Introduction
Stewart-Treves syndrome (STS) is a rare but fatal
tumor that has been associated with lymphedema (LE), with a 5-year
survival rate of 5–35% and a median survival time of 2.5 years
(1–6).
If patients refuse treatment, the survival time is reduced to 5–8
months. Although STS exhibits a poor prognosis, an early diagnosis
and prompt surgery are considered to be the main methods of
prolonging survival (7,8). Typically, chronic extremity LE leads to
cutaneous hyperkeratosis that may mask the symptoms of early tumors
involving the skin, but STS may also be mistaken for limb LE caused
by exogenous factors, such as trauma (9). STS is also difficult to diagnose as
invasive tumors almost always exhibit the appearance of benign
tumors on magnetic resonance imaging (MRI), and this often leads to
a delayed diagnosis. In addition, it has been hypothesized that STS
exhibits multifocal nodules and a tendency for early metastasis
(10), but, to the best of our
knowledge, no studies have validated this. Studies on the use of
MRI in STS are limited, due to the costs of this technique. In
previous studies (8,9), STS were observed by MRI; however, no
information was provided regarding cluster of differentiation 31
(CD31) and D2-40. In the present study, clinical data on 5 STS
cases was collected, including MRI, light microscopy examination
and immunohistochemical results (including CD31 and D2-40)
(8,9).
Although, the diagnostic term ‘STS’ was first used in 1948 and the
pathological term ‘lymphangiosarcoma’ (LAS) was initially used in
the same study (1). Subsequently,
angiosarcoma (AS) and LAS were typically used interchangeably, and
applied and interpreted as a synonymous diagnostic term (8,11,12).
The present study aimed at analyzing the MRI data of
AS and compare them with the pathological results of STS, in order
to provide a tool that may aid the diagnosis of the syndrome.
Patients and methods
Patients
Between July 2008 and March 2015, a total of 4,289
female cases (age range, 42–78 years; mean ± standard deviation,
60.6±16.5 years) of secondary upper limb LE that were treated at a
single center (Beijing Friendship Hospital, Beijing, China) were
included in the present study. Of the studied population, 5 women
(range, 56–78 years; median, 65 years) with a diagnosis of STS who
underwent modified-radical post mastectomy followed by
radio-chemotherapy, and whose diagnosis was confirmed
pathologically, were included in the analysis. These accounted for
0.12% of the total study population. The patients' ages ranged
between 56 and 78 years, with a median age of 62 years. The range
of duration of LE was 9–17 years, with a median duration of 13
years. A total of 4 patients with STS had no obvious pathogenic
causes and 1 patient presented with a history of trauma in the
elbow joint that occurred 2 months previously. A total of 2
patients presented with STS in the dermis. The patients were
treated by wide excision and exhibited a survival time of >15
and >18 months, respectively. The other 3 patients presented
with STS located in the dermis and subcutaneous tissue (s-ct) which
was defined from the sub-dermis to the surface of muscle. The 2
patients with STS type I (AS) were treated by disarticulation and
exhibited short survival times (6 and 9 months, respectively). The
l patient with STS type II [mixed LAS (mLAS)] was treated by
amputation, including the elbow joint, and exhibited a longer
survival time of >24 months (Table
I). The study protocol was approved by Beijing Friendship
Hospital (Beijing, China).
 | Table I.Clinical data and surgical history in
patients with Stewart-Treves syndrome. |
Table I.
Clinical data and surgical history in
patients with Stewart-Treves syndrome.
| Patient | Age, years | Prior treatment | Duration of
lymphedema, years | Surgery | Survival time,
months |
|---|
| 1 | 78 | Mastectomy and
radio-chemotherapy | 17 | Disarticulation | 6 |
| 2 | 60 | Mastectomy and
radio-chemotherapy | 11 | Disarticulation | 9 |
| 3 | 65 | Mastectomy and
radio-chemotherapy | 10 | Wide excision | >15 |
| 4 | 62 | Mastectomy and
radio-chemotherapy | 13 | Wide excision | >18 |
| 5 | 56 | Mastectomy and
radio-chemotherapy | 14 | Amputation | >24 |
Instruments and methods
The GE Signa 1.5T MRI unit (GE Healthcare, Chicago,
IL, USA) with a 16-channel body surface coil was the MRI imaging
system used. The regions of interest of the limb LE were placed
into the center coil as possible. The conventional MRI, including
T1-weighted image (WI)/axial image (AXI) used a repetition time
(TR) of 500 msec and an echo time (TE) of 15 msec, with a slice
thickness of 8 mm and a slice gap of 0.8 mm. The field of view
(FOV) was 38×30 cm, the matrix was 320×224 and the number of
excitations (NEX) was 2. The T2WI/AXI use a TR of 3,600 msec/TE 110
msec, with a slice thickness of 8 mm and a slice gap of 0.8 mm. The
FOV was 38×30 cm, the matrix was 384×288 and the NEX was 2. The
short TI inversion recovery (STIR)/AXI used a TR of 4,000 msec/TE
110 msec/time inversion recovery 155 msec, with a slice thickness
of 8 mm and a slice gap of 0.8 mm. The FOV was 40×40/38×30 cm, the
matrix was 320×224 and the NEX was 4. The STIR sequence was
selectable (only in patients in a good condition). All cases
underwent contrast-enhanced (ce)MRI; the contrast agent,
gadopentetic acid (Consun Pharmaceutical Co., Ltd., Guangzhou,
China), was injected through the median cubital vein, at a dose of
0.1 mmol/kg body weight, using a high pressure syringe at a flow
rate of 2.0 ml/sec. All patients provided written informed
consent.
