Introduction
Soft tissue sarcomas (STSs) are a heterogeneous
group of rare tumors of mesenchymal origin, accounting for ~1% of
all adult malignancies (1).
Approximately 20% of all STS arise in the chest wall (2).
At present, there have been several analyses of the
prognostic factors influencing survival in patients with soft
tissue and bone sarcomas of the chest wall (2–6). Among
these factors, histological grade, age, tumor size, depth and
histological subtype are considered the most significant. The
majority of these analyses involved heterogeneous patient cohorts
due to varying inclusion criteria. Certain studies evaluated the
outcome of chest wall STS together with STS of the abdominal wall,
pelvis and extremities (6–8). Numerous other studies only included
patients who underwent full-thickness chest wall resections;
however, no rationale for the exclusion of patients with
superficial chest wall STS was provided (9–11). Thus,
patients with STS that infiltrated the bony chest wall structures
were assessed in these previous studies, resulting in a specific
patient population with advanced local disease. Furthermore, these
studies mixed patients with soft tissue and bone sarcomas in the
analyses, although chondrosarcomas and other bone sarcomas exhibit
different clinical behaviors compared with STS (12). Furthermore, only certain studies have
specifically focused on the outcomes of patients with chest wall
STS (13).
Although negative margins are commonly sought in the
surgical treatment of chest wall STS, the prognostic significance
of surgical margins remains controversial. None of the large
studies on chest wall STS assessed the prognostic significance of
microscopic surgical margins; instead these studies compared the
outcomes of patients with wide and marginal excisions as defined by
the surgeons rather than the pathologists (5,13). Thus,
the effect of microscopic surgical margins on the outcome of chest
wall STS warrants evaluation, and the question remains whether wide
resections with clear margins at any cost or more conservative
resections should be performed.
To further understand the clinical behavior of chest
wall STS, the present study reviewed the demographic, tumor and
treatment characteristics of 110 patients who underwent surgical
treatment with curative intent at the BG-University Hospital
Bergmannsheil (Bochum, Germany). The potential prognostic
indicators of survival and with focus on the effect of surgical
margins on disease outcome were assessed.
Patients and methods
Patients
A total of 122 patients with chest wall STS were
treated consecutively at the BG-University Hospital Bergmannsheil
between June 1999 and May 2016. Only patients presenting with
primary chest wall STS and no simultaneous distant metastases were
included in the present study. All patients underwent surgical
treatment with curative intent. Chest wall tumors extending into
the region bordered superiorly by the clavicles and inferiorly by
the rib margins were included. From this cohort, 3 patients were
excluded due to essential data regarding the initial surgical
procedure, including tumor size or margin status, not being
available. Furthermore, 9 patients, including those from other
countries, were lost to follow-up. Thus, the analyses were
restricted to 110 participants with full information available on
the outcome, histology and surgical margins of the initial
procedures. Patient follow-up results were obtained from the
BG-University Hospital Bergmannsheil database, medical records and
patient correspondence. The study was approved by the BG-University
Hospital Bergmannsheil Ethics Committee with the registration no.
4782-13.
Treatment
Preoperatively, computed tomography scans and/or
contrast-enhanced magnetic resonance imaging (MRI) of the chest
wall and the tumor site were routinely performed. The goal of
surgical treatment for all patients was resection of the primary
tumor with negative margins. A lateral clear margin of 2 cm of
healthy tissue was ensured wherever possible. In epifascial
lesions, a deep clear margin of one fascial layer was intended.
Full-thickness chest wall resections were performed on lesions
infiltrating the ribs or intercostal space. Plastic reconstructive
surgery involving skin grafts and local flaps was performed for
coverage of resulting soft tissue defects following partial- and
full-thickness chest wall resections.
Several patients received adjuvant radiation and/or
chemotherapy. The indication for adjuvant radiation or chemotherapy
was given at the discretion of the interdisciplinary tumor board of
the BG-University Hospital Bergmannsheil or referral
institutions.
Following surgical treatment, the follow-up
management for all patients included clinical examinations, chest
X-rays and contrast-enhanced MRIs every 3 months for the first 2
years, and then every six months for the next 3 years. The decision
of whether follow-up MRIs and chest X-rays would be continued
following five years for every six or twelve months was based on
previous tumor behavior and the decision of the informed
patient.
Histopathological classification
All STS were diagnosed and classified according to
the French Federation of Cancer Centers and the most recent World
Health Organization guidelines (14,15).
Surgical margins were assessed following fixation of the
pathological specimen (sample thickness 5 µm) with formalin (10%)
and staining the surface with ink (TMD™ Tissue Marking Dye, Blue;
General Data Company, Inc., Cincinnati, OH, USA). All pathological
slides and the according surgical margin widths were analyzed or
reviewed for consensus diagnosis by an experienced soft tissue
pathologist at the BG-University Hospital Bergmannsheil.
Statistical analysis
All patients were retrospectively analyzed regarding
possible prognostic factors influencing survival (Tables I and II). Overall survival (OS) was defined as
the time period between the date of surgery for primary disease and
the date of mortality from any cause or the date of the last
follow-up assessment in living patients. The local recurrence-free
survival (LRFS) was calculated as the time period between the date
of surgery for the primary disease and the date of first recurrence
or the date of the last follow-up assessment in recurrence-free
patients. Survival rates were estimated according to the
Kaplan-Meier method with 95% confidence intervals (CIs), and were
compared using the log-rank test. Multivariate analyses and
regression analysis of surgical margin widths were performed using
Cox's proportional hazards model and the Wald test. Variables that
were associated with P<0.05 in the univariate analysis were
included in the multivariate regression analysis to assess
independent prognostic factors for LRFS and OS. Data analyses were
performed using Stata software (version 11.2; StataCorp LP, College
Station, TX, USA). Mean data are presented. P<0.05 was
considered to indicate a statistically significant difference.
 | Table I.Results of the univariate analyses to
determine factors predictive of LRFS. |
Table I.
