Introduction
Acute promyelocytic leukemia (APL) is the M3 subtype
of acute myeloid leukemia (AML) that results from an arrest of the
terminal differentiation of promyelocytes into granulocytes
(1,2).
APL accounts for 5–15% of all cases of AML and is frequently
associated with the expression of the oncogenic promyelocytic
leukemia-retinoic acid receptor α fusion gene (3,4).
Clinically, APL is now considered a fatal disease with a high rate
of early mortality, often due to hemorrhage from disseminated
intravascular coagulation (DIC) or hyperfibrinolysis (5). Chemotherapy with anthracyclines,
including daunorubicin, idarubicin and cytarabine arabinoside,
which are the frontline treatments of APL, resulted in complete
remission in 75–80% of patients (6,7). However,
this standard treatment regimen was associated with a high early
mortality rate due to the exacerbation of pre-existing DIC
(5,7,8). Despite
high sensitivity to anthracycline, only 35–45% of patients with APL
are cured by standard chemotherapy alone (9). Therefore, it is urgently required to
develop novel therapeutic agents for patients with APL to overcome
the high early mortality rate.
Centratherum anthelminticum
commonly termed kalajiri, somraj, black cumin or
bitter cumin, is a robust leafy plant belonging to the Asteraceae
family of flowering plants (10). The
plant has additional scientific synonyms, including Vernonia
anthelmintica and Conyza anthelmintica. It is widely
used as an herbal medicine against cough, fever and diarrhea in a
number of regions, including India, the Himalaya mountains, Khasi
Hills, Sri Lanka and Afghanistan (11). The extracts from the seeds of C.
anthelminticum have been demonstrated to possess various
pharmacological properties, including analgesic, antipyretic,
anti-inflammatory, antiviral and anti-filarial effects (12–15). A
previous study reported that the chloroform fraction, but not the
hexane or methanol fractions, from C. anthelminticum seeds
exhibited antioxidant properties of inhibiting tumor necrosis
factor-α-induced human cancer cell growth by interrupting the
activation of nuclear factor-κB activation (16). Vernodalin is an active compound
isolated through bioassay-guided fractionation, which can induce
apoptosis in human breast cancer cells via the caspase pathway
(10). In addition, vernodalin can
induce cell cycle arrest and apoptosis in breast cancer cells
through forkhead box O3a (11).
Vernodalol is similar to vernodalin, and both can be extracted from
C. anthelminticum (10).
However, the effects of vernodalol on carcinoma cells have not been
studied.
The present study investigated the antitumor effects
of vernodalol on APL cells. The effect of vernodalol on the APL
cell cycle and apoptosis was assessed, and the associated molecular
mechanisms were investigated. Results of the present study
indicated that vernodalol may be utilized as a potent medicine for
the treatment of APL.
Materials and methods
Reagents and cell culture
Vernodalol was obtained from Sigma-Aldrich (Merck
KGaA, Darmstadt, Germany). Antibodies specific for caspase-9 (cat.
no. CST 9502), caspase-3 (cat. no. CST 9662), cleaved-poly (ADP
ribose) polymerase (PARP; cat. no. CST 9542), B-cell lymphoma-2
(Bcl-2; cat. no. CST 2872), Bcl-2-associated X protein (Bax; cat.
no. CST 2774), Bcl-2-associated death promoter (Bad; cat. no. CST
9292) and Bcl-2 homologous antagonist killer (Bak; cat. no. CST
3814) were obtained from Cell Signaling Technology, Inc. (Danvers,
MA, USA). Antibodies specific for phosphatase and tensin homolog
(PTEN; sc-7974), myeloid cell leukemia-1 (Mcl-1; sc-12756),
Bcl-2-like protein 11 (Bim; sc-130511), cytochrome c
(sc-13561) and second mitochondria-derived activator of
caspase/direct IAP-binding protein with low pI (Smac/DIABLO;
sc-22766) were purchased from Santa Cruz Biotechnology, Inc.
(Dallas, TX, USA). Antibodies specific for phosphorylated
(p)-protein kinase B (Akt; ab38449), Akt (ab8805), phosphoinositide
3-kinase (PI3K; ab86714) and mechanistic target of rapamycin (mTOR;
ab2732) were purchased from Abcam (Cambridge, MA, USA). Anti-GAPDH
antibody was obtained from Sigma-Aldrich (Merck KGaA; G9545). The
Annexin V/propidium iodide (PI) binding kit was purchased from BD
Biosciences (San Jose, CA, USA). All other chemicals of analytical
grade were obtained from Sigma-Aldrich (Merck KGaA). Human APL cell
lines, KG-1a, NB-4 and HL-60, were obtained from Shanghai Centre of
Cell Resource, Chinese Academy of Sciences (Shanghai, China). All
cell lines were maintained at 37°C in RPMI-1640 (Gibco; Thermo
Fisher Scientific, Inc.) supplemented with 10% heat-inactivated
fetal bovine serum (Sigma-Aldrich; Merck KGaA) and 1%
penicillin/streptomycin in a humidified atmosphere containing 5%
CO2. All cells were passaged every 3 days.
Cell viability assay
The effects of vernodalol on viability of human APL
cells were analyzed by MTT assay (Sigma-Aldrich; Merck KGaA).
Briefly, 5×103 cells per well were plated on 96-well
plates and treated with vernodalol at various concentrations (20,
40, 60, 80 and 100 µM) for the indicated times (24, 48 or 72 h).
The RPMI-1640 with the compounds or 0.5% dimethyl sulfoxide (DMSO;
as the control treatment) was then replaced with 180 µl of fresh
media with 20 µl of MTT solution (MTT dissolved in PBS at 5 mg/ml)
per well and incubated at 37°C for 4 h. The MTT-containing medium
was discarded, and DMSO (150 µl/well) was added to dissolve the
newly formed formazan crystals. The absorbance of each well was
determined by a microplate reader (Synergy H4; BioTek China,
Beijing, China) at a wavelength of 590 nm.
Cell cycle analysis
The cells were cultured on 6-well plates at 37°C to
reach 70–80% confluence with RPMI-1640 and then treated with
vernodalol at various concentrations (25, 50 or 100 µM) for the
indicated time. The vernodalol-treated and control cells were
harvested by centrifugation for 5 min at 377 × g and room
temperature and fixed in 4 ml ice-cold 75% ethanol at 4°C
overnight. The cells were stained with 200 µl PI (50 µg/ml;
Sigma-Aldrich; Merck KGaA) at 37°C for 10 min and incubated with 20
µl RNase (1 mg/ml; Sigma-Aldrich; Merck KGaA) for removal of RNA in
a 37°C water bath for 15–20 min. The cells were then analyzed by
flow cytometry (FACScan; BD Biosciences). The results are presented
as mean values from three independent experiments.