The 5 patients with STS were randomly assigned to 2
senior radiologists. The location, number, outline and signal
intensity of nodules (Φ ≥4 mm) were screened and analyzed by
conventional MRI and ceMRI. If the two radiologists held a
different view about any nodule, it was excluded. The ceMRI
facilitated the diagnosis of infiltrative and confluent growth. As
the fiber cable in LE and the deep fascia demonstrate a line shape,
if the fiber cable and/or deep fascia became abnormally thickened
and enhanced beside the nodule, these signs indicated that the
nodules exhibited significant infiltrative growth. If the outline
of the nodules was irregular and visible as a notch appearance, it
indicated a confluent nodule.
The removed surgical specimens were fixed in 10%
formalin within 12 h of collection and embedded in paraffin for
light microscopy examination (magnification, ×200). The tissue
sections (thickness, 6 µm) were stained with hematoxylin and eosin
(H&E; Zhongshan Biotechnology, Guangdong Province, China), as
described previously (8,12). The immunohistochemical staining
processes of the pathological specimens were performed using a Dako
Automated Staining instrument (cat. no. ZYB/USA2016-2012; Dako;
Agilent Technologies, Inc., Santa Clara, CA, USA) according to the
manufacturer's protocol. All immunohistochemical processes were
performed at 37°C. Subsequently, according to the expression of the
endothelial marker CD31 (anti-CD31; cat. no. JC/70A; dilution,
1:80; Dako; Agilent Technologies, Inc.) and lymphatic marker D2-40
(anti-D2-40; cat. no. M3619; dilution, 1:60; Dako; Agilent
Technologies, Inc.), the cases were divided into the following 3
types: STS type I, strongly positive for CD31 and weakly positive
for D2-40; STS type II 1 (pure LAS; pLAS), strongly positive for
D2-40 and weakly positive for CD31; and STS type II 2 (mLAS),
moderately positive for D2-40 and CD31. The analysis of expression
in immunohistochemical results was performed by two experienced
pathologists. If disagreement between the two pathologists
occurred, after reaching the consensus, the results of analysis
were included in this study.
Results
Pathological types
STS types I and II are presented in Figs. 1 and 2,
respectively. In the STS I type, including 37 nodules (Tables II and III), the H&E staining revealed a
densely hypercellular tumor and the tendency of infiltration
(Fig. 1A). The expression of CD31 was
positive (Fig. 1B) and the expression
of D2-40 was negative (Fig. 1C) or
weakly positive (Table II). No
nodules of STS type II 1 were included in this study. In STS type
II 2 including 6 nodules (Tables II
and III), H&E staining revealed
more densely hypercellular tumor than the STS I type, and lack of
vessels (Fig. 2A). The expression of
CD31 was positive (Fig. 2B), as was
the expression of D2-40 (Fig. 2C),
which was classified as STS type II 2 (mLAS). The expression
indices of Ki-67 and cellular tumor antigen p53 (p53) were also
detected. (Table II).
