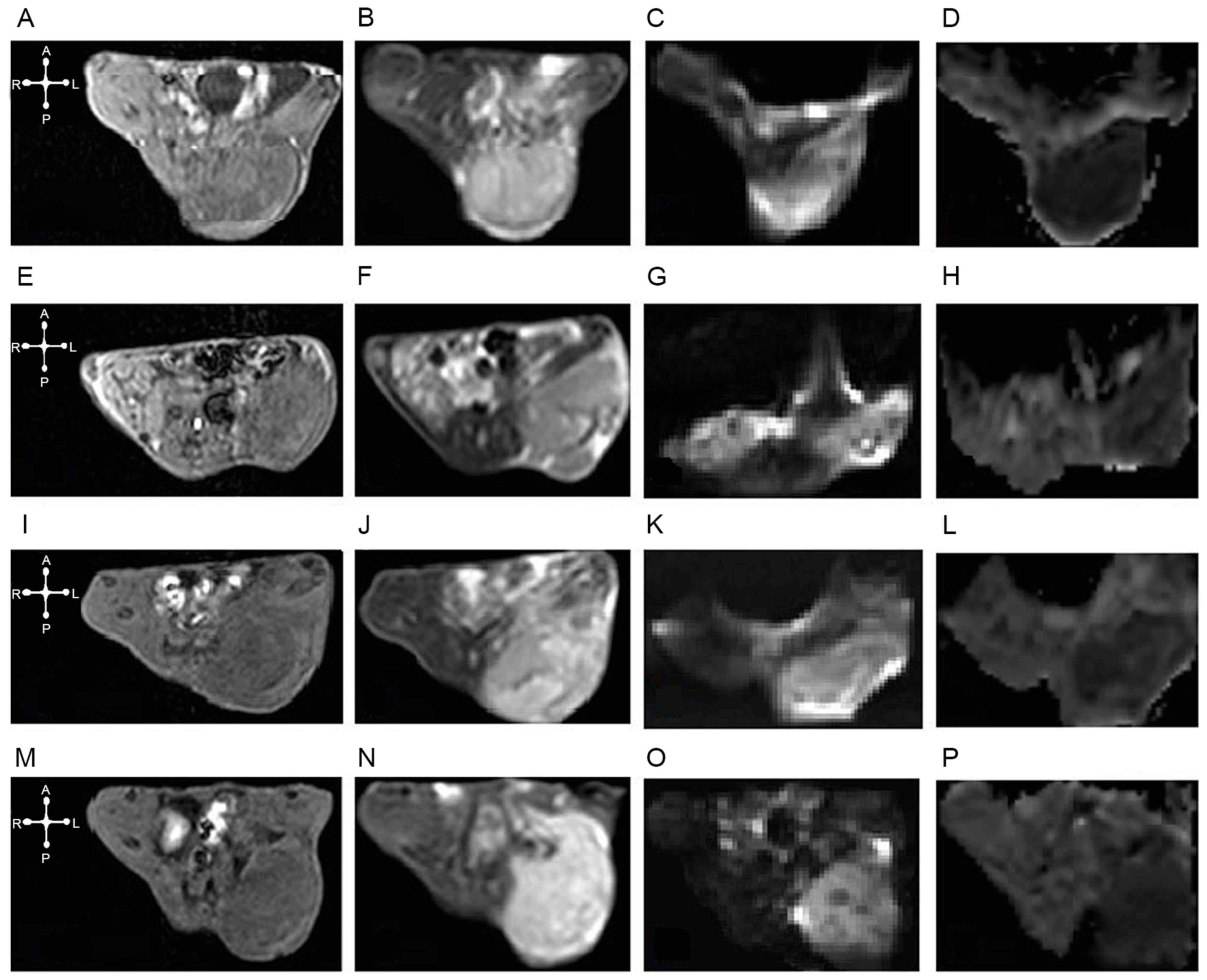 | Figure 2.ADC and DWI maps, high-resolution
axial T1 and T2WI from tumors prior to irradiation and at various
times post-irradiation. In the non-irradiation group, U14 cervical
cancer was hypo-isointense on (A) axial T1WI, hyperintense on (B)
axial T2WI and (C) DWI (b=800) and diffusion restricted in the
corresponding (D) ADC map (ADC mean=0.574×10−3
mm2/sec). In the irradiation groups: (6 h following
irradiation), (E) T1WI and (F) T2WI images, (G) DWI and (H) ADC
maps (ADC mean=0.509×10−3 mm2/sec); (24 h
following irradiation) (I) T1WI and (J) T2WI images, and (K) DWI
and (L) ADC maps (ADC mean=0.642×10−3
mm2/sec); and (72 h following irradiation) (M) T1WI and
(N) T2WI images, and (O) DWI and (P) ADC maps (ADC
mean=0.748×10−3 mm2/sec), the U14 cervical