Cell apoptosis analysis
The cells were cultured (5×105) in each
well of 6-well plates to 70–80% confluence with RPMI-1640 and then
treated with vernodalol at various concentrations (0, 50, 75 or 100
µM) for the indicated time (24 h). The vernodalol-treated and
control cells (treated with DMSO only) were harvested by
centrifugation for 5 min at 377 × g and room temperature and washed
twice with cold 1X PBS. The cells were then stained with 5 µl
Annexin V and PI (BD Biosciences) for 15 min in dark conditions at
room temperature according to the manufacturer's protocol and then
subjected to analysis by flow cytometry (FACS Calibur; BD
Biosciences). Data were analyzed using Flowjo software version 10.0
(Tree Star, Inc., Ashland, OR, USA). Early apoptosis was evaluated
based on the percentage of cells with Annexin
V+/PI− staining, and late apoptosis was
evaluated based on the percentage of cells with Annexin
V+/PI+ staining.
Preparation of subcellular
fractions
To separate the cytosolic and mitochondrial
fractions, the cells were washed with ice-cold PBS. The cells were
then lysed using Cell Lysis and Mitochondria Intact buffer (250 mM
sucrose, 80 mM KCl and 50 µg/ml digitonin in PBS) on ice for 5 min.
The cell suspension was centrifuged at 377 × g for 5 min at 4°C.
The supernatant was removed and stored at −20°C as the cytosolic
fraction.
Western blot analysis
The cells were treated with vernodalol at different
concentrations (0, 50, 75 or 100 µM) for the indicated time (24 h).
The cells were extracted and lysed with CHAPS lysis buffer
(Beyotime Institute of Biotechnology, Beijing, China) for 30 min on
ice and then centrifuged at 12,000 × g for 15 min at 4°C. The total
protein concentration was determined with bicinchoninic acid
protein assay kit (Beyotime Institute of Biotechnology). Equal
amounts (30 µg per lane) of protein samples were subjected to 8–12%
SDS-PAGE electrophoresis and transferred to polyvinylidene fluoride
membranes (EMD Millipore, Billerica, MA, USA). Each membrane was
blocked with 10% non-fat milk at room temperature for 2 h and
incubated with primary antibodies (caspase-9, caspase-3, PARP,
Bcl-2, Bax, Bcl-2, Bad, Bak, PTEN, Bim, cytochrome c,
Smac/DIABLO, Akt, PI3K, mTOR and GAPDH) at 4°C overnight, followed
by incubation with goat anti-mouse (cat. no. SZ200) or anti-rabbit
(cat. no. SZ2004) secondary antibodies (1:1,000 dilution; Santa
Cruz Biotechnology, Inc.) conjugated to horseradish peroxidase at
room temperature for 2 h. The relative protein expression levels
were quantified using Image-Pro Plus software version 6.0 (Media
Cybernetics, Inc., Rockville, MD, USA) and normalized to GAPDH.
Statistical analysis
Statistical analysis was performed using GraphPad
Prism version 5.01 (GraphPad Software, Inc., La Jolla, CA, USA) and
expressed as the mean ± standard deviation. The values of
half-maximal inhibitory concentration (IC50) were fitted
using a nonlinear regression model with a sigmoidal dose response.
The values were evaluated by one-way analysis of variance followed
by Tukey's multiple comparison, which was utilized to determine the
significance of controls and treated groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
Vernodalol inhibits the growth of
human acute promyelocytic leukemia cells
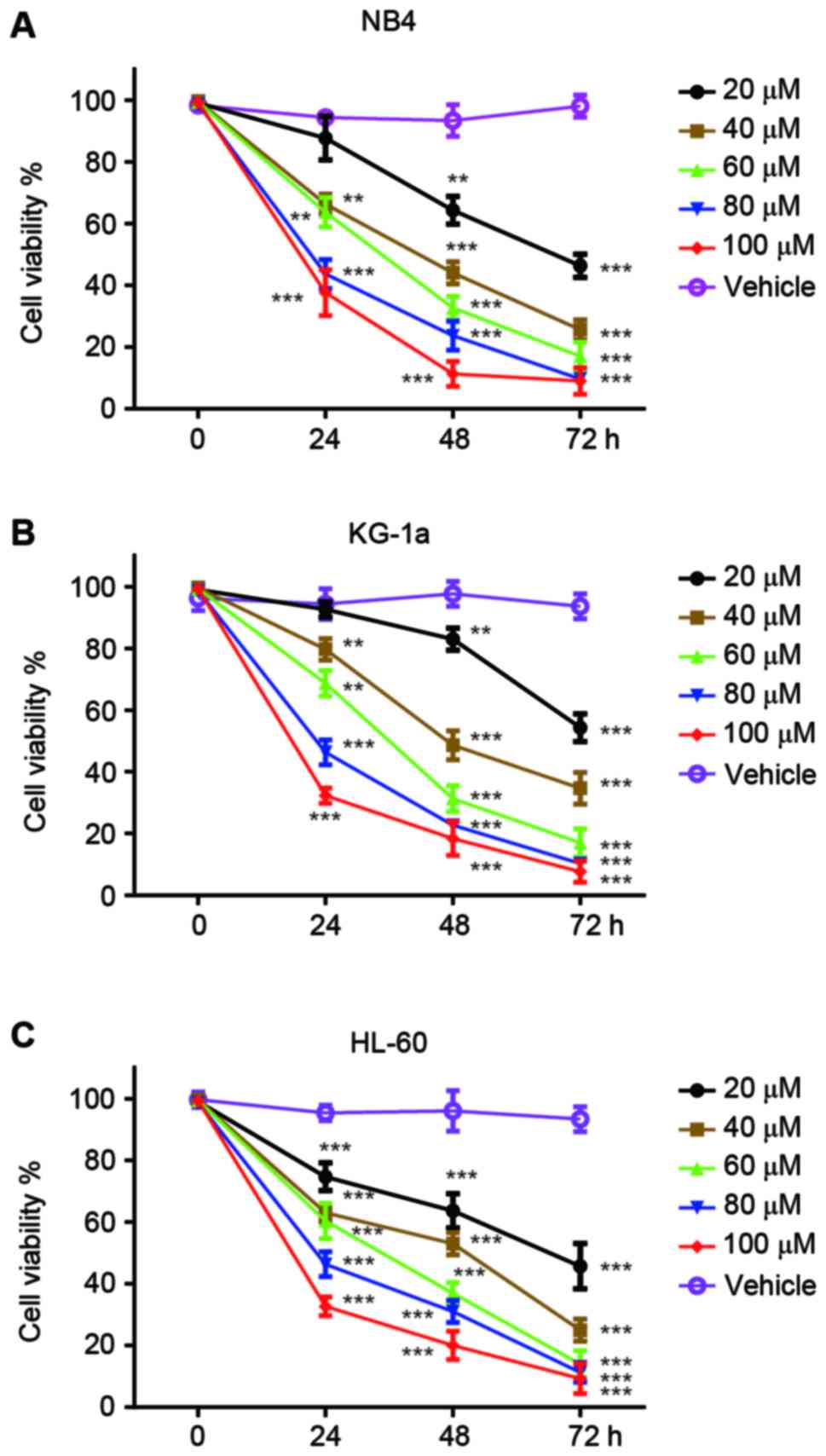
The antitumor effects of vernodalol were initially
tested using three human APL cell lines, NB4, KG-1a and HL-60.