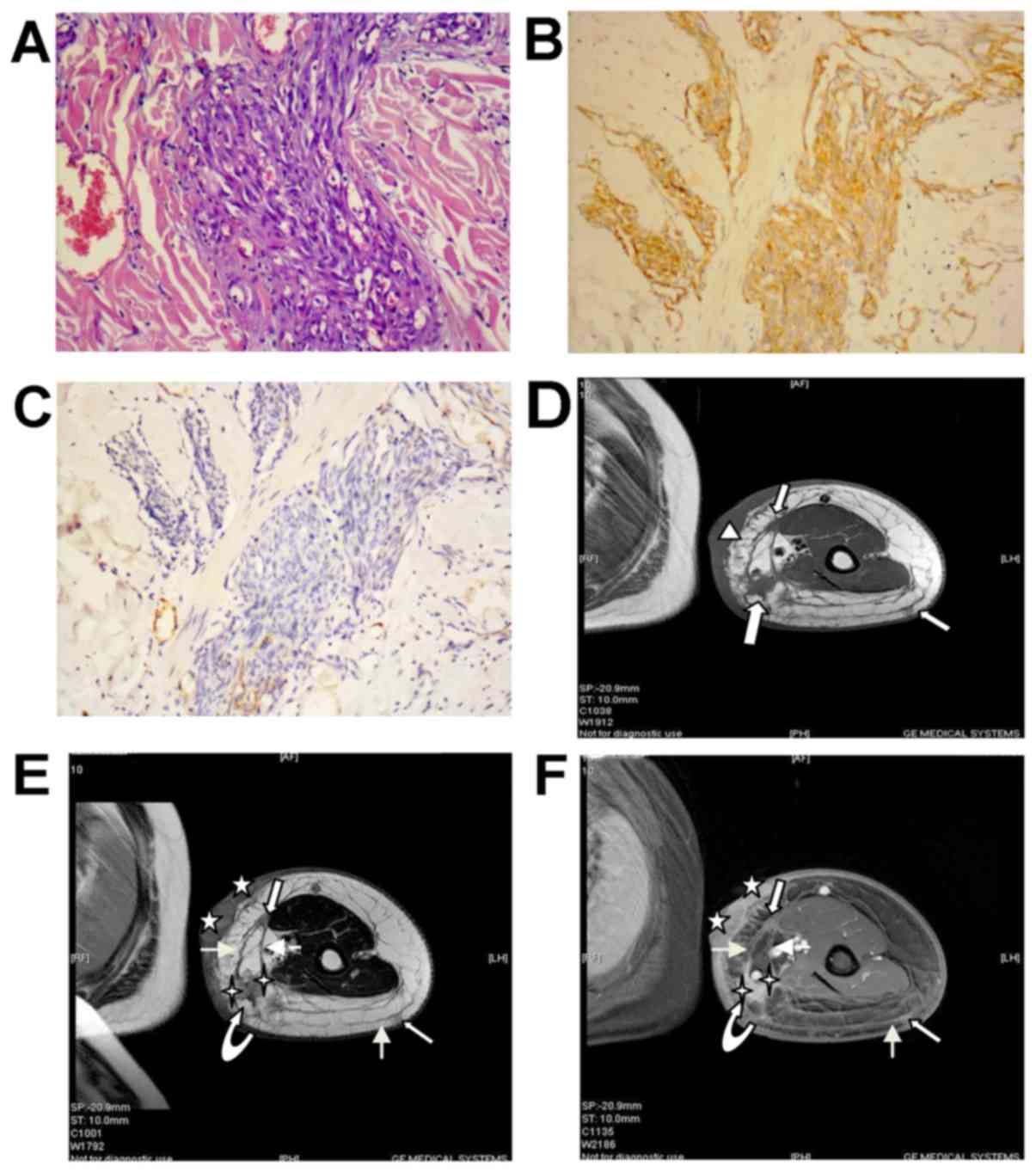 | Figure 1.Angiosarcoma (STS-I type). (A)
Hematoxylin and eosin histopathology at original magnification ×200
revealed that the tumor was composed predominantly of atypical
epithelioid cells with a moderate degree of nuclear atypia, and
also exhibited invasive growth. There were a high number of
immature vessels diffused throughout the tumor and small mature
vessels in collagen fiber with red blood cells. (B) Cluster of
differentiation 31 staining was positive (magnification, ×200). (C)
D2-40 staining was negative (magnification, ×200). (D) Axial T1WI
demonstrates skin thickening in a large range of the patient's limb
with regional processes (indicated by triangle). It indicates at
least three nodules (indicated by outlined arrows) in the s-ct
which was defined from the subdermis to the surface of muscle, with
markedly thickened adjacent fibers. Also noted is the extensive
circumference of the upper arm, consistent with LE. (E)
Corresponding axial FSE T2WI demonstrates more tumor nodules with
well-defined outlines. Scans indicate that the SI of the 2 hidden
dermal nodules (indicated by stars) and 4 subcutaneous nodules is
decreased slightly compared with the LE tissue, and is higher
compared with that of the underlying muscle with normal SI. The
larger nodule (indicated by curved arrow) in the s-ct is 1 nodule
made up of two fused smaller nodules (indicated by 4-pointed star),
and the outline is irregular with the appearance of visible
incisura with markedly thickened adjacent fibers (indicated by
plain arrows). The outlined white arrows indicate tumor nodules in
the s-ct. The deep thickened fascia between the 2 nodules was
observed. The above signs indicate that the tumor is exhibiting
invasive growth. The extensive LE was apparent. (F) Corresponding
axial post-gadolinium T1WI demonstrates diffuse contrast
enhancement within the tumor nodules. Adjacent thickened skin is
markedly enhanced. The 5-pointed stars indicate the 2 hidden dermal
nodules. The 4-pointed stars indicate the two fused smaller nodules
in the s-ct. The curved outline arrow indicates the larger nodule
in the s-ct. The plain white arrows indicate thickened adjacent
fibers. The outlined white arrows indicate the tumor nodules in the
s-ct. LE, lymphedema; WI, weighted image; SI, signal intensity;
