Introduction
Oxaliplatin (OXA) is a third-generation platinum
compound and OXA-based chemotherapy is a widely used treatment for
solid organ malignancies. The combination of OXA with other
chemotherapy agents, including 5-fluorouracil/folic acid (FOLFOX)
and capecitabine, is a first-line therapy for colorectal cancer
(1). Despite its utility, OXA-based
chemotherapy is associated with chemotherapy-associated liver
injury. Rubbia-Brandt et al (2) reported that 78% of patients with
metastatic colorectal cancer receiving OXA-based chemotherapy
experience varying degrees of sinusoidal injury to the liver. A
number of other studies have also suggested that OXA can cause
liver injury (2,3). FOLFOX is associated with the development
of sinusoidal obstruction syndrome (SOS) and nodular regenerative
hyperplasia (3). Soubrane et
al (4) revealed that liver
histopathological changes occur in ~59% of patients who have
received OXA-based preoperative chemotherapy followed by hepatic
resection for colorectal liver metastases. In addition, OXA-based
chemotherapy is associated with increased peri-operative morbidity,
including post-hepatectomy liver failure and prolonged prothrombin
time (5–7). Furthermore, 10–60% of patients receiving
OXA-based chemotherapy have abnormal liver function which can cause
chemotherapy delays and necessitate dose reduction, as well as
increase the incidence of irregular events during chemotherapy
(6,8).
Currently, the underlying mechanism of OXA-induced
liver toxicity is unclear. One hypothesis is that OXA-induced liver
damage may be associated with oxidative stress (9–11). In a
mouse model of OXA-induced liver injury, Robinson et al
(10) observed that the expression
levels of certain oxidative stress-related genes, including
metallothionein 1 (Mt1), heme oxygenase 1 (HO1) and superoxide
dismutase 3 (SOD3), were all upregulated. This indicates that
oxidative stress may serve a central role in FOLFOX-induced SOS
that can be prevented by the administration of the antioxidant
butylated hydroxyanisole (10).
Schwingel et al (11)
determined that the antioxidative compounds resveratrol, quercetin
(QT) and quercetin nanoemulsion (NQT) can effectively alleviate
OXA-induced liver toxicity in a murine model. In addition, several
antioxidative compounds can ameliorate steatohepatitis and
OXA-induced neurotoxicity through reducing oxidative stress
(11–13).
However, prior clinical and animal studies have
focused on studying chronic liver injuries caused by long-term use
(4–8 weeks) of OXA-based chemotherapy. Currently, few studies are
performed using animal models of OXA-induced acute liver injury
(ALI). In addition, there are limited reports available regarding
the pathological changes in patients with ALI receiving OXA-based
chemotherapy. Due to ethical issues and unwillingness of patients
to receive a liver needle biopsy, it is difficult to perform
clinical studies on OXA-induced ALI.
At present, there is no standard clinical treatment
for OXA-induced ALI. Clinicians can only use experience to select
one or a combination of various hepatoprotective drugs, one of
which is reduced glutathione (GSH). GSH is a bioactive peptide and
important non-enzymatic antioxidant widely present in living
organisms (14). The highest levels
of GSH appear in the liver, which is the major organ for GSH
synthesis and metabolism. GSH can promote the metabolism of sugar,
fat and protein, and maintain normal cell metabolism and cell
membrane integrity. It can bind toxic substances, such as
electrophilic radicals and oxygen free radicals, and has extensive
antioxidative effects (14).
Currently, GSH preparations are widely used for treating certain
liver diseases, including viral hepatitis, liver cirrhosis and
drug-induced liver injury (14,15).
Although GSH is empirically selected for the prevention and
treatment of OXA-induced liver injury, the protective role of GSH
and its underlying mechanism in OXA-induced ALI remain unclear, and
associated studies are rare. Due to the aforementioned challenges,
it is often problematic to obtain liver histological specimens from
patients with cancer and OXA-induced ALI, which restricts the
prospects of studies on OXA-induced ALI associated with
hepatoprotective therapies. Therefore, an animal model of
OXA-induced ALI was established, in order to study the role of
oxidative stress in and the hepatoprotective function of GSH
treatment on OXA-induced ALI.
Materials and methods
Ethical statement
All animal studies were performed according to the
guidelines of the Chinese Council on Animal Care and were approved
by the Affiliated Tumor Hospital of Guangxi Medical University
Committees on Animal Experimentation (Nanning, China).
Drugs and reagents
OXA for injection (no. 13092615; Jiangsu Hengrui
Medicine Co., Ltd.); alanine aminotransferase (ALT) kit (no.
2014007; Changchu Huli Biotech Co., Ltd.); aspartate
aminotransferase (AST) kit, GSH kit, SOD kit, glutathione
peroxidase (GSH-px) kit, malondialdehyde (MDA) kit and total
protein quantification kit (BCA method) (all 6 kits are no.
20140402; Jiangcheng Bioengineering Institute (Nanjing, China).
In vivo chemotherapy model
Twenty male KM mice (aged 8–10 weeks and weighing
26–28 g) were purchased from Beijing Vital River Laboratory Animal
Technology Co., Ltd. (Beijing, China). All mice were housed under
standardized conditions with one cage for every 5 mice, ad
libitum access to a standard chow and water, and 1 week to
adapt to the laboratory environment prior to manipulation. The room
temperature was 22–25°C with 45–55% humidity and a 12-h light-dark
diurnal cycle (lights on between 7:00 a.m. and 7:00 p.m.). Mice
were treated with 8 mg/kg OXA (0.5 ml), administered via
intraperitoneal injection (i.p.), for 4 days. The drug regimen was
based on previously published studies (10,11) and
the preliminary dose exploration experiment. Control animals only
received 5% glucose (10 ml/kg, i.p.). There were 10 animals per
treatment group. Mice were randomly culled by cardiac puncture
under isoflurane anesthesia 12 h after OXA injection until the end
of the experiment. Mice were anesthetized separately using 2%
isoflurane and an incision was made in the middle of the abdomen,
prior to samples (blood and liver tissue) being collected for
further analysis. The characteristics of the mice (mental state and
hair color) and the body weights were examined every day for
abnormalities. Pathological examination was performed following
hematoxylin and eosin (H&E) staining of the liver tissue
sections. To assess the impact of GSH treatment on OXA-induced ALI,
mice (n=10 per group) were treated with OXA (10 mg/kg, i.p.) and
GSH (400 mg/kg, i.p., 30 min prior to first OXA injection) for 4
days (once daily until the end of the experiment). Mice were
euthanized via deep anesthesia with isoflurane 3 days after the
final dose of chemotherapy. Samples (blood and liver tissues) were
collected for further analysis.