Cells were treated with different concentrations of vernodalol or
vehicle (DMSO) for 24, 48 and 72 h, and the cell viability was
determined by MTT assay. As shown in Fig.
1, the treatment of three APL cell lines with vernodalol
resulted in a dose- and time-dependent attenuation of cell
viability. IC50 values were calculated and are listed in
Table I. The results showed that
vernodalol inhibits the growth of APL cells in a dose- and
time-dependent manner (P<0.01). Among these three APL cell
lines, HL-60 was more sensitive to vernodalol compared with NB4 and
KG-1a cell lines. Therefore, HL-60 was selected for subsequent
experiments.
 | Table I.IC50 values of vernodalol
on NB4, KG-1a and HL-60 cell lines. |
Table I.
IC50 values of vernodalol
on NB4, KG-1a and HL-60 cell lines.
|
| IC50,
µM |
|---|
|
|
|
|---|
| Cell lines | 24 h | 48 h | 72 h |
|---|
| NB4 | 65.72±4.4 | 36.6±4.5 | 17.06±4.0 |
| KG-1a | 76.4±1.5 | 42.53±4.5 | 23.78±2.9 |
| HL-60 | 67.83±2.9 | 38.36±3.4 | 16.43±3.5 |
Vernodalol induces G2/M growth
arrest
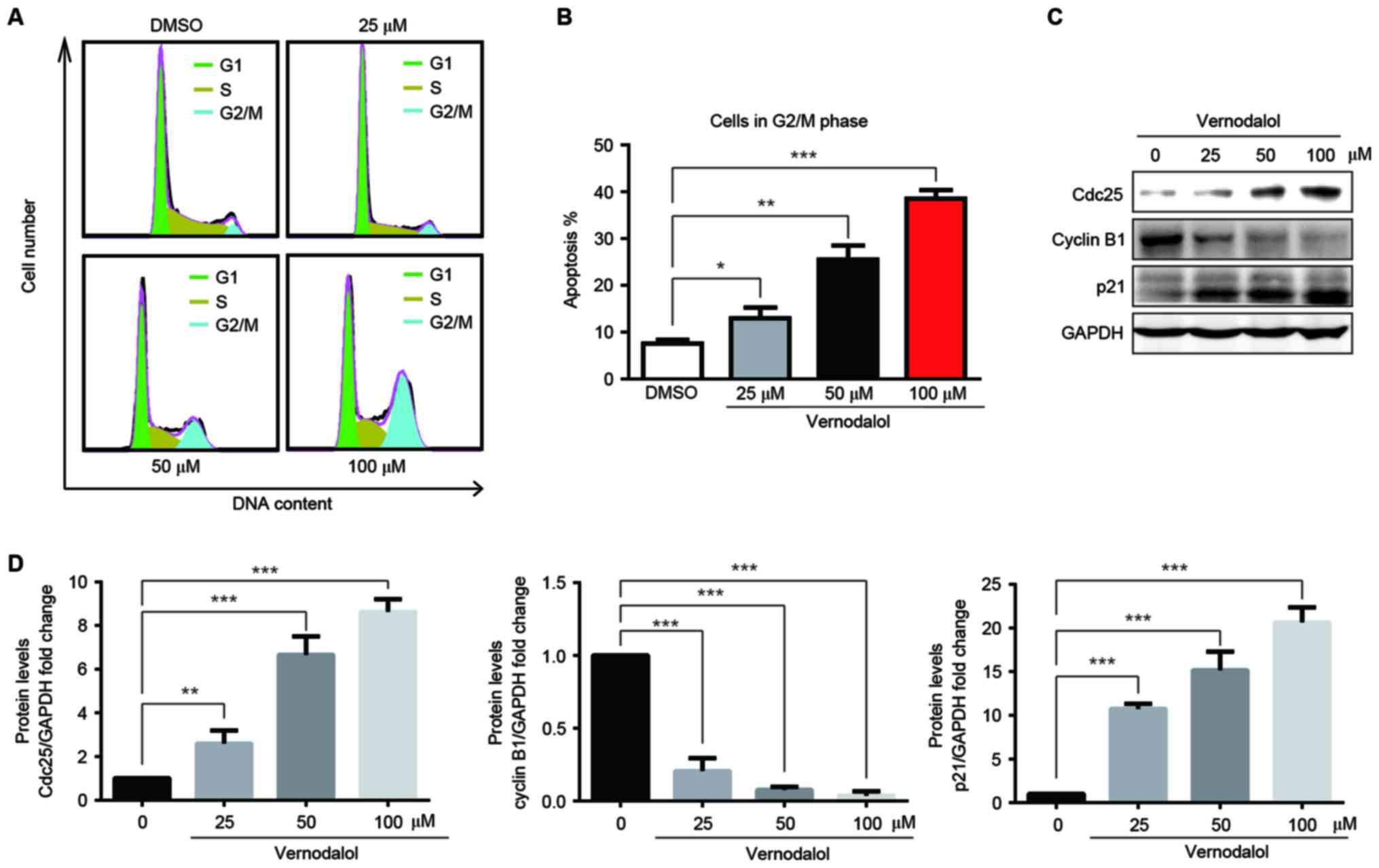
To examine whether vernodalol inhibited
proliferation through the induction of cell cycle arrest, the
effect of vernodalol on the cell cycle was assessed. HL-60 cells
were treated with different concentrations of vernodalol for 24 h,
and the DNA-based cell cycle was analyzed by flow cytometry
following PI staining. Vernodalol caused a marked accumulation of
cells in the G2/M phase in a dose-dependent manner (Fig. 2A and B). Western blot analysis was
performed to examine the effect of vernodalol on possible cell
cycle-associated targets, including p21, cyclin B1 and Cdc25. As
shown in Fig. 2C and D, vernodalol
induced the upregulation of p21 and Cdc25 and the downregulation of
cyclin B1 in a dose-dependent manner. These data indicated that
vernodalol may induce G2/M cell cycle arrest via cell cycle
regulatory molecules in APL cells.
