Introduction
Leukemia is a kind of malignant tumor of the
hemopoietic system posing a great threat to human health (1). The mortality rate ranks sixth in males
and eighth in females among all malignant tumors in China in all
age groups, and first among children and adults under 35 years of
age (2). There is a higher number of
patients with acute myeloid leukemia (AML) than with acute
lymphoblastic leukemia (ALL) (AML:ALL=2.67:1.00); AML is mostly
reported in young adults (76.2%) and has a negative effect on
health (3). In contrast to solid
tumors, leukemia is a kind of ‘liquid tumor’ that cannot be removed
by surgery (4). At present,
chemotherapy is still the most widely used method in the treatment
of patients with leukemia due to the difficulty in matching bone
marrow for transplantation and the high price of molecular targeted
therapy (5). However, the current
research focus is to identify novel chemotherapy drugs with strong
activity and low toxicity from natural drugs that are able to
overcome chemotherapeutic resistance and adverse side reactions
(6).
Following cell apoptosis, autophagy has become the
hot research topic as type-II programmed death in recent years
(5). Autophagy involves the formation
of autophagic vacuoles, their integration with lysosomes and the
degradation of the contents of these autophagosomes (7). In contrast to cell apoptosis, autophagy
has a dual regulatory function in cell survival, which serves an
important role in the occurrence and development of tumors
(8). Previous studies have shown that
intracellular autophagy in certain tumor cells can kill these cells
and maintain the normal internal body environment in the early
stages of cancer (9,10). However, upon the formation of tumors,
autophagy promotes apoptosis by removing damaged organelles, so as
to reduce the growth of tumor cells (11,12).
Therefore, researching the mechanism of autophagy development has a
good prospect in clinical application for the prevention and
treatment of cancer (13).
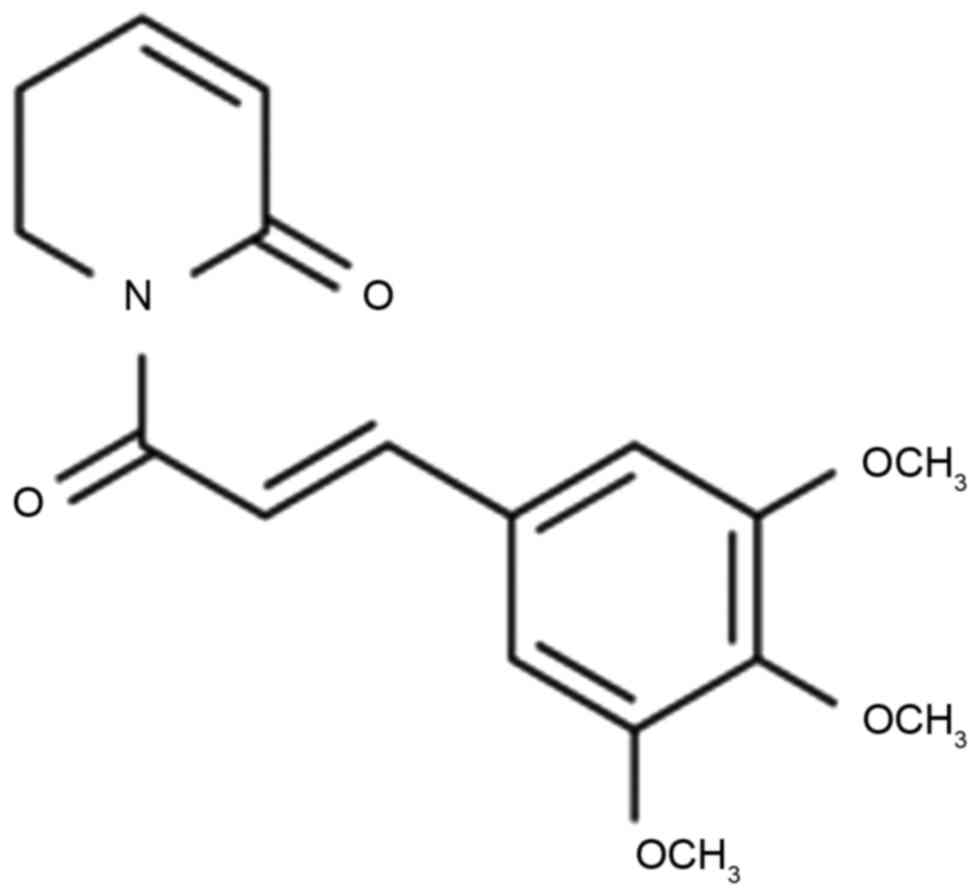
Piperlongumine (Fig.
1) belongs to the family of alkaloids, and exhibits a variety
of pharmacological effects and pharmacological activities,
including antiplatelet aggregation, analgesia and antifungal
properties (14). In addition,
piperlongumine has a marked cytotoxic effect on tumor cells and can
regulate blood lipid metabolism in hyperlipidemic rats (14). Previous studies have shown that
piperlongumine has a specific cytotoxic effect on a variety of
tumor cells, but exhibits low toxicity towards normal cells
(14,15). Therefore, piperlongumine is an extract
of a herb used in traditional Chinese medicine, which has the
potential to selectively kill tumors (14). The pharmacological effects of
piperlongumine mainly include antitumor, antiplatelet aggregation,
analgesic, antifungal, anti-schistosomiasis, anti-anxiety and
anti-depression effects (16). The
aim of the present study was to investigate whether piperlongumine
induces apoptosis and autophagy in leukemic cells, and to explore
the underlying molecular mechanisms.
Materials and methods
Cell lines and mice
Leukemic monocytic lymphoma U937 cells were
purchased from the Cell Bank of Type Culture Collection of the
Chinese Academy of Sciences (Shanghai, China) and cultured in
RPMI-1640 medium (pH 6.8) supplemented with 10% fetal bovine serum
(both from Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) and antibiotics (50 IU/ml penicillin G and 50 µg/ml
streptomycin), and were incubated at 37°C in a humidified
atmosphere containing 5% CO2.