Pathological examination of mouse
liver tissues
Liver tissues were fixed in 4% paraformaldehyde, and
then embedded in paraffin. After sectioning, the liver specimens
were stained with H&E. As observed via optical microscopy, the
pathological changes associated with liver injury included liver
cell turbidity and degeneration, balloon-like changes and necrosis.
According to the coverage of abnormal liver cells, liver injuries
were graded as follows: Level 0, normal, no liver cell
degeneration; level 1, mild, the ratio of hepatic lobule lesion
<1/3; level 2, moderate, the ratio of hepatic lobule lesion was
between 1/3 and 2/3; level 3, serious, the ratio of hepatic lobule
lesion >2/3 (+++).
Analysis of serum ALT and AST
levels
Blood samples from the mice were centrifuged at 300
× g for 8 min at 37°C, and the supernatants were measured using an
alanine aminotransferase (ALT) kit (HuiLi Biotech Co., Ltd.,
Changchun, China) and an aspartate aminotransferase (AST) kit
(Jiancheng Bioengineering Institute, Nanjing, China), according to
the manufacturer's protocols. The results are represented as
units/l.
Analysis of oxidative stress
indicators
Proteins were extracted from whole liver tissues in
RIPA buffer and quantified using a Bradford assay (Nanjing
Jiangcheng Bioengineering Institute). The GSH, GSH-Px, SOD and MDA
content of liver tissues were detected using the kits obtained from
the Nanjing Jiangcheng Bioengineering Institute, according to the
protocols provided by the manufacturer.
Statistical analysis
All statistical analyses were performed using SPSS
version 10 (SPSS, Inc., Chicago, IL, USA). All experiments were
performed using 3–5 mice per experimental group and repeated at
least three times to assess reproducibility. Differences were
analyzed using Student's t-test or one-way analysis of variance,
followed by Tukey's post hoc test. Cumulative survival time was
calculated using the Kaplan-Meier method and was analyzed by the
log-rank test. Data are presented as the mean ± standard deviation.
P<0.05 was considered to indicate a statistically significant
difference.
Results
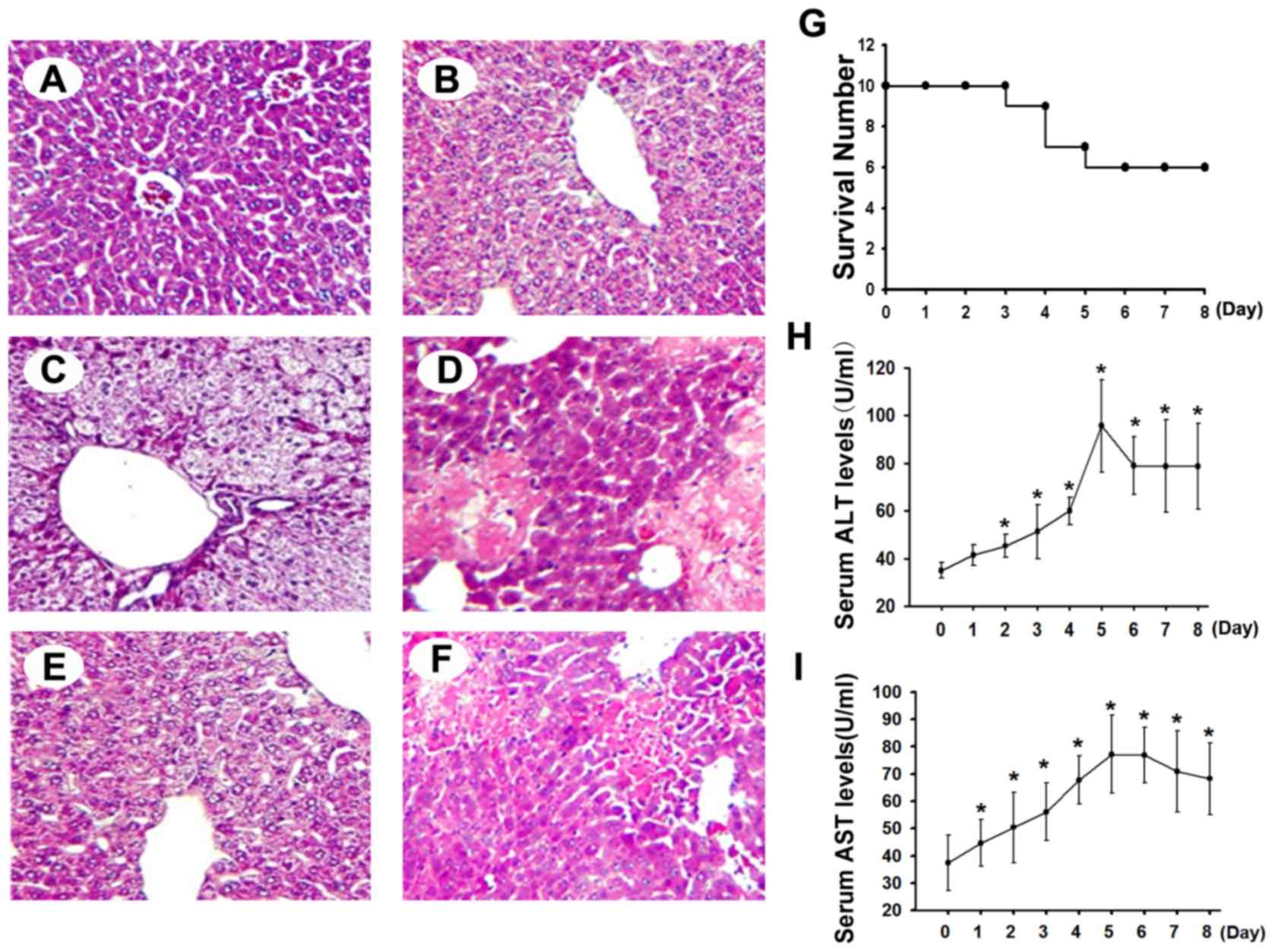
A mouse model of OXA-induced ALI was
successfully established
To establish a mouse model of OXA-induced ALI, KM
mice were treated with OXA (i.p.) for 4 days. Following 2 days of
OXA treatment, mice exhibited a reduced appetite and mild diarrhea,
which were aggravated with an increase in OXA treatment. A number
of mice experienced severe diarrhea, and ultimately died. No
abnormal pathological changes were observed in the control mice
(Fig. 1A), while liver injuries,
including mild liver cell swelling, liver cell turbidity and
degeneration, and loose cellular structure, were observed following
3 days of OXA treatment in the OXA group (Fig. 1B). Varying degrees of liver cell
turbidity and degeneration (Fig.