Vernodalol induces apoptosis through
the mitochondrial apoptosis pathway
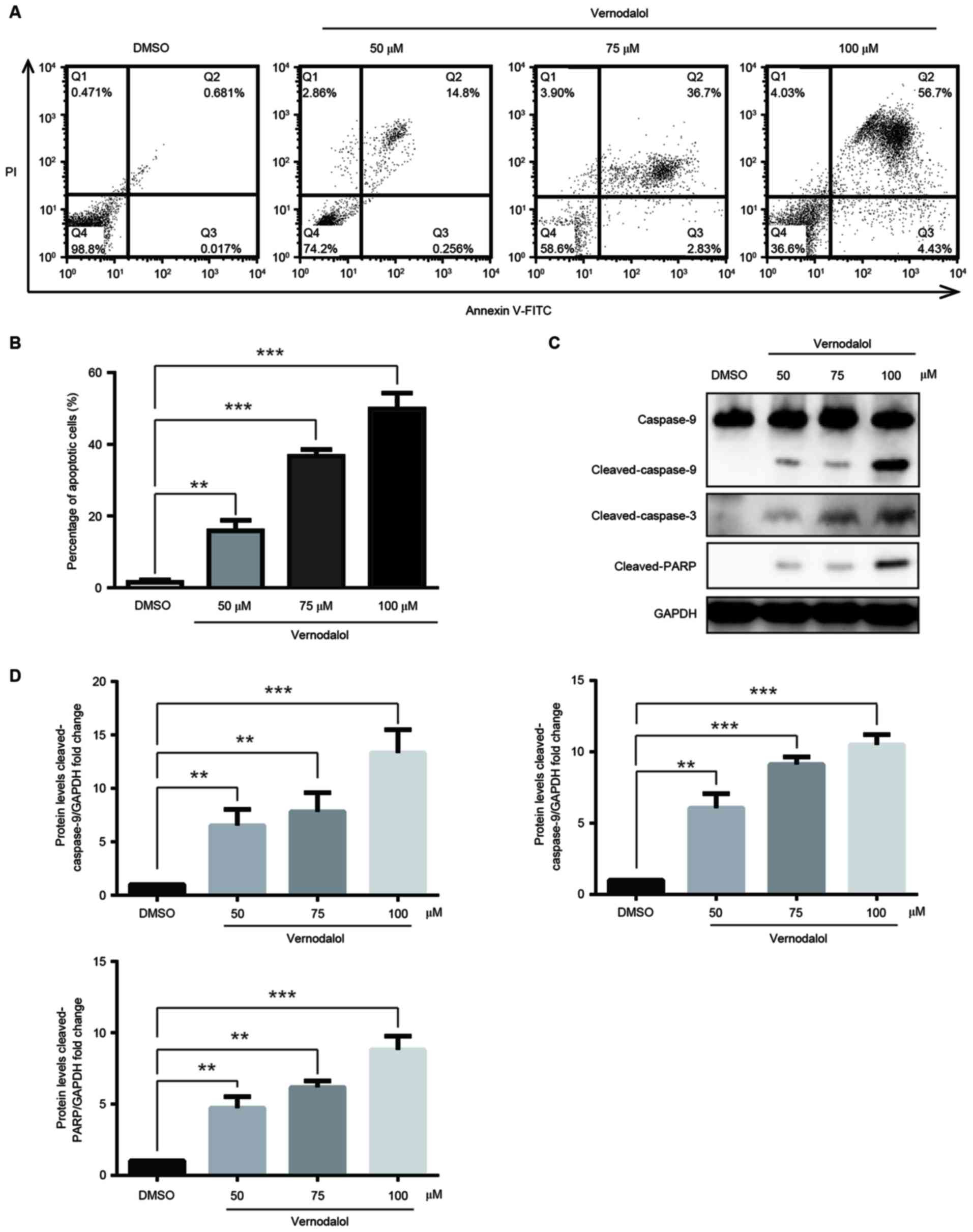
Next, it was examined whether vernodalol had an
effect on cell apoptosis. HL-60 cells were stained with Annexin
V/PI subsequent to being treated with different concentrations of
vernodalol for 24 h and subjected to flow cytometric analysis. As
shown in Fig. 3A and B, the
proportion of cells in early apoptosis (Annexin
V+/PI−; right lower quadrant), as well as
late apoptosis (Annexin V+/PI+; right upper
quadrant), was increased with the different concentrations of
vernodalol in a dose-dependent manner. These data indicated that
vernodalol is able to induce cell apoptosis in human APL cells.
To elucidate the mechanism by which vernodalol
induced cell apoptosis, apoptosis-associated proteins were examined
following treatment with vernodalol. The results indicated that
vernodalol caused cleavage of caspase-9 and −3 in HL-60 cells
(Fig. 3C and D). In addition, cleaved
PARP was also detected, which is a marker of cells undergoing
apoptosis (Fig. 3C and D). This
suggested that the caspase cascade and PARP inactivation are
involved in vernodalol-mediated apoptosis. As demonstrated in
Fig. 4, vernodalol downregulated the
expression of Bcl-2 and Mcl-1 but upregulated Bim, Bax and Bad
expression in a dose-dependent manner. In addition, it was
investigated whether the mitochondrial apoptosis pathway was
involved in vernodalol-induced apoptosis. The release of cytochrome
c and Smac into the cytosol was measured following cell
treatment with vernodalol. Vernodalol caused a significant increase
in the expression of cytochrome c and Smac/DIABLO in the
cytosol (Fig. 4B and C). These
results indicated that vernodalol induces cell apoptosis through
the mitochondrial apoptosis pathway in APL cells.