Cell viability assay and
immunofluorescence
The effect of piperlongumine on the viability of
U937 cells was evaluated. U937 cells (2.5–5.0×104
cells/200 µl of RPMI-1640 medium/well) were seeded in 96-well
tissue culture plates and incubated with piperlongumine (0–20 µM)
for 48 h at 37°C in the presence of 5% CO2. MTT (2.0
mg/ml) and phenazine methyl sulfate (0.92 mg/ml) were added into
every well and incubated at 37°C for 3 h. The absorbance was
measured at 490 nm in an ELISA reader (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). Cells (at 60–70% confluence) were seeded in
6-well tissue culture plates and treated with piperlongumine (0–20
µM) for 48 h at 37°C in the presence of 5% CO2.
U937 cells treated with piperlongumine (0, 5, 10 and
20 µM) were washed with PBS and fixed using 4% paraformaldehyde for
15 min at room temperature. Cells were incubated with Triton X-100
(0.1%) for 10 min at room temperature and then incubated with
anti-LC3 antibodies (cat. no. 3868; 1:3,000; Cell Signaling
Technology, Inc., Beverly, MA, USA) at 4°C overnight. Cells were
incubated with Alexa Fluor 488-labeled goat anti-rabbit secondary
antibody (Thermo Fisher Scientific, Inc.) for 1 h at room
temperature in darkness. Cells were observed using a Nikon Eclipse
E800 fluorescence microscope and NIS-Elements 4.0 software (all
from Nikon; Tokyo, Japan).
Apoptosis assay
U937 cells (2.5×105 cells/ml) were
incubated with piperlongumine (0–20 µM) for 48 h at 37°C in the
presence of 5% CO2. Cells were washed twice with PBS and
resuspended in 100 µl of Annexin V binding buffer (pH 7.4) (BD
Biosciences, Franklin Lakes, NJ, USA). Then, annexin V-fluorescein
isothiocyanate (BD Biosciences) was added and incubated for 15 min
under dark conditions. Propidium iodide (0.1 µg/ml; Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany) was added just prior to signal
acquisition. The apoptosis rate was acquired using a FACSAria flow
cytometer (BD Biosciences) and analyzed with FACSDiva 7.6.1
software (BD Biosciences).
Western blotting
Total cellular proteins were isolated from U937
cells (2.5×105 cells/ml) incubated with piperlongumine
(0–20 µM) for 48 h at 37°C in the presence of 5% CO2.
Cells were lysed using radioimmunoprecipitation assay (RIPA) buffer
(Beyotime Institute of Biotechnology, Jiangsu, China) and
centrifuged for 10 min at 4°C at 10,000 × g. Protein concentration
was estimated using the bicinchoninic acid method (Beyotime
Institute of Biotechnology). Electrophoretic separations were
carried out on 10% SDS-PAGE, and the proteins were then
electrotransferred onto a polyvinylidene fluoride membrane. Blots
were blocked for 1 h at 37°C in TBS containing 0.01% Tween-20
(TBST) and 5% skimmed milk, and probed overnight at 4°C with
appropriate primary antibodies: Anti-microtubule-associated protein
1A/1B-light chain 3 (LC3-I; cat. no. 3868; 1:3,000),
anti-phosphorylated (p)-Akt (cat. no. 4228; 1:3,000), anti-Akt
(cat. no. 6211; 1:3,000), anti-p-mechanistic target of rapamycin
(mTOR; cat. no. 2974; 1:3,000), anti-p-p38 (cat. no. 4511, 1:2,000)
and anti-β-actin (cat. no. 4970, 1:2,000) (all from Cell Signaling
Technology, Inc., Beverly, MA, USA) antibodies. Next, the membranes
were washed with TBST and incubated with anti-rabbit IgG,
HRP-linked antibody (cat. no. 7074, 1:5,000, Cell Signaling
Technology, Inc.) at 37°C for 1 h. Proteins were visualized using
BeyoECL Plus (Beyotime Institute of Biotechnology) and analyzed
uisng Bio-Rad Laboratories Quantity One software 3.0 (Bio-Rad
Laboratories, Inc., Hercules, CA, USA).
Measurement of caspase activity
U937 cells (2.5–5.0×104 cells/200 µl of
RPMI-1640 medium/well) were seeded in 96-well tissue culture plates
and incubated with piperlongumine (0–20 µM) for 48 h at 37°C in the
presence of 5% CO2. Cells were lysed using RIPA buffer
(Beyotime Institute of Biotechnology) and centrifuged for 10 min at
4°C at 10,000 × g. Protein concentration was estimated using the
BCA Protein Assay kit (Beyotime Institute of Biotechnology). U937
cell lysates (5 µg) were incubated in 50 µl of reaction buffer and
acetyl-Asp-Glu-Val-Asp p-nitroanilide (p-NA) (Beyotime Institute of
Biotechnology) at 37°C for 5 h for determination of caspase-3
activity. The emission of pNA was measured at 405 nm in an ELISA
reader (Bio-Rad Laboratories, Inc.).
Statistical analysis
All experiments were repeated independently at least
three times using SPSS 17.0 (SPSS, Inc., Chicago, IL, USA). The
values were expressed as mean ± standard error of the mean, and
statistical analyses were performed with a two-way analysis of
variance followed by the Student-Newman-Keuls test.