cancer tumors were hyperintense on T2WI and DWI, and hypointense on
T1WI with a relatively high mean ADC value. ADC, apparent diffusion
coefficient; DWI, diffusion-weighted image. |
Histological changes and MMP-9
expression
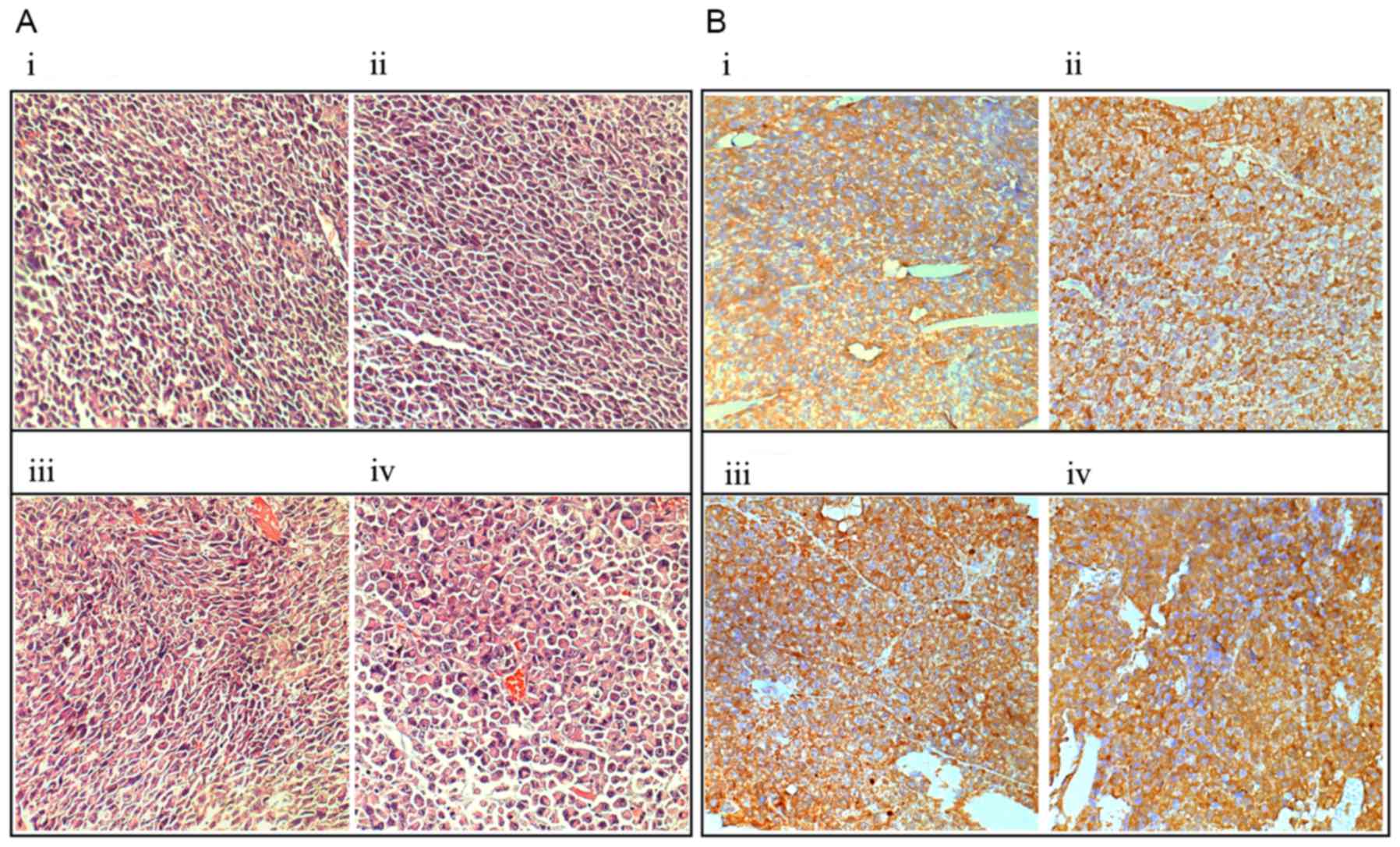
Histological examination of HE staining revealed
that no marked necrosis was present in the irradiated and control
tumors, but the irradiated tumors exhibited cellular edema,
swelling, increases in size, cell layer loosening and extracellular
space dilatation (Fig. 4Ai-iv);
immunohistochemical staining demonstrated that the expression
levels of extracellular/cell-surface MMP-9 were markedly increased
in the irradiated tumors at 6 h subsequent to irradiation, as
compared with in the control tumors (Fig.