s-ct, subcutaneous tissue; FSE, fast spin echo. |
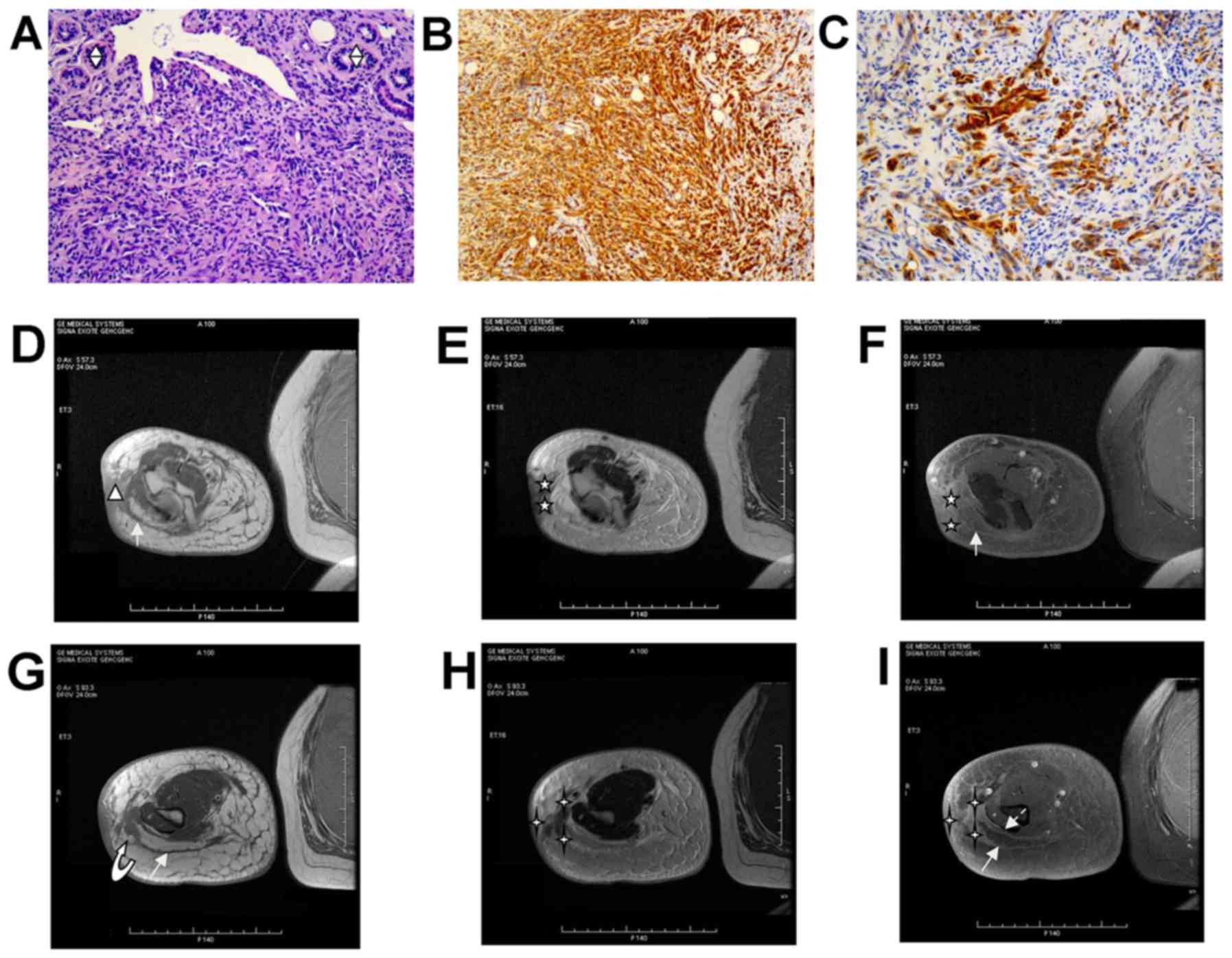 | Figure 2.Lymphangiosarcoma (STS-II 2 type). (A)
Hematoxylin and eosin histopathology (magnification, ×200) revealed
a compact tumor with invasive growth that was composed of densely
atypical epithelioid cells. The tumor cells exhibit a moderate
degree of nuclear atypia. There are a lack of vessels. In certain
areas, these atypical epithelioid cells formed clefts and numerous,
anastomosing or markedly dilated, slit-like erythrocyte-free
channels that occasionally contained proteinaceous fluid and a
small number of lymphocytes. Skin adnexal structures were visible
(indicated by split diamonds). (B) Cluster of differentiation 31
staining was positive (magnification, ×200). (C) D2-40 staining was
partially positive (magnification, ×200). (D) Axial T1WI
demonstrates the focal thickening skin with an irregular outline
(triangle), and with extension into s-ct by 9 mm. It demonstrates
markedly thickened adjacent fibers (plain white arrow), with a
burr-like projection. (E) Corresponding axial FSE T2WI demonstrates
that that the 2 tumor nodules (indicated by stars) are hidden in
the triangle-shape region of the signal, in which the SI of tumor
nodules is markedly decreased compared with the LE tissues, and is
closer to the SI of the underlying muscle tissue. The outlines are
relatively well-defined. (F) Corresponding axial post-gadolinium
T1WI demonstrates contrast enhancement in the tumor nodules, and
the adjacent thickened skin is more enhanced compared with the
tumor nodules. The 5-pointed angle stars indicate the 2 hidden
dermal nodules. |
 | Table II.Results of immunohistochemistry and
pathological types in patients with STS. |
Table II.
Results of immunohistochemistry and
pathological types in patients with STS.
|
| STS type I | STS type II |
|---|
|
|
|
|
|---|
| Cell marker | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 |
|---|
| CD31 | + | + | + | + | + |
| D2-40 | − | − | ± | − | + |
| Ki-67, % | 90–95 | 60 | 20 | 50 | 30 |
| p53 |
+a |
+a | − |
±a | − |
 | Table III.Magnetic resonance imaging data in
patients with Stewart-Treves syndrome. |
Table III.
Magnetic resonance imaging data in
patients with Stewart-Treves syndrome.
| Patient no. | Location of