Results
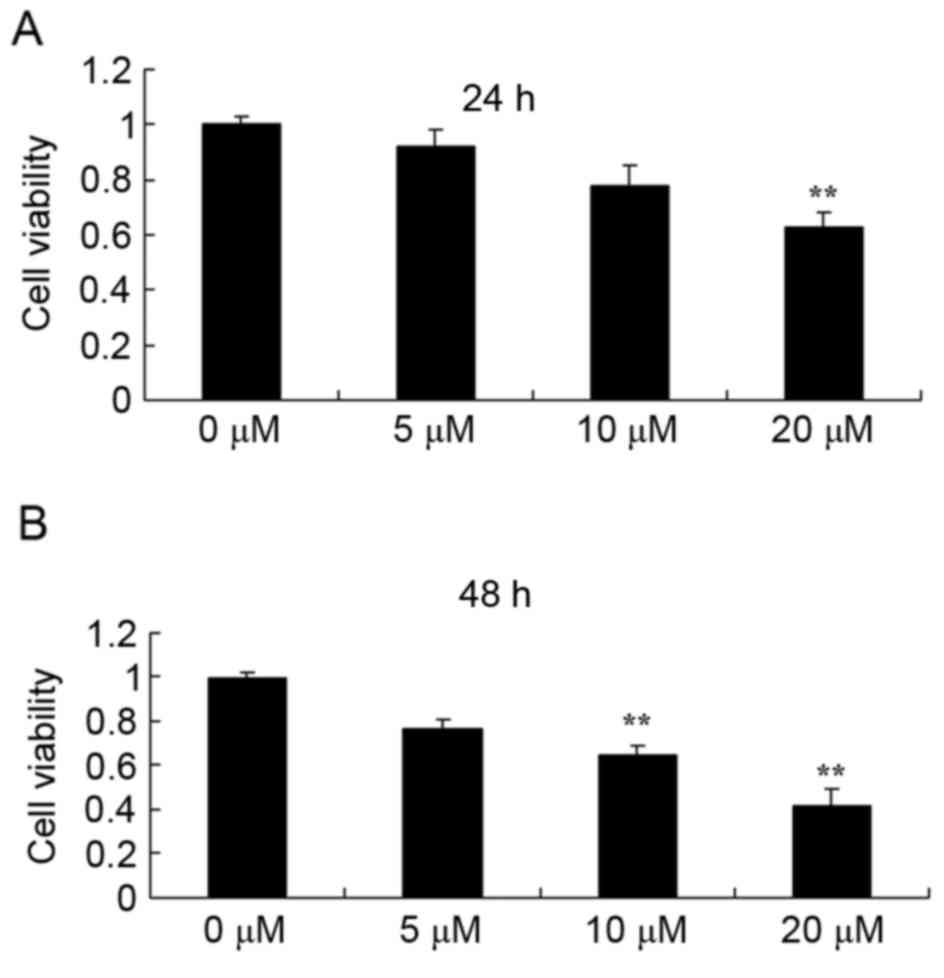
Piperlongumine suppresses cell
proliferation in leukemic cells
The anticancer effect of piperlongumine in terms of
suppressing the proliferation of leukemic cells was evaluated. An
MTT assay was used to analyze the change in cell proliferation in
U937 cells. As shown in Fig. 2, 0–20
µM of piperlongumine could suppress the proliferation of U937 cells
in a time- and dose-dependent manner. At 20 µM, piperlongumine
significantly suppressed the proliferation of U937 cells at 24 h,
while 10 or 20 µM of piperlongumine significantly suppressed the
proliferation of U937 cells at 48 h (Fig.
2).
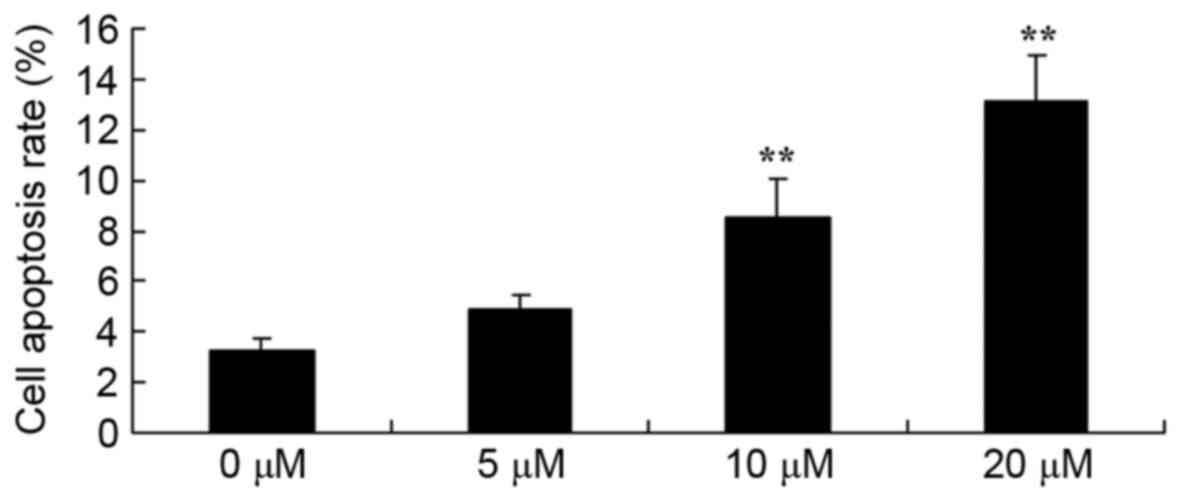
Piperlongumine induces apoptosis in
leukemic cells
To further confirm the role of apoptosis in the
effect of piperlongumine on leukemic cells, the apoptosis rate was
evaluated using flow cytometry. The results revealed that 0–20 µM
of piperlongumine induced the apoptosis of U937 cells in a
dose-dependent manner (Fig. 3).
Treatment with 10 or 20 µM of piperlongumine significantly induced
the apoptosis of U937 cells, compared with that of U937 cells
incubated with 0 µM of piperlongumine (Fig. 3).