4Bi-iv).
Associations between ADC values, TV
and MMP-9 expression
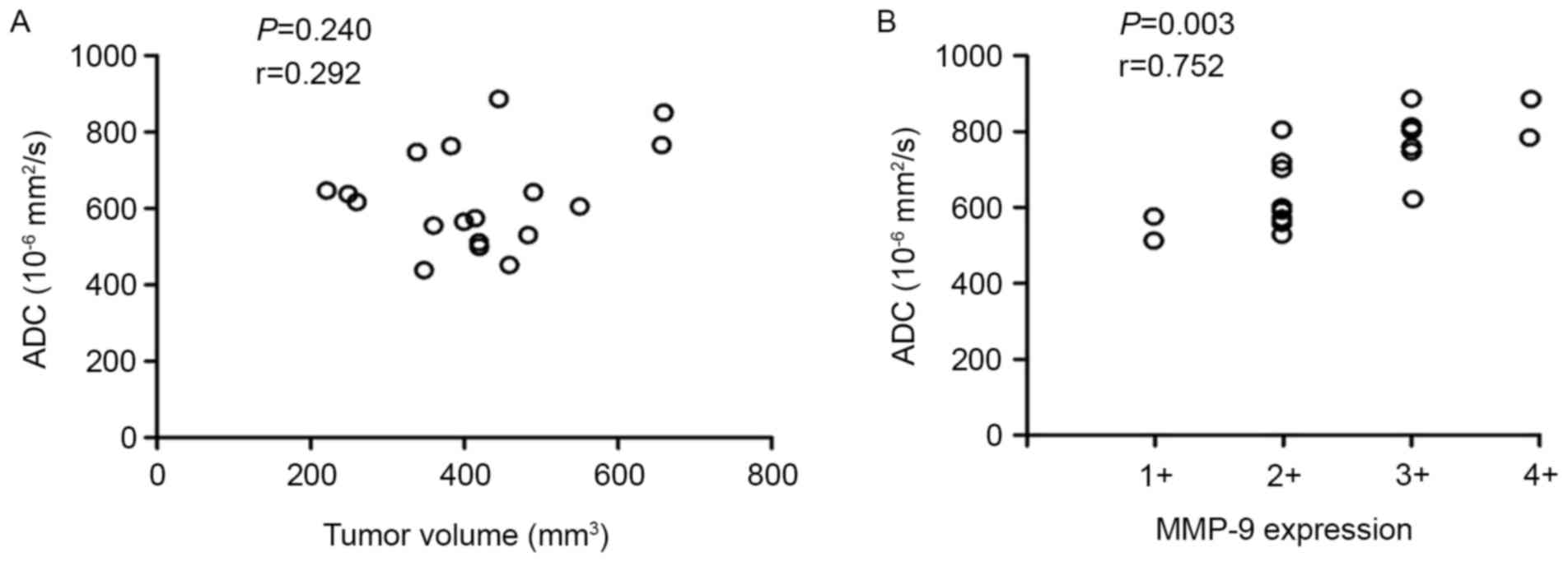
As indicated in Fig.
