Introduction
Granulocyte-colony stimulating factor (G-CSF) is a
glycoprotein associated with the proliferation and maturation of
neutrophils (1,2). Since the first case of G-CSF-producing
lung carcinoma reported by Asano et al in 1977 (3), similar carcinomas have been reported in
various organs (4,5). However, G-CSF-producing carcinomas of
the pancreas are relatively rare (6–10).
Mucinous cystic neoplasms (MCN) are defined as cyst-forming
epithelial neoplasms that arise in the pancreas (11). MCNs have a female predominance, and
they arise in the body and tail of the pancreas, without
communication with the pancreatic duct system (12). The most specific pathological feature
of MCNs is ovarian-type subepithelial stroma in the cyst wall
(12). According to previous reports,
the ovarian-like stroma of MCNs exhibits immunohistochemically
positive progesterone receptor (PgR) and estrogen receptor (ER)
(13). The size of MCNs ranges
between 2 and 35 cm in diameter, and patients with MCNs with an
associated invasive carcinoma are 5–10 years older compared with
those with non-invasive MCNs (11).
These findings indicate that the progression from non-invasive MCN
to invasive carcinoma occurs over a period of years. The histology
of G-CSF-producing neoplasms of the pancreas, including
unconventional tumors such as adenosquamous carcinoma or anaplastic
carcinoma, has been previously described (6,7,9,10,14,15).
However, to the best of our knowledge, there are no reports
concerning MCNs, and this is the first study to identify positive
G-CSF immunostaining in a patient with MCN with an associated
invasive carcinoma.
Case report
A 65-year-old woman presenting with abdominal pain,
constipation and lingering fever was referred to Department of
Medicine, Omuta City Hospital (Omuta, Japan) for further
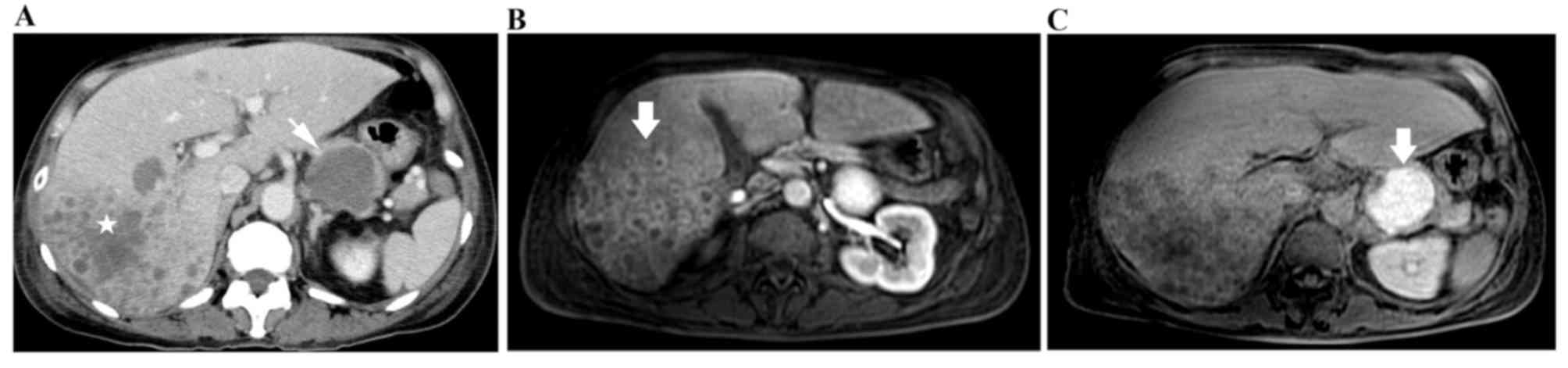
examination in April 2015. Abdominal computed tomography indicated
multiple hepatic nodules, a pancreatic cyst and ascites (Fig. 1A). The abdominal magnetic resonance
imaging indicated multiple ring-enhanced lesions (Fig. 1B), and a pancreatic tail cyst
exhibited high intensity on a fat suppression T1-weighted image
(Fig. 1C). Laboratory tests revealed
marked leukocytosis [white blood cell count, 39,640/µl (normal,
3,500–9,100/µl); neutrophils, 94.1% (normal, 32–79%); monocytes,
3.2% (normal, 0–8%); lymphocytes, 2.3% (normal, 18–59%);
eosinophils, 0.3% (normal, 0–6%); basophils, 0.1% (normal, 0–2%);
and no blasts] and elevated serum C-reactive protein level (20.4
mg/dl; normal, <0.3 mg/dl). Serum tumor markers, including
carbohydrate antigen 19-9 (10.5 U/ml; normal 0–37 U/ml),
carcinoembryonic antigen (3.2 ng/ml; normal 0–5 ng/ml) and
α-fetoprotein (1.8 ng/ml; 0–10 ng/ml), were within normal limits.