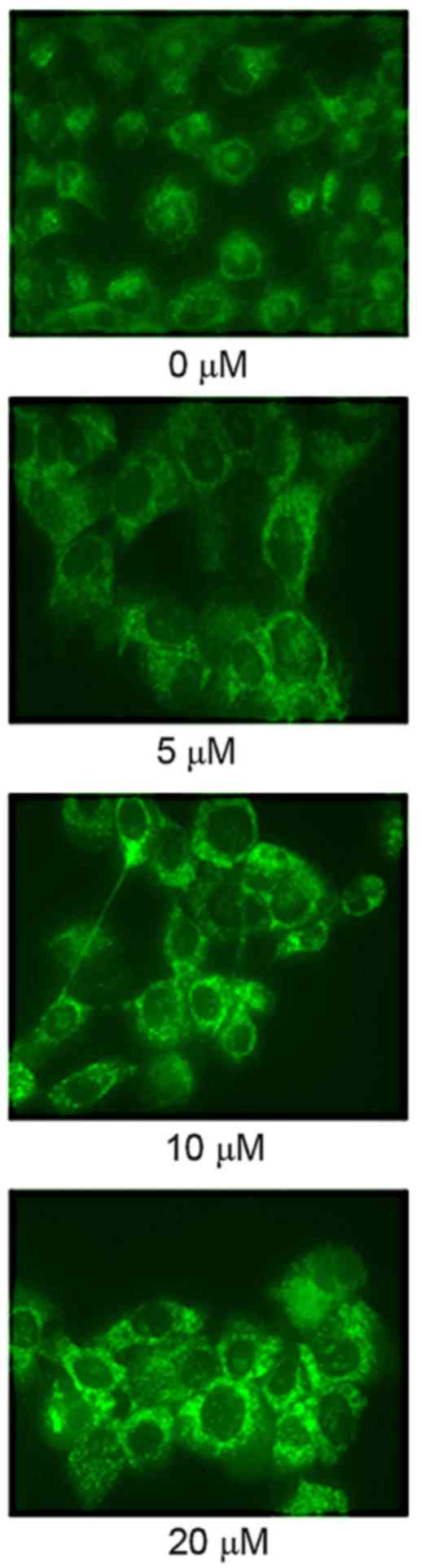
Piperlongumine induces autophagy in
leukemic cells
The autophagy of U937 cells was measured by
fluorescence microscopy targeted at LC3. An increase in the
autophagy of U937 cells incubated with 0–20 µM of piperlongumine
was observed (Fig. 4). Treatment with
10 or 20 µM of piperlongumine activated the autophagy of U937
cells, compared with that of U937 cells incubated with 0 µM of
piperlongumine (Fig. 4).
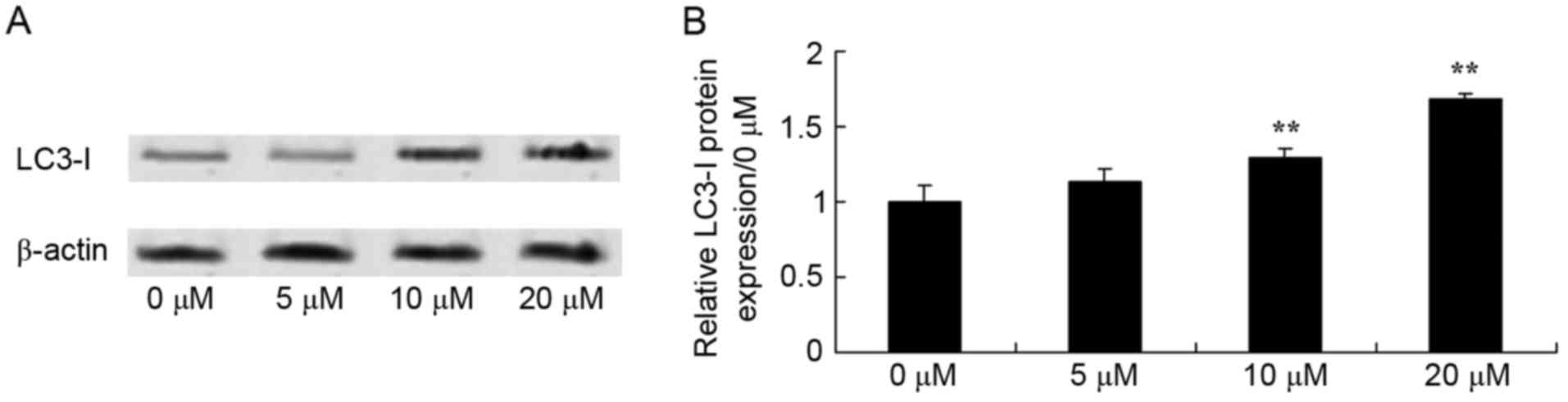
Piperlongumine induces LC3-I
expression in leukemic cells
Upon co-incubation of U937 cells with 0–20 µM of
piperlongumine for 48 h, the protein expression of LC3-I was
explored using western blotting. The protein expression of LC3-I
was induced by 0–20 µM of piperlongumine in a dose-dependent manner
(Fig. 5). In particular, 10 or 20 µM
of piperlongumine significantly increased LC3-I protein expression
in U937 cells, compared with that of U937 cells incubated with 0 µM
of piperlongumine (Fig. 5).