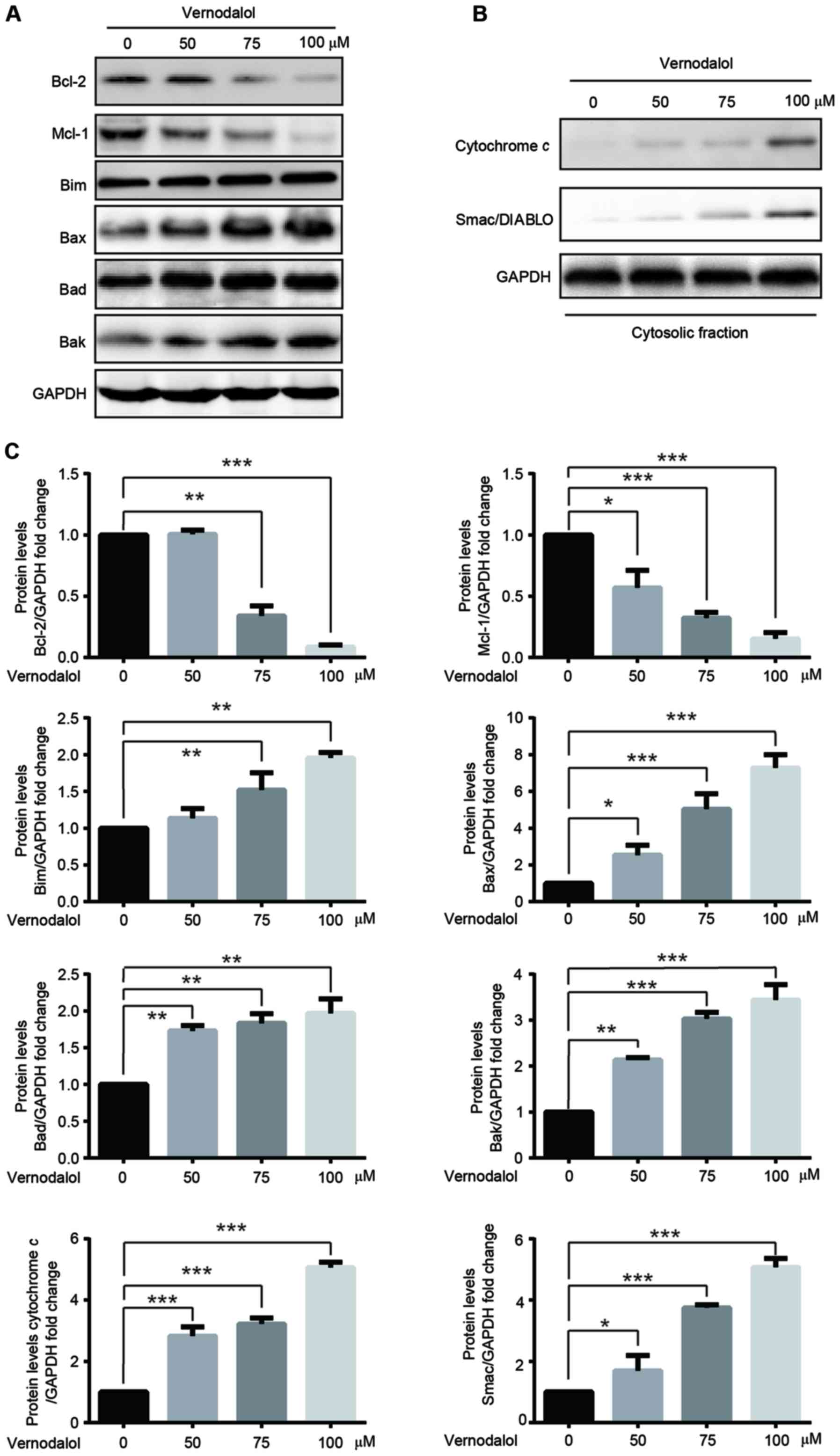 | Figure 4.Vernodalol affects the expression of
apoptosis-associated proteins. (A) HL-60 cells were treated with
various concentrations of vernodalol. Cellular extracts were
analyzed by western blot analysis with the indicated antibodies.
(B) Cytosolic fractions were analyzed by western blot analysis with
the indicated antibodies. (C) Quantification of Bcl-2, Mcl-1, Bim,
Bax, Bad, cytochrome c and Smac/DIABLO protein expression was
normalized to GAPDH using a densitometer. Data are presented as the
mean ± standard deviation (n=3). *P<0.05, **P<0.01 and
***P<0.001 vs. vehicle control. Bcl-2, B-cell lymphoma-2; Bax,
Bcl-2-associated X protein; Bad, Bcl-2-associated death promoter;
Mcl-1, myeloid cell leukemia-1; Bim, Bcl-2-like protein 11;
Smac/DIABLO, second mitochondria-derived activator of
caspase/direct IAP-binding protein with low pl. |
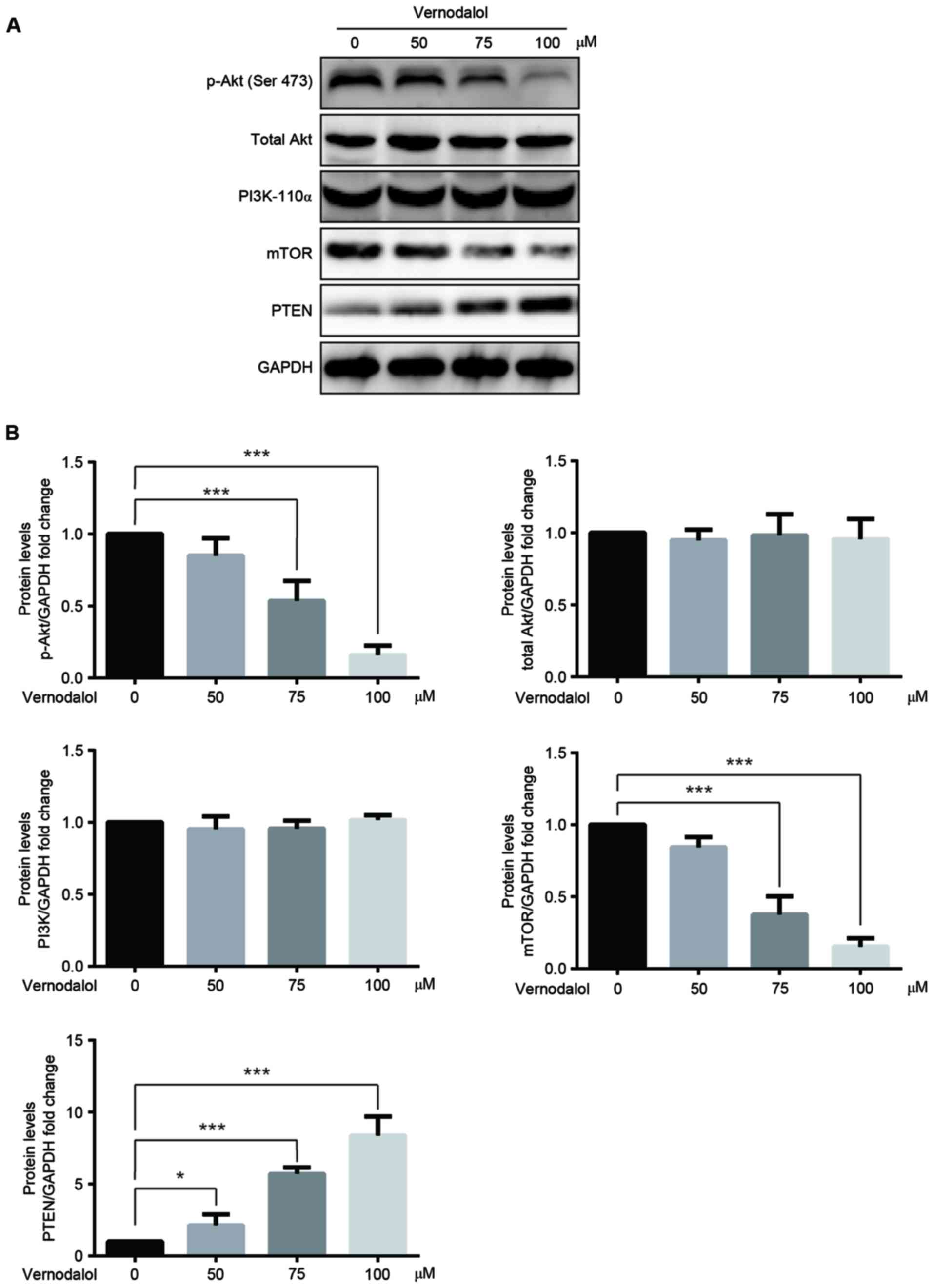
Vernodalol inhibits the PI3K/Akt
signaling pathway
It has been reported that the inhibition of the
PI3K/Akt/mTOR signaling pathway has a vital role in the inhibition
of tumors (17). Therefore, western
blot analysis was performed to investigate whether the
PI3K/Akt/mTOR signaling pathway was involved in the
vernodalol-mediated anticancer effects. The results demonstrated
that the phosphorylation of Akt at Ser473 as well as the expression
of mTOR, a well-known downstream target of Akt, were downregulated
in cells treated with vernodalol in a dose-dependent manner
(Fig. 5). Furthermore, there were
little changes in the levels of PI3K-p110α and total Akt.
Additionally, PTEN, a key negative regulator of the PI3K/Akt
pathway, was upregulated in the treated cells (Fig. 5) compared with the untreated cells.
These results provide compelling preliminary data that vernodalol
may exert its antitumor function through inhibition of the
PI3K/Akt/mTOR signal pathway.
Discussion
APL is a distinct subtype of acute leukemia
(9). Patients with APL exhibit a
tendency to bleed severely and prognosis is poor with a fatal
course in only weeks (5). Although
the clinical outcomes of refractory, relapsed and newly diagnosed
patients with APL have been greatly improved, the mortality rate
remains high (18). The present study
demonstrated that vernodalol isolated from C. anthelminticum
exerted antitumor effects in human APL cells. Vernodalol suppressed
growth of human APL cells by inducing apoptosis through the
mitochondrial pathway and causing cell cycle arrest in the G2/M
phase. In addition, the results suggested that the strong
cytokinetic effects of vernodalol were in part mediated via
inhibition of the PI3K/Akt signaling pathway.
Vernodalol is a sesquiterpene lactone isolated from
the seeds of C. anthelmintica (19). To date, a limited number of studies,
which investigate the anti-cancer effects of vernodalol in human