Ascites cytology was negative for malignancy. Bone marrow
aspiration revealed hypercellular marrow with excessive myeloid
cells. Considering the clinical symptoms, radiological studies and
laboratory results, liver abscess was suspected as an infective
focus. However, neither arterial infusion therapy of
imipenem/cilastatin (1 g/day for 4 days) nor administration of
tazobactam/piperacillin (13.5 g/day for 10 days), metronidazole
(1,500 mg/day for 10 days) and amphotericin B (2 g/day for 4 days)
elicited any response in the patient. Upon further examination, the
cystic lesion in the pancreas was suspected to be a malignant
neoplasm, and tumor-associated leukocytosis was considered. The
serum concentration of G-CSF was elevated (98.8 pg/ml; normal
<39.0 pg/ml). The physical status of the patient deteriorated
rapidly, with multiple liver nodules and aggravation of jaundice
and ascites. At 6 weeks after admission, the patient succumbed to
liver failure. Written informed consent was obtained from the
patient's family following mortality.
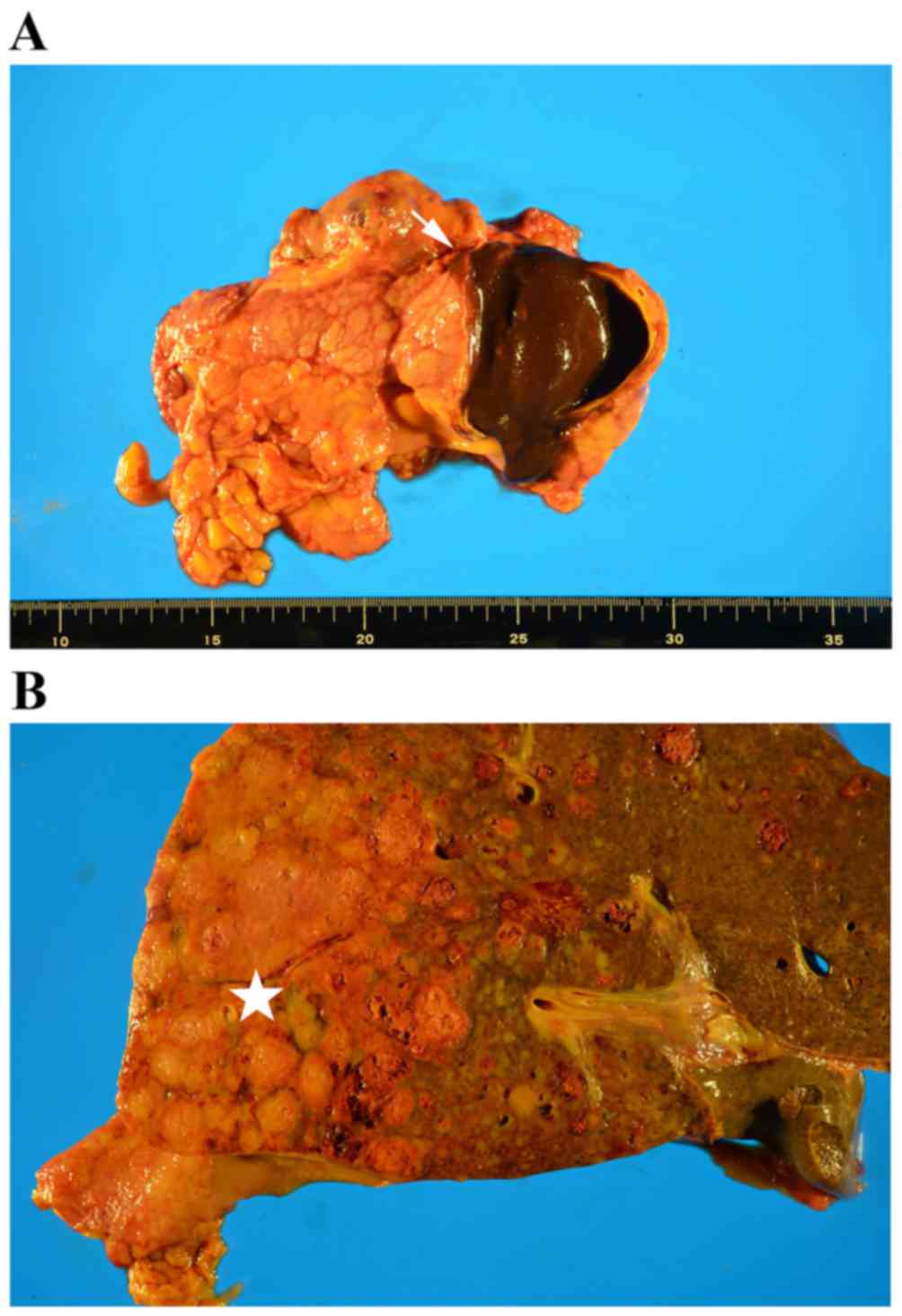
At autopsy, a cystic lesion (size, 4×3 cm) was noted
in the pancreatic tail. This lesion had an elastic hard wall and
contained bloody necrotic fluid (Fig.
2A). Multiple liver nodules, located predominantly in the right
lobe, were observed (Fig. 2B).