Results of the univariate analyses to
determine factors predictive of LRFS.
| Clinicopathological
characteristic | N | No. of local
recurrences | 1-year LRFS (95%
CI) | 2-year LRFS (95%
CI) | 5-year LRFS (95%
CI) | P-value
(log-rank) |
|---|
| All patients | 110 | 55 | 77.3 (68.1–84.2) | 70.4 (60.6–78.2) | 60.6 (50.3–69.4) |
|
| Age, years |
|
|
|
|
| 0.116 |
| ≤50 | 39 | 18 | 81.6 (65.3–90.8) | 79.0 (62.4–88.9) | 70.4 (52.9–82.5) |
|
|
>50 | 71 | 37 | 74.9 (62.7–83.6) | 65.3 (52.4–75.4) | 54.7 (41.5–66.1) |
|
| Gender |
|
|
|
|
| 0.007 |
|
Female | 57 | 35 | 74.1 (60.1–83.8) | 64.3 (49.9–75.6) | 49.2 (34.7–62.2) |
|
| Male | 53 | 20 | 80.7 (67.1–89.1) | 76.6 (62.4–86.0) | 72.3 (57.7–82.6) |
|
| Tumor size, cm |
|
|
|
|
| 0.104 |
| ≤5 | 37 | 17 | 89.1 (73.5–95.8) | 83.4 (66.6–92.2) | 73.9 (55.6–85.6) |
|
|
>5 | 73 | 38 | 71.0 (58.7–80.2) | 63.4 (50.7–73.6) | 53.4 (40.6–64.7) |
|
| Tumor depth |
|
|
|
|
| 0.109 |
|
Epifascial | 52 | 26 | 87.8 (74.9–94.3) | 81.7 (67.8–90.0) | 71.1 (56.1–81.8) |
|
|
Subfascial | 58 | 29 | 68.1 (54.3–78.6) | 60.3 (46.1–71.8) | 51.1 (36.6–63.8) |
|
| Grading |
|
|
|
|
|
<0.001a |
| G1 | 32 | 8 | 100 (−) | 96.8 (79.2–99.5) | 89.5 (70.8–96.5) |
|
| G2 | 32 | 18 | 77.4 (58.4–88.5) | 67.3 (47.7–80.9) | 53.5
(34.3–69.3) |
|
| G3 | 46 | 29 | 60.7
(44.5–73.5) | 52.8
(36.7–66.6) | 44.1
(28.3–58.8) |
|
| Subtype |
|
|
|
|
|
|
|
NOS | 31 | 18 | 64.5
(45.2–78.5) | 57.9
(38.7–73.0) | 57.9
(38.7–73.0) | 0.166 |
|
Angiosarcoma | 21 | 17 | 73.7
(47.9–88.1) | 57.9
(33.2–76.3) | 26.3
(9.6–46.8) | <0.001 |
|
Liposarcoma | 19 | 4 | 88.1
(60.2–96.9) | 88.1
(60.2–96.9) | 88.1
(60.2–96.9) | 0.007 |
|
Leiomyosarcoma | 10 | 4 | 80.0
(40.9–94.6) | 80.0
(40.9–94.6) | 66.7
(27.2–88.1) | 0.361 |
| Margin status |
|
|
|
|
| 0.275 |
| (Primary
tumor) |
|
|
|
|
|
|
| R0 | 96 | 47 | 76.7
(66.8–84.0) | 71.2
(60.8–79.2) | 61.5
(50.6–70.7) |
|
|
R1/R2 | 14 | 8 | 82.5
(46.1–95.3) | 61.9
(27.0–83.9) | 49.5
(16.9–75.7) |
|
| Full-thickness
resection received |
|
|
|
|
| 0.523 |
| No | 89 | 45 | 80.3
(70.3–87.3) | 71.9
(61.0–80.2) | 62.7
(51.2–72.2) |
|
|
Yes | 21 | 10 | 64.6
(39.6–81.4) | 64.6
(39.6–81.4) | 52.2
(27.7–72.0) |
|
| Wound closure |
|
|
|
|
| 0.917 |
| Primary
closure | 90 | 46 | 80.5
(70.5–87.4) | 72.0
(61.2–80.3) | 63.1
(51.7–72.5) |
|
| Plastic
surgical tissue transfer | 20 | 9 | 63.3
(38.1–80.6) | 63.3
(38.1–80.6) | 47.5
(22.1–69.3) |
|
| Adjuvant
radiotherapy received (Primary tumor) |
|
|
|
|
| 0.799 |
| No | 73 | 38 | 77.0
(65.3–85.3) | 72.6
(60.5–81.6) | 61.2
(48.4–71.8) |
|
|
Yes | 37 | 17 | 78.2
(61.1–88.5) | 65.9
(47.7–79.1) | 59.5
(41.1–73.8) |
|
| Adjuvant
chemotherapy received (Primary tumor) |
|
|
|
|
| 0.519 |
| No | 96 | 47 | 78.2
(68.3–85.4) | 71.4
(60.8–79.5) | 63.8
(52.7–72.9) |
|
|
Yes | 14 | 8 | 71.4
(40.6–88.2) | 63.5
(33.1–83.0) | 39.7
(14.8–64.0) |
|
 | Table II.Results of the univariate analyses to
determine factors predictive of OS. |
Table II.
Results of the univariate analyses to
determine factors predictive of OS.