5, no significant correlation between the mean ADC value and
the TV was observed (P=0.240; r=0.292; Fig. 5A), but there was a significant
correlation between the mean ADC value and the MMP-9 expression
level (P=0.003; r=0.752; Fig. 5B).
These data suggest that radiation-induced increased MMP-9
expression may contribute to the elevation of mean ADC values in
irradiated tumors with a larger ECS by degrading the ECM.
Discussion
As a cancer treatment response technique, DW-MRI
provides information about microscopic structures, such as cell
density and integrity. It is sensitive to macromolecular and
microstructural changes, which may occur at the cellular level
relatively earlier when compared with anatomical changes during
therapy (1). Studies have
demonstrated that the therapeutic response to concurrent
chemoradiation in several tumor types, including cervical cancer,
may be detected by measuring early changes in ADC values using
DW-MRI (19–24). It is well known that the restriction
of water diffusion in biological tissues is associated with tissue
cellularity and cell membrane integrity. Factors that affect the
diffusion of water molecules, including edema and differences in
cellularity, have been identified to be associated with changes in
ADC values (25). However, the
underlying mechanisms remain unknown.
ADC values increase with reducing cellularity and
barriers to water diffusion in biological tissues, and are
negatively correlated with tumor cellularity (26–30). The
determinants of diffusion in the tumor ECS include ECM composition,
ECS size and geometry (31). ECM and
tumor cell interactions serve critical roles in tumor cellularity,
which alters diffusion in tumors, thus the ADC values increase as
cellularity decreases in DW-MRI (6,28). In the
present study, the results demonstrated that irradiation does
reduce U14 tumor cellularity with a corresponding increase in the
ADC value at 24 h following irradiation (Fig. 3A and C and Fig. 4Ai-iv), suggesting that an early change
in the ADC value reflects tumor cellularity following irradiation.
ECS diffusion parameters are affected by a loss of cellularity and
degradation of the ECM (31). It is
known that ADC values decrease due to pericellular ECM degradation
caused by MMPs, and that increased ADC values are associated with
the expression and activity of MMP-9 localized within the
intercellular spaces (32). MMP-9 is
a matrix protein involved in the degradation of ECM that is active
within 24 h of the onset of stress-induced tissue injury, and may
mediate collagen IV degradation in the BM and pericellular ECM,
intercellular space dilatation and cellularity reduction (33,34). MMP-9
is activated by various stimuli, including irradiation and human
papillomavirus (HPVs) in tumor tissues (15–16).
Sub-lethal doses of radiation may enhance MMP-9 promoter activity
and expression through the phosphoinositide 3-kinase/protein kinase
B/NF-κB signal transduction pathways (13,35). An
early and significant increase in MMP-9 expression induced by
irradiation facilitates ECM degradation (36). It has been suggested that gene
knockdown of MMP-9 or RNA interference-mediated downregulation of
radiation-induced MMP-9 may significantly reverse ADC reduction,
and that increased expression of MMP-9 facilitates ECM degradation,
leading to a decrease in cellularity and an increase in the water
diffusion and ADC values of tumors (35). These data indicate that a high tumor
ADC value reflects the low tumor cellularity involved in
MMP-9-mediated degradation of the ECM. In the present study, it was
also identified that DW-MRI identified regions in irradiated U14
tumors with increased signal on ADC maps (Fig. 2A-P), and that the increased ADC
corresponded with increased MMP-9 expression in U14 tumors within
72 h of irradiation (Fig. 5B).