1C-E), and even balloon-like changes and focal necrosis, were
observed in the liver tissues following OXA withdrawal; these liver
pathological changes were most evident at 2 days following OXA
withdrawal. The major liver pathological changes present in the
deceased mice were moderate cell turbidity and degeneration and
focal necrosis (Fig. 1F). Survival
curve analysis revealed that mortality occurred following 4 days of
OXA treatment in the OXA group, and the survival rate in this group
was 60% (6/10) 7 days after the final dose of OXA was administered
(Fig. 1G).
To evaluate OXA-induced liver toxicity in the mouse
model, changes in the serum AST and ALT levels were detected.
Compared with the control mice, OXA-treated mice showed
significantly elevated serum ALT and AST levels (P<0.05) after 2
days and 1 day of OXA treatment, respectively. With the increase in
the number of OXA treatments, these elevations were enhanced, and
the high serum AST and ALT levels persisted for 4 days following
OXA withdrawal (Fig. 1H and I).
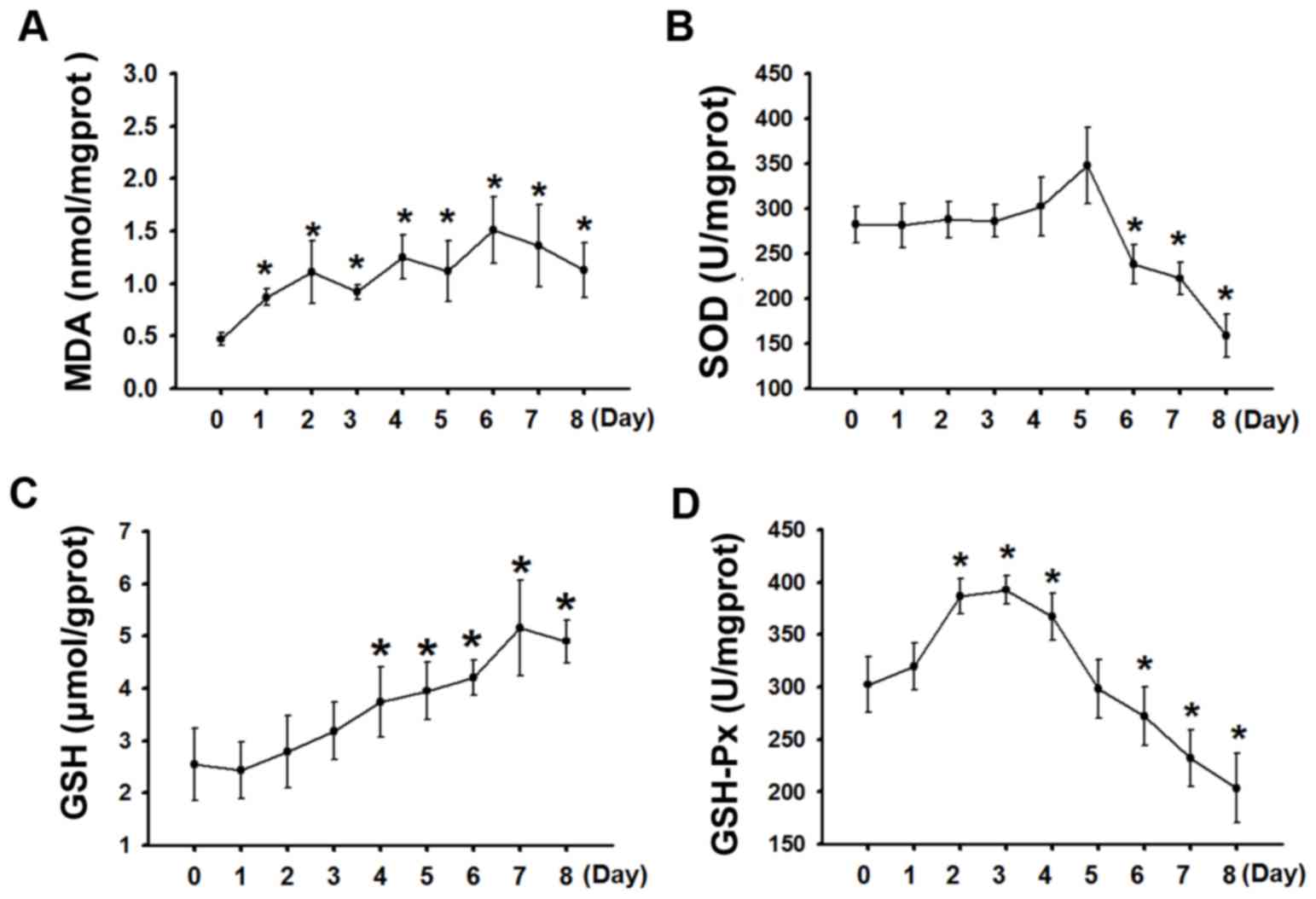
Oxidative stress in OXA-induced
ALI
Evidence from various patient studies suggests that
liver injuries induced by OXA-based chemotherapy, including
FOLFOX-induced SOS, are associated with increased oxidative stress
in the liver (10). To elucidate the
role of oxidative stress in OXA-induced ALI, the oxidative
indicator MDA and the antioxidative indicators SOD, GSH and GSH-Px,
were analyzed. As presented in Fig.