Sections (3-µm thick) were fixed with 10% formalin and
paraffin-embedded at room temperature for 2 days. The sections were
stained with hematoxylin for 5 min and with eosin for 3 min at room
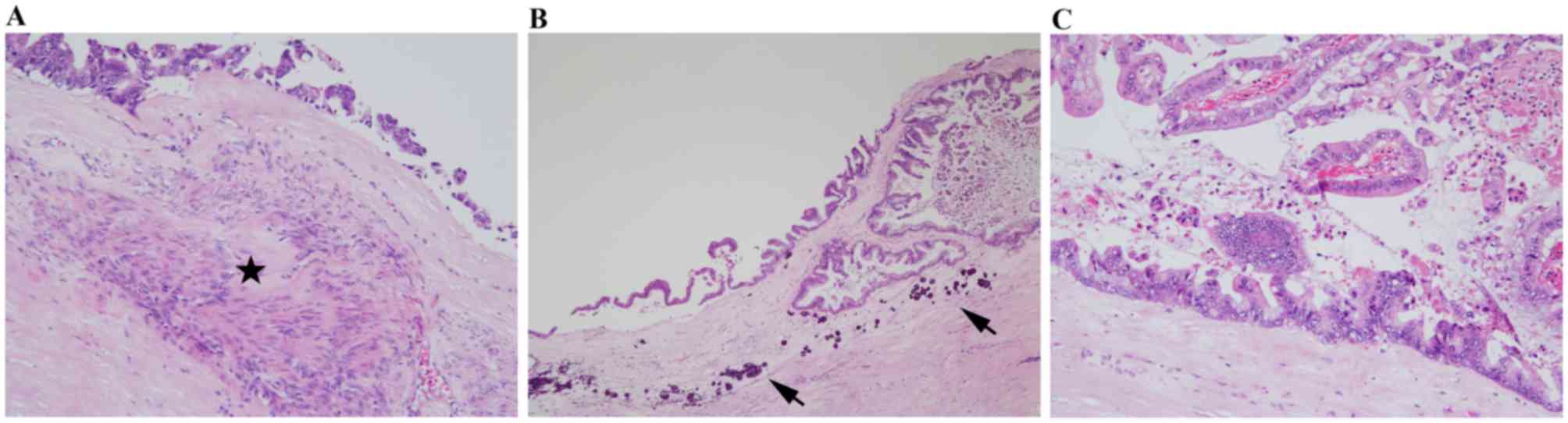
temperature. Histologically, the pancreatic tumor was a cystic
lesion that had ovarian-type subepithelial stroma with focal
calcification and was lined by columnar mucinous epithelium with
high-grade dysplasia (Fig. 3A-C).
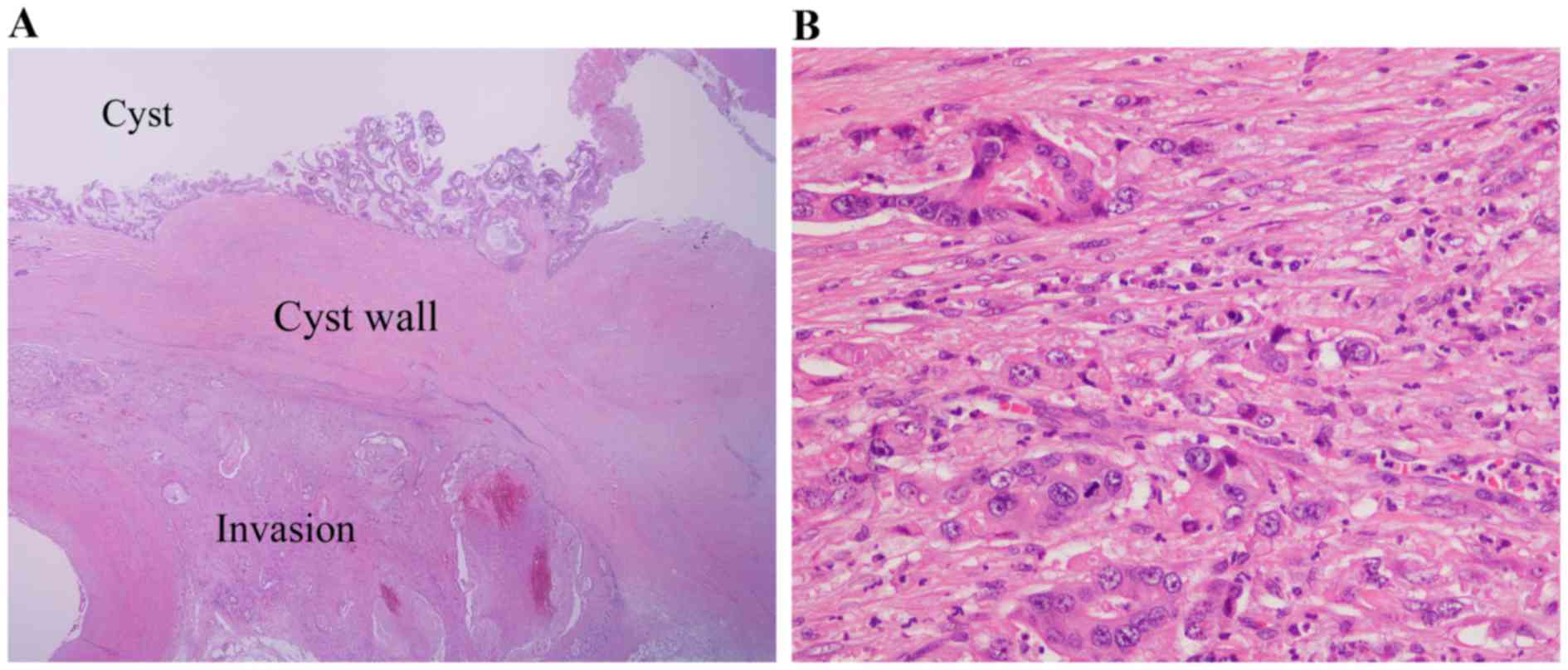
Adenocarcinoma, which extended into pancreatic acinus and invaded
splenic vein, exhibited irregular glandular structures and poorly
cohesive cell clusters (Fig. 4A and
B). The parenchyma was infiltrated by considerable neutrophils
(Fig. 4B).