| Clinicopathological
characteristic | N | No. of
mortalities | 1-year OS (95%
CI) | 2-year OS (95%
CI) | 5-year OS (95%
CI) | P-value
(log-rank) |
|---|
| All patients | 110 | 44 | 90.7
(83.3–94.9) | 80.0
(71.0–86.5) | 66.0
(55.9–74.3) |
|
| Age, years |
|
|
|
|
| 0.003 |
|
≤50 | 39 | 8 | 94.8
(80.8–98.7) | 89.4
(74.1–95.9) | 80.9
(64.0–90.4) |
|
|
>50 | 71 | 36 | 88.3
(77.9–94.0) | 74.6
(62.3–83.4) | 57.6
(44.7–68.5) |
|
| Gender |
|
|
|
|
| 0.267 |
|
Female | 57 | 25 | 87.1
(74.8–93.6) | 75.6
(61.7–85.1) | 59.5
(44.8–71.5) |
|
|
Male | 53 | 19 | 94.3
(83.5–98.1) | 84.5
(71.4–91.9) | 72.5
(58.0–82.7) |
|
| Tumor size, cm |
|
|
|
|
| 0.022 |
| ≤5 | 37 | 10 | 94.6
(80.1–98.6) | 89.2
(73.7–95.8) | 80.5
(63.3–90.2) |
|
|
>5 | 73 | 34 | 88.6
(78.5–94.1) | 75.0
(62.8–83.7) | 58.0
(45.2–68.8) |
|
| Tumor depth |
|
|
|
|
| 0.098 |
|
Epifascial | 52 | 18 | 96.0
(85.1–99.0) | 86.0
(72.9–93.1) | 75.6
(60.9–85.3) |
|
|
Subfascial | 58 | 26 | 85.9
(73.8–92.7) | 74.6
(60.8–84.1) | 57.3
(43.0–69.3) |
|
| Grading |
|
|
|
|
| 0.003a |
| G1 | 32 | 7 | 100 (−) | 100 (−) | 88.9
(69.3–96.3) |
|
| G2 | 32 | 13 | 87.5
(70.0–95.1) | 84.3
(66.2–93.1) | 64.0
(44.4–78.3) |
|
| G3 | 46 | 24 | 86.3
(72.0–93.6) | 62.3
(46.0–75.0) | 50.3
(34.5–64.2) |
|
| Subtype |
|
|
|
|
|
|
|
NOS | 31 | 18 | 64.5
(45.2–78.5) | 57.9
(38.7–73.0) | 57.9
(38.7–73.0) | 0.166 |
|
Angiosarcoma | 21 | 17 | 73.7
(47.9–88.1) | 57.9
(33.2–76.3) | 26.3
(9.6–46.8) | <0.001 |
|
Liposarcoma | 19 | 4 | 88.1
(60.2–96.9) | 88.1
(60.2–96.9) | 88.1
(60.2–96.9) | 0.007 |
|
Leiomyosarcoma | 10 | 4 | 80.0
(40.9–94.6) | 80.0
(40.9–94.6) | 66.7
(27.2–88.1) | 0.361 |
| Margin status
(Primary tumor) |
|
|
|
|
| 0.046 |
| R0 | 96 | 36 | 92.5
(85.0–96.4) | 82.6
(73.1–88.9) | 69.9
(59.2–78.3) |
|
|
R1/R2 | 14 | 8 | 76.9
(44.2–91.9) | 61.5
(30.8–81.8) | 38.5
(14.1–62.8) |
|
| Full-thickness
resection |
|
|
|
|
| 0.025 |
| No | 89 | 32 | 96.5
(89.6–98.9) | 84.5
(74.7–90.7) | 70.7
(59.5–79.3) |
|
|
Yes | 21 | 12 | 66.7
(42.5–82.5) | 61.9
(38.1–78.8) | 46.4
(24.4–65.9) |
|
| Wound closure |
|
|
|
|
| 0.352 |
| Primary
closure | 90 | 35 | 94.3
(86.9–97.6) | 82.5
(72.7–89.1) | 68.0
(56.9–76.9) |
|
| Plastic
surgical tissue transfer | 20 | 9 | 73.7
(47.9–88.1) | 68.4
(42.8–84.4) | 55.8
(30.2–75.2) |
|
| Adjuvant
radiotherapy received (Primary tumor) |
|
|
|
|
| 0.383 |
| No | 73 | 27 | 90.0
(80.2–95.1) | 79.8
(68.3–87.5) | 70.5
(58.0–79.9) |
|
|
Yes | 37 | 17 | 91.9
(76.9–97.3) | 80.4
(63.2–90.2) | 57.4
(39.6–71.8) |
|
| Adjuvant
chemotherapy received (Primary tumor) |
|
|
|
|
| 0.479 |
| No | 96 | 37 | 89.3
(81.0–94.1) | 80.4
(70.7–87.2) | 66.6
(55.7–75.3) |
|
|
Yes | 14 | 7 | 100 (−) | 76.9
(44.2–91.9) | 61.5
(30.8–81.8) |
|
Results
Patient and tumor characteristics
The median age of patients at the time of primary
diagnosis was 59.8 years (range, 17.3–91.7) for the entire cohort.
There were 57 female (51.8%) and 53 male (48.2%) individuals. Only
patients with primary STS of the chest wall were included in the
present study. A total of 61 patients (55.5%) presented with
untreated primary tumors at the BG-University Hospital
Bergmannsheil. However, several patients were referred to the
BG-University Hospital Bergmannsheil following ‘whoops’ procedures.
A total of 49 patients (44.5%) underwent previous inadequate
resections of their primary tumors. Of these, 38 (77.6%) underwent
previous R1 resections and 4 patients (8.1%) underwent resections
with R2 margins. In the remaining 7 patients (14.3%) the margin
status was unclear. All of these 49 patients underwent subsequent
re-excisions at the BG-University Hospital Bergmannsheil.
A total of 55 patients (50.0%) developed ≥1 local
recurrence, whereas 30 patients (27.3%) had ≥2 local recurrences
(range, 2–16) during the course of disease. Over time, 27 patients
(24.5%) developed distant metastases. Of these patients, 15
presented with pulmonary metastases. The median survival time
following diagnosis of the initial metastasis was 0.9 years (95%
CI, 0.3–1.7).
The distribution of the histological grading was as
follows: G1 in 32 cases (29.1%); G2 in 32 cases (29.1%); G3 in 46
cases (41.8%). Primary tumors were located epifascially in 52
patients (47.3%), while 58 patients (52.7%) presented with
subfascial tumors. In 73 patients (66.4%), the primary tumors were
>5 cm. Among the entire cohort, the most frequent histotypes
were as follows: 31 sarcoma not otherwise specified (sarcoma NOS;
28.2%); 21 angiosarcoma (19.1%); 19 liposarcoma (17.3%).
Treatment characteristics
Surgical resection of the primary tumor in one or
two steps resulted in microscopically negative margins (R0) in 96
patients (87.3%), whereas 10 patients (9.1%) exhibited
microscopically positive margins (R1) and 4 patients (3.6%)
exhibited macroscopically positive margins (2). In the patients with positive margins,
tumors infiltrated critical anatomical structures or were too
advanced and widespread for complete resection, which may have
resulted in increased morbidity or more extensive surgery. Thus,
positive margins were tolerated consensually in these patients.
Continuous follow-ups were performed in these patients to monitor
tumor progression.
Full-thickness chest wall resections were performed
in 21 patients (19.1%) presenting with primary tumors. Following
the resection of the primary tumor, soft tissue defects had to be
covered in 16 patients (14.5%) with local flaps, while 4 patients
(3.6%) underwent transplantation with split-thickness skin grafts
due to skin defects.