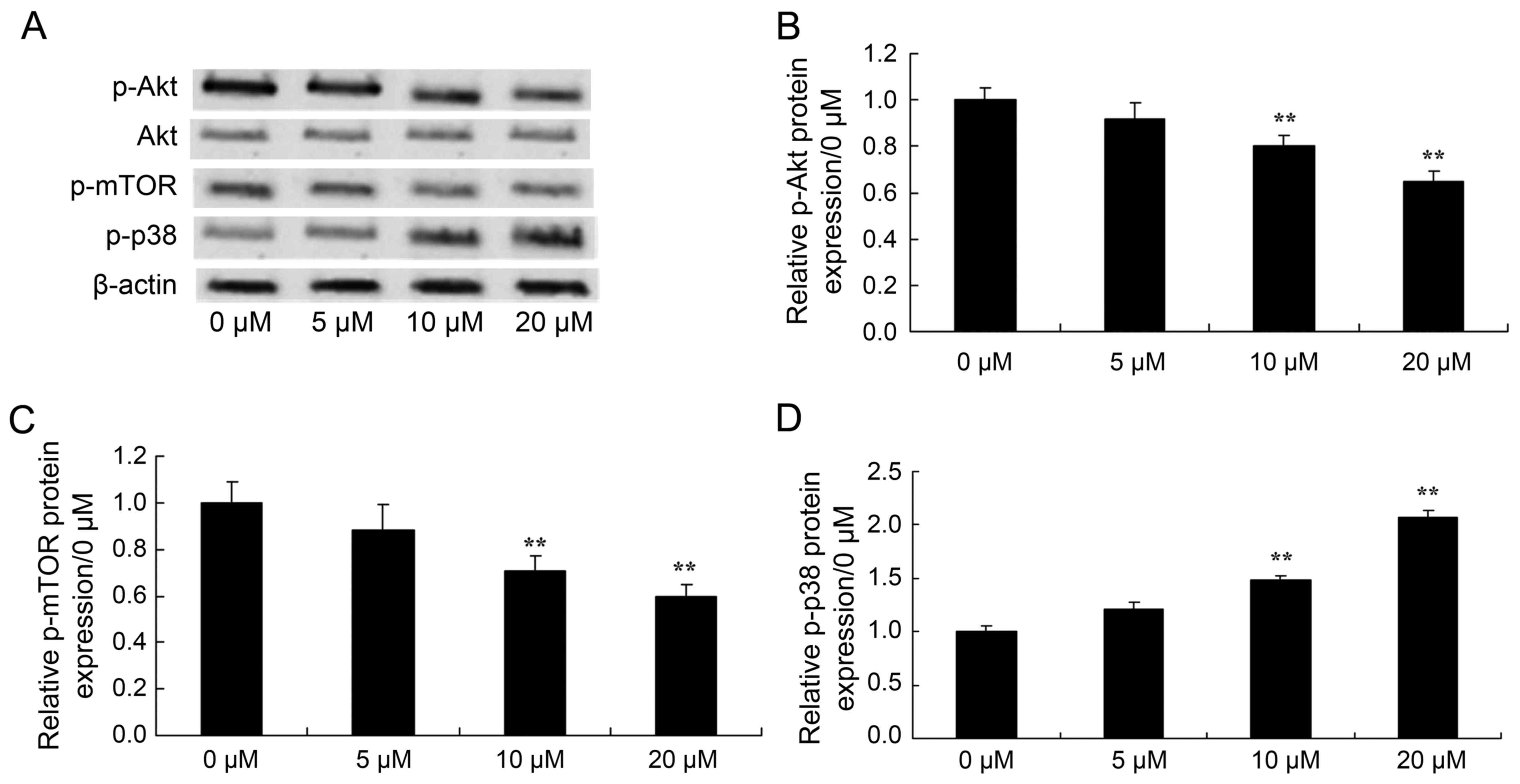
Piperlongumine reduces Akt levels in
leukemic cells
To analyze the mechanism of piperlongumine on
autophagy, the phosphorylation of Akt in U937 cells was determined
by western blotting. As shown in Fig.
6, a dose-dependent decrease in the ratio of p-Akt/Akt protein
expression was detected in U937 cells following treatment with 0–20
µM of piperlongumine. Particularly, 10 or 20 µM of piperlongumine
significantly reduced the p-Akt/Akt ratio in U937 cells, compared
with that of U937 cells incubated with 0 µM of piperlongumine
(Fig. 6A and B).
Piperlongumine reduces mTOR levels in
leukemic cells
To further analyze the mechanism of piperlongumine
on autophagy, the protein expression of p-mTOR was measured.
Fig. 6 shows that 0–20 µM of
piperlongumine inhibited p-mTOR protein expression in U937 cells in
a dose-dependent manner. Treatment with 10 or 20 µM of
piperlongumine significantly inhibited p-mTOR protein expression in
U937 cells, compared with that of the 0 µM of piperlongumine group
(Fig. 6A and C).
Piperlongumine induces the
phosphorylation of p38 protein in leukemic cells
To investigate the protein expression of p38, U937
cells were incubated with piperlongumine (0–20 µM) for 48 h.
Treatment with 0–20 µM of piperlongumine induced the protein
expression of p-p38 in U937 cells (Fig.
6A and D). Treatment with 10 or 20 µM of piperlongumine
significantly induced p-p38 protein expression in U937 cells,
compared with that of U937 cells incubated with 0 µM of
piperlongumine (Fig. 6A and D).