2A, the MDA levels in OXA-treated mice were significantly
increased 1 day following OXA injection (P<0.05), a difference
that was enhanced as the OXA injection dose increased (P>0.05).
MDA was maintained at high levels even several days following the
termination of OXA treatment. Compared with the control group, no
significant change in SOD levels was observed during OXA treatment,
but decreased SOD levels were observed 2 days following OXA
withdrawal (P<0.05; Fig. 2B). GSH
levels did not significantly change during early OXA treatment
(P>0.05), but continuously increased during later OXA treatment
and the early period following OXA withdrawal (P<0.05; Fig. 2C). In OXA-treated mice, GSH-Px levels
were significantly increased following OXA injection (P<0.05;
Fig. 2D), but was decreased 2 days
following OXA withdrawal and thereafter remained at low levels.
GSH attenuates OXA-induced ALI
To examine whether GSH therapy has a protective
effect on OXA-induced ALI, OXA-treated mice received GSH treatment
30 min prior to each OXA injection for 4 days. Optical microscopy
and H&E staining indicated clear liver cell injury in
OXA-treated mice, including liver cell swelling and degeneration
(mainly moderate and severe), balloon-like changes and focal
necrosis (Fig. 3A). Compared with the
OXA group mice, GSH group mice exhibited alleviated liver cell
injury, which demonstrated mild turbidity and swelling, and no
notable hepatocyte necrosis (Fig.
3A). In addition, the serum AST and ALT levels in the GSH group
mice were markedly decreased, compared with those in the OXA group
mice (P<0.05; 46.77±7.64 vs. 72.17±15.34, 42.37±15.83 vs.
60.78±24.94 for ALT and AST, respectively), but were still higher
than those in the control mice (P<0.05; Fig. 3B and C). However, in the GSH-treated
group, GSH did not significantly alleviate the OXA-induced reduced
appetite, decreased body weight and diarrhea (data not presented).
Body weight increased over time in the control mice, but
significantly decreased in the OXA and GSH groups (P<0.05).
There was no significant difference between the OXA group and GSH
group (P>0.05) with respect to body weight (Fig. 3D). In addition, GSH therapy did not
increase the survival rate of the GSH group (Fig. 3E) compared with the OXA group (60 vs.
60%).
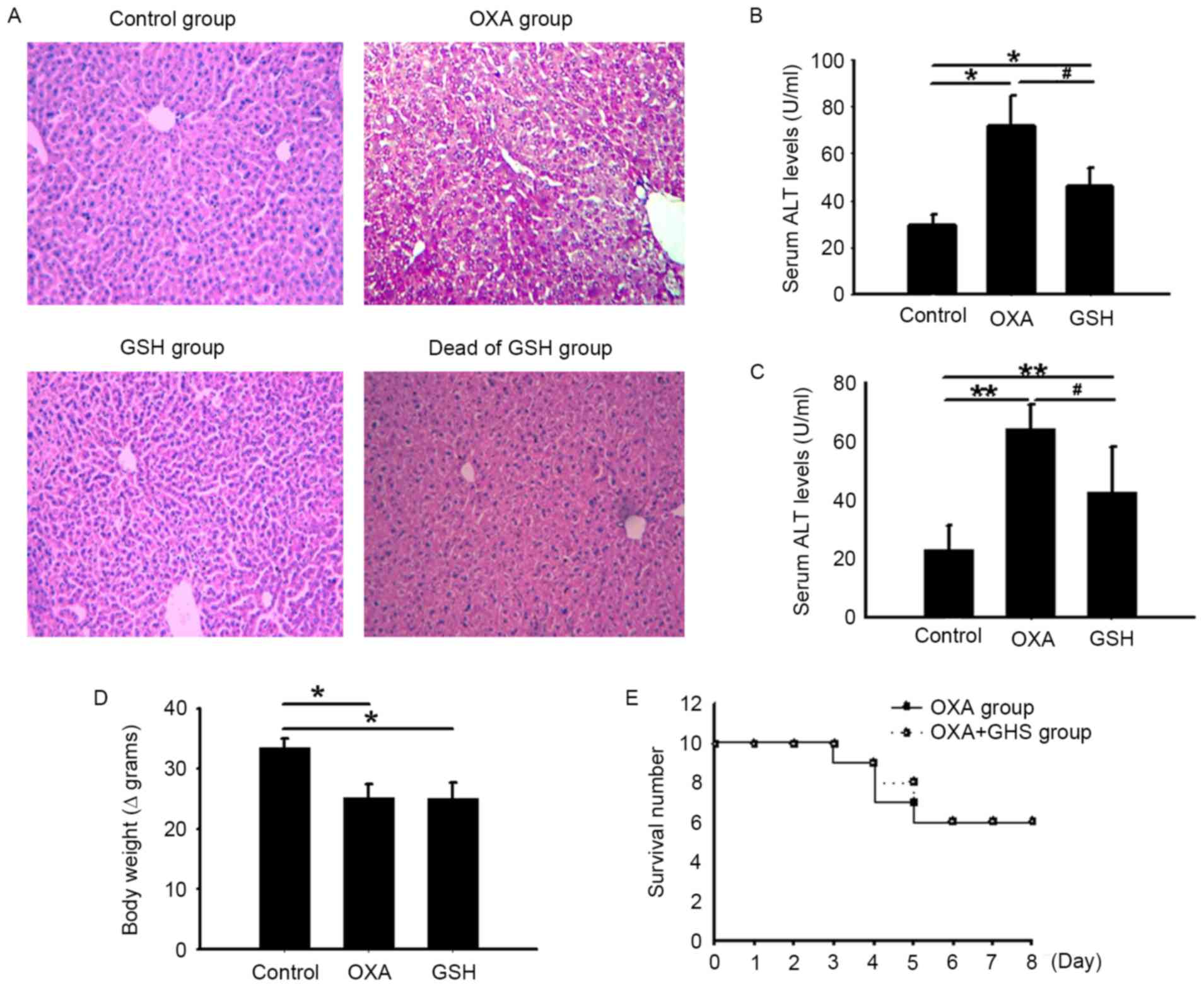 | Figure 3.Treatment with GSH attenuated
OXA-induced ALI in mice. The OXA group were treated with OXA for 4
days, the GSH group were treated with OXA for 4 days and with GSH
every day from the first day of OXA administration until the end of
the experiment, and the control group were administered with 5%
glucose (i.p.) for 4 days. The samples (blood and liver tissue)
from each group were collected 3 days after the final dose of OXA.