A total of 37 patients (33.7%) received adjuvant
radiotherapy following resection of their primary tumor, with a
median overall dose of 57.6 Gy (range, 44.8–70.0). Of these 37
patients, 18 (48.6%) had G3 lesions while 17 (45.9%) had G2 tumors.
A total of 2 patients (5.4%) with R1-resected G1 tumors were also
radiated postoperatively. No patients were treated preoperatively
with radiation.
A total of 14 patients received adjuvant
anthracycline-based chemotherapy following resection of the primary
tumor. A total of 10 of these patients (71.4%) had G3 tumors and 4
patients (28.6%) had G2 tumors. All patients treated with adjuvant
chemotherapy had subfascially localized tumors >5 cm.
Follow-up
As of June 2016 (cut-off date), the median follow-up
was 5.4 years and the reverse Kaplan-Meier estimate of the median
follow-up following primary diagnosis was 9.6 years (95% CI,
7.2–10.5) (16,17). At the cut-off date, 61 patients
(55.5%) had no evidence of disease whereas 5 patients (4.5%) were
alive with residual localized disease. During follow-up, 44
patients (40.0%) had succumbed to the disease.
Univariate analysis of LRFS
The 5-year rate of LRFS was 60.6% (95% CI,
50.3–69.4) for the entire cohort. Female gender, histological
grade, and the angiosarcoma and liposarcoma subtypes were the only
factors with a statistically significant effect on LRFS following
univariate analysis (Table I). Female
patients exhibited a significantly decreased LRFS compared with
male patients [5-year LRFS, 49.2% (34.7–62.2) vs. 72.3%
(57.7–82.6); P=0.007). Regarding the histological grade, G1 tumors
had a significantly more favorable local outcome compared with G2
and G3 lesions [5-year LRFS, G1 89.5% (70.8–96.5) vs. G2 53.5%
(34.3–69.3) vs. G3 44.1% (28.3–58.8); P<0.001].
Angiosarcomas were associated with a significantly
diminished LRFS [5-year LRFS, 26.3% (9.6–46.8) vs. 69.2%
(58.1–77.9); P<0.001]. By contrast, liposarcomas displayed the
lowest rates of local recurrence [5-year LRFS, 88.1% (60.2–96.9)
vs. 55.6% (44.4–65.5); P=0.007].
When analyzing the treatment characteristics, the