nodule | Number of nodules,
dermis/s-ct | T2WIa | T2WIb |
|---|
| 1 | Forearm;
dermis/s-ctc | 5/15 | Slightly
decreased | Higher |
| 2 | Upper arm;
dermis/s-ctc | 4/8 | Slightly
decreased | Higher |
| 3 | Upper arm;
dermis | 3/0 | Slightly
decreased | Higher |
| 4 | Forearm; dermis | 2/0 | Slightly
decreased | Higher |
| 5 | Lateral elbow and
forearm; dermis/s-ctc | 2/4 | Markedly
decreased | Slightly
higher |
MRI data
A total of 43 nodules were studied, of which 16
nodules were located in the dermis, and 27 nodules in the s-ct. The
2 patients with AS exhibited tumors that were located in the
dermis, with 5 nodules. Of the 3 patients with tumors of mixed
location, 2 patients with AS presented with 32 nodules (9 nodules
in the dermis and 23 nodules in the s-ct) (Fig. 1D-F) and 1 patient with mLAS presented
with 7 nodules (2 nodules in the dermis and 5 nodules in the s-ct).
In the patient with mLAS, there was a triangular region (Fig. 2D and E) with slightly low signal
intensity (SI) compared with the muscle signals, in the lateral
dermis of the elbow. But in the ceMRI, 2 enhanced nodules in the
triangular region were detected (Fig.
2F). Therefore, the 2 nodules were included into the total
count of dermis nodules.
Outline of nodules
As the nodules in the dermis exhibited an appearance
of fusion growth and cutaneous hyperkeratosis, their outline was
not satisfactorily detected in the T2WI sequence. However, in
ceMRI, the outline of all 16 nodules was determined satisfactorily.
The nodules in the s-ct demonstrated a clear outline against LE in
the T2WI scans (Figs. 1E and 2H). Of all the 27 nodules in the s-ct, 23
nodules exhibited the appearance of infiltrative growth, with
abnormally thickened fiber cables and/or deep fascia. There were 2
confluent nodules in the patient with AS and 1 confluent nodule in
the patient with mLAS. These confluent nodules exhibited abnormally
thickened and enhanced close fiber cables and deep fascia (Figs. 1F, 2F
and 2I). There were 13 non-confluent
nodules; 10 of these only exhibited abnormally thickened close
fiber cables, while the other 3 only exhibited abnormally thickened
close deep fascia. The diameters of 13 non-confluent nodules ranged
between 5–8 mm. The mean diameter of the 4 nodules without
infiltrative growth or fusion was 4 mm.