(A) The liver histopathology was examined in each group (H&E
staining, original magnification, ×100). (B) The serum ALT and AST
levels of each group 3 days after the final dose of OXA. (C) The
body weights of each group 3 days after the final dose of OXA. For
(B) and (C), the results are presented as the means ± standard
deviation from five mice in each group. *P<0.05 and **P<0.01,
compared with the control group. #P<0.05, compared
with the OXA group. (D) The survival rates of the three groups were
observed. OXA, oxaliplatin; GSH, glutathione; ALI, acute liver
injury; ALT, alanine aminotransferase; AST, aspartate
aminotransferase levels; H&E, hematoxylin and eosin. |
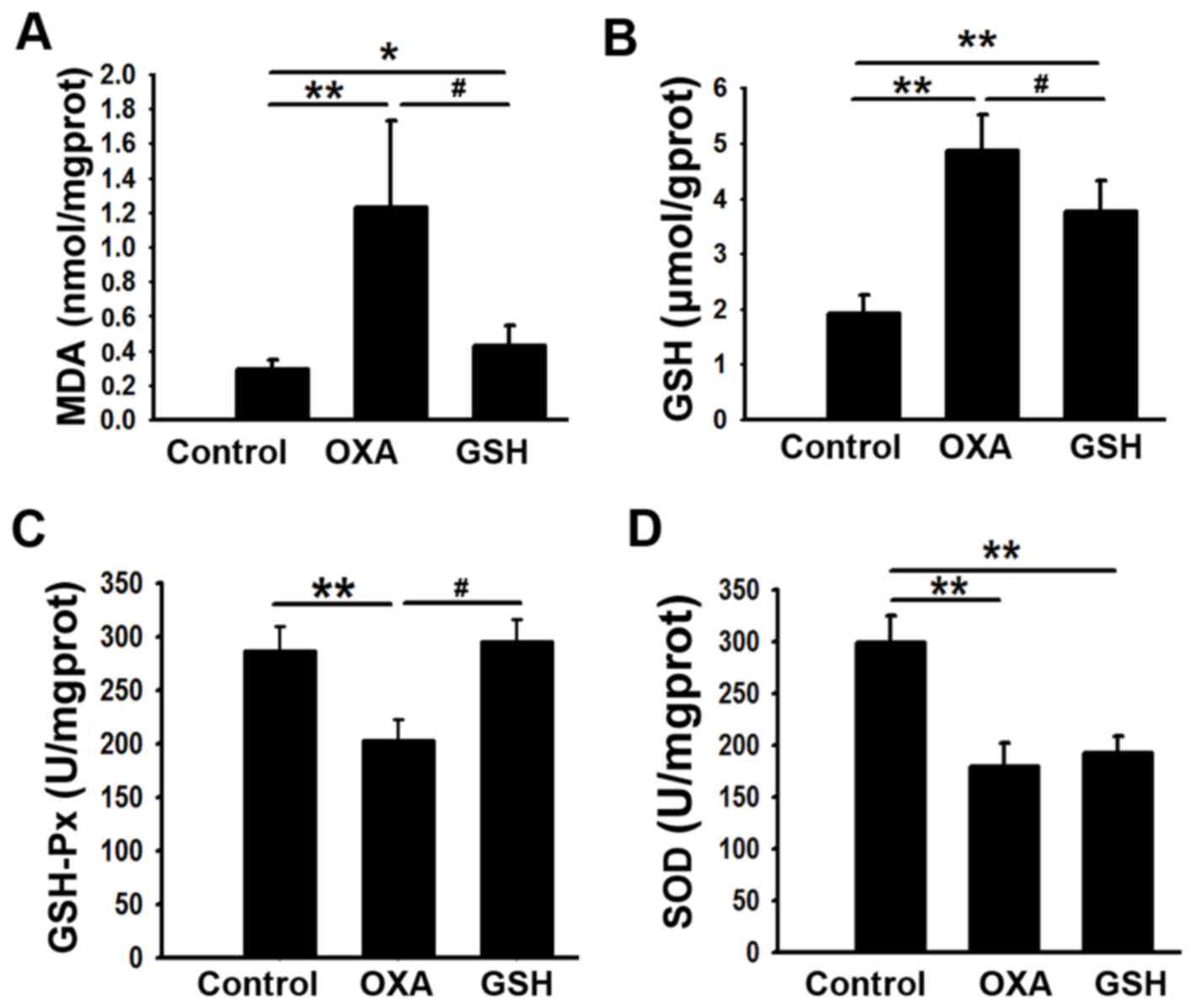
GSH suppresses OXA-induced oxidative
liver injury
The antioxidative effect of GSH on liver injury was
investigated. As presented in Fig. 4A and
B, GSH administration decreased the liver MDA and GSH levels in
the GSH group, compared with the OXA group (P<0.05; 0.43±0.12
vs. 1.23±0.50, 3.77±1.25 vs. 4.87±0.64 for MDA and GSH,
respectively). Compared with in the control group, liver GSH-PX
activity was significantly decreased (P<0.01) in the OXA group,
and this was reversed by GSH administration (Fig. 4C). However, no significant difference
in SOD activity was observed between the OXA and GSH groups
(Fig. 4D).