surgically attained margin status had no statistically significant
effect on LRFS [5-year LRFS, R0 61.5% (50.6–70.7) vs. R1/R2 49.5%
(16.9–75.7); P=0.275]. Adjuvant radiation (P=0.799) and
chemotherapy (P=0.519) also had no significant influence on
LRFS.
Univariate analysis of OS
Overall, the 5-year estimate of the OS rate was
66.0% (95% CI, 55.9–74.3). Age, tumor size, histological grade and
full-thickness resections were demonstrated to be statistically
significant predictors of OS in the univariate analysis (Table II). Patients >50 years old at the
time of primary diagnosis had significantly reduced OS compared
with younger patients [5-year OS, 57.6% (44.7–68.5) vs. 80.9%
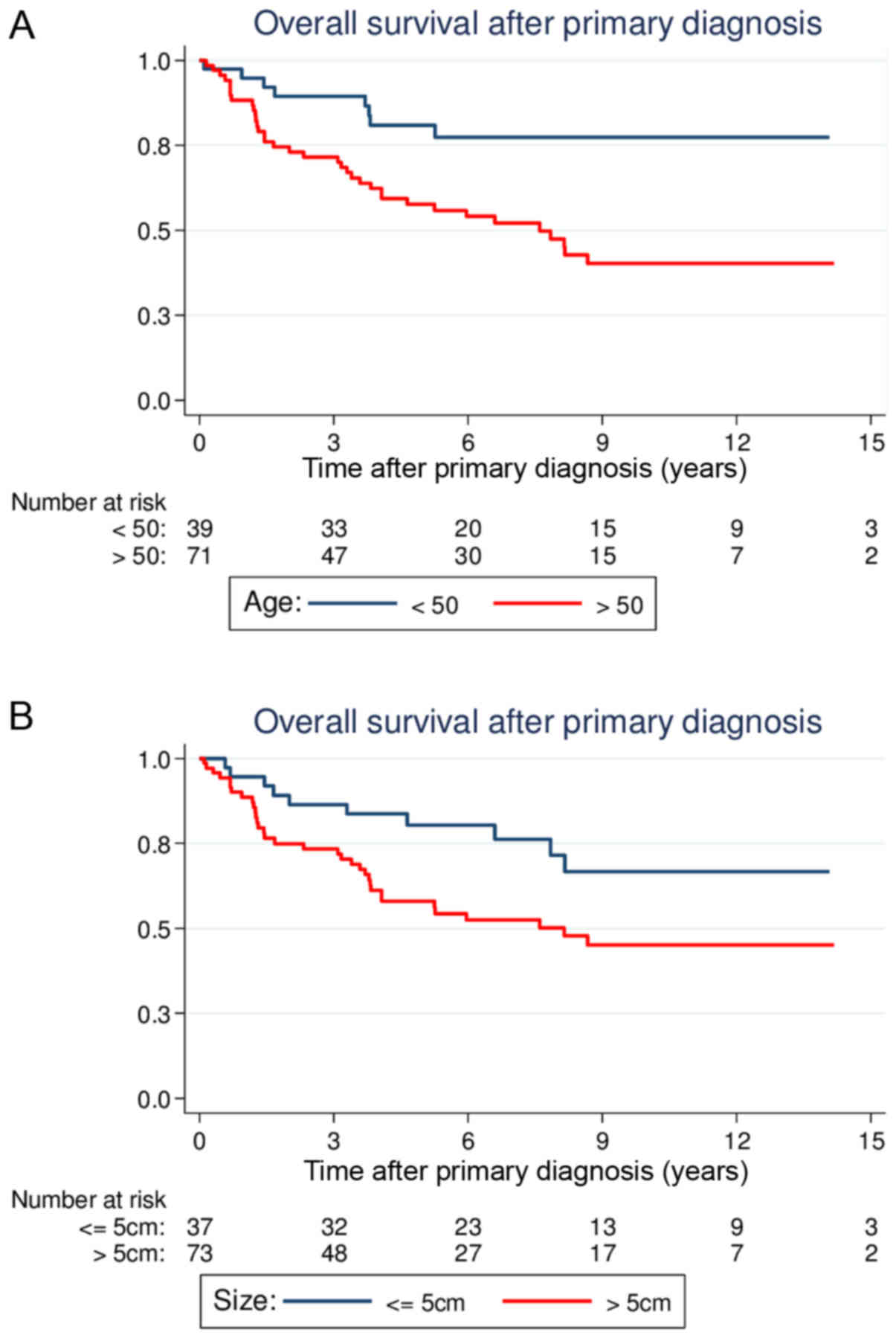
(64.0–90.4); P=0.003; Fig. 1A]. Large
tumor size (>5 cm) was also associated with a significantly
reduced OS compared with smaller tumor sizes [5-year OS, 58.0%
(45.2–68.8) vs. 80.5% (63.3–90.2); P=0.022; Fig. 1B]. Similar to the results for LRFS,
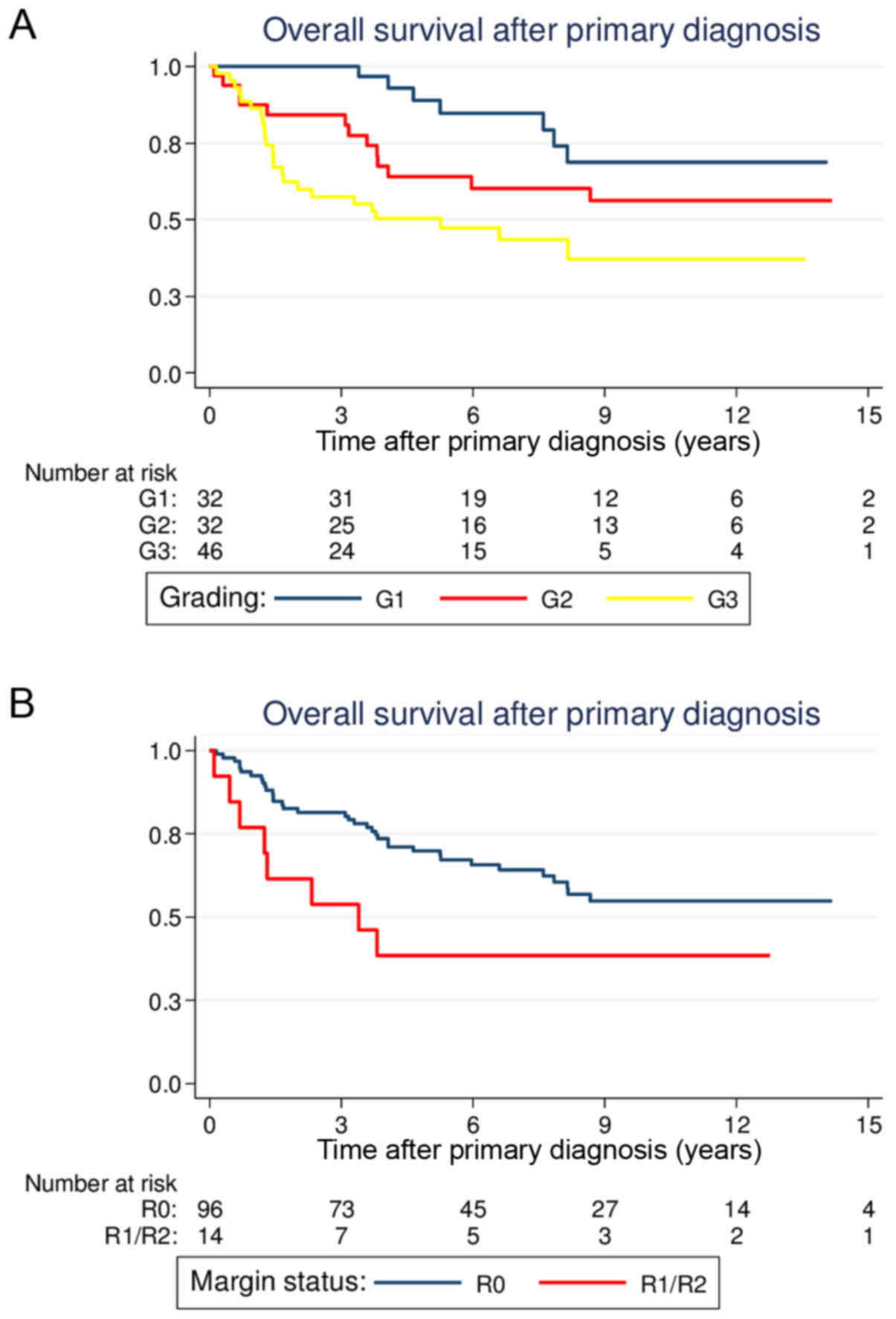
patients with G1 lesions had more favorable prognoses compared with
patients with G2 or G3 lesions [5-year OS, G1 88.9% (69.3–96.3) vs.
G2 64.0% (44.4–78.3) vs. G3 50.3% (34.5–64.2); P=0.003; Fig. 2A]. Regarding the different
histological subsets, patients with liposarcomas tended to have a
more favorable OS (P=0.007), whereas patients with angiosarcomas
had reduced survival (P<0.001). The other histological subtypes
were not associated with a significantly altered outcome.
The surgical margin status following treatment of
the primary tumor reached prognostic significance in the univariate
analysis. Patients with R0 margins had a significantly better OS
compared with patients with positive margins [5-year OS, 69.9%
(59.2–78.3) vs. 38.5% (14.1–62.8); P=0.046; Fig. 2B]. Notably, patients who underwent
full-thickness chest wall resections for the primary tumors had a
significantly diminished OS [5-year OS, 46.4% (24.4–65.9) vs. 70.7%
(59.5–79.3); P=0.025]. Similar to the results for LRFS, adjuvant
radiation (P=0.383) and chemotherapy (P=0.479) did not
significantly alter OS.