Increases in MMP-9 activity induced by irradiation and decreases in
cellularity due to the degradation of ECM in tumor tissues are
associated not only with increases in the intercellular space, but
also with the dilatation of the ECS, which in turn increases ADC
values (37). The dilatation of the
ECS is characterized by a loss of cellularity, degradation of the
ECM, morphological changes such as cell-drink and occupancy effect,
and inactivation of Na+/K+/ATP enzyme (38,39). It
has been previously demonstrated that ECM degradation is associated
with the increased mobility of ECM macromolecules, and that
macromolecule ADCs offer potential sensitive and early markers for
ECM degradation and the prospect of directly monitoring ECM
degradation processes in vivo in clinical settings at the
molecular and microstructural levels (36). These results support the hypothesis
that ECS is crucial for determining the ADC values of tumors: The
extracellular ADC values increased with increases in the ECS due to
MMP-9-mediated degradation of the ECM following radiation
treatment.
To conclude, ECM degradation in tumors following
exposure to ionizing radiation may reflect the specialized role of
MMP-9 in the ECS, and indicate that radiation-induced increased
expression of MMP-9 is a potential mechanism underlying early
changes in ADC values observed in cervical tumors.
Radiation-enhanced changes in ADC values, including increased
expression and activation of MMP-9 in tumors, may be used as a
variable for early assessment of the radiation-treatment response
of patients with cervical cancer. However, as changes in ADC values
are associated with spatio-temporal dynamics of tumor responses to
radiation, ADC values and MMP-9 may be candidate biomarkers of the
early response to radiotherapy, though this requires further
investigation with respect to clinical outcomes.
Acknowledgements
The present study was supported by the Science and
Technology Program Project Funds of Sichuan Province (grant no.
2015SZ0053) and Applied Basic Research Programs of Science and
Technology Foundation of Sichuan Province (grant no.
2016JY0135).
Glossary
Abbreviations
Abbreviations:
|
ADC
|
apparent diffusion coefficient
|
|
DW-MRI
|
diffusion-weighted magnetic resonance
imaging
|
|
MMPs
|
matrix metalloproteinases
|
|
MMP-9
|
matrix metalloproteinase-9
|
|
HE
|
hematoxylin-eosin
|
|
ECM
|
extracellular matrix
|
|
ECS
|
extracellular space
|
|
BM
|
basement membranes
|
|
ROI
|
region of interest
|
|
TV
|
tumor volume
|
|
TR
|
repetition time
|
|
TE
|
echo time
|
References
|
1
|
Thoeny HC and Ross BD: Predicting and
monitoring cancer treatment response with diffusion-weighted MRI. J
Magn Reson Imaging. 32:2–16. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rudin M: Imaging readouts as biomarkers or
surrogate parameters for the assessment of therapeutic
interventions. Eur Radiol. 17:2441–2457. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Padhani AR, Liu G, Koh DM, Chenevert TL,
Thoeny HC, Takahara T, Dzik-Jurasz A, Ross BD, Van Cauteren M,
Collins D, et al: Diffusion-weighted magnetic resonance imaging as
a cancer biomarker: Consensus and recommendations. Neoplasia.
11:102–125. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Koh DM and Collins DJ: Diffusion-weighted
MRI in the body: Applications and challenges in oncology. AJR Am J
Roentgenol. 188:1622–1635. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Szafer A, Zhong J, Anderson AW and Gore
JC: Diffusion-weighted imaging in tissues: Theoretical models. Nmr
Biomed. 8:289–296. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Matsumoto Y, Kuroda M, Matsuya R, Kato H,
Shibuya K, Oita M, Kawabe A, Matsuzaki H, Asaumi J, Murakami J, et
al: In vitro experimental study of the relationship between the
apparent diffusion coefficient and changes in cellularity and cell
morphology. Oncol Rep. 22:641–648. 2009.PubMed/NCBI
|
|
7
|
Lyng H, Haraldseth O and Rofstad EK:
Measurement of cell density and necrotic fraction in human melanoma
xenografts by diffusion weighted magnetic resonance imaging. Magn
Reson Med. 43:828–836. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sadeghi N, Camby I, Goldman S, Gabius HJ,
Balériaux D, Salmon I, Decaesteckere C, Kiss R and Metens T: Effect
of hydrophilic components of the extracellular matrix on
quantifiable diffusion-weighted imaging of human gliomas:
Preliminary results of correlating apparent diffusion coefficient
values and hyaluronan expression level. AJR Am J Roentgenol.