Sections (3-µm thick) were fixed with 10% formalin
and paraffin-embedded at room temperature for 2 days. Onboard
heat-induced antigen retrieval was performed on a fully automated
BOND-III system (Leica Microsystems Ltd., Milton Keynes, UK) with a
BOND Epitope Retrieval Solution 2 (cat. no. AR9640; Leica
Biosystems, Inc., Buffalo Grove, IL, USA) for 20 min at 99°C, and
sections were incubated with the following primary antibodies for
15 min at room temperature: Mucin (MUC)1 (cat. no. NCL-MUC-1;
dilution, 1:300; clone Ma695; Novocastra; Leica Biosystems, Inc.),
MUC2 (cat. no. NCL-MUC-2; dilution, 1:200; clone Ccp58; Novocastra;
Leica Biosystems, Inc.), MUC5AC (cat. no. NCL-MUC-5AC; dilution,
1:200; clone CLH2; Novocastra; Leica Biosystems, Inc.), cytokeratin
AE1/AE3 (cat. no. N3515; dilution, 1:300; clone AE1/AE3; Dako;
Agilent Technologies, Inc., Santa Clara, CA, USA), Vimentin (cat.
no. M0725; dilution 1:9; clone V9; Dako; Agilent Technologies,
Inc.) and G-CSF (cat. no. GF05; dilution 1:2,000; clone 5.24;
Calbiochem; EMD Millipore, Billerica, MA, USA). Peroxide Blocking
Reagent (3–4%) (cat. no. DS9800; Leica Biosystems, Inc.) was
applied for 5 min at room temperature, according to the
manufacturer's protocols. The automated system used a Bond Polymer
Refine Detection kit (cat. no. DS9800; Leica Biosystems, Inc.;
containing an immunoglobulin G linker, a horseradish
peroxidase-linked polymer and 3,3′-diaminobenzidine (DAB) to detect
the bound primary antibodies. Incubation with the secondary
antibody was performed for 30 min at room temperature.
ER (cat. no. 107925; ready to use; clone SP1;
Ventana Medical Systems, Inc., Tucson, AZ, USA) and PgR (cat. no.
102333; ready to use; clone 1E2; Ventana Medical Systems, Inc.)
staining for 32 min at room temperature was performed using
BenchMark ULTRA (Ventana Medical Systems, Inc.). The automated
system used the streptavidin-biotin complex method with DAB as a
chromogen (cat. no. 109431; Ventana iVIEW DAB Detection kit;
Ventana Medical Systems, Inc.). An Olympus BX51 optical microscope
was used to view all slides under ×12.5–400 magnification.