SI
The nodules of the 2 STS types were isointense
compared with the normal muscle in the same sections in the T1WI
sequences, yet there was no marked signal difference between the 2
STS types (Figs. 1D, 2D and 2G).
Their SI was decreased by various degrees compared with LE in T2WI
sequence, but they demonstrated a visually recognizable signal
difference. The SI in the patients with mLAS was more markedly
decreased (Fig. 2E and 2H) compared with that of the patients with
AS (Fig. 1E). When comparing the SI
of the normal muscle to the tumor nodules in the same slice, the SI
of mLAS was closer to that of the muscle compared with the SI of
AS. The SI of AS was slightly decreased even in comparison with LE,
and was higher compared with that of muscle. The nodules were
markedly enhanced following intravenous contrast in the 2 types of
STS (Table III).
Discussion
Chronic LE is an essential and important aspect of
the pathogenesis of STS (1–7). There are numerous factors that
contribute to this pathogenesis, including but not limited to tumor
radical surgery (radical mastectomy or radical hysterectomy), lymph
node excision, trauma and inflammation. However, there are also
cases of idiopathic LE (8–12). The identified patients with STS
involving the upper limb following radical mastectomy with or
without radiotherapy account for ~90% of the total cases described
(6,11). Nascimento et al (12) hypothesized that the incidence of STS
has markedly decreased due to the improvements in surgery and
radiation therapy techniques, and the popularity of breast
conservation therapy. The incidence of STS in the present study was
0.12% and was therefore consistent with previous studies (2,6). The
duration of LE and the age of STS onset were consistent with those
suggested in the literature (2,3). There was
1 patient with impact trauma 2 months previously, in whom nodules
in the dermis and s-ct were detected using ceMRI. This appearance
was described in previous studies (9,10), and
therefore it was not discussed. It was not considered possible that
the impact trauma was the direct causal factor of STS, despite of
the 2 nodules in the dermis. It is possible that following a trauma
in the fragile region of LE, it becomes difficult to repair the
local damage, and that a state of immunodeficiency would then occur
gradually and latently in this region, ultimately leading to STS.
It was reasoned that the 4 cases of STS without apparent causes
were closely associated with the occurrence of immunodeficiency in
LE (12–16).
In 1948, Stewart and Treves (1) first used the term LAS in a pathological
setting. In the following decades, AS and LAS were often used
interchangeably and applied and interpreted as a diagnostic term
synonymous for angiosarcoma, until 2011 when Yu and Yang (17) clearly explained the pathological
diagnosis standards of pLAS, and determined that AS and LAS were
actually misnomers. D2-40 is an available selective marker for the
lymphatic endothelium, and the sensitivity and the positive
predictive value of the diagnosis of LAS were shown to be excellent
(18–20). CD31 is an adequate and approved marker
for vascular endothelial markers in long-term clinical application
(21), and it is able to identify
tumor cells derived from the vascular endothelial cells. In a study
investigating pLAS (17), CD31 was
weakly positive or negative, and D2-40 was positive. While in the
present study, CD31 was positive in all 5 cases, this meant that no
cases were classed as pLAS, but each of the tumors consisted of
vascular endothelial tumor cells. In total, 1 out of 5 patients
exhibited positive expression of D2-40. The present study followed
previous advice (17) that all tumors
that express a mixed immunophenotype of CD31 and D2-40 should be
classified as a subset of LAS. It has been previously suggested
that tumor cells demonstrate at least partial differentiation along
the lymphatic endothelial lineage (18–20). As
such, the present patient, who demonstrated a mixed
immunophenotype, was diagnosed with mLAS. The other 4 cases were
classified as AS.
It was observed that the tumors demonstrated a
visually recognizable signal difference in the T2WI scans in the 2
STS types. The SI of AS and LAS was decreased compared with that of
LE, but the SI of mLAS was decreased more markedly compared with
that of AS. If compared with the normal muscle tissues, the SI of
mLAS was closer to that of muscle tissues compared with that of AS,
and the SI of AS was higher compared with that of muscle tissue.
Through analyzing and comparing MRI signals with the
histopathological results, it was identified that the tumors were
composed predominantly of dense atypical cells with oval nuclear
contours, prominent nucleoli and eosinophilic cytoplasm in the 2
STS types (Figs. 1A and 2A). In the AS tissues, the collagen fibrils
were abundantly distributed between the tumor nests. The structure
of dense tumor cells with or without collagen fibrils is the basis
of the tumor entity. In certain areas of the mLAS tumors, specific
clefts and slit-like channels were identified, occasionally with
specific sections that contained proteinaceous fluid and a small
number of lymphocytes (Fig. 2A).