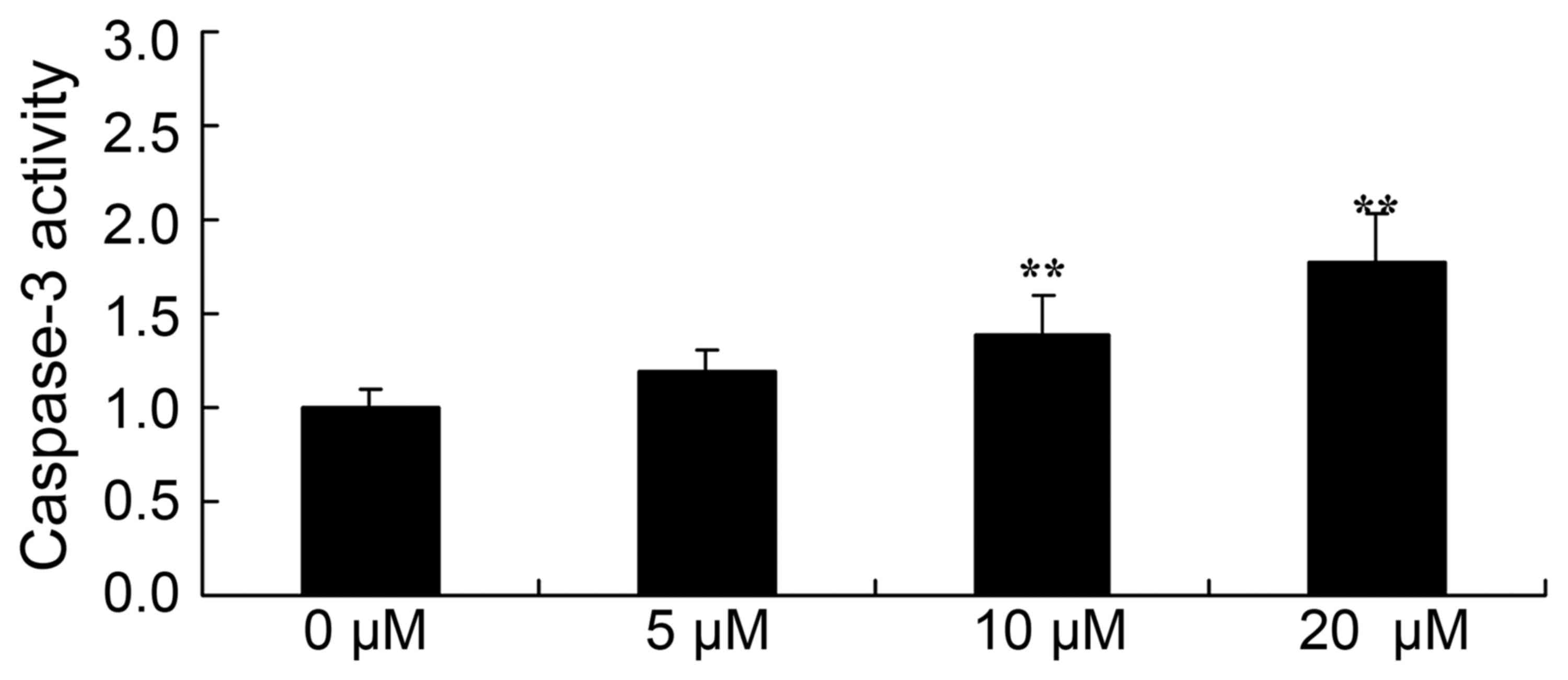
Piperlongumine induces caspase-3
activity in leukemic cells
In order to assess the role of caspase-3 activity in
U937 cells, the cells were co-incubated with 0–20 µM of
piperlongumine for 48 h. As shown in Fig.
7, 0–20 µM of piperlongumine increased the caspase-3 activity
of U937 cells in a dose-dependent manner. Particularly, treatment
with 10 or 20 µM of piperlongumine significantly increased the
caspase-3 activity of U937 cells, compared with that of U937 cells
incubated with 0 µM of piperlongumine (Fig. 7).
Discussion
Leukemia is a type of malignant tumor of the
hemopoietic system posing a severe threat to human health, and its
mortality rate ranks first among all malignant tumors among
children and adults under 35 years of age (17). Its morbidity and mortality are
increasing, and it is urgently required to identify novel
therapeutics against leukemic cells (18). Dhillon et al reported that
piperlongumine induces pancreatic cancer cell death (19). The present data clearly demonstrated
that piperlongumine significantly suppresses cell proliferation and
induces apoptosis in U937 cells.
Autophagy is a conserved self-degradation system in
eukaryotic cells, which is involved in numerous physiological and
pathological processes (13). The
autophagosome is the typical characteristic of autophagy, and the
regulation of the formation and degradation of the autophagosome is
the main regulation of autophagy (8).
Due to the dual characteristics of autophagy in promoting both cell
growth and death, autophagy determines cell survival to certain
extent (20). Autophagy is closely
associated with tumors, and is involved in tumor development,
metastasis and drug resistance (12).
Targeted autophagy may become a new strategy for the treatment of
cancer and drug resistance (21).
Further study of autophagy in leukemic cells will clarify the
mechanism of induction and regulation of autophagy in leukemic
cells, and may provide a new treatment target and strategy for
leukemic cells (22). In the present
study, it was demonstrated that piperlongumine induces autophagy
and induces expression of LC3-I protein in leukemic cells. Makhov
et al demonstrated that piperlongumine promotes autophagy in
a xenograft mouse model through inhibition of Akt/mTOR signaling,
and mediates cancer cell death (23).
Wang et al (24) showed that
Piperlongumine induces autophagy of cancer cell by targeting p38
signaling.
The main function of mTOR is to inhibit the
occurrence of self-autophagy via two mechanisms: i) mTOR regulates
the transcription and translation of autophagy-associated genes
through the activation of downstream effectors, which affects the
signal transduction pathway; and ii) inhibition of mTOR can induce
the occurrence of autophagy (25).
PI3K/Akt is the upstream signaling pathway that activates mTOR
(26). PI3K is important in cancer
development, while the serine-threonine protein kinase Akt is the
downstream effector of PI3K and is involved in the regulation of
various biological processes, including cell metastasis, growth,
development, apoptosis, and regulation of gene transcription,
protein synthesis and nutritional metabolism (27). Akt may be key to the inhibition and
survival signal pathway and inhibit autophagy by phosphorylating
mTOR and contacting PI3K/Akt (27).
The activated mTOR signal transduction pathway can inhibit the
apoptosis and autophagy induced by various factors, which leads to
cell cycle progression, cell growth and proliferation. It is also
associated with angiogenesis, thus serving an important role in the
formation, invasion and metastasis of tumors (28,29).
Numerous tumors exhibit mutated genes coding for proteins involved
in mTOR signaling, and the resulting over-activated mTOR signaling
pathway is mainly caused by abnormal expression of these proteins
(28,29). Previous studies have demonstrated that
breast cancer, leukemia, small cell lung cancer, urinary system
tumors and other diseases progress through PI3K/Akt/mTOR signaling
(22,27). The present study has demonstrated that
piperlongumine significantly reduces Akt/mTOR signaling in U937
cells. Wang et al previously demonstrated that
piperlongumine induces apoptosis and autophagy through inhibition
of the PI3K/Akt/mTOR signaling pathway in human lung cancer cells
(16).