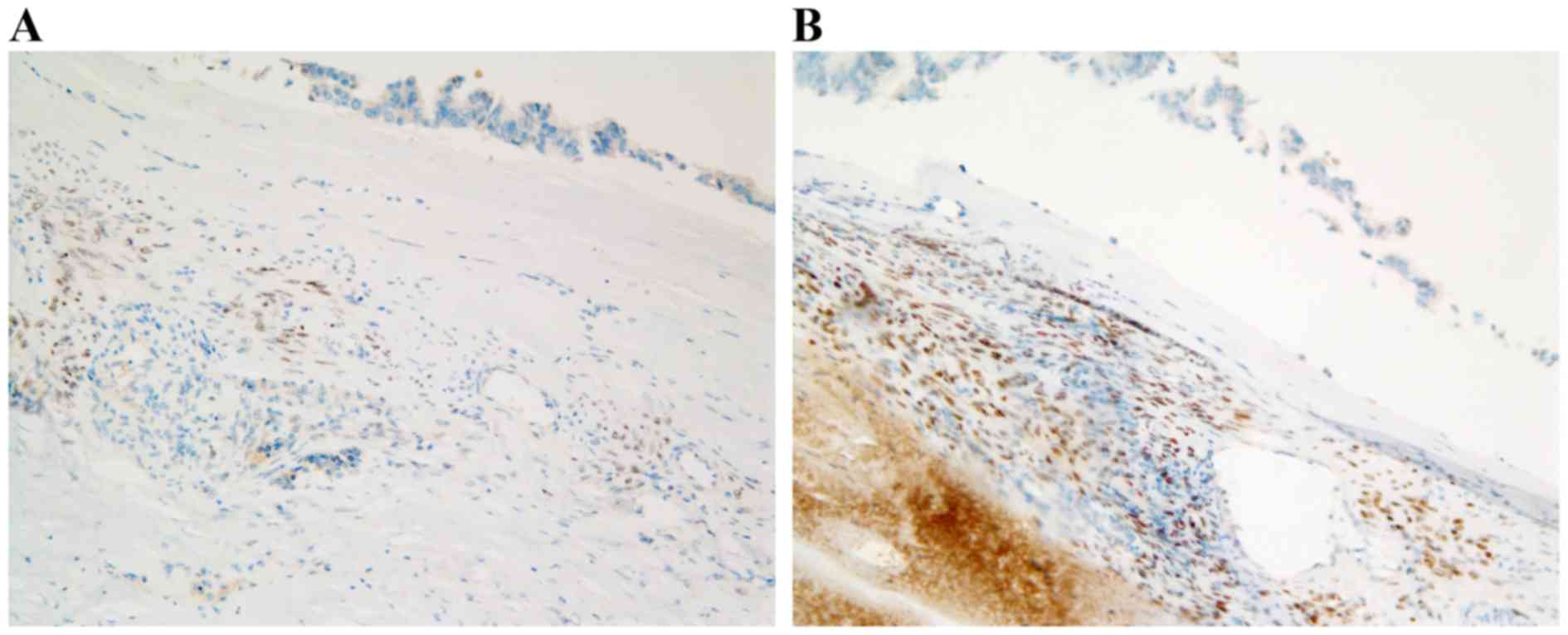
The tumor cells exhibited positive staining for
MUC5AC, and negative staining for MUC1 and MUC2. The ovarian-type
subepithelial stroma exhibited positive staining for vimentin, ER
and PgR (Fig. 5A and B). On the basis
of these findings, the tumor was diagnosed as a MCN with an
associated invasive carcinoma of the pancreas.
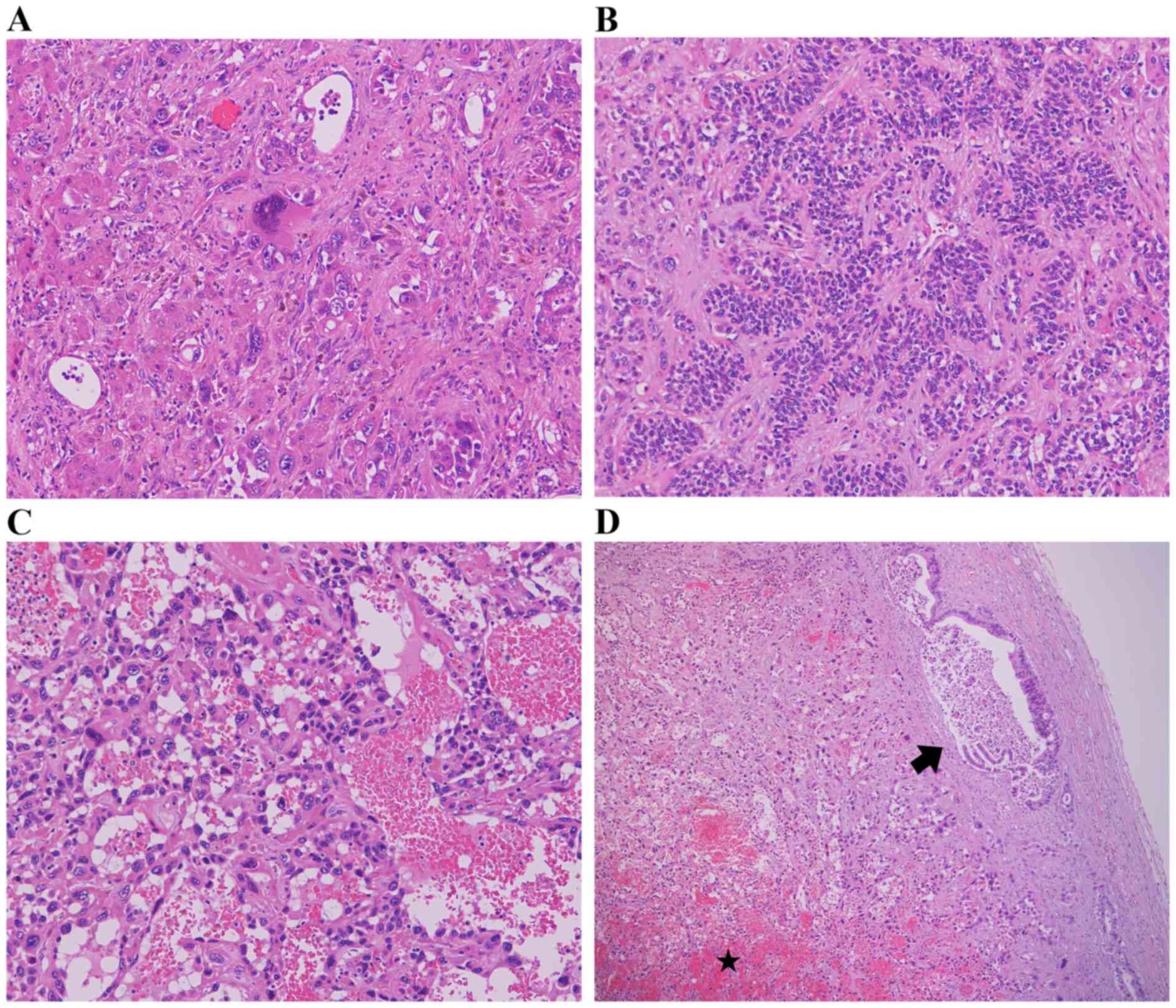
Lymph nodes of the hepatic portal region were
swollen and white/off-white masses (size, 2–3 mm) were identified
scattered in the lungs. Microscopically, all of the lymph nodes
exhibited moderately to poorly differentiated adenocarcinoma. By
contrast, the hepatic nodules consisted of pleomorphic cells,
represented by anaplastic giant cells, a trabecular growth pattern
and an angiosarcoma-like appearance, along with small
adenocarcinoma foci beneath the liver capsule and a massive
necrotic background (Fig. 6A-D). The
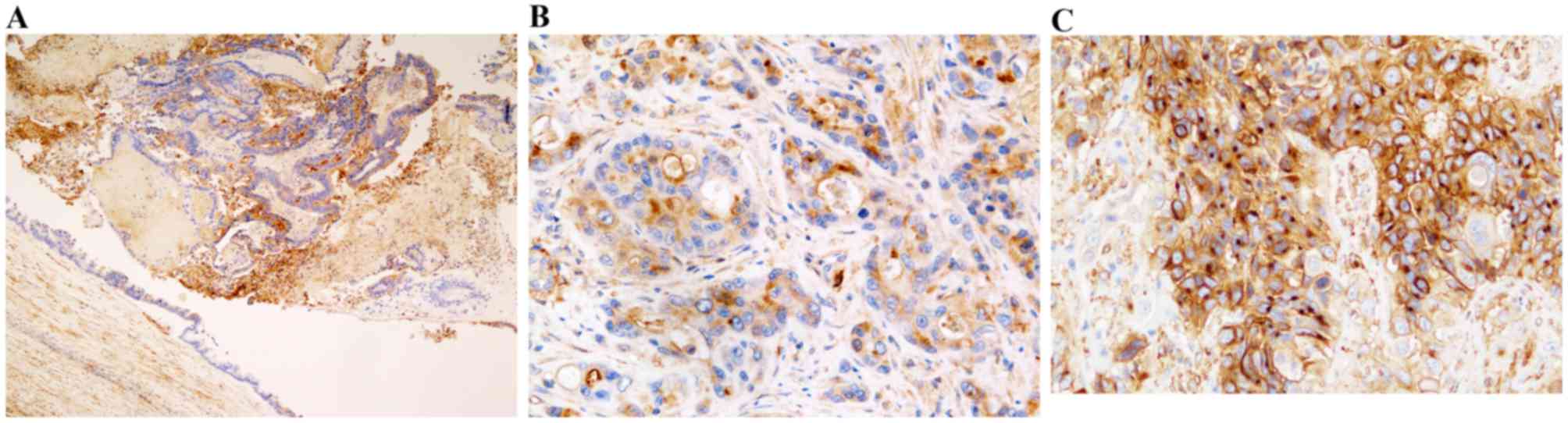
immunological profile of the adenocarcinoma was identical to that
of the pancreatic MCN. The angiosarcomatous area was positive for
cytokeratin (AE1/AE3) and vimentin. The pancreatic and hepatic
cancer cells were confirmed to be positive for immunostaining of
G-CSF (Fig. 7A-C).
Discussion
Following the purification of human G-CSF from tumor
cell lines (16) and its utilization
for immunoperoxidase staining of paraffin-embedded sections
(17), several G-CSF-secreting
pancreatic carcinomas have been confirmed using
immunohistochemistry (6). The
diagnostic criteria for G-CSF-producing tumors are as follows: i)
Extreme leukocytosis, ii) elevated G-CSF activity, iii) a decreased
white blood cell count following tumor resection, or iv) proof of
G-CSF production in the tumor (9).
The detection of G-CSF by immunostaining is difficult as the G-CSF
protein is generally retained in the cytoplasm for a short time and
the antigenicity is inconstant (18).
Although, the intensity of G-CSF immunostaining varied by location
in the case discussed in the present study, positive staining was
easily recognized in intraepithelial neoplastic cells, invaded
adenocarcinoma and liver metastatic cells. Thus, it was concluded
that present case is a rare, G-CSF-producing tumor.