Conversely, in the AS tumors, numerous clefts and channels were
present and were markedly dilated, and always contained free
erythrocytes. It was also observed that in the AS tumors there were
a number of immature vessels in the tumor nests and mature vessels
in the collagen fibrils (Fig. 1A).
The large number of vascular vessels may suggest that there was an
increased proportion of water molecules in the AS tumors. However,
the mLAS tumors clearly lacked these. It was hypothesized that the
overall structure in all types of tumors caused the SI of the tumor
to be decreased in comparison with LE areas. The different
proportions of vascular vessels caused the changes in SI by various
degrees compared with either normal muscle or LE tissues in the
T2WI sequences. A potential reason for this is that the tumors
demonstrated a visually recognizable difference in signals in the
two STS types. It is hypothesized that there are two reasons why
there are SI alterations in MRIs: One is the proportion of tumor
cells and blood vessels in the tumor (22,23), and
the other is due to the abnormal amount of fat and fibrous tissues
in LE (23). A similar difference
occurred in the present study too. Therefore, it is suggested that
the SI of normal muscle should be used as the internal reference,
in order to reduce the random subjective visual interference and to
obtain a unified idea of understanding of the SI.
Although there is trend and potential for multifocal
growth and early transformation in STS, confirmatory studies are
extremely rare (10). According to
current knowledge, the MRI data concerning these factors have not
been described. In the present study, there were not only 3 cases
of multifocal distribution, but also 13 nodules and 3 confluent
nodules with a diameter of >5 mm that exhibited a clear
infiltration growth trend in the s-ct. There were greater numbers
of transferred nodules (23 nodules) in the 2 patients with AS in
the s-ct compared with the numbers in the patient with mLAS (4
nodules). This was hypothesized to be associated with the existing
vascular lumen structure in the patients with AS and that the
vascular vessels provided blood supply for the growth of tumor and
establish tumor transfer along the vascular luminal structure,
acting as a ‘highway’. However, 1 mLAS case exhibited a lack of a
vascular lumen structure. The expression Ki-67 and p53 indices are
closely associated with the survival time; as suggested by the 2
patients with AS and 1 patient with mLAS. The expression Ki-67 and
p53 indices in the patients with AS were markedly higher compared
with that of the patient with mLAS, but the survival time
demonstrated the opposite outcome. This difference indicates that
the degree of malignancy of AS with no clear cause is higher
compared with that of mLAS with exogenous causal factors, such as
trauma, and also suggested the hypothesis that oncogenesis is
closely associated with the region of focal immunodeficiency in LE
(12–16). The reasons were as follows: Although
the expression of Ki-67 and p53 was not mentioned in the previous
studies investigating STS, it was hypothesized that the expression
of each index was actually associated with prognosis, not with age.
There were two unavoidable limitations to the present study namely
that the observation time was short and that the number of enrolled
cases was only 5.
In conclusion, the data of the present study suggest
that: i) Although it is difficult to clinically estimate the degree
of focal immunodeficiency in LE, it is feasible to perform MRI
scans for patients who have exhibited LE for >10 years. It is
necessary for patients to avoid trauma in the limb with LE to
reduce the number of patients with STS. ii) For the patients with
STS only located in the dermis, it may be beneficial to perform a
wide excision surgery, which may have a good prognosis. For
multifocal STS with early metastasis, the prognosis of LAS may be
improved compared with that of AS. If the number of metastatic
nodules in the s-ct is >8 in AS, even amputation may result in a
poor prognostic outcome. iii) In conventional MRI scans, it is easy
to detect a visually recognizable signal difference between the two
STS types compared with histological examination. Obtaining a
unified understanding of the tumor SI by use of the normal muscle
and LE SIs as references will also assist this. The MRI scans
provide a more accurate evaluation of multifocality and stages of
early transformation.
It is hypothesized that MRI scans are a useful tool
for evaluating STS, and that they also provide important diagnostic
information in a clinical setting. A chronic case of LE, in which
MRI scans demonstrate the nodules in the LE, suggest a diagnosis of
STS.