breast cancer cells and skin cancer models have been performed
(10,20). These studies demonstrated the
cytotoxic activity of vernodalol on melanoma and ovarian cancer
cell lines, and human nasopharyngeal carcinoma (21,22).
However, the effect and the precise mechanism of vernodalol on
human APL cells, remains unclear. To the best of our knowledge,
this is the first study investigating the function and mechanism of
vernodalol in human APL cells.
Cell cycle dysregulation contributes to tumor
initiation and progression. Therefore, cell cycle arrest has become
the major focus of anticancer treatments (23). The majority of chemotherapeutic agents
cause cell cycle arrest either at the G0/G1 or G2/M stage (24,25). In
the present study, downregulation of cyclin B1 expression and
upregulation of p21 and Cdc25, was observed following vernodalol
treatment. p21, which belongs to the Cip/Kip family of
cyclin-dependent kinase (CDK) inhibitors, binds to and inhibits the
kinase activity of CDK2 and CDK1, leading to growth arrest at
specific stages of the cell cycle (26). Cdc25C triggers G2 progression to
mitosis by dephosphorylation of the cyclin B1/Cdk1 complex
(26). Cyclin B1 is also a key cell
cycle regulator of the G2/M phase transition, and the prototypical
cyclin B1 reaches maximum levels in G2 when it enters the nucleus
to form a complex with Cdk1 in a phosphorylation-dependent manner
(27). It has been reported that the
overexpression of cyclin B1 causes uncontrolled cell growth and may
even promote the malignant transformation required for the
initiation of mitosis (28). Taken
together, the present data suggest a novel cell cycle regulating
property of vernodalol via G2-phase arrest in human APL cells.
Apoptosis, a complex program of cell death, is
regulated by numerous molecular signaling pathways (29). A study has shown that the
mitochondrial pathway performs a key role in the apoptotic process
(10). In this pathway, there is an
increase in the release of apoptogenic factors (cytochrome c
and Smac/DIABLO) from the outer mitochondrial membrane space into
the cytosol due to changes in the mitochondrial membrane potential
(Dym). The Bcl-2 family is a major regulator of programmed cell
death (30). A previous study
reported that Bcl-2 can block cell death in mitochondria by
inhibiting the apoptosis-associated release of cytochrome c
from the mitochondria (30). Results
of the present study indicated a decrease in the levels of
anti-apoptotic proteins Bcl-2 and Mcl-1 and an increase in
pro-apoptotic proteins Bim, Bax and Bad following treatment with
vernodalol (Fig. 4A). Furthermore,
the levels of cytochrome c and Smac/DIABLO were increased in
the cytosol following treatment with vernodalol. Therefore, the
present findings indicated that treatment with vernodalol induces
apoptosis in a manner that is dependent on activation of the
mitochondrial apoptosis pathway.