Discussion
Chemotherapy-associated liver injury can include
steatosis, liver cell necrosis, severe steatohepatitis and SOS.
Distinct types of liver injuries may be associated with specific
chemotherapy drugs (16,17). In patients with colon cancer receiving
multi-cycle OXA-based chemotherapy, liver injury pathological
changes include steatosis and sinusoidal injury, in addition to
elevated AST and phosphatase levels (6). SOS is the most typical histological
change, and is characterized by impaired sinusoidal wall integrity,
sinusoidal hyperemia and blockage, sinusoidal fibrosis, fibroid
blockage in the lobular central vein and nodular hyperplasia or
hemacelinosis (18). Similar
pathological changes to the liver are also observed in animal
models of OXA or OXA-based chemotherapies. Schwingel et al
(11) treated BALB/c mice with OXA
(10 mg/kg/week, i.p.), and reported the appearance of
steatohepatitis after 6 weeks of OXA treatment. Keizman et
al (19) treated C57BL/6 mice
with OXA (10 mg/kg/week, i.p.) for 4 weeks, and established a mouse
model of OXA-induced steatohepatitis. Robinson et al
(10) treated mice with FOLFOX (10
mg/kg/week, i.p.) for 6 weeks, and successfully established an
animal model of OXA-induced SOS.
A mouse model of OXA-induced ALI was successfully
established in the current study. In this model, elevated ALT and
AST levels characterized OXA-induced ALI during the early stage of
OXA treatment. Hepatic histopathology of the OXA-induced ALI
demonstrated varying degrees of liver cell turbidity and
degeneration, even balloon like changes and focal necrosis, and
sinusoidal hemorrhage in certain individuals. These hepatic
pathological changes in OXA-induced ALI were different from the
pathology of chronic liver injuries induced by multi-cycle
OXA-based chemotherapy reported in clinical observation and animal
studies, in which the primary characteristics of liver injury are
liver sinusoidal injury and SOS (2,3).
Therefore, liver sinusoidal injury and SOS are the pathological
characteristics of long-term OXA chemotherapy (18), while OXA-induced ALI is characterized
by varying degrees of liver cell degeneration, such as
turbidity-like degeneration and balloon-like degeneration.
Recently, it has been suggested that oxidative
stress is an important contributing factor to hepatotoxicity
induced by long-term OXA chemotherapy (9,10).
Oxidative stress is the overproduction of highly active molecules,
such as ROS, and when liver cells are exposed to certain noxious
stimuli, leading to an imbalance between the oxidative and
antioxidative systems, liver injury occurs (20). In the present study, it was revealed
that the level of oxidative indicator MDA is increased in
OXA-treated mice. MDA is a lipid peroxidation product, and its
level can reflect the extent of oxidative stress-associated injury
caused by free radicals (21). In the
OXA-induced ALI model, elevated MDA levels indicate that OXA can
increase free radicals in the liver. Excessive MDA in liver tissue
will consume a large amount of antioxidative factors, such as SOD
and GSH, which can protect liver from the attacks of free radicals,
but once the balance is broken, SOD and GSH will be unable to
protect liver against the excessively increased MDA (22,23). The
present study demonstrated that, although GSH levels are
continuously increased following OXA withdrawal and liver MDA
levels are continuously increased, GSH-Px and SOD levels are
consistently decreased and are accompanied by elevated ALT and AST
levels. Additionally, pathological examination of the liver
revealed an increase in liver injury following OXA administration.
Furthermore, an increase in mouse mortality was also observed
following an increase in the number of OXA treatments. These
results indicate that the OXA-induced increase in liver free
radicals, massive depletion of SOD and the insufficient
compensation of GSH-Px and GSH syntheses all lead to the occurrence
of ALI. Therefore, the results suggest that oxidative stress may
serve an important role in the pathogenesis of OXA-induce ALI.
Under physiological conditions, the liver can resist
oxidative stress through GSH synthesis in hepatocytes. In the
present study, mice treated with OXA and GSH exhibited high GSH-Px
levels and low MDA levels, which indicated a reduction of oxidative
stress and is accompanied by decreased tissue injury, ALT and AST
levels. GSH can directly scavenge radicals and peroxides via mixed
disulfide formation or oxidization to generate oxidized glutathione
(14–15,24). GSH
can resist oxidative stress by serving as a substrate for
antioxidative enzymes, including GSH-Px which converts
hydroperoxide into less harmful fatty acids, water and GSH
disulfide (24). Therefore, GSH can
resist OXA-induced oxidative stress, and attenuate OXA-induced
liver injury.