181:235–241. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Vargová L, Homola A, Zámecnik J, Tichý M,
Benes V and Syková E: Diffusion parameters of the extracellular
space in human gliomas. Glia. 42:77–88. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pope WB, Mirsadraei L, Lai A, Eskin A,
Qiao J, Kim HJ, Ellingson B, Nghiemphu PL, Kharbanda S, Soriano RH,
et al: Differential gene expression in glioblastoma defined by ADC
histogram analysis: Relationship to extracellular matrix molecules
and survival. AJNR Am J Neuroradiol. 33:1059–1064. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Stamenkovic I: Extracellular matrix
remodelling: The role of matrix metalloproteinases. J Pathol.
200:448–464. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kessenbrock K, Plaks V and Werb Z: Matrix
metalloproteinases: Regulators of the tumor microenvironment. Cell.
141:52–67. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu CH, You Z, Liu CM, Kim YR, Whalen MJ,
Rosen BR and Liu PK: Diffusion-weighted magnetic resonance imaging
reversal by gene knockdown of matrix metalloproteinase-9 activities
in live animal brains. J Neurosci. 29:3508–3517. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Libra M, Scalisi A, Vella N, Clementi S,
Sorio R, Stivala F, Spandidos DA and Mazzarino C: Uterine cervical
carcinoma: Role of matrix metalloproteinases (review). Int J Oncol.
34:897–903. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nirmala C, Jasti SL, Sawaya R, Kyritsis
AP, Konduri SD, Ali-Osman F, Rao JS and Mohanam S: Effects of
radiation on the levels of MMP-2, MMP-9 and TIMP-1 during
morphogenic glial-endothelial cell interactions. Int J Cancer.
88:766–771. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chou CH, Teng CM, Tzen KY, Chang YC, Chen
JH and Cheng JC: MMP-9 from sublethally irradiated tumor promotes
Lewis lung carcinoma cell invasiveness and pulmonary metastasis.
Oncogene. 31:458–468. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim HS, Kim CK, Park BK, Huh SJ and Kim B:
Evaluation of therapeutic response to concurrent chemoradiotherapy
in patients with cervical cancer using diffusion-weighted MR
imaging. J Magn Reson Imaging. 37:187–193. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Spielmann H: FRAME annual lecture.
International co-operation: An essential requirement for replacing
animal toxicity tests. Altern Lab Anim. 29:637–648. 2001.PubMed/NCBI
|
|
19
|
Rhodes LV, Short SP, Neel NF, Salvo VA,
Zhu Y, Elliott S, Wei Y, Yu D, Sun M, Muir SE, et al: Cytokine
receptor CXCR4 mediates estrogen-independent tumorigenesis,
metastasis, and resistance to endocrine therapy in human breast
cancer. Cancer Res. 71:603–613. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cui Y, Zhang XP, Sun YS, Tang L and Shen
L: Apparent diffusion coefficient: Potential imaging biomarker for
prediction and early detection of response to chemotherapy in
hepatic metastases. Radiology. 248:894–900. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Seierstad T, Folkvord S, Røe K, Flatmark
K, Skretting A and Olsen DR: Early changes in apparent diffusion
coefficient predict the quantitative antitumoral activity of
capecitabine, oxaliplatin, and irradiation in HT29 xenografts in
athymic nude mice. Neoplasia. 9:392–400. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Makino H, Kato H, Furui T, Morishige K and
Kanematsu M: Predictive value of diffusion-weighted magnetic
resonance imaging during chemoradiotherapy for uterine cervical
cancer. J Obstet Gynaecol Res. 40:1098–1104. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Song I, Kim CK, Park BK and Park W:
Assessment of response to radiotherapy for prostate cancer: Value
of diffusion-weighted MRI at 3 T. AJR Am J Roentgenol.
194:W477–W482. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Park JJ, Kim CK, Park SY, Simonetti AW,
Kim E, Park BK and Huh SJ: Assessment of early response to
concurrent chemoradiotherapy in cervical cancer: Value of
diffusion-weighted and dynamic contrast-enhanced MR imaging. Magn
Reson Imaging. 32:993–1000. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen J, Xia J, Zhou YC, Xia LM, Zhu WZ,
Zou ML, Feng DY and Wang CY: Correlation between magnetic resonance
diffusion weighted imaging and cell density in astrocytoma.