Carcinomas that produce G-CSF are known to be highly
malignant tumors (8,10). G-CSF functions as not only a
hematopoietic growth factor but also as an autocrine growth factor
associated with the proliferation of tumor cells (19,20). G-CSF
released by tumor cells may bind to G-CSF receptors in the tumor
cells, triggering proliferation, invasion, migration via an
autocrine mechanism, and the transformation of the epithelial
elements into a more immature or high-grade phenotype (16,19).
Regardless of the therapeutic efforts, the prognosis of patients
G-CSF-producing pancreatic tumors is extremely poor, which is
supported by the fact that all referred cases, regardless of
surgical treatment or chemotherapy, succumbed to disease within 8
months of the initial consultation (12).
Histologically, anaplastic carcinoma (7,10,14,15),
poorly differentiated adenocarcinoma (8), and adenosquamous carcinoma (6,9) are the
dominant subtypes of G-CSF-producing pancreatic tumor. To the best
of our knowledge, an incidence of MCN with associated invasive
carcinoma of the pancreas has not been reported in the English or
Japanese literature. MCNs typically present as a single, spherical
mass with a smooth surface and a fibrous pseudocapsule of variable
thickness with occasional calcification (21). Judging from the scattered
calcification and scarce ovarian-type subepithelial stroma, the
case discussed in the present study is a long-term MCN, from which
malignant transformation of lining columnar cells and invasive
carcinoma may develop.
Metastatic cancer cells in the liver, the majority
of which were positive for G-CSF immunostaining, exhibited
pleomorphism. It is unclear why anaplastic change was observed only
in the liver. Liver biopsy was not performed in the initial period
of admission due to the presence of ascites and a risk of
hemorrhage. Although metastatic liver cancer was indicated by
radiological findings, arterial infusion of antibiotics was
conducted on the presumptive diagnosis of liver abscess. It is
arguable whether the antibiotic therapy contributed to the
alternation of cellular morphology. For example, the procedure
propagated the sinusoidal spread of carcinoma cells and formed the
angiosarcomatous vasoformative architecture. However, the tumor
cells were positive for cytokeratin and vimentin. As for anticancer
drugs, the majority of sarcomatoid changes in hepatocellular
carcinoma are associated with repeated chemotherapy or
transarterial chemoembolization (22). However, to the best of our knowledge,
the same transformation induced by antibiotics has not been
reported. It can therefore be concluded that anaplastic change was
associated with nature of the tumor rather than the procedure.
Unlike those with non-invasive MCNs, patients with
invasive MCNs are considered to have a poor prognosis (23), and also in the present case, the
invasive cancer led to mortality following an aggressive clinical
course. A case report alone may not be sufficient to draw a firm
conclusion. However, G-CSF-producing MCN with an associated
invasive carcinoma of the pancreas may be at type of pancreatic
cancer with a poor prognosis, particularly when poorly
differentiated carcinoma or sarcomatous changes are observed.
Glossary
Abbreviations
Abbreviations:
|
G-CSF
|
granulocyte colony-stimulating
factor
|
|
MCN
|
mucinous cystic neoplasm
|
|
PgR
|
progesterone receptor
|
|
ER
|
estrogen receptor
|
References
|
1
|
Lieschke GJ and Burgess AW: Granulocyte
colony-stimulating factor and granulocyte-macrophage
colony-stimulating factor (1). N Engl J Med. 327:28–35. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lieschke GJ and Burgess AW: Granulocyte
colony-stimulating factor and granulocyte-macrophage
colony-stimulating factor (2). N Engl J Med. 327:99–106. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Asano S, Urabe A, Okabe T, Sato N and
Kondo Y: Demonstration of granulopoietic factor(s) in the plasma of
nude mice transplanted with a human lung cancer and in the tumor
tissue. Blood. 49:845–852. 1977.PubMed/NCBI
|
|
4
|
Toyoda M, Chikamatsu K, Sakakura K, Fukuda
Y, Takahashi K, Miyashita M, Shimamura K and Furuya N: A case of
squamous cell carcinoma of the head and neck producing
granulocyte-colony stimulating factor with marked leukocytosis.
Auris Nasus Larynx. 34:267–271. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nakayama K, Takahashi T, Tsuyuoka R, Ueda
Y, Suzuki A, Okuno Y, Ihara Y, Seko S, Okada T, Kumagai N, et al:
Identification of colony-stimulating factor activity in patients
with malignant tumors associated with excessive leukocytosis. Jpn J
Clin Oncol. 21:395–399. 1991.PubMed/NCBI
|
|
6
|
Ohtsubo K, Mouri H, Sakai J, Akasofu M,
Yamaguchi Y, Watanabe H, Gabata T, Motoo Y, Okai T and Sawabu N:
Pancreatic cancer associated with granulocyte-colony stimulating
factor production confirmed by immunohistochemistry. J Clin
Gastroenterol. 27:357–360. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gotohda N, Nakagohri T, Saito N, Ono M,
Sugito M, Ito M, Inoue K, Oda T, Takahashi S and Kinoshita T: A
case of anaplastic ductal carcinoma of the pancreas with production
of granulocyte-colony stimulating factor. Hepatogastroenterology.