In the present study, MAD levels in the GSH
treatment group remained higher than in the control group, and no
significant impact on SOD level downregulation was observed
following GSH treatment. Therefore, although GSH treatment exerted
a significant protective effect against OXA-induced liver injury in
the present study, hepatic oxidative stress continues to occur. In
addition, the ALT and AST levels in OXA and GSH-treated mice did
not recover to within the normal range, indicating that GSH alone
is insufficient for suppressing oxidative stress during OXA-induced
ALI. Perhaps combining GSH with other drugs, such as antioxidants,
may further alleviate OXA-induced liver injury. Indeed, various
endogenous of dietary antioxidants are capable of ameliorating
steatohepatitis and OXA-induced neurotoxicity via reducing
oxidative stress. Besides oxidative stress, prior studies
determined that other mechanisms are also involved in OXA-induced
liver injury. These mechanisms include the activation of
inflammation-associated pathways (10,25,26), the
activation of cellular hypoxia (27)
and the upregulation of genes involved in coagulation (particularly
PAI-1 and vWF) (3,10,28).
Studies have also detected the upregulation of
angiogenesis-associated genes, including VEGF-A, VEGF-C and VEGF-D
in OXA-induced SOS (10,27,29).
Concordantly, prior clinical observations suggested that
bevacizumab is effective in reducing the incidence and severity of
SOS associated with OXA-based chemotherapy (28,30,31).
Therefore, to further alleviate OXA-induced liver injury, it is
essential to consider other potential mechanisms that contribute to
liver injury, which will be examined in subsequent studies.
As observed in the present study, GSH treatment
alone cannot reduce OXA-induced mortality. Histopathological
examination detected no liver failure, and the cause of mortality
was determined to be severe diarrhea. Compared with the OXA-treated
mice, OXA and GSH-treated mice exhibited no significant difference
in body weight loss, appetite reduction and diarrhea (data not
presented), indicating that GSH treatment has no significant
ameliorative effect on OXA-induced liver injury. Therefore, during
treatment of the liver injury caused by OXA chemotherapy, other
OXA-induced toxicities, including neurotoxicity, gastrointestinal
toxicity and hematological toxicity, must also be considered.
In summary, an animal model of OXA-induced ALI was
successfully established. The results suggest that oxidative stress
serves an important role in the pathogenesis of OXA-induced ALI,
and that GSH treatment can attenuate OXA-induced ALI by suppressing
oxidative stress in the liver.
Acknowledgements
The present study was partially supported by the
Guangxi Natural Science Foundation (grant no. 2016GXNSFBA380218),
the Guangxi Key Laboratory of Molecular Medicine in Liver Injury
and Repair (grant no. 16-140-46-18), the Guangxi Basic Ability
Promotion Project of Middle-aged and Young Teachers in Colleges and
Universities (grant no. 2017KY0121), the Youth Science Foundation
of Guangxi Medical University (grant no. GXMUYSF201336), the
Self-Raised Funds of Guangxi Health Department (grant no. Z2016438
and grant no. Z2013423), The Medication and Health Care Research
Program of Guangxi (grant no. S201418-03) and the Key Planning
Development Research Program of Guangxi (grant no.
guikeAB16380215).
References
|
1
|
Goldstein DA, Zeichner SB, Bartnik CM,
Neustadter E and Flowers CR: Metastatic colorectal cancer: A
systematic review of the value of current therapies. Clin
Colorectal Cancer. 15:1–6. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rubbia-Brandt L, Audard V, Sartoretti P,
Roth AD, Brezault C, Le Charpentier M, Dousset B, Morel P, Soubrane
O, Chaussade S, et al: Severe hepatic sinusoidal obstruction
associated with oxaliplatin-based chemotherapy in patients with
metastatic colorectal cancer. Ann Oncol. 15:460–466. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tajima H, Ohta T, Miyashita T, Nakanuma S,
Matoba M, Miyata T, Sakai S, Okamoto K, Makino I, Kinoshita J, et
al: Oxaliplatin-based chemotherapy induces extravasated platelet
aggregation in the liver. Mol Clin Oncol. 3:555–558. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Soubrane O, Brouquet A, Zalinski S, Terris
B, Brézault C, Mallet V, Goldwasser F and Scatton O: Predicting
high grade lesions of sinusoidal obstruction syndrome related to
oxaliplatin-based chemotherapy for colorectal liver metastases:
Correlation with post-hepatectomy outcome. Ann Surg. 251:454–460.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nakano H, Oussoultzoglou E, Rosso E,
Casnedi S, Chenard-Neu MP, Dufour P, Bachellier P and Jaeck D:
Sinusoidal injury increases morbidity after major hepatectomy in
patients with colorectal liver metastases receiving preoperative
chemotherapy. Ann Surg. 247:118–124. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nalbantoglu IL, Tan BR Jr, Linehan DC, Gao
F and Brunt EM: Histological features and severity of
oxaliplatin-induced liver injury and clinical associations. J Dig
Dis. 15:553–560. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Vreuls CP, Van Den Broek MA, Winstanley A,
Koek GH, Wisse E, Dejong CH, Damink Olde SW, Bosman FT and Driessen
A: Hepatic sinusoidal obstruction syndrome (SOS) reduces the effect
of oxaliplatin in colorectal liver metastases. Histopathology.
61:314–318. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Vincenzi B, Daniele S, Frezza AM, Berti P,
Vespasiani U, Picardi A and Tonini G: The role of
S-adenosylmethionine in preventing oxaliplatin-induced liver
toxicity: A retrospective analysis in metastatic colorectal cancer
patients treated with bevacizumab plus oxaliplatin-based regimen.
Support Care Cancer. 20:135–139. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Santoro V, Jia R, Thompson H, Nijhuis A,
Jeffery R, Kiakos K, Silver AR, Hartley JA and Hochhauser D: Role
of reactive oxygen species in the abrogation of oxaliplatin
activity by cetuximab in colorectal cancer. J Natl Cancer Inst.