Zhonghua Zhong Liu Za Zhi. 27:309–311. 2005.(In Chinese).
PubMed/NCBI
|
|
26
|
Humphries PD, Sebire NJ, Siegel MJ and
Olsen ØE: Tumors in pediatric patients at diffusion-weighted MR
imaging: Apparent diffusion coefficient and tumor cellularity.
Radiology. 245:848–854. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen L, Liu M, Bao J, Xia Y, Zhang J,
Zhang L, Huang X and Wang J: The correlation between apparent
diffusion coefficient and tumor cellularity in patients: A
meta-analysis. PLoS One. 8:e790082013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen L, Zhang J, Chen Y, Wang W, Zhou X,
Yan X and Wang J: Relationship between apparent diffusion
coefficient and tumour cellularity in lung cancer. PLoS One.
9:e998652014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kishimoto K, Tajima S, Maeda I, Takagi M,
Ueno T, Suzuki N and Nakajima Y: Endometrial cancer: Correlation of
apparent diffusion coefficient (ADC) with tumor cellularity and
tumor grade. Acta Radiol. 57:1021–1028. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Manenti G, Di Roma M, Mancino S,
Bartolucci DA, Palmieri G, Mastrangeli R, Miano R, Squillaci E and
Simonetti G: Malignant renal neoplasms: Correlation between ADC
values and cellularity in diffusion weighted magnetic resonance
imaging at 3 T. Radiol Med. 113:199–213. 2008.(In English,
Italian). View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Verkman AS: Diffusion in the extracellular
space in brain and tumors. Phys Biol. 10:450032013. View Article : Google Scholar
|
|
32
|
Sood R, Yang Y, Taheri S, Candelario-Jalil
E, Estrada EY, Walker EJ, Thompson J and Rosenberg GA: Increased
apparent diffusion coefficients on MRI linked with matrix
metalloproteinases and edema in white matter after bilateral
carotid artery occlusion in rats. J Cereb Blood Flow Metab.
29:308–316. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lee WH, Warrington JP, Sonntag WE and Lee
YW: Irradiation alters MMP-2/TIMP-2 system and collagen type IV
degradation in brain. Int J Radiat Oncol Biol Phys. 82:1559–1566.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Keating M, Kurup A, Alvarez-Elizondo M,
Levine AJ and Botvinick E: Spatial distributions of pericellular
stiffness in natural extracellular matrices are dependent on
cell-mediated proteolysis and contractility. Acta Biomater.
57:304–312. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cheng JC, Chou CH, Kuo ML and Hsieh CY:
Radiation-enhanced hepatocellular carcinoma cell invasion with
MMP-9 expression through PI3K/Akt/NF-kappaB signal transduction
pathway. Oncogene. 25:7009–7018. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang AM, Cao P, Yee A, Chan D and Wu EX:
Detection of extracellular matrix degradation in intervertebral
disc degeneration by diffusion magnetic resonance spectroscopy.
Magn Reson Med. 73:1703–1712. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Matsumoto Y, Kuroda M, Matsuya R, Kato H,
Shibuya K, Oita M, Kawabe A, Matsuzaki H, Asaumi J, Murakami J, et
al: In vitro experimental study of the relationship between the
apparent diffusion coefficient and changes in cellularity and cell
morphology. Oncol Rep. 22:641–648. 2009.PubMed/NCBI
|
|
38
|
Zhang H and Verkman AS: Microfiberoptic
measurement of extracellular space volume in brain and tumor slices
based on fluorescent dye partitioning. Biophys J. 99:1284–1291.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yoon JH, Son JW, Chung H, Park CH, Kim YJ,
Chang HJ, Hong GR, Kim TH, Ha JW, Choi BW, et al: Relationship
between myocardial extracellular space expansion estimated with
post-contrast T1 mapping MRI and left ventricular remodeling and
neurohormonal activation in patients with dilated cardiomyopathy.
Korean J Radiol. 16:1153–1162. 2015. View Article : Google Scholar : PubMed/NCBI
|