The p38/mitogen-activated protein kinase (MAPK)
signaling pathway is involved in the activation of autophagy in
macrophages (30). MAPK p38 mainly
inhibits autophagy, and the effect of the p38/MAPK signaling
pathway is markedly complex in the development of cells (31). The activation of this pathway leads to
the inhibition of cell proliferation. p38 can also induce the
arrest of the cell cycle into the stationary phase and promote DNA
repair against the DNA damage induced by chemotherapy (32). In particular, p38 has been reported to
exhibit anti-apoptotic properties in various cell lines, and may
have a direct effect on tumor infiltration and metastasis (33). The present study noticed that
piperlongumine significantly induced p-p38 protein expression and
increased caspase-3 activity in U937 cells. Xiong et al
reported that piperlongumine induces the autophagic death of
primary myeloid leukemia cells through the p38/c-Jun N-terminal
kinase signaling pathway (34).
In summary, the present study has demonstrated that
piperlongumine significantly suppresses cell proliferation and
induces apoptosis in U937 cells. Preferentially, piperlongumine
significantly induced the autophagy of U937 cells through targeting
the PI3K/Akt/mTOR and p38 signaling pathways. The present data
suggest that piperlongumine could be applied in the treatment of
leukemic cells.
References
|
1
|
Hefazi M, Siddiqui M, Patnaik M, Wolanskyj
A, Alkhateeb H, Zblewski D, Elliott M, Hogan W, Litzow M and
Al-Kali A: Prognostic impact of combined NPM1+/FLT3-genotype in
patients with acute myeloid leukemia with intermediate risk
cytogenetics stratified by age and treatment modalities. Leuk Res:
Sep. 3:2015.(Epub ahead of print).
|
|
2
|
Ong E, Szedlak A, Kang Y, Smith P, Smith
N, McBride M, Finlay D, Vuori K, Mason J, Ball ED, et al: A
scalable method for molecular network reconstruction identifies
properties of targets and mutations in acute myeloid leukemia. J
Comput Biol. 22:266–288. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Urtishak KA, Edwards AY, Wang LS, Hudome
A, Robinson BW, Barrett JS, Cao K, Cory L, Moore JS, Bantly AD, et
al: Potent obatoclax cytotoxicity and activation of triple death
mode killing across infant acute lymphoblastic leukemia. Blood.
121:2689–2703. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang F, Liu Z, Zeng J, Zhu H, Li J, Cheng
X, Jiang T, Zhang L, Zhang C, Chen T, et al: Metformin
synergistically sensitizes FLT3-ITD-positive acute myeloid leukemia
to sorafenib by promoting mTOR-mediated apoptosis and autophagy.
Leuk Res. 39:1421–1427. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kim Y, Eom JI, Jeung HK, Jang JE, Kim JS,
Cheong JW, Kim YS and Min YH: Induction of cytosine
arabinoside-resistant human myeloid leukemia cell death through
autophagy regulation by hydroxychloroquine. Biomed Pharmacother.
73:87–96. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zauli G, Celeghini C, Melloni E, Voltan R,
Ongari M, Tiribelli M, di Iasio MG, Lanza F and Secchiero P: The
sorafenib plus nutlin-3 combination promotes synergistic
cytotoxicity in acute myeloid leukemic cells irrespectively of FLT3
and p53 status. Haematologica. 97:1722–1730. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Martelli AM, Lonetti A, Amadori S,
McCubrey JA and Chiarini F: Enhancing the effectiveness of
nucleoside analogs with mTORC1 blockers to treat acute myeloid
leukemia patients. Cell Cycle. 12:1815–1816. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu C, Xu P, Chen D, Fan X, Xu Y, Li M,
Yang X and Wang C: Roles of autophagy-related genes Beclin-1 and
LC3 in the development and progression of prostate cancer and
benign prostatic hyperplasia. Biomed Rep. 1:855–860.
2013.PubMed/NCBI
|
|
9
|
Liu L, He J, Wei X, Wan G, Lao Y, Xu W, Li
Z, Hu H, Hu Z, Luo X, et al: MicroRNA-20a-mediated loss of
autophagy contributes to breast tumorigenesis by promoting genomic
damage and instability. Oncogene. Jun 19–2017.(Epub ahead of
print). View Article : Google Scholar
|
|
10
|
Qiu S, Sun L, Jin Y, An Q, Weng C and
Zheng J: Silencing of BAG3 promotes the sensitivity of ovarian
cancer cells to cisplatin via inhibition of autophagy. Oncol Rep.
38:309–316. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Brigger D, Proikas-Cezanne T and Tschan
MP: WIPI-dependent autophagy during neutrophil differentiation of
NB4 acute promyelocytic leukemia cells. Cell Death Dis.
5:e13152014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xie N, Zhong L, Liu L, Fang Y, Qi X, Cao
J, Yang B, He Q and Ying M: Autophagy contributes to
dasatinib-induced myeloid differentiation of human acute myeloid
leukemia cells. Biochem Pharmacol. 89:74–85. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang SP, Niu YN, Yuan N, Zhang AH, Chao
D, Xu QP, Wang LJ, Zhang XG, Zhao WL, Zhao Y and Wang JR: Role of
autophagy in acute myeloid leukemia therapy. Chin J Cancer.
32:130–135. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jin HO, Park JA, Kim HA, Chang YH, Hong
YJ, Park IC and Lee JK: Piperlongumine downregulates the expression
of HER family in breast cancer cells. Biochem Biophys Res Commun.
486:1083–1089. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bullova P, Cougnoux A, Abunimer L, Kopacek
J, Pastorekova S and Pacak K: Hypoxia potentiates the cytotoxic
effect of piperlongumine in pheochromocytoma models. Oncotarget.