108:djv3942015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Robinson SM, Mann J, Vasilaki A, Mathers
J, Burt AD, Oakley F, White SA and Mann DA: Pathogenesis of FOLFOX
induced sinusoidal obstruction syndrome in a murine chemotherapy
model. J Hepatol. 59:318–326. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Schwingel TE, Klein CP, Nicoletti NF, Dora
CL, Hadrich G, Bica CG, Lopes TG, da Silva VD and Morrone FB:
Effects of the compounds resveratrol, rutin, quercetin, and
quercetin nanoemulsion on oxaliplatin-induced hepatotoxicity and
neurotoxicity in mice. Naunyn Schmiedebergs Arch Pharmacol.
387:837–848. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Azevedo MI, Pereira AF, Nogueira RB, Rolim
FE, Brito GA, Wong DV, Lima-Júnior RC, de Albuquerque Ribeiro R and
Vale ML: The antioxidant effects of the flavonoids rutin and
quercetin inhibit oxaliplatin-induced chronic painful peripheral
neuropathy. Mol Pain. 9:532013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Carozzi VA, Marmiroli P and Cavaletti G:
The role of oxidative stress and anti-oxidant treatment in
platinum-induced peripheral neurotoxicity. Curr Cancer Drug
Targets. 10:670–682. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen Y, Dong H, Thompson DC, Shertzer HG,
Nebert DW and Vasiliou V: Glutathione defense mechanism in liver
injury: Insights from animal models. Food Chem Toxicol. 60:38–44.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Balendiran GK, Dabur R and Fraser D: The
role of glutathione in cancer. Cell Biochem Funct. 22:343–352.
2004. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Khan AZ, Morris-Stiff G and Makuuchi M:
Patterns of chemotherapy-induced hepatic injury and their
implications for patients undergoing liver resection for colorectal
liver metastases. J Hepatobiliary Pancreat Surg. 16:137–144. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Raschi E and De Ponti F: Drug- and
herb-induced liver injury: Progress, current challenges and
emerging signals of post-marketing risk. World J Hepatol.
7:1761–1771. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fan CQ and Crawford JM: Sinusoidal
obstruction syndrome (hepatic veno-occlusive disease). J Clin Exp
Hepatol. 4:332–346. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Keizman D, Maimon N, Ish-Shalom M, Buchbut
D, Inbar M, Klein B, Bernheim J, Goldiner I, Leikin-Frenkel A and
Konikoff F: An animal model for chemotherapy-associated
steatohepatitis and its prevention by the oral administration of
fatty acid bile acid conjugate. Cancer. 116:251–255.
2010.PubMed/NCBI
|
|
20
|
de Andrade KQ, Moura FA, Dos Santos JM, de
Araújo OR, de Farias Santos JC and Goulart MO: Oxidative stress and
inflammation in hepatic diseases: Therapeutic possibilities of
N-acetylcysteine. Int J Mol Sci. 16:30269–30308. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nielsen F, Mikkelsen BB, Nielsen JB,
Andersen HR and Grandjean P: Plasma malondialdehyde as biomarker
for oxidative stress: Reference interval and effects of life-style
factors. Clin Chem. 43:1209–1214. 1997.PubMed/NCBI
|
|
22
|
Xing H, Jia K, He J, Shi C, Fang M, Song
L, Zhang P, Zhao Y, Fu J and Li S: Establishment of the tree shrew
as an alcohol-induced Fatty liver model for the study of alcoholic
liver diseases. PLoS One. 10:e01282532015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Curry-McCoy TV, Osna NA, Nanji AA and
Donohue TM Jr: Chronic ethanol consumption results in atypical
liver injury in copper/zinc superoxide dismutase deficient mice.
Alcohol Clin Exp Res. 34:251–261. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bułdak RJ, Bułdak Ł, Kukla M, Gabriel A
and Zwirska-Korczala K: Significance of selected antioxidant
enzymes in cancer cell progression. Pol J Pathol. 65:167–175. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Marzano C, Cazals-Hatem D, Rautou PE and
Valla DC: The significance of nonobstructive sinusoidal dilatation
of the liver: Impaired portal perfusion or inflammatory reaction
syndrome. Hepatology. 62:956–963. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Robinson SM, Mann DA, Manas DM, Oakley F,
Mann J and White SA: The potential contribution of tumour-related
factors to the development of FOLFOX-induced sinusoidal obstruction
syndrome. Br J Cancer. 109:2396–2403. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rubbia-Brandt L, Tauzin S, Brezault C,
Delucinge-Vivier C, Descombes P, Dousset B, Majno PE, Mentha G and
Terris B: Gene expression profiling provides insights into pathways
of oxaliplatin-related sinusoidal obstruction syndrome in humans.
Mol Cancer Ther. 10:687–696. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nishigori N, Matsumoto M, Koyama F,
Hayakawa M, Hatakeyayama K, Ko S, Fujimura Y and Nakajima Y: von
Willebrand factor-rich platelet thrombi in the liver cause
sinusoidal obstruction syndrome following oxaliplatin-based
chemotherapy. PLoS One. 10:e01431362015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Paré-Brunet L, Sebio A, Salazar J,
Berenguer-Llergo A, Río E, Barnadas A, Baiget M and Páez D: Genetic
variations in the VEGF pathway as prognostic factors in metastatic
colorectal cancer patients treated with oxaliplatin-based
chemotherapy. Pharmacogenomics J. 15:397–404. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Imai K, Emi Y, Iyama KI, Beppu T, Ogata Y,
Kakeji Y, Samura H, Oki E, Akagi Y, Maehara Y, et al: Splenic
volume may be a useful indicator of the protective effect of
bevacizumab against oxaliplatin-induced hepatic sinusoidal
obstruction syndrome. Eur J Surg Oncol. 40:559–566. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Arakawa Y, Shimada M, Utsunomiya T, Imura
S, Morine Y, Ikemoto T, Hanaoka J, Kanamoto M, Iwahashi S, Saito Y,
et al: Bevacizumab improves splenomegaly and decreases production
of hyaluronic acid after L-OHP based chemotherapy. Anticancer Res.
34:1953–1958. 2014.PubMed/NCBI
|