Multivariate analysis of LRFS
In the Cox's hazard regression model, the
significant and independent prognostic factors for the local
outcome were identified as histological grade and angiosarcoma
subtype (Table III). The hazard
ratio (HR) for local recurrence was 2.65 (95% CI, 1.15–6.11;
P=0.022) for G2 and 3.99 (95% CI, 1.86–8.54; P<0.001) for G3
lesions. Angiosarcomas presented an HR of 1.96 (95% CI, 1.02–3.78;
P=0.043).
 | Table III.Results of multivariate analysis on
LRFS according to Cox's proportional hazards model. |
Table III.
Results of multivariate analysis on
LRFS according to Cox's proportional hazards model.
| Category:
(reference) | Hazard ratio for
recurrence | 95% confidence
interval | P-value |
|---|
| Gender: Female (vs.
male) | 1.16 | 0.58–2.30 | 0.679 |
| Histological grade:
G2 (vs. G1) | 2.65 | 1.15–6.11 | 0.022 |
| Histological grade:
G3 (vs. G1) | 3.99 | 1.86–8.54 | <0.001 |
| Histological
subtype: Angiosarcoma (vs. other) | 1.96 | 1.02–3.78 | 0.043 |
| Histological
subtype: Liposarcoma (vs. other) | 0.36 | 0.10–1.27 | 0.113 |
Multivariate analysis of OS
Multivariate analysis revealed age, histological
grade, tumor size and angiosarcoma subtype as independent
prognostic factors of OS (Table IV).
The HR for mortality was 2.84 (95% CI, 1.27–6.39; P=0.011) in
patients >50 years. Regarding the prognostic significance of
tumor size and histological grade, the HR for mortality was 2.43
(95% CI, 1.13–5.23; P=0.023) for tumors >5 cm and 3.02 (95% CI,
1.35–6.76; P=0.0007) for G3 tumors when compared with G1 lesions.
Angiosarcomas presented a HR of 2.32 (95% CI, 1.20–4.44;
P=0.012).
 | Table IV.Results of multivariate analysis on
OS according to Cox's proportional hazards model. |
Table IV.
Results of multivariate analysis on
OS according to Cox's proportional hazards model.
| Clinicopathological
characteristic: (reference) | Hazard ratio for
mortality | 95% confidence
interval | P-value |
|---|
| Age: >50 years
(vs. ≤50 years) | 2.84 | 1.27–6.39 | 0.011 |
| Margin status:
R1/R2 (vs. R0) | 1.50 | 0.62–3.64 | 0.370 |
| Histological grade:
G2 (vs. G1) | 1.40 | 0.61–3.20 | 0.423 |
| Histological grade:
G3 (vs. G1) | 3.02 | 1.35–6.76 | 0.007 |
| Tumor size: >5
cm (vs. ≤5 cm) | 2.43 | 1.13–5.23 | 0.023 |
| Histological
subtype: Angiosarcoma (vs. other) | 2.31 | 1.20–4.44 | 0.012 |
| Full-thickness
resection: Yes (vs. no) | 1.59 | 0.66–3.84 | 0.303 |
Although margin status and full-thickness resection
were found to be statistically significant predictors of OS in
univariate analysis, they failed to reach statistical significance
in multivariate analysis because they were dependent on
histological grade and tumor size. In other words, positive margins
and full-thickness resections were more frequent in those cases
where tumors were large and high-grade. Therefore, none of the
assessed treatment characteristics was an independent predictor of
OS in multivariate analysis.
Regression analysis of non-categorized
surgical margin width
In the subgroup of patients with R0 margins, the
impact of negative margin widths was assessed. The closest negative
margin width (median, 0.6 cm) was assessed histologically at the
BG-University Hospital Bergmannsheil for 59/96 patients with
R0-resected tumors. Cox's regression analysis was performed to
evaluate the prognostic significance of non-categorized clear
margin widths in the R0 subgroup, which identified that the closest
surgical margin width did not significantly influence OS. The HR
for mortality following the Wald test was 0.52 (95% CI, 0.23–1.21)
for wide margins, which did not reach statistical significance
(P=0.129). LRFS was also unaffected by the surgical margin width.
The HR for tumor recurrence was 0.45 (95% CI, 0.18–1.08) for wide
margins (P=0.074). Thus, close and wide negative margins resulted
in similar OS and LRFS.
Discussion
In the present study, the outcome of 110 patients
who underwent surgical resection of primary chest wall STS with
curative intent was analyzed. NOS (28.2%), angiosarcoma (19.1%) and
liposarcoma (17.3%) were the most frequent histological subtypes in
our series. The majority of the tumors were high-grade (G3, 41.8%)
and large (>5 cm, 66.4%). Surgical treatment of the primary
tumor resulted in microscopic negative margins in 87.3% of all
patients. Despite surgical resection, 24.5% developed distant
metastases during the disease course. The median survival time
following the diagnosis of metastasis was 0.9 years. In the
multivariate analysis, age, tumor size, histological grade and
angiosarcoma subtype were identified as independent predictors of
OS. Several previous studies also confirmed the prognostic
significance of histological grade with respect to OS, although
these studies involved smaller patient cohorts (2–5). Regarding
the local outcome, angiosarcoma subtype and histological grade were
identified as independent prognostic factors. In the univariate
analysis, female gender was demonstrated to be a statistically
significant predictor of LRFS, but did not reach significance in
the multivariate analysis because it demonstrated a dependency
towards the angiosarcoma subtype. In the present study, all 21
patients with angiosarcoma were female.
One of the main aims of the present study was to
determine the prognostic significance of surgical margins. To the
best of our knowledge, this is the first study to analyze the
prognostic effects of surgical margins on survival in >100
patients with chest wall STS. Notably, margins were revealed to be
significant predictors of OS in univariate analysis whereby the
5-year OS rate was 69.9% for patients with microscopic negative
margins and 38.5% for those with positive margins. However,
surgical margins did not to reach statistical significance in
multivariate analysis as they demonstrated a dependency towards
being independent predictors of OS. The data of the present study
suggest that it may have been the factor ‘R0 resectability’ and not
the R0 resection itself that resulted in the improved outcome.
Conversely, tumors that could not be completely resected were
larger and exhibited more aggressive biological features compared
with completely resectable tumors. More specifically, it was the
inherent aggressiveness of the tumor itself that influenced the
surgically attainable margin status and the final outcome.
Subsequently, a positive margin status may be a result rather than
a cause of biological aggressiveness and may not influence the
outcome directly.