7:40531–40545. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang F, Mao Y, You Q, Hua D and Cai D:
Piperlongumine induces apoptosis and autophagy in human lung cancer
cells through inhibition of PI3K/Akt/mTOR pathway. Int J
Immunopathol Pharmacol. 28:362–373. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Malkan UY, Gunes G, Isik A, Eliacik E,
Etgul S, Aslan T, Balaban MS, Haznedaroglu IC, Demiroglu H, Goker
H, et al: Rebound thrombocytosis following induction chemotherapy
is an independent predictor of a good prognosis in acute myeloid
leukemia patients attaining first complete remission. Acta
Haematol. 134:32–37. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
O'Hear C, Inaba H, Pounds S, Shi L, Dahl
G, Bowman WP, Taub JW, Pui CH, Ribeiro RC, Coustan-Smith E, et al:
Gemtuzumab ozogamicin can reduce minimal residual disease in
patients with childhood acute myeloid leukemia. Cancer.
119:4036–4043. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dhillon H, Chikara S and Reindl KM:
Piperlongumine induces pancreatic cancer cell death by enhancing
reactive oxygen species and DNA damage. Toxicol Rep. 1:309–318.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yu J, Lan L, Lewin SJ, Rogers SA, Roy A,
Wu X, Gao P, Karanicolas J, Aubé J, Sun B and Xu L: Identification
of novel small molecule Beclin 1 mimetics activating autophagy.
Oncotarget. May 18–2017.(Epub ahead of print).
|
|
21
|
Tanios R, Bekdash A, Kassab E, Stone E,
Georgiou G, Frankel AE and Abi-Habib RJ: Human recombinant arginase
I(Co)-PEG5000 [HuArgI(Co)-PEG5000]-induced arginine depletion is
selectively cytotoxic to human acute myeloid leukemia cells. Leuk
Res. 37:1565–1571. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu LL, Long ZJ, Wang LX, Zheng FM, Fang
ZG, Yan M, Xu DF, Chen JJ, Wang SW, Lin DJ and Liu Q: Inhibition of
mTOR pathway sensitizes acute myeloid leukemia cells to aurora
inhibitors by suppression of glycolytic metabolism. Mol Cancer Res.
11:1326–1336. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Makhov P, Golovine K, Teper E, Kutikov A,
Mehrazin R, Corcoran A, Tulin A, Uzzo RG and Kolenko VM:
Piperlongumine promotes autophagy via inhibition of Akt/mTOR
signalling and mediates cancer cell death. Br J Cancer.
110:899–907. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang Y, Wang JW, Xiao X, Shan Y, Xue B,
Jiang G, He Q, Chen J, Xu HG, Zhao RX, et al: Piperlongumine
induces autophagy by targeting p38 signaling. Cell Death Dis.
4:e8242013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang KF, Yang H, Jiang WQ, Li S and Cai
YC: Puquitinib mesylate (XC-302) induces autophagy via inhibiting
the PI3K/AKT/mTOR signaling pathway in nasopharyngeal cancer cells.
Int J Mol Med. 36:1556–1562. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Huang WR, Chiu HC, Liao TL, Chuang KP,
Shih WL and Liu HJ: Avian reovirus protein p17 functions as a
nucleoporin Tpr suppressor leading to activation of p53, p21 and
PTEN and inactivation of PI3K/AKT/mTOR and ERK signaling pathways.
PLoS One. 10:e01336992015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhou ZW, Li XX, He ZX, Pan ST, Yang Y,
Zhang X, Chow K, Yang T, Qiu JX, Zhou Q, et al: Induction of
apoptosis and autophagy via sirtuin1- and PI3 K/Akt/mTOR-mediated
pathways by plumbagin in human prostate cancer cells. Drug Des
Devel Ther. 9:1511–1554. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tsai JP, Lee CH, Ying TH, Lin CL, Lin CL,
Hsueh JT and Hsieh YH: Licochalcone A induces autophagy through
PI3K/Akt/mTOR inactivation and autophagy suppression enhances
licochalcone A-induced apoptosis of human cervical cancer cells.
Oncotarget. 6:28851–28866. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sun H, Wang Z and Yakisich JS: Natural
products targeting autophagy via the PI3K/Akt/mTOR pathway as
anticancer agents. Anticancer Agents Med Chem. 13:1048–1056. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li JP, Yang YX, Liu QL, Pan ST, He ZX,
Zhang X, Yang T, Chen XW, Wang D, Qiu JX and Zhou SF: The
investigational Aurora kinase A inhibitor alisertib (MLN8237)
induces cell cycle G2/M arrest, apoptosis, and autophagy via p38
MAPK and Akt/mTOR signaling pathways in human breast cancer cells.
Drug Des Devel Ther. 9:1627–1652. 2015.PubMed/NCBI
|
|
31
|
Henson SM, Lanna A, Riddell NE, Franzese
O, Macaulay R, Griffiths SJ, Puleston DJ, Watson AS, Simon AK,
Tooze SA and Akbar AN: p38 signaling inhibits mTORC1-independent
autophagy in senescent human CD8+ T cells. J Clin Invest.
124:4004–4016. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu J, Chang F, Li F, Fu H, Wang J, Zhang
S, Zhao J and Yin D: Palmitate promotes autophagy and apoptosis
through ROS-dependent JNK and p38 MAPK. Biochem Biophys Res Commun.
463:262–267. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Qin Y, Zhou ZW, Pan ST, He ZX, Zhang X,
Qiu JX, Duan W, Yang T and Zhou SF: Graphene quantum dots induce
apoptosis, autophagy, and inflammatory response via p38
mitogen-activated protein kinase and nuclear factor-κB mediated
signaling pathways in activated THP-1 macrophages. Toxicology.
327:62–76. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Xiong XX, Liu JM, Qiu XY, Pan F, Yu SB and
Chen XQ: Piperlongumine induces apoptotic and autophagic death of
the primary myeloid leukemia cells from patients via activation of
ROS-p38/JNK pathways. Acta Pharmacol Sin. 36:362–374. 2015.
View Article : Google Scholar : PubMed/NCBI
|