References
|
1
|
Stewart FW and Treves N: Lymphangiosarcoma
in postmastectomy lymphedema; a report of six cases in
elephantiasis chirurgica. Cancer. 1:64–81. 1948. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fitzpatrick PJ: Lymphangiosarcoma and
breast cancer. Can J Surg. 12:172–177. 1969.PubMed/NCBI
|
|
3
|
Maldonado-Fernández N, López-Espada C,
Sánchez-Rodriguez J, Rodríguez-Morata A, Fernández-Quesada F,
Martínez-Gámez J, Moreno-Escobar J and García-Róspide V: Síndrome
de Stewart-Treves: Linfangiosarcoma en linfedema crónico
posmastectomía. Angiología. 54:467–471. 2002. View Article : Google Scholar
|
|
4
|
Young RJ, Brown NJ, Reed MW, Hughes D and
Woll PJ: Angiosarcoma. Lancet Oncol. 11:983–991. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sordillo PP, Chapman R, Hajdu SI, Magill
GB and Golbey RB: Lymphangiosarcoma. Cancer. 48:1674–1679. 1981.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Woodward AH, Ivins JC and Soule EH:
Lymphangiosarcoma arising in chronic lymphedematous extremities.
Cancer. 30:562–572. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Grobmyer SR, Daly JM, Glotzbach RE and
Grobmyer AJ III: Role of surgery in the management of
postmastectomy extremity angiosarcoma (Stewart-Treves syndrome). J
Surg Oncol. 73:182–188. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kerchner K, Fleischer A and Yosipovitch G:
Lower extremity lymphedema update: Pathophysiology, diagnosis, and
treatment guidelines. J Am Acad Dermatol. 59:324–331. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Borel Rinkes IH and de Jongste AB:
Lymphangiosarcoma in chronic lymphedema. Reports of 3 cases and
review of the literature. Acta Chir Scand. 152:227–230.
1986.PubMed/NCBI
|
|
10
|
Peyron N, Dandurand M and Guillot B:
Malignant tumors as complications of lymphedema. J Mal Vasc.
18:293–298. 1993.(In French). PubMed/NCBI
|
|
11
|
Martin MB, Kon ND, Kawamoto EH, Myers RT
and Sterchi JM: Postmastectomy angiosarcoma. Am Surg. 50:541–545.
1984.PubMed/NCBI
|
|
12
|
Nascimento AF, Raut CP and Fletcher CD:
Primary angiosarcoma of the breast: Clinicopathologic analysis of
49 cases, suggesting that grade is not prognostic. Am J Surg
Pathol. 32:1896–1904. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Futrell J and Meyers GH Jr: The regional
lymphatics and cancer immunity. Ann Surg. 177:1–7. 1973. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ruocco V, Brunetti G, Puca RV and Ruocco
E: The immunocompromised district: A unifying concept for
lymphoedematous, herpes-infected and otherwise damaged sites. J Eur
Acad Dermatol Venereol. 23:1364–1373. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ruocco V, Schwartz RA and Ruocco E:
Lymphedema: An immunologically vulnerable site for development of
neoplasms. J Am Acad Dermatol. 47:124–127. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ruocco V, Ruocco E, Schwartz RA and
Janniger CK: Kaposi sarcoma and quinine: A potentially overlooked
triggering factor in millions of Africans. J Am Acad Dermatol.
64:434–436. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yu L and Yang SJ: Lymphangiosarcoma of the
vocal cord: A rare entity defined by a D2-40 immunohistochemical
and ultrastructural study. J Clin Oncol. 29:e57–e61. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kahn HJ, Bailey D and Marks A: Monoclonal
antibody D2-40, a new marker of lymphatic endothelium, reacts with
Kaposi's sarcoma and a subset of angiosarcomas. Mod Pathol.
15:434–440. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fukunaga M: Expression of D2-40 in
lymphatic endothelium of normal tissues and in vascular tumours.
Histopathology. 46:396–402. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mankey CC, McHugh JB, Thomas DG and Lucas
DR: Can lymphangiosarcoma be resurrected? A clinicopathological and
immunohistochemical study of lymphatic differentiation in 49
angiosarcomas. Histopathology. 56:364–371. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ohsawa M, Naka N, Tomita Y, Kawamori D,
Kanno H and Aozasa K: Use of immunohistochemical procedures in
diagnosing angiosarcoma. Evaluation of 98 cases. Cancer.
75:2867–2874. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Schindera ST, Streit M, Kaelin U, Stauffer
E, Steinbach L and Anderson SE: Stewart-Treves syndrome: MR imaging
of a postmastectomy upper-limb chronic lymphedema with
angiosarcoma. Skeletal Radiol. 34:156–160. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nakazono T, Kudo S, Matsuo Y, Matsubayashi
R, Ehara S, Narisawa H and Yonemitsu N: Angiosarcoma associated
with chronic lymphedema (Stewart-Treves syndrome) of the leg: MR
imaging. Skeletal Radiol. 29:413–416. 2000. View Article : Google Scholar : PubMed/NCBI
|
