The PI3K/Akt signaling pathway is a cell survival
pathway that is important for normal cell growth and proliferation
(31). Numerous studies have
demonstrated that the PI3K/Akt signaling pathway is involved in
various cellular functions, including protein synthesis, cell cycle
progression, cell survival, apoptosis and angiogenesis (31,32). The
amplification of PI3K/Akt signal transduction is the main cause of
cellular growth, and the aberrant activation of PI3K/Akt due to
genetic variation in key genes has been observed in several types
of cancer, including leukemia and esophageal cancer (33,34). It
has been reported that the PI3K/Akt signaling axis is
constitutively activated in APL, and the addition of PI3K and mTOR
inhibitors to induction treatment regimens may provide therapeutic
benefits for APL (33,35). Akt is one of the major downstream
effectors of PI3K (36). Upon PI3K
activation, Akt is translocated to the inner membrane via its
pleckstrin homology domain where it is phosphorylated by
3-phosphoinositide-dependent kinase-1 on its activation loop
(37). This Akt modification is
sufficient to activate the mTOR complex 1, which was originally
identified as a crucial component of insulin receptor intracellular
signaling (38). PTEN, a tumor
suppressor phosphatase and tensin homolog, may antagonize PI3K/Akt
signaling (39). PTEN was identified
as a frequently mutated gene in numerous types of tumors,
particularly endometrium, skin, brain and prostate (40). A study has also revealed that the
downregulation of PI3K, Akt and mTOR and increased PTEN gene
expression induces apoptosis and inhibits cell cycle progression in
the HL-60 cell line (33). Therefore,
the abrogation of PI3K or Akt function may be crucial for APL
therapy. In the present study, western blot analysis demonstrated
that vernodalol attenuated the phosphorylation of Akt at Ser473 and
the expression of the downstream target mTOR and increased the
expression of tumor suppressor PTEN (Fig.
5A) in a dose-dependent manner in human APL cells. These
results suggest that vernodalol may induce apoptosis and inhibit
cell cycle G2/M arrest by suppressing the PI3K/Akt signaling
pathway. However, further investigation is required to validate
this hypothesis.
In conclusion, to the best of our knowledge, the
present study reported for the first time that vernodalol, an
active compound isolated from C. anthelminticum, has a
potential therapeutic function in human APL cells. Vernodalol
induces cell apoptosis and G2/M growth arrest. In addition, the
present findings also highlighted the ‘therapeutic value of the
repression of the PI3K/Akt pathway by vernodalol in human APL
cells. Therefore, vernodalol may be a potential candidate used for
the treatment of APL, subsequent to further investigation.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 30900533), the
Science Foundation of Traditional Medicine Zhejiang Province (grant
no. 2013ZB080) and the Science Foundation of National Health and
Family Planning Commission (grant no. 2014BAI09B12).
References
|
1
|
Bennett JM, Catovsky D, Daniel MT,
Flandrin G, Galton DA, Gralnick HR and Sultan C: Proposals for the
classification of the acute leukaemias. French-American-British
(FAB) co-operative group. Br J Haematol. 33:451–458. 1976.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Vardiman JW, Thiele J, Arber DA, Brunning
RD, Borowitz MJ, Porwit A, Harris NL, Le Beau MM,
Hellström-Lindberg E, Tefferi A and Bloomfield CD: The 2008
revision of the World Health Organization (WHO) classification of
myeloid neoplasms and acute leukemia: Rationale and important
changes. Blood. 114:937–951. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Castaigne S, Chomienne C, Daniel MT,
Ballerini P, Berger R, Fenaux P and Degos L: All-trans retinoic
acid as a differentiation therapy for acute promyelocytic leukemia.
I. Clinical results. Blood. 76:1704–1709. 1990.PubMed/NCBI
|
|
4
|
Chen Z, Tong JH, Dong S, Zhu J, Wang ZY
and Chen SJ: Retinoic acid regulatory pathways, chromosomal
translocations and acute promyelocytic leukemia. Genes Chromosomes
Cancer. 15:147–156. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jones ME and Saleem A: Acute promyelocytic
leukemia. A review of literature. Am J Med. 65:673–677. 1978.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cunningham I, Gee TS, Reich LM, Kempin SJ,
Naval AN and Clarkson BD: Acute promyelocytic leukemia: Treatment
results during a decade at Memorial Hospital. Blood. 73:1116–1122.
1989.PubMed/NCBI
|
|
7
|
Sanz MA, Jarque I, Martin G, Lorenzo I,
Martínez J, Rafecas J, Pastor E, Sayas MJ, Sanz G and Gomis F:
Acute promyelocytic leukemia. Therapy results and prognostic
factors. Cancer. 61:7–13. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fenaux P, Wang ZZ and Degos L: Treatment
of acute promyelocytic leukemia by retinoids. Curr Top Microbiol
Immunol. 313:101–128. 2007.PubMed/NCBI
|
|
9
|
Kamimura T, Miyamoto T, Harada M and
Akashi K: Advances in therapies for acute promyelocytic leukemia.
Cancer Sci. 102:1929–1937. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Looi CY, Arya A, Cheah FK, Muharram B,
Leong KH, Mohamad K, Wong WF, Rai N and Mustafa MR: Induction of
apoptosis in human breast cancer cells via caspase pathway
byvernodalol isolated from Centratherum anthelminticum (L.) seeds.
PLoS One. 8:e566432013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sadagopan SK Ananda, Mohebali N, Looi CY,
Hasanpourghadi M, Pandurangan AK, Arya A, Karimian H and Mustafa
MR: Forkhead box transcription factor (FOXO3a) mediates the
cytotoxic effect ofvernodalol in vitro and inhibits the breast
tumor growth in vivo. J Exp Clin Cancer Res. 34:1472015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ashok P, Koti BC, Thippeswamy AH, Tikare
VP, Dabadi P and Viswanathaswamy AH: Evaluation of antiinflammatory
activity of Centratherum anthelminticum (L) Kuntze seed. Indian J
Pharm Sci. 72:697–703. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Purnima A, Koti BC, Tikare VP,
Viswanathaswamy AH, Thippeswamy AH and Dabadi P: Evaluation of
analgesic and antipyretic activities of Centratherum anthelminticum
(L) Kuntze seed. Indian J Pharm Sci. 71:461–464. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sharma S and Mehta BK: In vitro
antimicrobial efficacy of Centratherum anthelminticum seeds
extracts. J Hyg Epidemiol Microbiol Immunol. 35:157–161.