The question remains whether an aggressive surgical
approach would result in a survival benefit and should generally be
required in the treatment of chest wall STS. Presently, to the best
of our knowledge, no prospective studies have assessed this
question regarding chest wall STS or extremity STS. In the current
study, the results from the multivariate analysis suggest that
tumor biology dictates survival and that the quality of surgery has
a minor effect on the final outcome. Hence, radical surgery with
the aim of clear margins at any price cannot be justified by the
presented findings in order to improve OS. However, the translation
of retrospective data into clinical decisions is only possible to a
limited extent and possesses several problems. On the one hand,
whether the achievement of negative margins at any cost would have
improved OS in those patients with positive margins cannot be
assessed. On the other hand, the outcome of those patients with
negative margins if they had been treated with inadequate margins
cannot be estimated. Nevertheless, given the diminished outcome of
patients left with positive margins, it appears reasonable that
surgical efforts should aim for complete resections with negative
margins wherever feasible.
Reviewing the literature on chest wall STS, several
retrospective studies were identified, but they involved varied
patient cohorts and were difficult to compare (Table V). In the largest specific study on
chest wall STS, Gross et al (3) analyzed the outcomes of 55 surgically
treated patients in the Hospital do Cancer in Sao Paulo. In this
study, histological grade and tumor size were identified to be
significant prognostic factors for OS in the univariate analysis;
however, only histological grade emerged as an independent
prognostic factor. Notably, a 5-year OS rate of 87% was reported,
which is higher compared with the rate of 66% identified in the
current study. The two studies reported comparable median follow-up
durations (52 vs. 65 months) and had similar rates of high-grade
sarcomas. However, the study by Gross et al (3) did not include any patients with
angiosarcoma, whereas these patients accounted for 19.1% of the
patient population in the present study. The study by Gross et
al (3), which reviewed patients
between 1964 and 1996, did not reflect the patient distribution
reported in the present study. During the last few years, the
incidence of secondary angiosarcomas has risen due to the increased
use of adjuvant radiation in the treatment of breast cancer
(18,19). In the present study, 14.5% of the
entire cohort and 76.2% of all patients with angiosarcoma had
secondary angiosarcomas, a previous history of breast cancer and
adjuvant radiation treatment. Furthermore, the median age was 47.5
years in the study of Gross et al (3), while that in the present study was 59.8
years. Although the two studies focused on chest wall STS without
bone sarcomas, they are not comparable.
 | Table V.Overview of retrospective analyses on
primary chest wall sarcomas. |
Table V.
Overview of retrospective analyses on
primary chest wall sarcomas.
| Authors | No. of
patients | Median follow-up
(months) | Sarcoma type | M (%) | 5-OS (%) | Microscopic margins
available | Prognostic effect
of microscopic margins on OS | (Refs.) |
|---|
| Present study | 110 | 65 | STS | 24 | 66 | + | + |
|
| Kachroo et
al | 51 | NR | STS/bone/AF | 23 | 66 | + | − | (2) |
| Oksuz et
al | 26 | 82 | STS | 42 | 69 | + | − | (4) |
| Tsukushi et
al | 44 | 57 | STS | NR | 89 | − | NA | (5) |
| Gross et
al | 55 | 52 | STS | 18 | 87 | − | NA | (3) |
| Van Geel et
al | 60 | 20 | STS/bone | 48 | 46 | + | − | (9) |
| Wouters et
al | 83 | 73 | STS/bone | 25 | 63 | + | NR | (10) |
| Gordon et
al | 149 | NA | STS/AF | 35 | 66 | − | NA | (13) |
| McMillan et
al | 192 | 51 | STS/AF | 15 | 73 | + | NR | (20) |
The remaining studies on chest wall STS by Oksuz
et al (4) and Tsukushi et
al (5) revealed age and
histological grade to be significant prognostic factors of survival
based on univariate analyses. Tsukushi et al (5) performed a study on 44 patients with
chest wall STS involving a high proportion of dermatofibrosarcoma
protuberans (27.2 vs. 7.5% in the current study), which rarely
metastasizes and, therefore, may have resulted in the high 5-year
OS rate of 89%. The distribution of histological subtypes in the
series of Oksuz et al (4) was
comparable with that in the patient population of the present
study, resulting in similar 5-year OS rates (69 vs. 66%). The study
by Oksuz et al (4) is the only
analysis on chest wall STS to date that determined the prognostic
effects of surgical margins. In the analysis of 26 patients,
microscopic negative margins failed to reach statistical
significance in a univariate analysis.
To date, there have been two studies with larger
patient cohorts than those in the present study. These two studies
were performed at the Memorial Sloan-Kettering Cancer Center
(MSKCC) in New York. The MSKCC study in 1991 reviewed the outcomes
of 189 patients; however, it did not delineate the prognostic role
of surgical margins (13). It is
notable that 32.2% of all patients included in the survival
analysis had aggressive fibromatosis, which is a semi-malignant
mesenchymal tumor that does not metastasize. The more recent MSKCC
study (2013) involving 192 patients also included a high proportion
of aggressive fibromatosis patients (17.2%) and only assessed the
local recurrence patterns (20). In
this study, an association between surgical margins and local
outcomes was not able to be established.
Finally, a large retrospective study was performed
by Wouters et al (10), in
which the outcomes of 83 patients with primary chest wall STS and
bone sarcomas who were treated at two institutions were analyzed.
Although data on surgical margins were available for the majority
of patients, Wouters et al (10) did not investigate the prognostic
impact of margins. Furthermore, patients with chondrosarcoma or
osteosarcoma constituted 43% of the patient population. As stated
previously, STS and bone sarcomas possess different clinical
behaviors. Regarding chondrosarcomas, there have been two
well-characterized retrospective studies that outlined the
prognostic significance of negative margins (21,22).
Finally, similar to many other retrospective
analyses of STS, the present study also had several limitations.
Although it was one of the largest analyses on chest wall STS to
date, the assessed subgroups in this study remained relatively
small. Despite the exclusion of bone sarcomas and aggressive
fibromatosis, the distribution of the histological subtypes
remained heterogeneous. Furthermore, the present analysis only
included patients with STS who were suitable for further surgical
treatment with curative intent. Patients with extensive tumors that
could not be approached surgically due to rapid disease progression
and, therefore, less favorable outcomes, were not assessed in this
study. Thus, the results of the current study are only applicable
to the group of patients in whom further surgical treatment was
possible and not to all patients with chest wall STS. This implies
a study selection bias that must be acknowledged.