53:957–959. 2006.PubMed/NCBI
|
|
8
|
Takami K, Miura K, Takeuchi H, Egawa S,
Moriya T, Nakamura Y, Tanabe A, Sugita J, Karasawa H, Unno M and
Sasaki I: Granulocyte-colony stimulating factor-producing
pancreatic cancer: Report of a case. Surg Today. 38:453–457. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Joshita S, Nakazawa K, Sugiyama Y, Kamijo
A, Matsubayashi K, Miyabayashi H, Furuta K, Kitano K and Kawa S:
Granulocyte-colony stimulating factor-producing pancreatic
adenosquamous carcinoma showing aggressive clinical course. Intern
Med. 48:687–691. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kitade H, Yanagida H, Yamada M, Satoi S,
Yoshioka K, Shikata N and Kon M: Granulocyte-colony stimulating
factor producing anaplastic carcinoma of the pancreas treated by
distal pancreatectomy and chemotherapy: Report of a case. Surg Case
Rep. 1:462015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kosmahl M, Pauser U, Peters K, Sipos B,
Luttges J, Kremer B and Klöppel G: Cystic neoplasms of the pancreas
and tumor-like lesions with cystic features: A review of 418 cases
and a classification proposal. Virchows Arch. 445:168–178. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tanaka M, Chari S, Adsay V, Fernandez-del
Castillo C, Falconi M, Shimizu M, Yamaguchi K, Yamao K and Matsuno
S; International Association of Pancreatology, : International
consensus guidelines for management of intraductal papillary
mucinous neoplasms and mucinous cystic neoplasms of the pancreas.
Pancreatology. 6:17–32. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fukushima N and Fukayama M: Mucinous
cystic neoplasms of the pancreas: Pathology and molecular genetics.
J Hepatobiliary Pancreat Surg. 14:238–242. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Murata T, Terasaki M, Sakaguchi K, Okubo
M, Fukami Y, Nishimae K, Kitayama Y and Hoshi S: A case of
anaplastic carcinoma of the pancreas producing granulocyte-colony
stimulating factor. Clin J Gastroenterol. 2:109–114. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nakajima A, Takahashi H, Inamori M, Abe Y,
Kobayashi N, Kubota K and Yamanaka S: Anaplastic carcinoma of the
pancreas producing granulocyte-colony stimulating factor: A case
report. J Med Case Rep. 2:3912008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nomura H, Imazeki I, Oheda M, Kubota N,
Tamura M, Ono M, Ueyama Y and Asano S: Purification and
characterization of human granulocyte colony-stimulating factor
(G-CSF). EMBO J. 5:871–876. 1986.PubMed/NCBI
|
|
17
|
Shimamura K, Fujimoto J, Hata J, Akatsuka
A, Ueyama Y, Watanabe T and Tamaoki N: Establishment of specific
monoclonal antibodies against recombinant human granulocyte
colony-stimulating factor (hG-CSF) and their application for
immunoperoxidase staining of paraffin-embedded sections. J
Histochem Cytochem. 38:283–286. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Takenaka M, Akiba J, Kawaguchi T, Niizeki
T, Arinaga-Hino T, Sata M, Nakashima O, Yano H and Kage M:
Intrahepatic cholangiocarcinoma with sarcomatous change producing
granulocyte-colony stimulating factor. Pathol Int. 63:233–235.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mueller MM, Herold-Mende CC, Riede D,
Lange M, Steiner HH and Fusenig NE: Autocrine growth regulation by
granulocyte colony-stimulating factor and granulocyte macrophage
colony-stimulating factor in human gliomas with tumor progression.
Am J Pathol. 155:1557–1567. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tachibana M, Miyakawa A, Tazaki H,
Nakamura K, Kubo A, Hata J, Nishi T and Amano Y: Autocrine growth
of transitional cell carcinoma of the bladder induced by
granulocyte-colony stimulating factor. Cancer Res. 55:3438–3443.
1995.PubMed/NCBI
|
|
21
|
Zamboni G, Fukushima N, Hruban RH and
Kloppel G: Mucinous cystic neoplasm of the pancreasWorld health
organization classification of tumors. WHO classification of tumor
of the digestive system. Bosman FT, Carneiro F, Hruban RH and
Theise ND: IARC Press; Lyon: pp. 300–303. 2010
|
|
22
|
Kojiro M, Sugihara S, Kakizoe S, Nakashima
O and Kiyomatsu K: Hepatocellular carcinoma with sarcomatous
change: A special reference to the relationship with anticancer
therapy. Cancer Chemother Pharmacol. 23 Suppl:S4–S8. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wilentz RE, Albores-Saavedra J, Zahurak M,
Talamini MA, Yeo CJ, Cameron JL and Hruban RH: Pathologic
examination accurately predicts prognosis in mucinous cystic
neoplasms of the pancreas. Am J Surg Pathol. 23:1320–1327. 1999.
View Article : Google Scholar : PubMed/NCBI
|