1991.PubMed/NCBI
|
|
15
|
Singhal KC, Sharma S and Mehta BK:
Antifilarial activity of Centratherum anthelminticum seed extracts
on Setaria cervi. Indian J Exp Biol. 30:546–548. 1992.PubMed/NCBI
|
|
16
|
Arya A, Achoui M, Cheah SC, Abdelwahab SI,
Narrima P, Mohan S, Mustafa MR and Mohd MA: Chloroform fraction of
Centratherum anthelminticum (L.) seed inhibits tumor necrosis
factor alpha and exhibits pleotropic bioactivities: Inhibitory role
in human tumor cells. Evid Based Complement Alternat Med.
2012:6272562012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Manning BD and Cantley LC: AKT/PKB
signaling: Navigating downstream. Cell. 129:1261–1274. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Petrie K, Zelent A and Waxman S:
Differentiation therapy of acute myeloid leukemia: Past, present
and future. Curr Opin Hematol. 16:84–91. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rabe T, Mullholland D and van Staden J:
Isolation and identification of antibacterial compounds from
Vernonia colorata leaves. J Ethnopharmacol. 80:91–94. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Looi CY, Moharram B, Paydar M, Wong YL,
Leong KH, Mohamad K, Arya A, Wong WF and Mustafa MR: Induction of
apoptosis in melanoma A375 cells by a chloroform fraction of
Centratherum anthelminticum (L.) seeds involves NF-kappaB, p53 and
Bcl-2-controlled mitochondrial signaling pathways. BMC Complement
Altern Med. 13:1662013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kasim LS, Ferro V, Odukoya OA, Ukpo GE,
Seidel V, Gray AI and Waigh R: Cytotoxicity of isolated compounds
from the extracts of Struchium sparganophora (Linn) Ktze
asteraceae. Pak J Pharm Sci. 24:475–478. 2011.PubMed/NCBI
|
|
22
|
Kupchan SM, Hemingway RJ, Karim A and
Werner D: Tumor inhibitors. XLVII. Vernodalol and vernomygdin, two
new cytotoxic sesquiterpene lactones from Vernonia amygdalina Del.
J Org Chem. 34:3908–3911. 1969. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Drexler HG: Review of alterations of the
cyclin-dependent kinase inhibitor INK4 family genes p15, p16, p18
and p19 in human leukemia-lymphoma cells. Leukemia. 12:845–859.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pan MH, Lin CL, Tsai JH, Ho CT and Chen
WJ: 3,5,3′,4′, 5′-pentamethoxystilbene (MR-5), a synthetically
methoxylated analogue of resveratrol, inhibits growth and induces
G1 cell cycle arrest of human breast carcinoma MCF-7 cells. J Agric
Food Chem. 58:226–234. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Weir NM, Selvendiran K, Kutala VK, Tong L,
Vishwanath S, Rajaram M, Tridandapani S, Anant S and Kuppusamy P:
Curcumin induces G2/M arrest and apoptosis in cisplatin-resistant
human ovarian cancer cells by modulating Akt and p38 MAPK. Cancer
Biol Ther. 6:178–184. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Abbas T and Dutta A: p21 in cancer:
Intricate networks and multiple activities. Nat Rev Cancer.
9:400–414. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Furnari B, Blasina A, Boddy MN, McGowan CH
and Russell P: Cdc25 inhibited in vivo and in vitro by checkpoint
kinases Cds1 and Chk1. Mol Biol Cell. 10:833–845. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Innocente SA, Abrahamson JL, Cogswell JP
and Lee JM: p53 regulates a G2 checkpoint through cyclin B1. Proc
Natl Acad Sci USA. 96:pp. 2147–2152. 1999; View Article : Google Scholar : PubMed/NCBI
|
|
29
|
McConkey DJ and Orrenius S: Signal
transduction pathways in apoptosis. Stem Cells. 14:619–311. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yang J, Liu X, Bhalla K, Kim CN, Ibrado
AM, Cai J, Peng TI, Jones DP and Wang X: Prevention of apoptosis by
Bcl-2: Release of cytochrome c from mitochondria blocked. Science.
275:1129–1132. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Martini M, De Santis MC, Braccini L,
Gulluni F and Hirsch E: PI3K/AKT signaling pathway and cancer: An
updated review. Ann Med. 46:372–383. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Vivanco I and Sawyers CL: The
phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev
Cancer. 2:489–501. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Han S, Zhang G, Li M, Chen D, Wang Y, Ye W
and Ji Z: L-securinine induces apoptosis in the human promyelocytic
leukemia cell line HL-60 and influences the expression of genes
involved in the PI3K/AKT/mTOR signaling pathway. Oncol Rep.
31:2245–2251. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hildebrandt MA, Yang H, Hung MC, Izzo JG,
Huang M, Lin J, Ajani JA and Wu X: Genetic variations in the
PI3K/PTEN/AKT/mTOR pathway are associated with clinical outcomes in
esophageal cancer patients treated with chemoradiotherapy. J Clin
Oncol. 27:857–871. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ma W, Wang DD, Li L, Feng YK, Gu HM, Zhu
GM, Piao JH, Yang Y, Gao X and Zhang PX: Caveolin-1 plays a key
role in the oleanolic acid-induced apoptosis of HL-60 cells. Oncol
Rep. 32:293–301. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Burgering BM and Coffer PJ: Protein kinase
B (c-Akt) in phosphatidylinositol-3-OH kinase signal transduction.
Nature. 376:599–602. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wick MJ, Dong LQ, Riojas RA, Ramos FJ and
Liu F: Mechanism of phosphorylation of protein kinase B/Akt by a
constitutively active 3-phosphoinositide-dependent protein
kinase-1. J Biol Chem. 275:40400–40406. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Aoki M, Blazek E and Vogt PK: A role of
the kinase mTOR in cellular transformation induced by the
oncoproteins P3k and Akt. Proc Natl Acad Sci USA. 98:pp. 136–141.
2001; View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ali IU, Schriml LM and Dean M: Mutational
spectra of PTEN/MMAC1 gene: A tumor suppressor with lipid
phosphatase activity. J Natl Cancer Inst. 91:1922–1932. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Li J, Yen C, Liaw D, Podsypanina K, Bose
S, Wang SI, Puc J, Miliaresis C, Rodgers L, McCombie R, et al:
PTEN, a putative protein tyrosine phosphatase gene mutated in human
brain, breast and prostate cancer. Science. 275:1943–1947. 1997.
View Article : Google Scholar : PubMed/NCBI
|