In conclusion, the present study provides long-term
follow-up data that may provide clinicians with a more detailed
insight into the clinical behavior and prognosis of patients with
chest wall STS. Adverse prognostic features identified include age
>50 years, tumor size >5 cm, high histological grade and an
angiosarcoma subtype. The data from this study was not able to
underscore the long-term benefit of negative margins achieved
following resection of the primary tumor. When the aim of achieving
negative margins requires extensive surgery with a high risk of
morbidity, the postoperative consequences should be clearly
discussed with the patient, as these can be highly subjective. The
final decision should be made in each case based on the
histological grade and progression of the tumor, the health status
of the patient and the decision of the informed patient.
References
|
1
|
Jemal A, Siegel R, Ward E, Murray T, Xu J
and Thun MJ: Cancer statistics, 2007. CA Cancer J Clin. 57:43–66.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kachroo P, Pak PS, Sandha HS, Lee C,
Elashoff D, Nelson SD, Chmielowski B, Selch MT, Cameron RB, Holmes
EC, et al: Single-institution, multidisciplinary experience with
surgical resection of primary chest wall sarcomas. J Thorac Oncol.
7:552–558. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gross JL, Younes RN, Haddad FJ,
Deheinzelin D, Pinto CA and Costa ML: Soft-tissue sarcomas of the
chest wall: Prognostic factors. Chest. 127:902–908. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Oksuz DC, Ozdemir S, Kaydihan N,
Dervisoglu S, Hiz M, Tuzun H, Mandel NM, Koca S and Dincbas FO:
Long-term treatment results in soft tissue sarcomas of the thoracic
wall treated with pre-or-postoperative radiotherapy-a single
institution experience. Asian Pac J Cancer Prev. 15:9949–9953.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tsukushi S, Nishida Y, Sugiura H,
Nakashima H and Ishiguro N: Soft tissue sarcomas of the chest wall.
J Thorac Oncol. 4:834–837. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Salas S, Bui B, Stoeckle E, Terrier P,
Ranchere-Vince D, Collin F, Leroux A, Guillou L, Michels JJ,
Trassard M, et al: Soft tissue sarcomas of the trunk wall (STS-TW):
A study of 343 patients from the French Sarcoma Group (FSG)
database. Ann Oncol. 20:1127–1135. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Maretty-Nielsen K, Aggerholm-Pedersen N,
Safwat A, Jørgensen PH, Hansen BH, Baerentzen S, Pedersen AB and
Keller J: Prognostic factors for local recurrence and mortality in
adult soft tissue sarcoma of the extremities and trunk wall: A
cohort study of 922 consecutive patients. Acta Orthop. 85:323–332.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Stojadinovic A, Leung DH, Hoos A, Jaques
DP, Lewis JJ and Brennan MF: Analysis of the prognostic
significance of microscopic margins in 2,084 localized primary
adult soft tissue sarcomas. Ann Surg. 235:424–434. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
van Geel AN, Wouters MW, Lans TE, Schmitz
PI and Verhoef C: Chest wall resection for adult soft tissue
sarcomas and chondrosarcomas: Analysis of prognostic factors. World
J Surg. 35:63–69. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wouters MW, van Geel AN, Nieuwenhuis L,
van Tinteren H, Verhoef C, van Coevorden F and Klomp HM: Outcome
after surgical resections of recurrent chest wall sarcomas. J Clin
Oncol. 26:5113–5118. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Walsh GL, Davis BM, Swisher SG, Vaporciyan
AA, Smythe WR, Willis-Merriman K, Roth JA and Putnam JB Jr: A
single-institutional, multidisciplinary approach to primary
sarcomas involving the chest wall requiring full-thickness
resections. J Thorac Cardiovasc Surg. 121:48–60. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Smith GM, Johnson GD, Grimer RJ and Wilson
S: Trends in presentation of bone and soft tissue sarcomas over 25
years: Little evidence of earlier diagnosis. Ann R Coll Surg Engl.
93:542–547. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gordon MS, Hajdu SI, Bains MS and Burt ME:
Soft tissue sarcomas of the chest wall. Results of surgical
resection. J Thorac Cardiovasc Surg. 101:843–854. 1991.PubMed/NCBI
|
|
14
|
Coindre JM: Grading of soft tissue
sarcomas: Review and update. Arch Pathol Lab Med. 130:1448–1453.
2006.PubMed/NCBI
|
|
15
|
Fletcher CD: The evolving classification
of soft tissue tumours-an update based on the new 2013 WHO
classification. Histopathology. 64:2–11. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Schemper M and Smith TL: A note on
quantifying follow-up in studies of failure time. Control Clin
Trials. 17:343–346. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Clark TG, Bradburn MJ, Love SB and Altman
DG: Survival analysis part I: Basic concepts and first analyses. Br
J Cancer. 89:232–238. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Coindre JM, Terrier P, Guillou L, Le
Doussal V, Collin F, Ranchère D, Sastre X, Vilain MO, Bonichon F
and Bui N'Guyen B: Predictive value of grade for metastasis
development in the main histologic types of adult soft tissue
sarcomas: A study of 1240 patients from the French Federation of
cancer centers sarcoma group. Cancer. 91:1914–1926. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mery CM, George S, Bertagnolli MM and Raut
CP: Secondary sarcomas after radiotherapy for breast cancer:
Sustained risk and poor survival. Cancer. 115:4055–4063. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
McMillan RR, Sima CS, Moraco NH, Rusch VW
and Huang J: Recurrence patterns after resection of soft tissue
sarcomas of the chest wall. Ann Thorac Surg. 96:1223–1228. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Marulli G, Duranti L, Cardillo G, Luzzi L,
Carbone L, Gotti G, Perissinotto E, Rea F and Pastorino U: Primary
chest wall chondrosarcomas: Results of surgical resection and
analysis of prognostic factors. Eur J Cardiothorac Surg.
45:e194–e201. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Widhe B and Bauer HC: Scandinavian Sarcoma
Group: Surgical treatment is decisive for outcome in chondrosarcoma
of the chest wall: A population-based Scandinavian Sarcoma Group
study of 106 patients. J Thorac Cardiovasc Surg. 137:610–614. 2009.
View Article : Google Scholar : PubMed/NCBI
|